Abstract
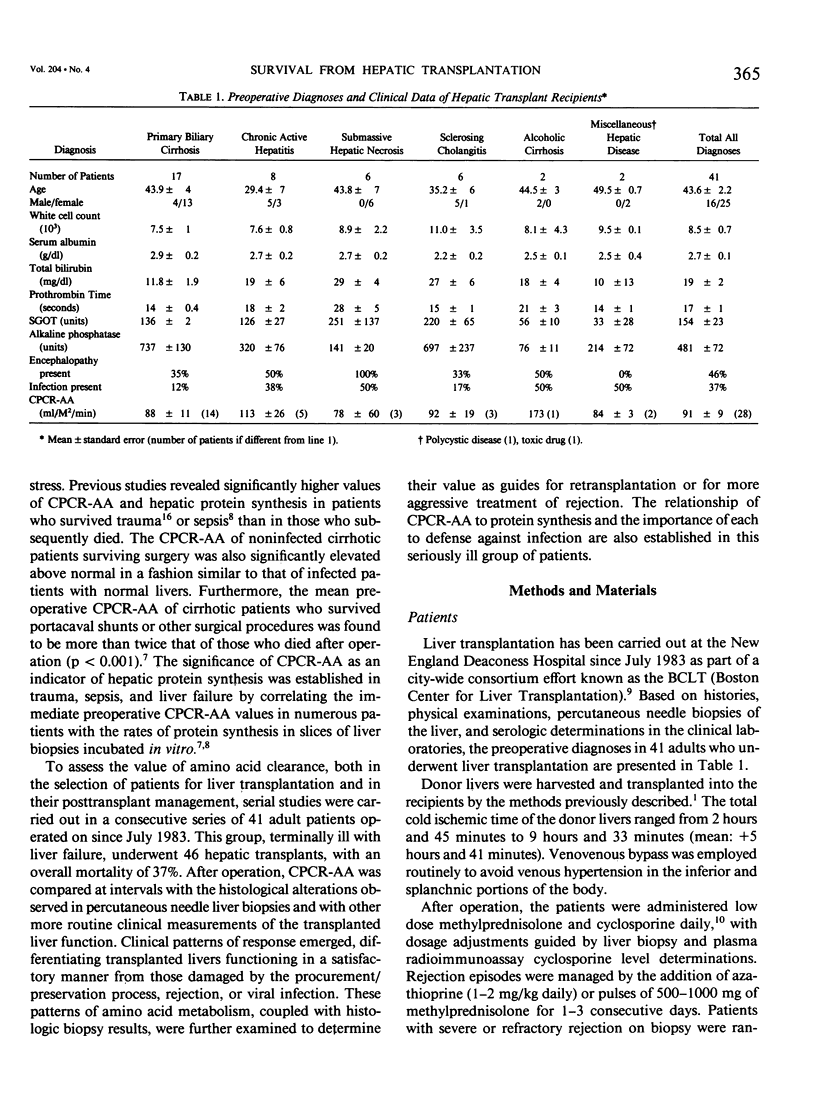
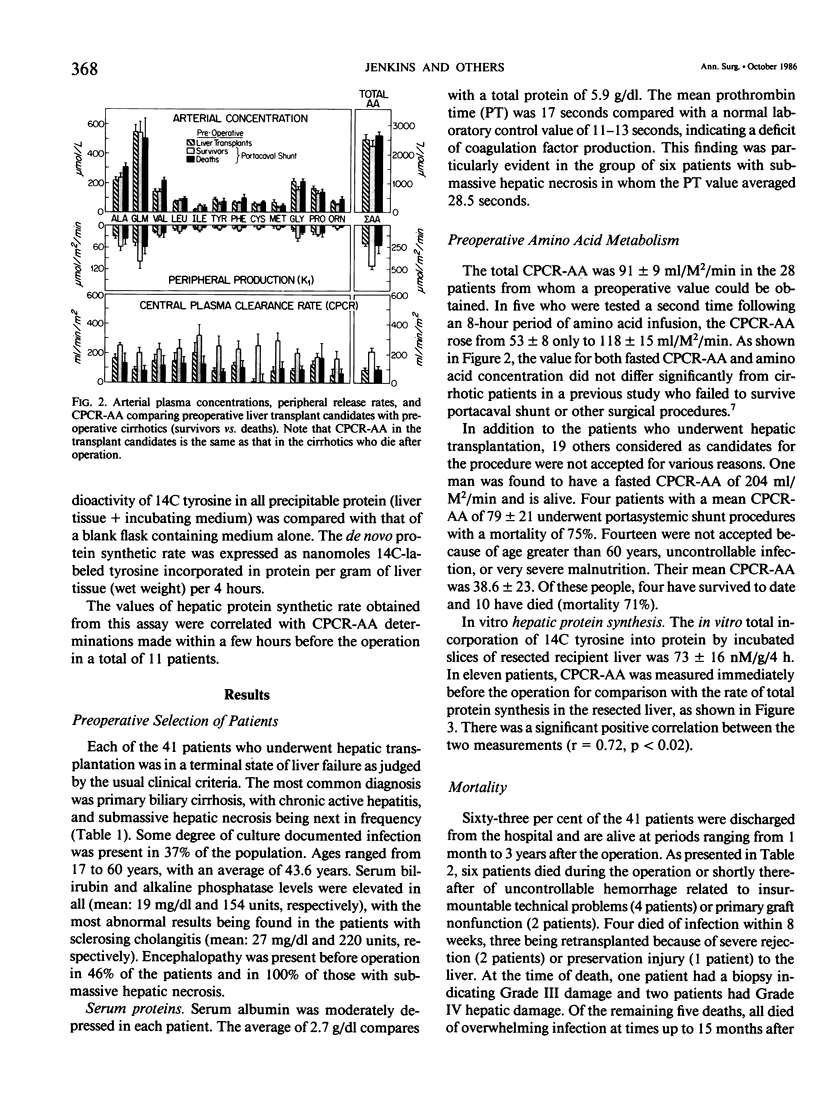
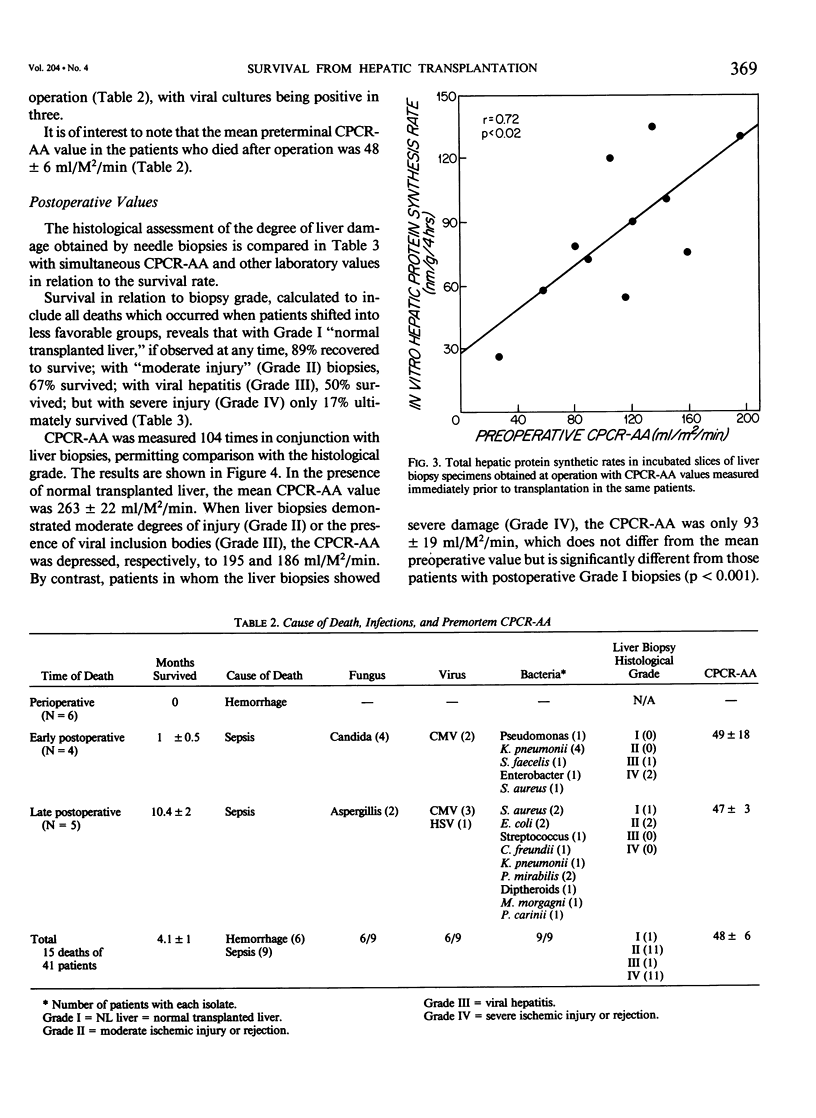
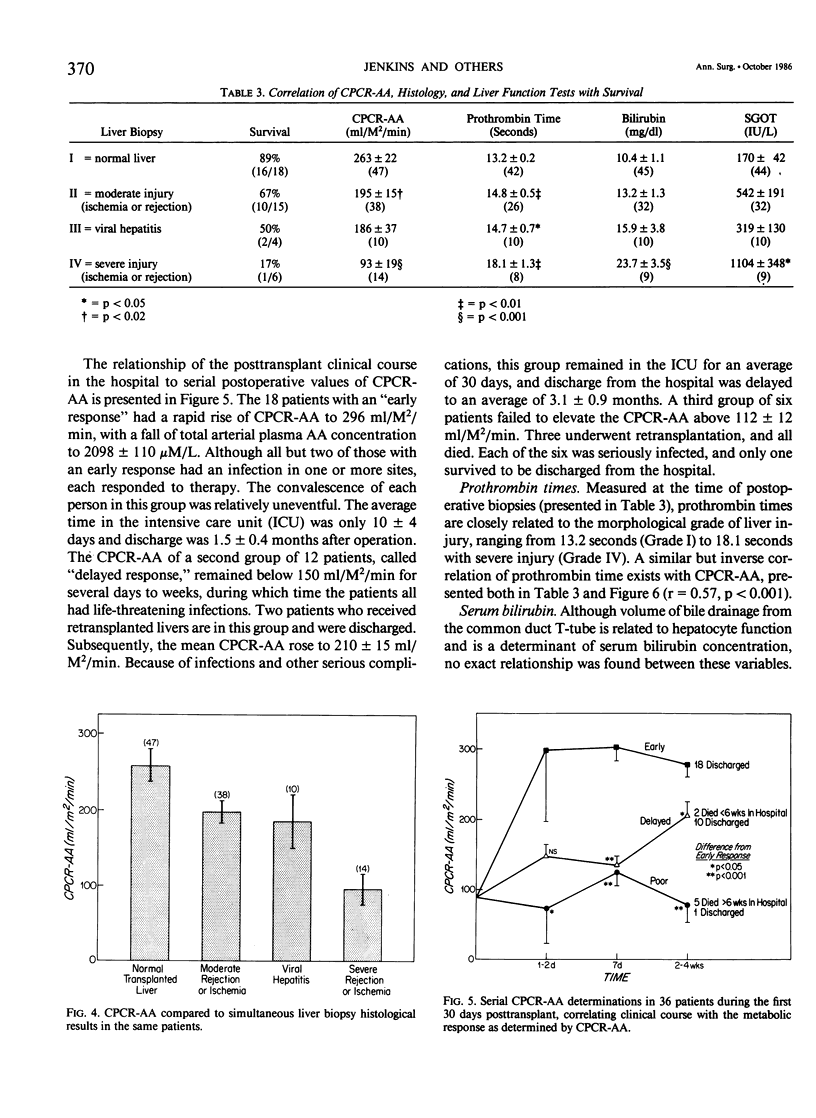
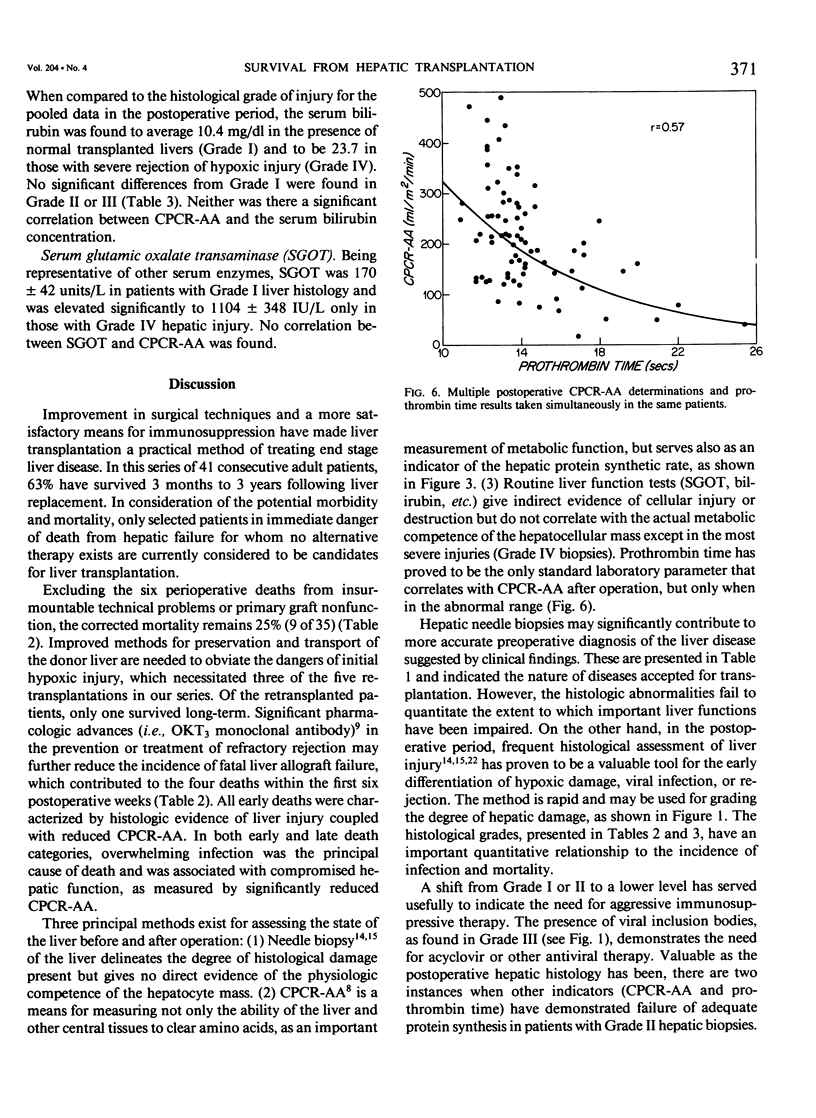
Forty-one patients, all in end stage hepatic failure, underwent 46 liver transplantations with a long-term survival rate of 63%. Six patients died of uncontrollable bleeding due to primary graft malfunction at or immediately after operation. Nine died early or late with overwhelming infection. In addition to clinical assessment, needle liver biopsy, central plasma clearance rate of amino acids (CPCR-AA), and routine "liver function tests" were employed to aid in selection of patients for transplantation and for guidance in postoperative management. Although liver biopsies usually afforded an exact diagnosis, neither they nor the routine liver function tests quantitated the extent to which hepatocyte function was impaired. CPCR-AA, which measures the rate of amino acid uptake by the liver and other central tissues for oxidation, gluconeogenesis, and protein synthesis was 91 +/- 9 ml/M2/min in the preoperative transplant group. This compares with a value of 97 +/- 16 in a previously studied series of cirrhotics who died following other forms of surgery and a CPCR-AA of 220 +/- 26 ml/m2/min in those who survived. In addition, the preoperative CPCR-AA was found to correlate with the in