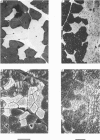
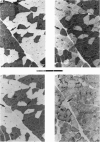
Abstract
1. Cat soleus (slow twitch) was cross-reinnervated with nerve to flexor hallucis or digitorum longus muscle (fast twitch). More than 3 years later motor unit isometric contractions and muscle immunohistochemistry and histochemistry were investigated. All muscles differed from normal fast or slow muscle.
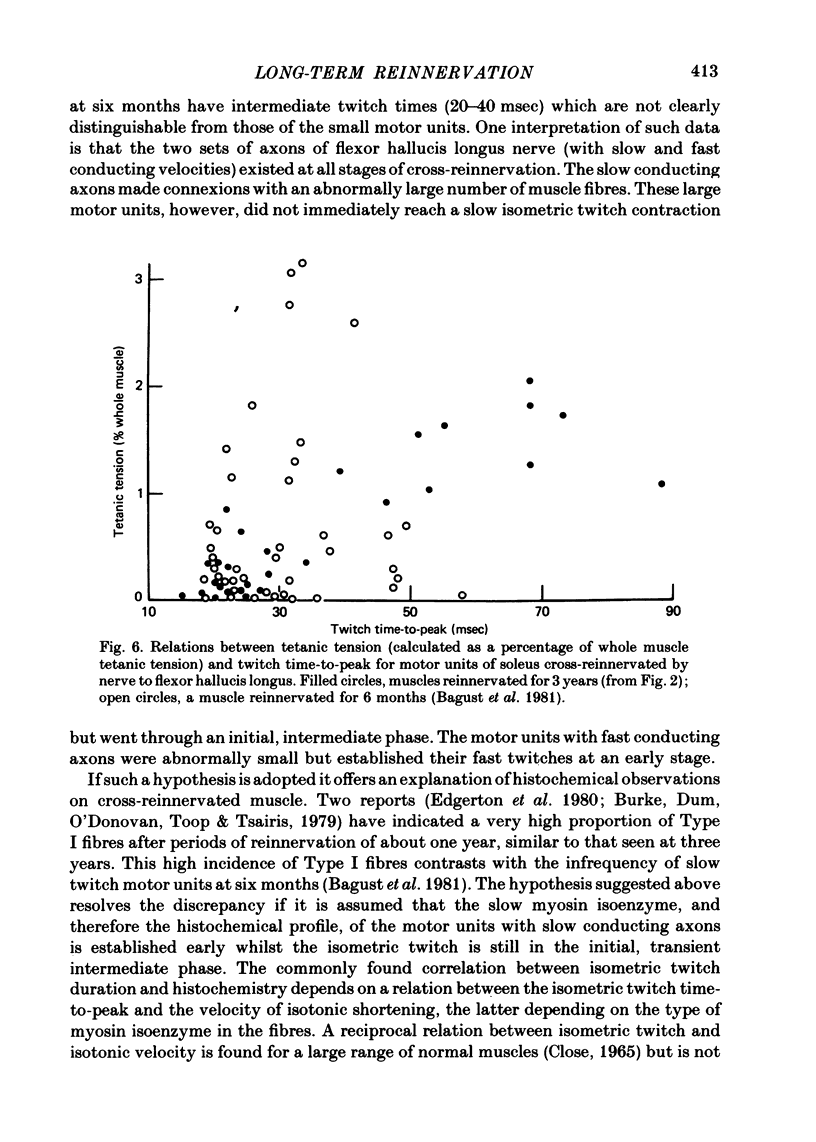
2. The motor units could be divided into two groups: one with fast twitches and low tetanic tension, the other with slow twitches and high tension. This is the reverse of the relation between motor unit twitch time and tetanic tension in normal muscle (fast or slow).
