Abstract
1. The action of adrenaline (in the presence of propranolol; 3 × 10-6 M), adenosine triphosphate (ATP) and carbachol on guinea-pig taenia caeci, and the interaction between these agonists, was studied by measuring changes in membrane potential using the sucrose-gap method in quiescent preparations at 22 °C.
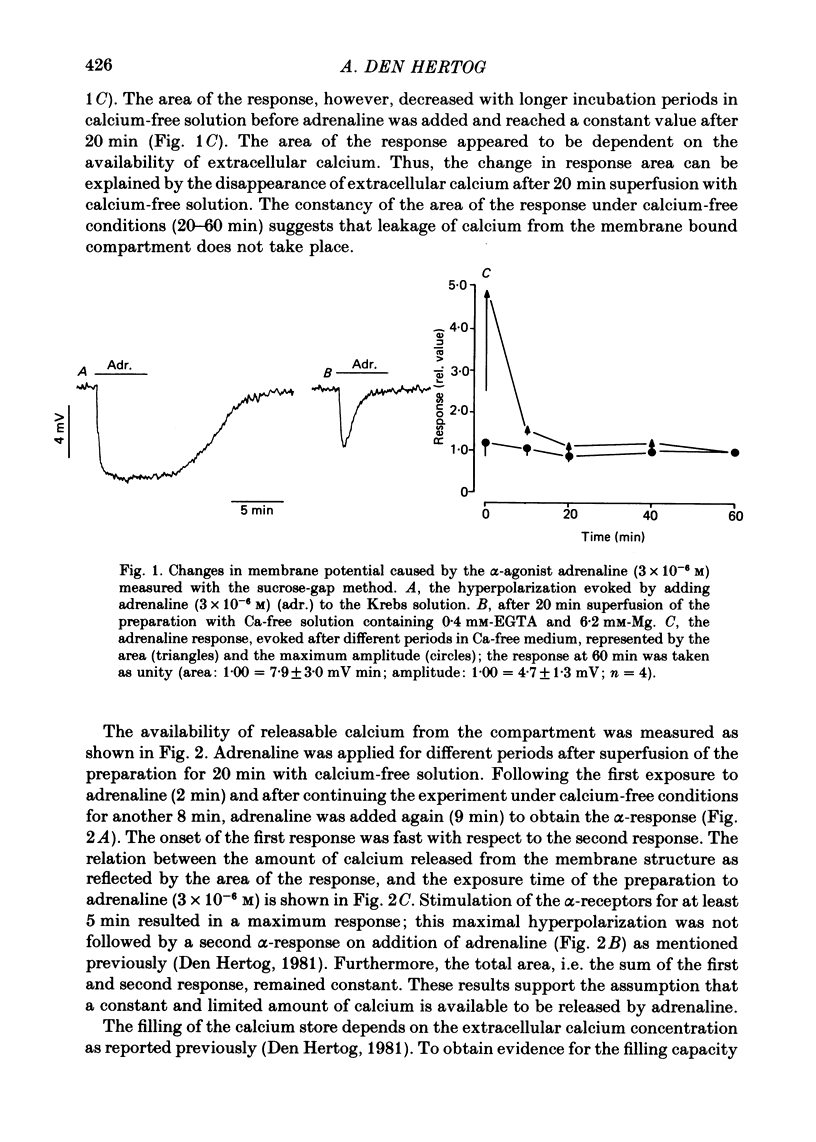
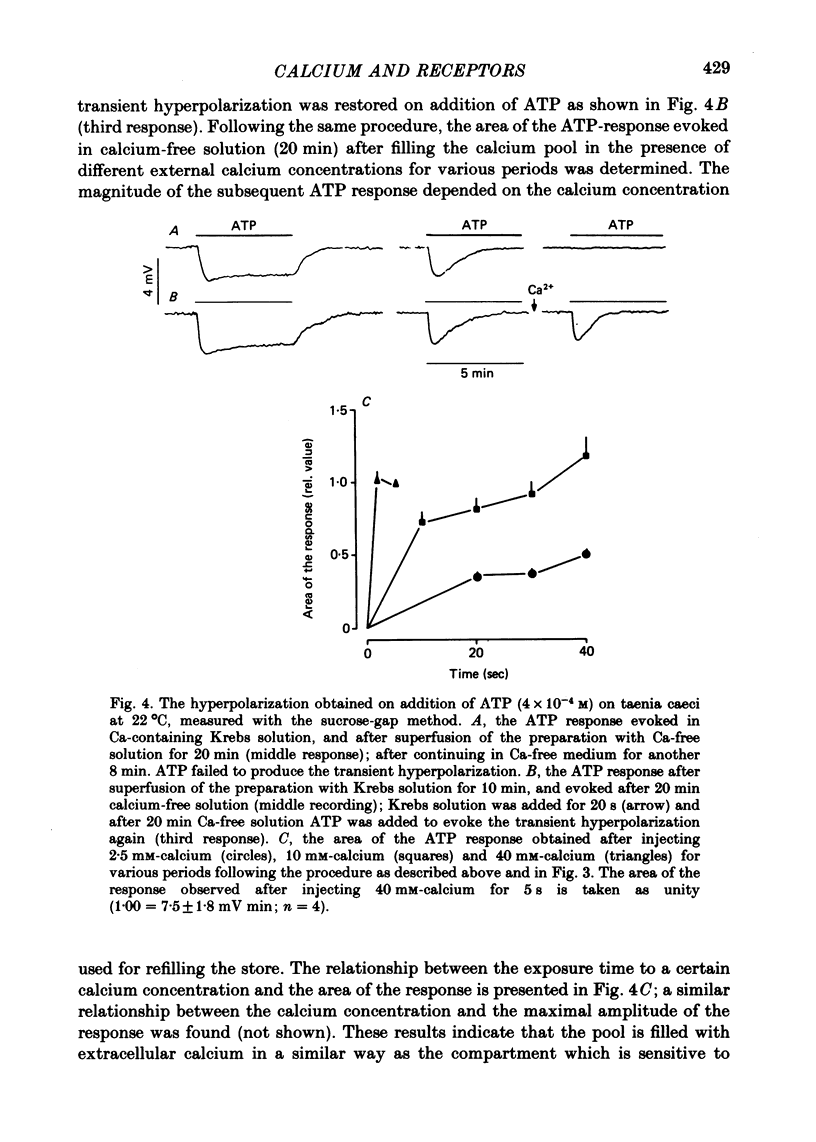
2. A sustained hyperpolarization was caused by addition of adrenaline (3 × 10-6 M) and by applying adenosine triphosphate (ATP; 4 × 10-4 M) for 5 min in Krebs solution. In calcium-free medium containing EGTA (0·4 mM) and high magnesium (6·2 mM), both the α-agonist and ATP