vitro hepatic protein synthetic rate of slices from the resected recipient liver (r = 0.72, p less than 0.02). After operation, serial hepatic needle biopsies were classified by histology into four grades of injury, ranging from normal liver transplant (Grade I) to mild hypoxic or rejection injury (Grade II), viral hepatitis (Grade III), and severe hypoxic or rejection injury (Grade IV). Significant relationships of the histological grades to ultimate mortality, CPCR-AA, and prothrombin times were found. CPCR-AA and prothrombin time correlate inversely (r = 0.57, p less than 0.001), further demonstrating the relationship of CPCR-AA to protein synthesis of clotting factors. These patterns of posttransplant response were delineated by serial CPCR-AA values. "Early" responders had values over 290 ml/M2/min and all survived. Twelve patients with delayed response were characterized by values of 150 +/- 12, rising to over 200 ml/M2/min after 2 weeks. Two who failed to increase CPCR-AA died. In six "poor" responders, CPCR-AA with Grade IV injury remained below 110 ml/M2/min. All died except for one whose CPCR-AA subsequently rose following retransplantation. It is concluded that percutaneous hepatic needle biopsies and CPCR-AA measurements in combination are of proven value, not only in understanding the nature of injury and functional impairment of the liver, but are also important as guides to selection of patients and for their posttransplant management.