Abstract
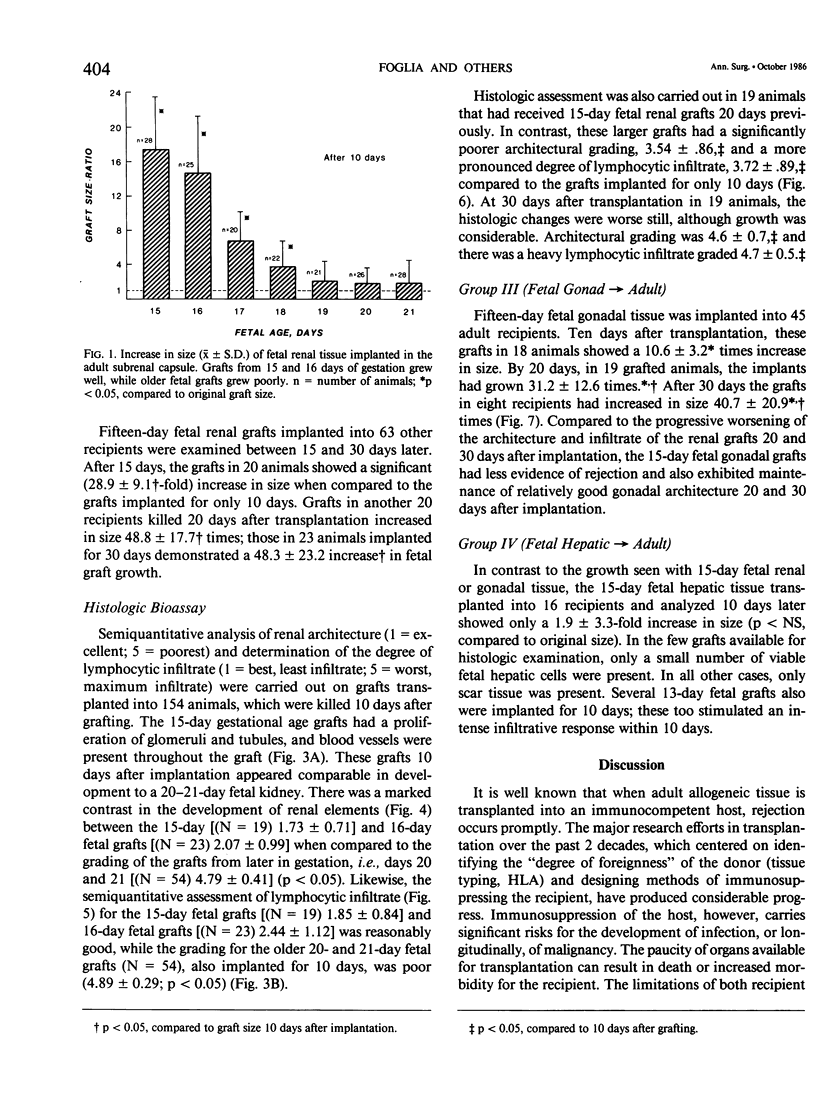
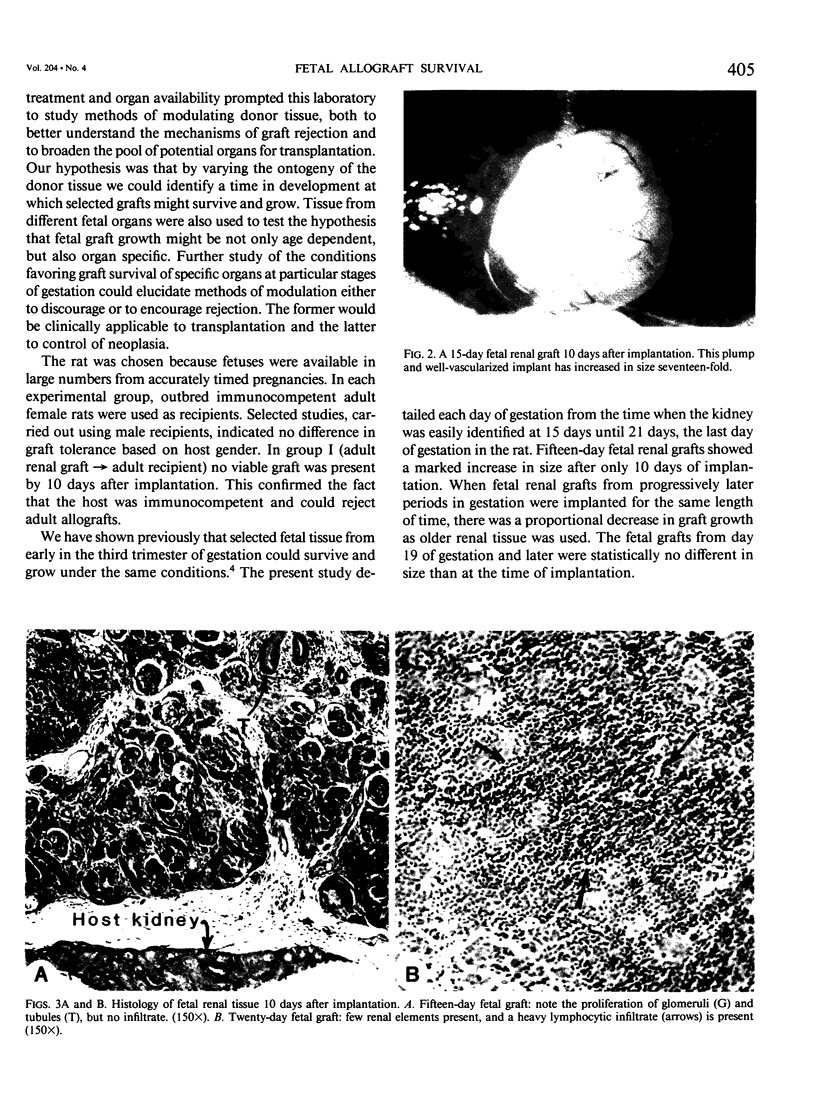
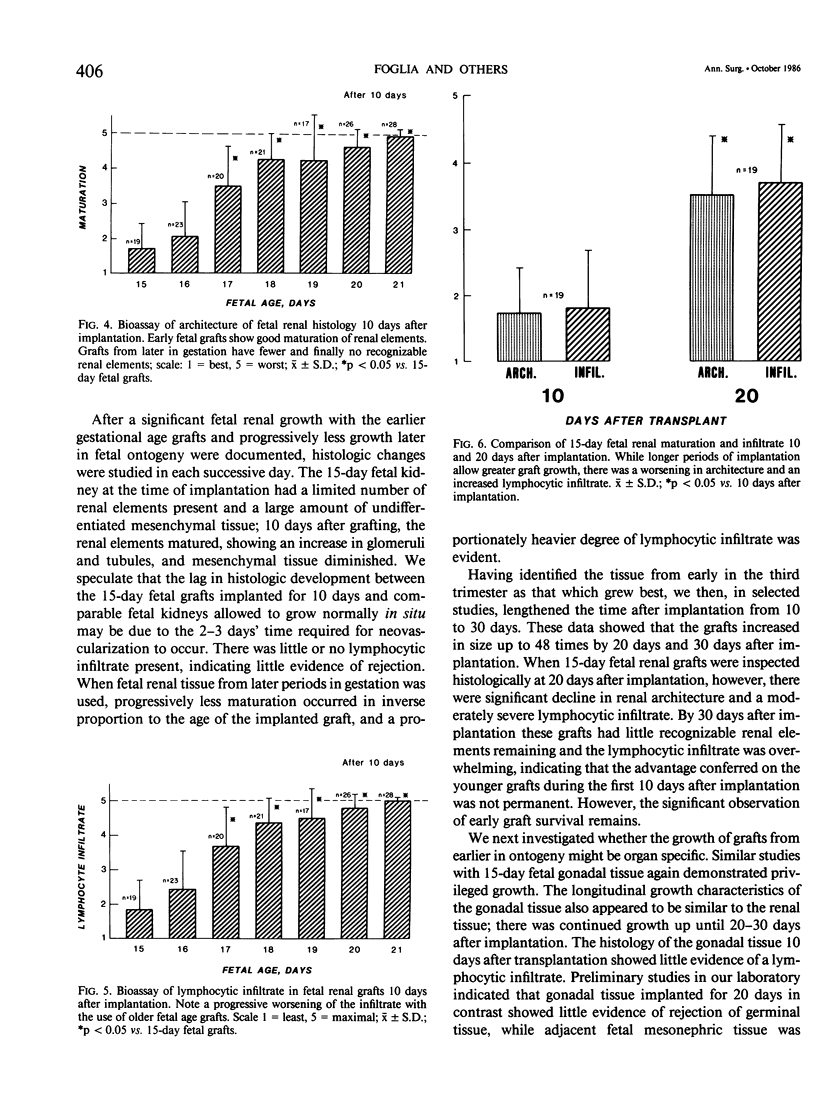
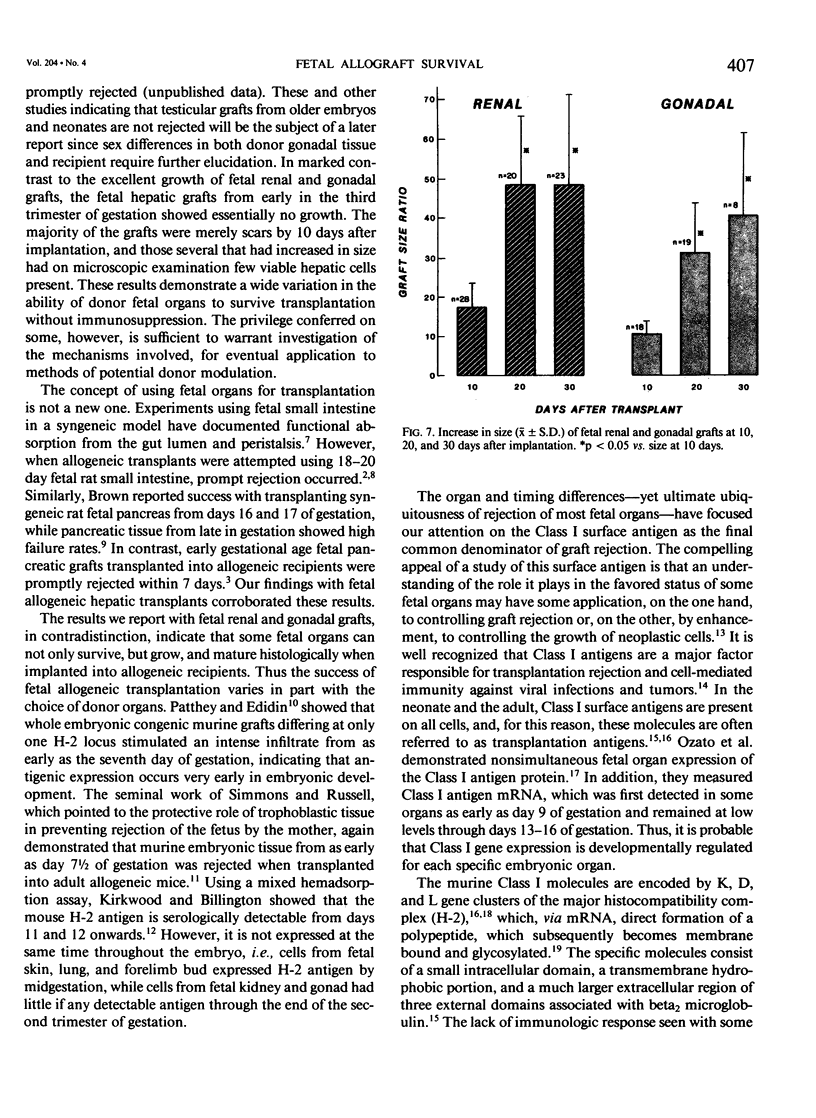
This study explores whether fetal allograft survival is age dependent and organ specific. Fetal rat tissue (renal, gonadal, hepatic) from the third trimester of gestation (days 15-21) was transplanted into 306 outbred adult rats for 10-30 days. Grafts were studied by morphometric and histologic analysis. Ten days after implantation, renal tissue (N = 75) from late gestation (days 19-21) showed no increase in size. In contrast, 17-day fetal grafts (N = 20) grew 6.8 +/- 3.4 times,* while 15-day fetal grafts (N = 28) grew 17.5 +/- 6.1* times. (The symbol "*" indicates p less than 0.05, compared to original size). Twenty days after implantation, these 15-day fetal grafts (N = 20) grew 48.8 +/- 17.7* times. Ten days after grafting, the younger fetal tissue showed excellent maturation of renal elements and no sign of rejection; older fetal grafts had poor renal architecture and a dense lymphocytic infiltrate. The 15-day fetal gonadal tissue (N = 18) showed a moderate 10.6 +/- 3.2* increase in size while the 15-day hepatic grafts (N = 16) were regularly rejected within 10 days. Selected fetal allografts from early in the third trimester can not only survive but can grow and mature in an immunocompetent recipient. This fetal graft growth appears to be both age dependent and organ specific. The use of fetal organs may broaden the potential pool for transplantation. However, further studies are needed to define the ontogeny of graft acceptance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Narayan P., DeWolf W. C. Major histocompatibility antigens are not detectable on post-meiotic human testicular germ cells. J Immunol. 