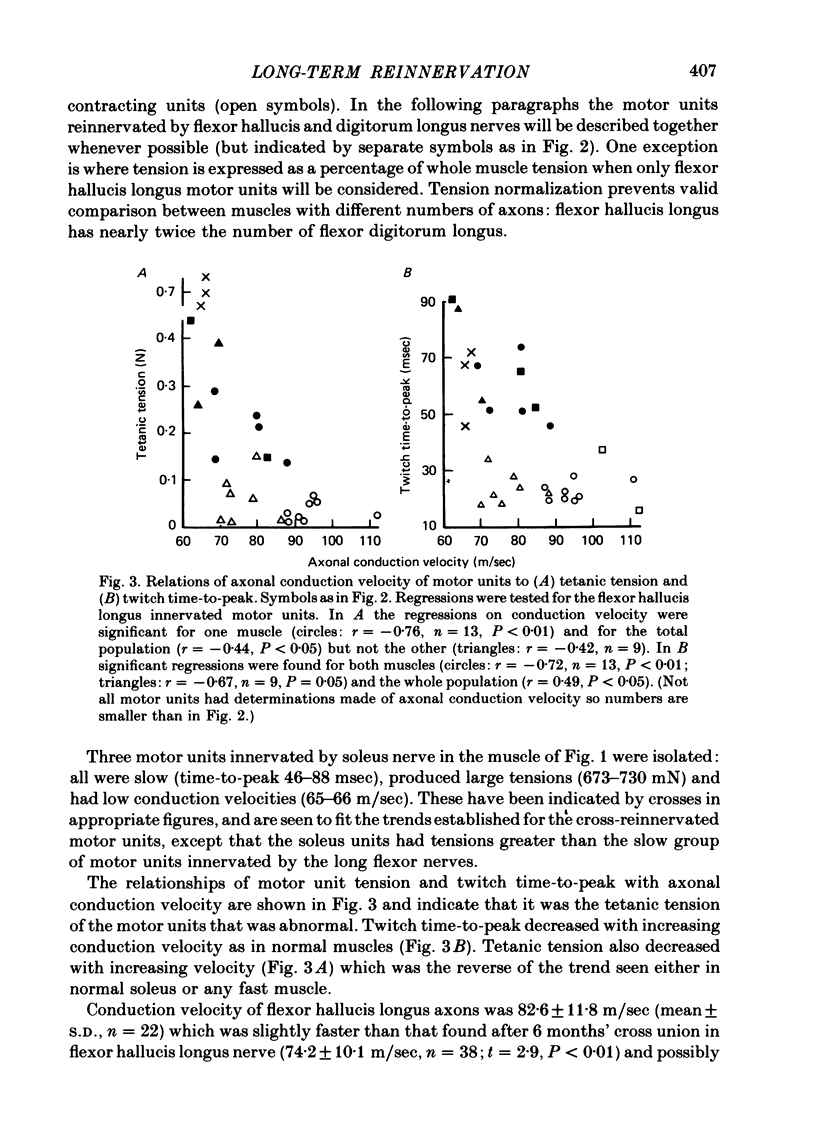
3. Motor unit twitch time to peak decreased with axonal conduction velocity, as in normal muscle, but so did tetanic tension, which is abnormal.