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessey P. Q., Watters J. M., Aoki T. T., Wilmore D. W. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984 Sep;200(3):264–281. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y., Rolles K., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Henderson R. G., Aziz S. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979 Nov 17;2(8151):1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Hirsch E., George B. C., Bigatello L. M., Mazuski J. E., Villee C. A., Jr Survival from sepsis. The significance of altered protein metabolism regulated by proteolysis inducing factor, the circulating cleavage product of interleukin-1. Ann Surg. 1985 Oct;202(4):446–458. doi: 10.1097/00000658-198510000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes G. H., Jr, McDermott W. V., Williams L. F., Loda M., Menzoian J. O., Pearl R. Amino acid clearance and prognosis in surgical patients with cirrhosis. Surgery. 1984 Oct;96(4):675–685. [PubMed] [Google Scholar]
- Clowes G. H., Jr, Randall H. T., Cha C. J. Amino acid and energy metabolism in septic and traumatized patients. JPEN J Parenter Enteral Nutr. 1980 Mar-Apr;4(2):195–205. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D. P. The disturbance of metabolism produced by bony and non-bony injury, with notes on certain abnormal conditions of bone. Biochem J. 1930;24(4):1244–1263. doi: 10.1042/bj0241244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath J. J., Ascher N. L., Konstantinides F. N., Bloomer J., Sharp H., Najarian J. S., Cerra F. B. Metabolism during hepatic transplantation: indicators of allograft function. Surgery. 1984 Oct;96(4):664–674. [PubMed] [Google Scholar]
- Flechner S. M., Lorber M., Van Buren C., Kerman R., Kahan B. D. The case against conversion to azathioprine in cyclosporine-treated renal recipients. Transplant Proc. 1985 Aug;17(4 Suppl 1):276–281. [PubMed] [Google Scholar]
- Hubscher S. G., Clements D., Elias E., McMaster P. Biopsy findings in cases of rejection of liver allograft. J Clin Pathol. 1985 Dec;38(12):1366–1373. doi: 10.1136/jcp.38.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. L., Benotti P. N., Bothe A. A., Rossi R. L. Liver transplantation. Surg Clin North Am. 1985 Feb;65(1):103–122. doi: 10.1016/s0039-6109(16)43535-8. [DOI] [PubMed] [Google Scholar]
- Jenkins R. L. The Boston Center for Liver Transplantation (BCLT). Initial experience of a new surgical consortium. Arch Surg. 1986 Apr;121(4):424–430. doi: 10.1001/archsurg.1986.01400040060009. [DOI] [PubMed] [Google Scholar]
- Loda M., Clowes G. H., Jr, Dinarello C. A., George B. C., Lane B., Richardson W. Induction of hepatic protein synthesis by a peptide in blood plasma of patients with sepsis and trauma. Surgery. 1984 Aug;96(2):204–213. [PubMed] [Google Scholar]