1984 Oct;133(4):1962–1965. [PubMed] [Google Scholar]
- BURNET F. M. The new approach to immunology. N Engl J Med. 1961 Jan 5;264:24–34. doi: 10.1056/NEJM196101052640107. [DOI] [PubMed] [Google Scholar]
- Bogden A. E., Cobb W. R., Lepage D. J., Haskell P. M., Gulkin T. A., Ward A., Kelton D. E., Esber H. J. Chemotherapy responsiveness of human tumors as first transplant generation xenografts in the normal mouse: six-day subrenal capsule assay. Cancer. 1981 Jul 1;48(1):10–20. doi: 10.1002/1097-0142(19810701)48:1<10::aid-cncr2820480105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Brown J., Molnar I. G., Clark W., Mullen Y. Control of experimental diabetes mellitus in rats by transplantation of fetal pancreases. Science. 1974 Jun 28;184(4144):1377–1379. doi: 10.1126/science.184.4144.1377. [DOI] [PubMed] [Google Scholar]
- Deutsch A. A., Arensman R., Levey R., Folkman J. The effect of antilymphocyte serum on fetal rat intestine transplanted as free subcutaneous homografts. J Pediatr Surg. 1974 Feb;9(1):29–34. doi: 10.1016/0022-3468(74)90006-2. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Donahoe P. K., Krane I., Bogdén A. E., Kamagata S., Budzik G. P. Subrenal capsule assay to test the viability of parenterally delivered müllerian inhibiting substance. J Pediatr Surg. 1984 Dec;19(6):863–869. doi: 10.1016/s0022-3468(84)80386-3. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Huber B., Wake C., Widera G. Organization and expression of the MHC of the C57 black/10 mouse. Immunol Rev. 1985 Jul;84:29–50. doi: 10.1111/j.1600-065x.1985.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Foglia R. P., LaQuaglia M., DiPreta J., Donahoe P. K. Can fetal and newborn allografts survive in an immunocompetent host? J Pediatr Surg. 1986 Jul;21(7):608–612. doi: 10.1016/s0022-3468(86)80415-8. [DOI] [PubMed] [Google Scholar]
- Gutmann D. H., Niederhuber J. E. Major histocompatibility complex regulation of the immune response. J Surg Res. 1985 Aug;39(2):172–181. doi: 10.1016/0022-4804(85)90175-1. [DOI] [PubMed] [Google Scholar]
- Guttman F. M., Nguyen L. T., Laberge J. M., Nguyen N. V., Seemayer T. A., Gibbons L. Fetal rat intestinal transplantation: cryopreservation and cyclosporin A. J Pediatr Surg. 1985 Dec;20(6):747–753. doi: 10.1016/s0022-3468(85)80038-5. [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Wood G. W. Interferon-gamma induces class I HLA and beta 2-microglobulin expression by human amnion cells. J Immunol. 1986 Jan;136(2):364–367. [PubMed] [Google Scholar]
- Kawata M., Parnes J. R., Herzenberg L. A. Transcriptional control of HLA-A,B,C antigen in human placental cytotrophoblast isolated using trophoblast- and HLA-specific monoclonal antibodies and the fluorescence-activated cell sorter. J Exp Med. 1984 Sep 1;160(3):633–651. doi: 10.1084/jem.160.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood K. J., Billington W. D. Expression of serologically detectable H-2 antigens on mid-gestation mouse embryonic tissues. J Embryol Exp Morphol. 1981 Feb;61:207–219. [PubMed] [Google Scholar]