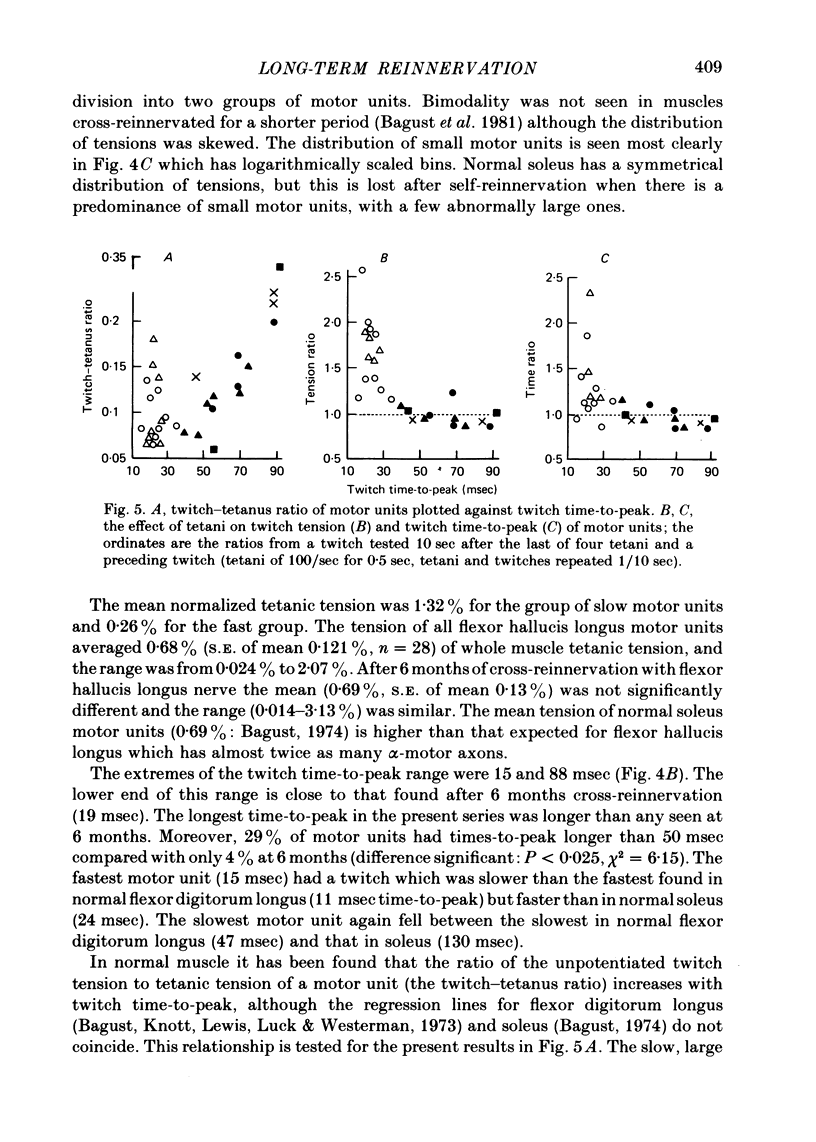
4. Twitch—tetanus ratio increased with twitch time to peak in the group of slow units but not in the fast group (although the range of ratios was as great).
5. A tetanus depressed the twitch tension of slow motor units and potentiated fast ones as in normal muscle but the potentiation was often accompanied by an abnormal prolongation of the twitch.