- Loda M., Clowes G. H., Jr, Nespoli A., Bigatello L., Birkett D. H., Menzoian J. O. Encephalopathy, oxygen consumption, visceral amino acid clearance, and mortality in cirrhotic surgical patients. Am J Surg. 1984 Apr;147(4):542–550. doi: 10.1016/0002-9610(84)90019-9. [DOI] [PubMed] [Google Scholar]
- McMenamy R. H., Birkhahn R., Oswald G., Reed R., Rumph C., Vaidyanath N., Yu L., Cerra F. B., Sorkness R., Border J. R. Multiple systems organ failure: I. The basal state. J Trauma. 1981 Feb;21(2):99–114. doi: 10.1097/00005373-198102000-00003. [DOI] [PubMed] [Google Scholar]
- Pearl R. H., Clowes G. H., Jr, Hirsch E. F., Loda M., Grindlinger G. A., Wolfort S. Prognosis and survival as determined by visceral amino acid clearance in severe trauma. J Trauma. 1985 Aug;25(8):777–783. doi: 10.1097/00005373-198508000-00008. [DOI] [PubMed] [Google Scholar]
- Pittiruti M., Siegel J. H., Sganga G., Coleman B., Wiles C. E., 3rd, Belzberg H., Wedel S., Placko R. Increased dependence of leucine in posttraumatic sepsis: leucine/tyrosine clearance ratio as an indicator of hepatic impairment in septic multiple organ failure syndrome. Surgery. 1985 Sep;98(3):378–387. [PubMed] [Google Scholar]
