Abstract
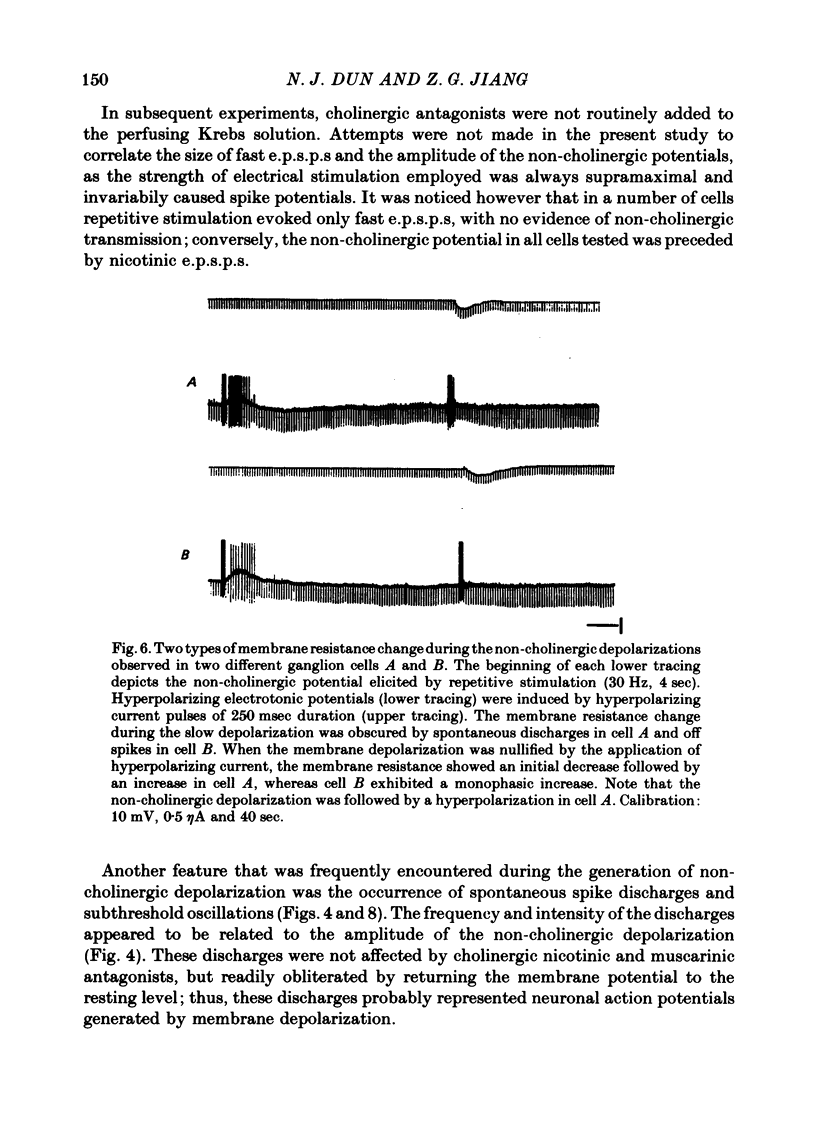
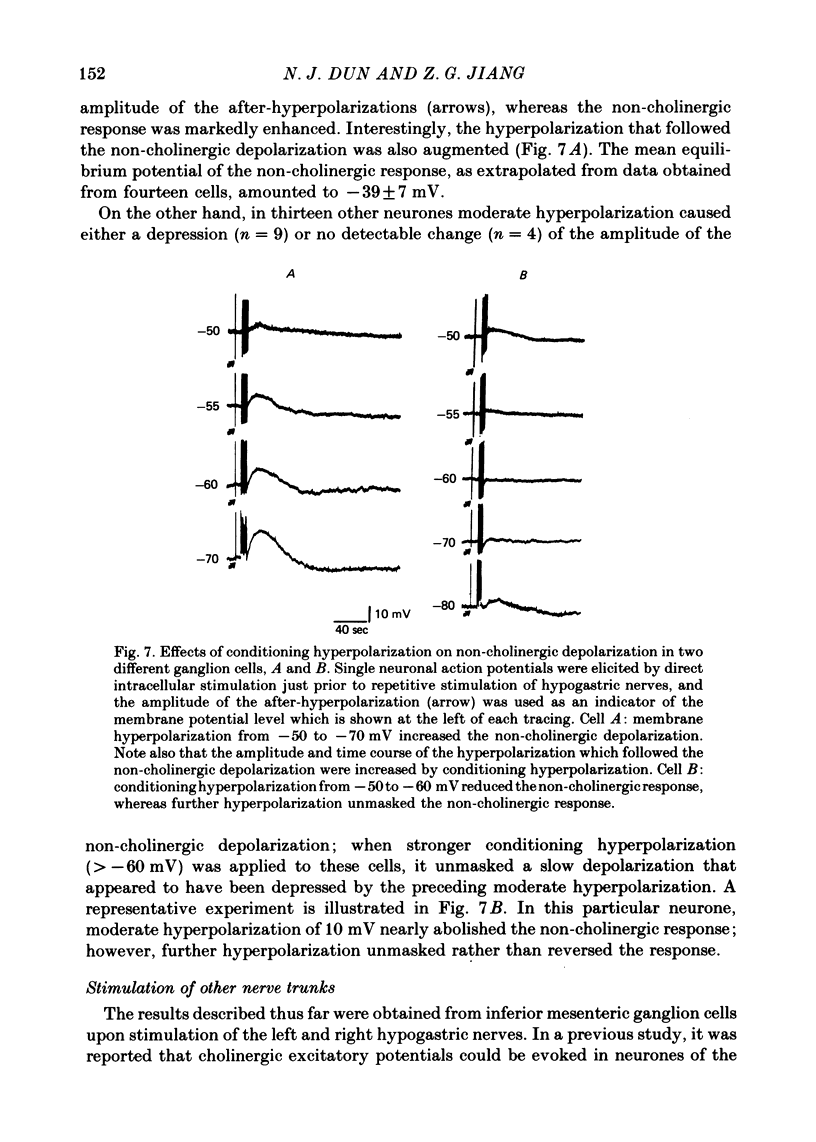
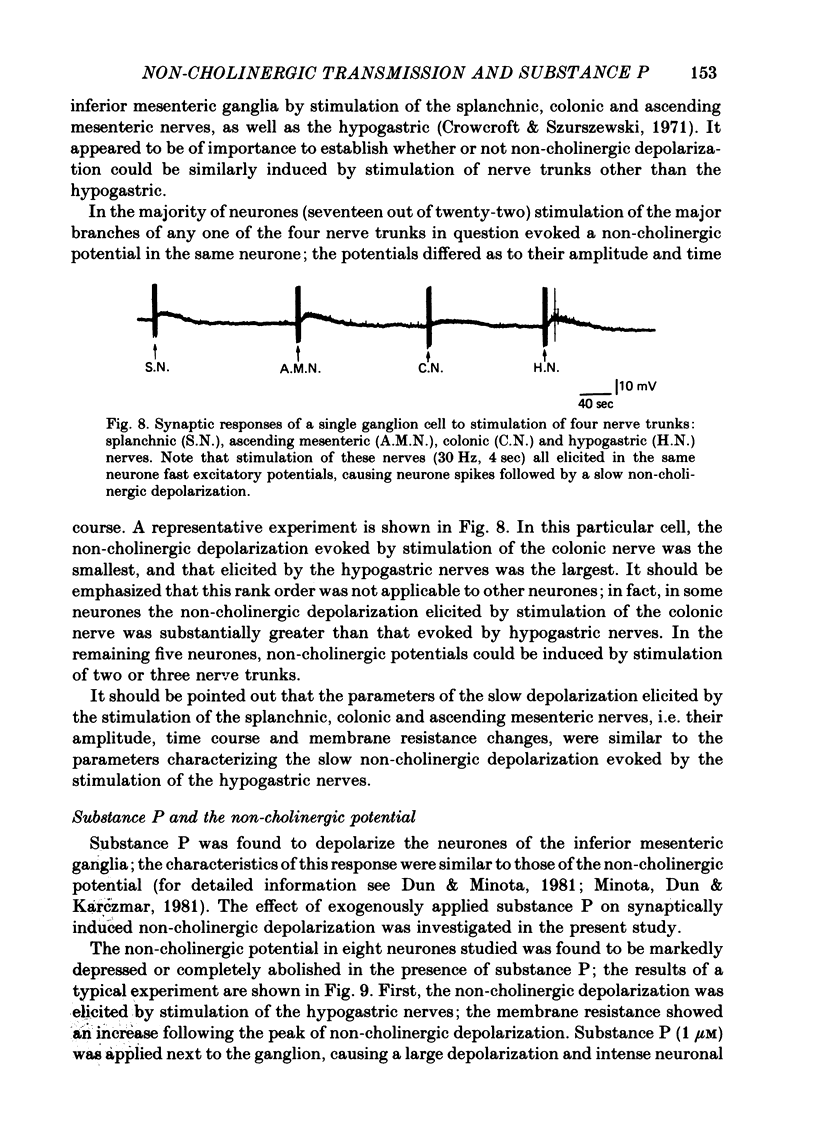
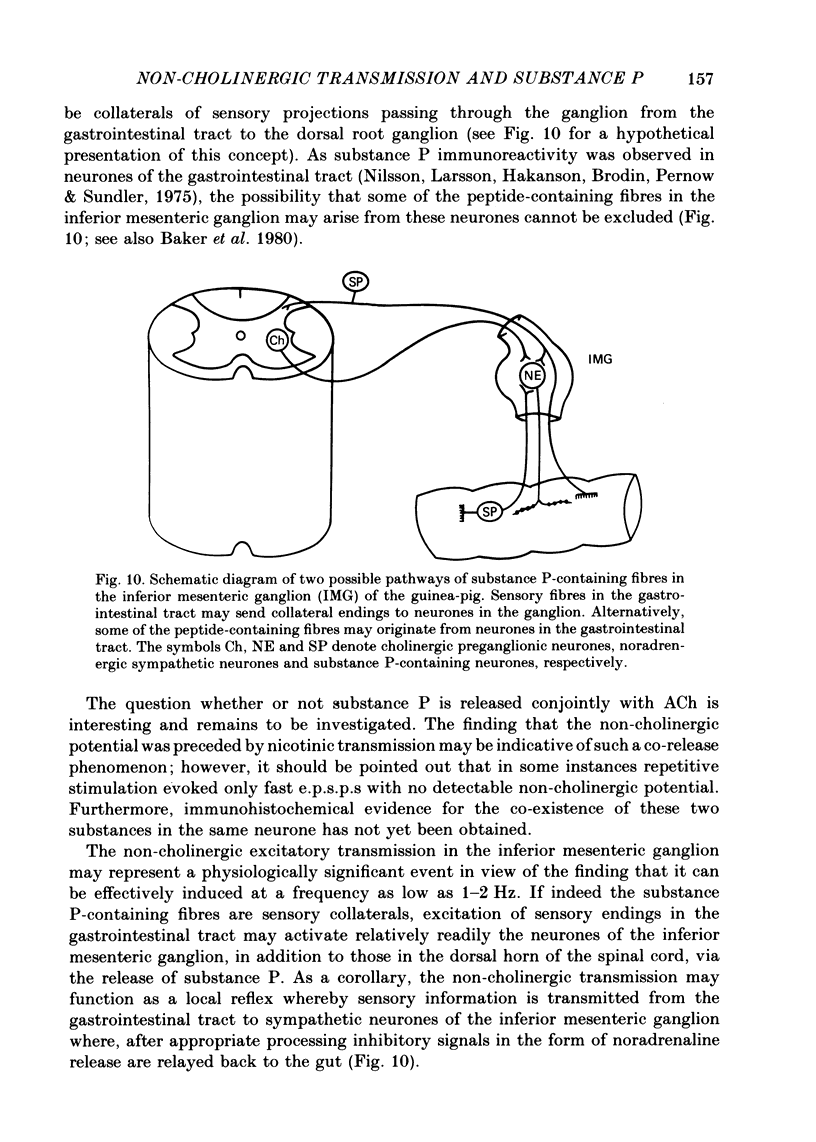
1. Repetitive stimulation of guinea-pig hypogastric nerves elicited, in addition to the fast cholinergic excitatory potential, a slow depolarization lasting for seconds to minutes in neurones of the isolated inferior mesenteric ganglion. 2. The slow depolarization which could be elicited at a frequency as low as 1-2 Hz for several seconds was not blocked by cholinergic antagonists, but was eliminated in a low Ca2+ solution; it was termed henceforth the non-cholinergic excitatory potential. 3. When the membrane potential was manually clamped, the non-cholinergic potential was associated with three types of membrane resistance change: an increase, a delayed increase and a biphasic change consisting of an initial decrease followed by an increase. 4. In the majority of neurones, conditioning hyperpolarization augmented the non-cholinergic depolarization; in a few neurones, moderate hyperpolarization depressed the latter, whereas stronger hyperpolarization unmasked a low depolarization. 