6. The mean conduction velocity of axons was slightly higher than at 6 months' reinnervation but below the normal value for fast muscle.
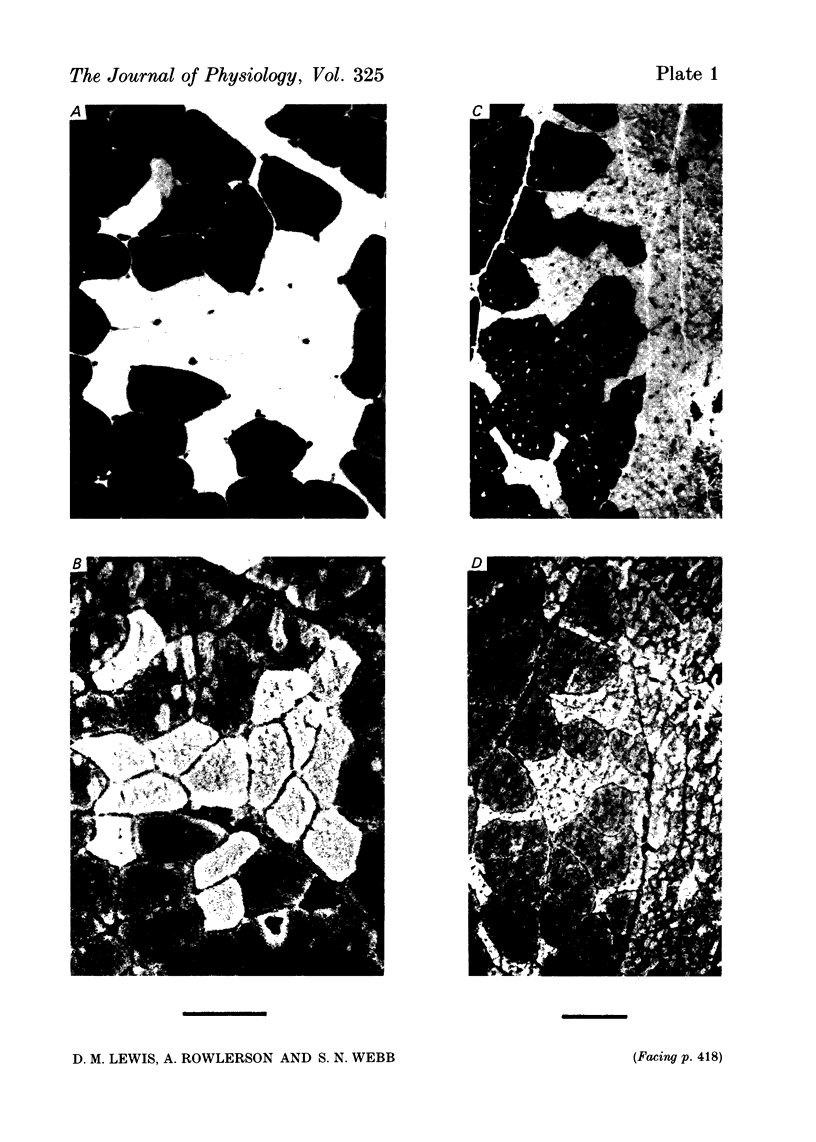
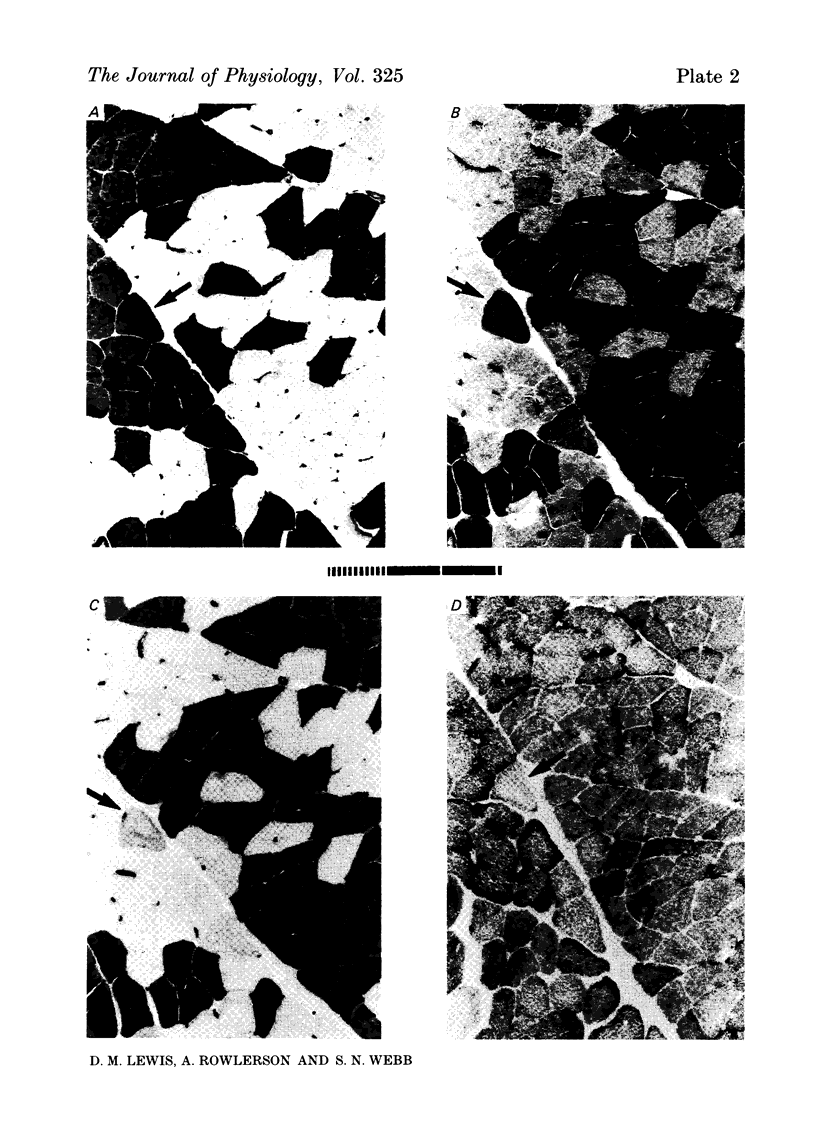
7. Antibody to slow myosin was bound strongly to Type I fibres but not to Type II fibres, confirming the histochemical division of fibres into Types I and II.