- Lau H., Reemtsma K., Hardy M. A. Prolongation of rat islet allograft survival by direct ultraviolet irradiation of the graft. Science. 1984 Feb 10;223(4636):607–609. doi: 10.1126/science.6420888. [DOI] [PubMed] [Google Scholar]
- Leapman S. B., Deutsch A. A., Grand R. J., Folkman J. Transplantation of fetal intestine: survival and function in a subcutaneous location in adult animals. Ann Surg. 1974 Jan;179(1):109–114. doi: 10.1097/00000658-197401000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O. Regulation of the immune response by the major histocompatibility system. N Engl J Med. 1980 Dec 25;303(26):1514–1517. doi: 10.1056/NEJM198012253032606. [DOI] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey H., Edidin M. Evidence for the time of appearance of H-2 antigens in mouse development. Transplantation. 1973 Feb;15(2):211–214. doi: 10.1097/00007890-197302000-00004. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- SIMMONS R. L., RUSSELL P. S. The immunologic problem of pregnancy. Am J Obstet Gynecol. 1963 Mar 1;85:583–593. doi: 10.1016/0002-9378(63)90305-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Sutherland J., Mannoni P., Rosa F., Huyat D., Turner A. R., Fellous M. Induction of the expression of HLA class I antigens on K562 by interferons and sodium butyrate. Hum Immunol. 1985 Feb;12(2):65–73. doi: 10.1016/0198-8859(85)90344-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- UCLA Conference. Pancreas transplantation for diabetes mellitus. Ann Intern Med. 1978 Dec;89(6):951–965. doi: 10.7326/0003-4819-89-6-951. [DOI] [PubMed] [Google Scholar]