5. The non-cholinergic response was markedly attenuated in the presence of exogenously applied substance P; it was partially suppressed by luteinizing hormone-releasing hormone. 6. Non-cholinergic depolarization could be elicited in the same neurone by stimulation of all four nerve trunks associated with the ganglion. 7. It is suggested that substance P, a peptide, may be the transmitter responsible for the generation of the non-cholinergic potential and that it may be released from collateral endings of primary sensory neurones, thus providing a functional connexion between sensory and autonomic neurones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980 Mar;68(3):353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Marsh S. Angiotensin mimics the action of muscarinic agonists on rat sympathetic neurones. Brain Res. 1980 Jul 14;193(2):614–619. doi: 10.1016/0006-8993(80)90200-0. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Actions of substance P on sympathetic neurons. Neuropharmacology. 1979 Feb;18(2):215–218. doi: 10.1016/0028-3908(79)90064-9. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N., Nishi S. Effects of dopamine on the superior cervical ganglion of the rabbit. J Physiol. 1974 May;239(1):155–164. doi: 10.1113/jphysiol.1974.sp010560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfvin L. G., Dalsgaard C. J. Retrograde axonal transport of horseradish perioxidase in afferent fibers of the inferior mesenteric ganglion of the guinea pig. Identification of the cells of origin in dorsal root ganglia. Brain Res. 1977 Apr 22;126(1):149–153. doi: 10.1016/0006-8993(77)90221-9. [DOI] [PubMed] [Google Scholar]
- Gamse R., Wax A., Zigmond R. E., Leeman S. E. Immunoreactive substance P in sympathetic ganglia: distribution and sensitivity towards capsaicin. Neuroscience. 1981;6(3):437–441. doi: 10.1016/0306-4522(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Evidence that substance P does not mediate slow synaptic excitation within the myenteric plexus. Nature. 1979 Jun 21;279(5715):720–721. doi: 10.1038/279720a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Schultzberg M., Goldstein M., Nilsson G. On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res. 1977 Aug 19;132(1):29–41. doi: 10.1016/0006-8993(77)90704-1. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Ionic mechanism of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Brain Res. 1974 Dec 6;81(2):338–342. doi: 10.1016/0006-8993(74)90949-4. [DOI] [PubMed] [Google Scholar]
- Minota S., Dun N. J., Karczmar A. G. Substance P-induced depolarization in sympathetic neurons: not simple K-inactivation. Brain Res. 1981 Jul 6;216(1):224–228. doi: 10.1016/0006-8993(81)91294-4. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Katayama Y. Evidence that substance P is a neurotransmitter in the myenteric plexus. Nature. 1980 Sep 11;287(5778):151–152. doi: 10.1038/287151a0. [DOI] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Larsson L. I., Håkanson R., Brodin E., Pernow B., Sundler F. Localization of substance P-like immunoreactivity in mouse gut. Histochemistry. 1975;43(1):97–99. doi: 10.1007/BF00490158. [DOI] [PubMed] [Google Scholar]