8. More than 95% of the fibres were oxidative, with Type I predominating over Type II a in the ratio of about 2:1.
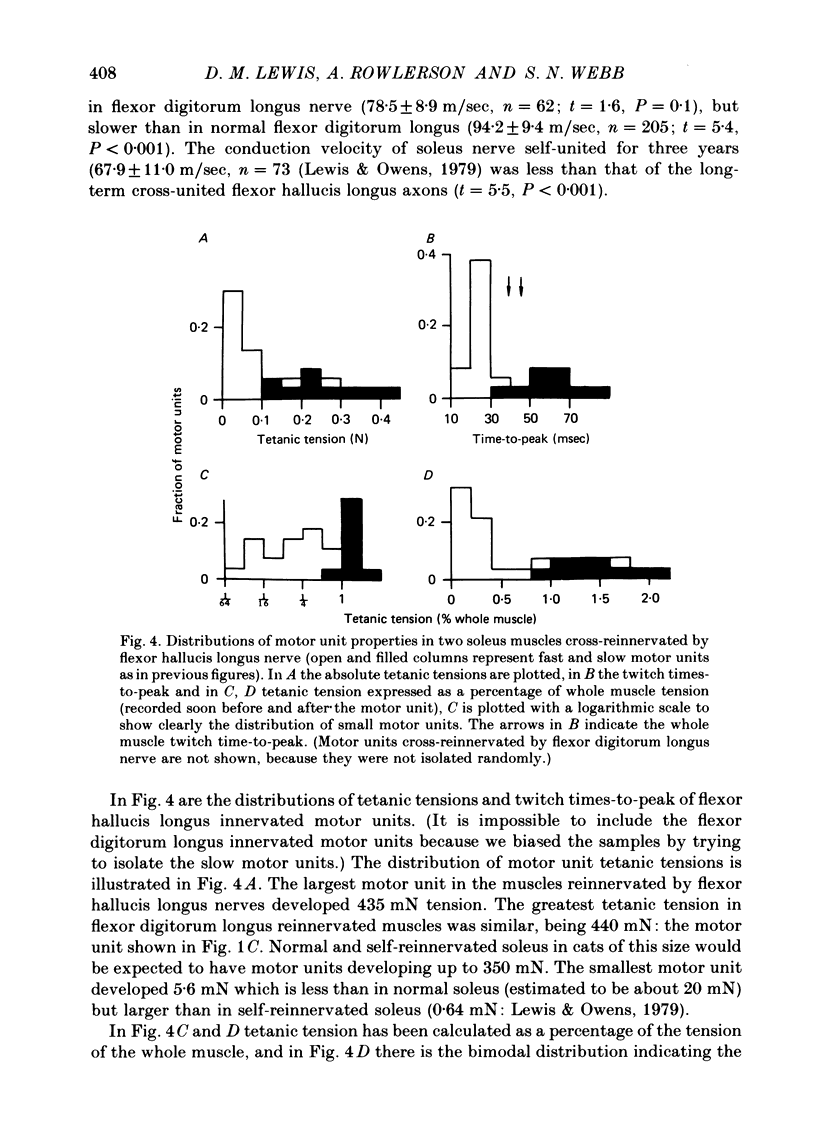
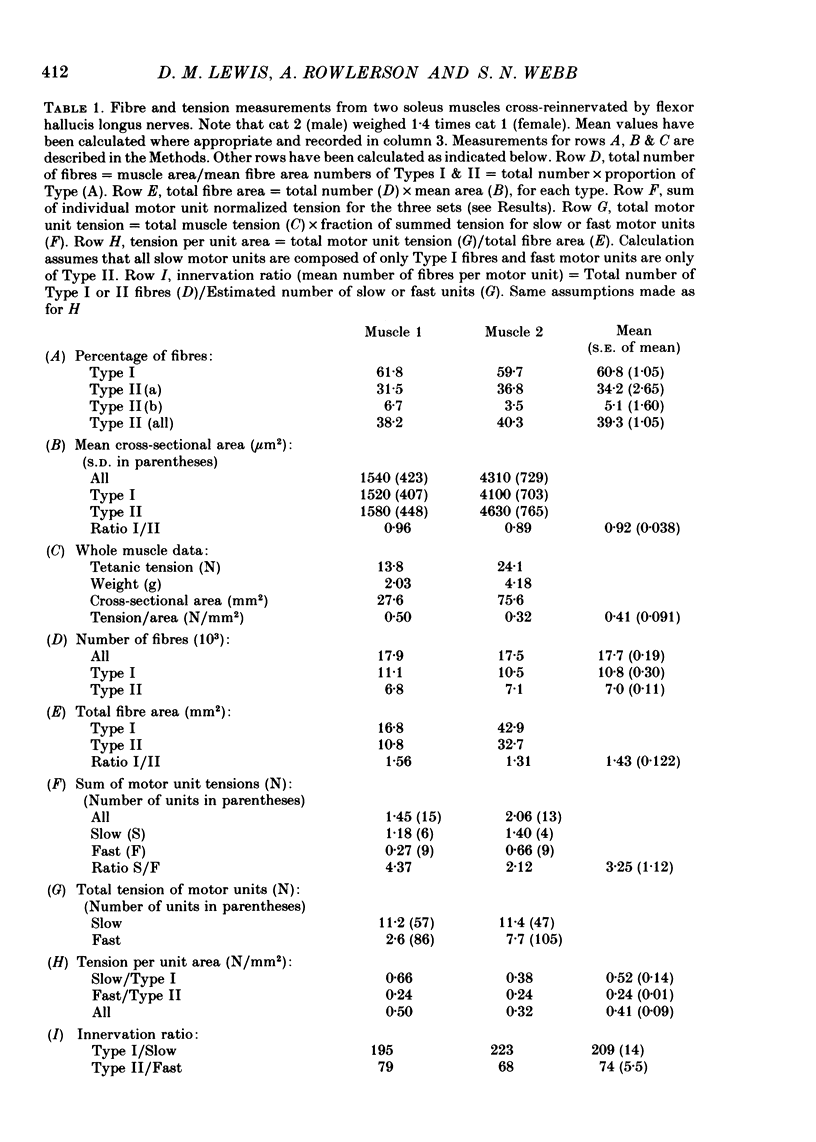
9. The higher tension of the slow motor units was the result of three factors: the number of fibres per motor unit was at least three times that in the fast; Type I fibres had cross-sectional areas little less than Type II (a and b together) and were estimated to develop more tension per unit area. All three findings were different from those in normal fast muscle.