- Shaw B. W., Jr, Martin D. J., Marquez J. M., Kang Y. G., Bugbee A. C., Jr, Iwatsuki S., Griffith B. P., Hardesty R. L., Bahnson H. T., Starzl T. E. Venous bypass in clinical liver transplantation. Ann Surg. 1984 Oct;200(4):524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snover D. C., Sibley R. K., Freese D. K., Sharp H. L., Bloomer J. R., Najarian J. S., Ascher N. L. Orthotopic liver transplantation: a pathological study of 63 serial liver biopsies from 17 patients with special reference to the diagnostic features and natural history of rejection. Hepatology. 1984 Nov-Dec;4(6):1212–1222. doi: 10.1002/hep.1840040620. [DOI] [PubMed] [Google Scholar]
- Stakeberg H., Gustafson A., Scherstén T. Incorporation rate of leucine into proteins in human liver slices. Eur J Clin Invest. 1974 Dec 5;4(6):393–398. doi: 10.1111/j.1365-2362.1974.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Iwatsuki S., Van Thiel D. H., Gartner J. C., Zitelli B. J., Malatack J. J., Schade R. R., Shaw B. W., Jr, Hakala T. R., Rosenthal J. T. Evolution of liver transplantation. Hepatology. 1982 Sep-Oct;2(5):614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Porter K. A., Iwatsuki S., Schröter G. P. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981 Jul 30;305(5):266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W., Peters T. G., Vera S. R., Britt L. G., van Voorst S. J., Haggitt R. C. Biopsy-directed immunosuppression following hepatic transplantation in man. Transplantation. 1985 Jun;39(6):589–596. doi: 10.1097/00007890-198506000-00003. [DOI] [PubMed] [Google Scholar]