10. One flexor hallucis longus muscle was self-reinnervated and examined histochemically. This muscle was abnormal in that a large majority of the fibres were Type I.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN J. J., GUTH L. Nonselectivity in establishment of neuromuscular connections following nerve regeneration in the rat. Exp Neurol. 1961 Sep;4:262–275. doi: 10.1016/0014-4886(61)90047-4. [DOI] [PubMed] [Google Scholar]
- BULLER A. J., LEWIS D. M. FURTHER OBSERVATIONS ON MAMMALIAN CROSS-INNERVATED SKELETAL MUSCLE. J Physiol. 1965 May;178:343–358. doi: 10.1113/jphysiol.1965.sp007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Knott S., Lewis D. M., Luck J. C., Westerman R. A. Isometric contractions of motor units in a fast twitch muscle of the cat. J Physiol. 1973 May;231(1):87–104. doi: 10.1113/jphysiol.1973.sp010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M. Isometric contractions of motor units in self-reinnervated fast and slow twitch muscles of the cat. J Physiol. 1974 Feb;237(1):91–102. doi: 10.1113/jphysiol.1974.sp010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Luck J. C. Post-tetanic effects in motor units of fast and slow twitch muscle of the cat. J Physiol. 1974 Feb;237(1):115–121. doi: 10.1113/jphysiol.1974.sp010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Westerman R. A. Motor units in cross-reinnervated fast and slow twitch muscle of the cat. J Physiol. 1981;313:223–235. doi: 10.1113/jphysiol.1981.sp013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J. Relationships between motor nerve conduction velocities and motor unit contraction characteristics in a slow twitch muscle of the cat. J Physiol. 1974 Apr;238(2):269–278. doi: 10.1113/jphysiol.1974.sp010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I., Luff A. R. Dynamic properties of inferior rectus muscle of the rat. J Physiol. 1974 Jan;236(2):259–270. doi: 10.1113/jphysiol.1974.sp010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh G., Gallego R., Kudo N., Kuno M. Evidence for the maintenance of motoneurone properties by muscle activity. J Physiol. 1978 Aug;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton V. R., Goslow G. E., Jr, Rasmussen S. A., Spector S. A. Is resistance of a muscle to fatigue controlled by its motoneurones? Nature. 1980 Jun 19;285(5766):589–590. doi: 10.1038/285589a0. [DOI] [PubMed] [Google Scholar]
- Edjtehadi G. D., Lewis D. M. Histochemical reactions of fibres in a fast twitch muscle of the cat. J Physiol. 1979 Feb;287:439–453. doi: 10.1113/jphysiol.1979.sp012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edjtehadi G., Lewis D. M. Structural features of muscle fibres from a fast and a slow twitch muscle in the kitten during postnatal development. J Anat. 1974 Nov;118(Pt 2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Finol H. J., Lewis D. M., Owens R. The effects of denervation on contractile properties or rat skeletal muscle. J Physiol. 1981;319:81–92. doi: 10.1113/jphysiol.1981.sp013893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar P., Kuno M., Kudo N., Miyata Y. Reaction of intact spinal motoneurones to partial denervation of the muscle. J Physiol. 1977 Feb;265(1):175–191. doi: 10.1113/jphysiol.1977.sp011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean C. J., Lewis D. M., McGarrick J. D. Dynamic properties of denervated fast and slow twitch muscle of the cat. J Physiol. 1974 Feb;237(1):103–113. doi: 10.1113/jphysiol.1974.sp010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. M., Bagust J., Webb S. N., Westerman R. A., Finol H. J. Axon conduction velocity modified by reinnervation of mammalian muscle. Nature. 1977 Dec 22;270(5639):745–746. doi: 10.1038/270745a0. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Parry D. J., Rowlerson A. Isometric contractions of motor units and immunohistochemistry of mouse soleus muscle. J Physiol. 1982 Apr;325:393–401. doi: 10.1113/jphysiol.1982.sp014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Development of muscle fiber specialization in the rat hindlimb. J Cell Biol. 1981 Jul;90(1):128–144. doi: 10.1083/jcb.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Amood W. S., Pope R. A comparison of the structural features of muscle fibres from a fast- and a slow-twitch muscle of the pelvic limb of the cat. J Anat. 1972 Oct;113(Pt 1):49–60. [PMC free article] [PubMed] [Google Scholar]