caused a transient hyperpolarization which passed off within 5 min, although the agonist was still present.
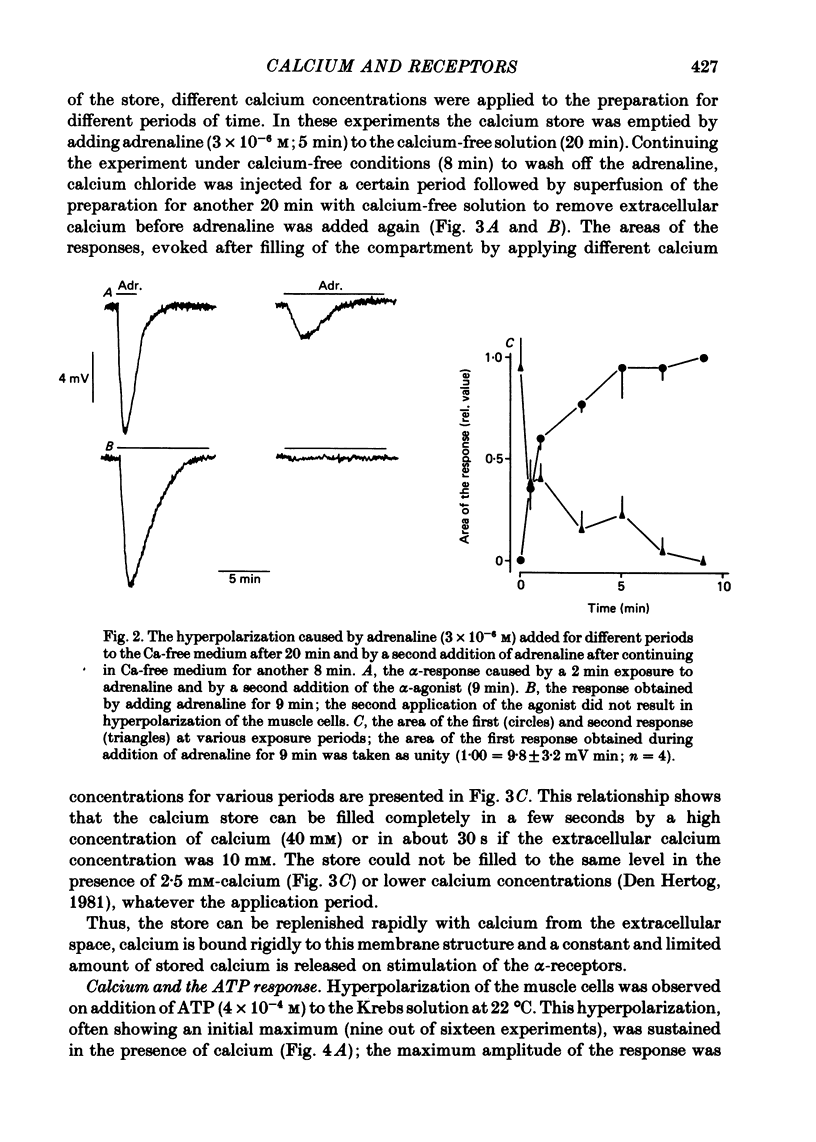
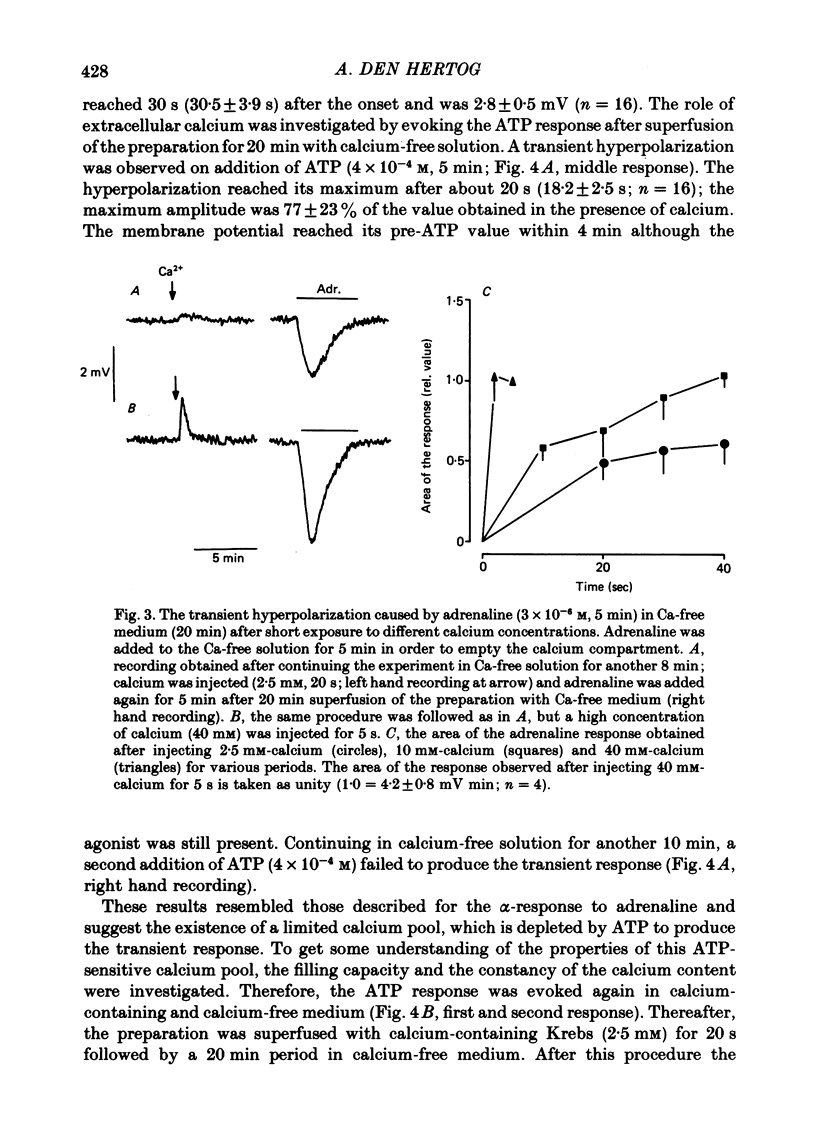
3. The transient hyperpolarization evoked by these agonists in the absence of calcium could be evoked only once. The response was restored after exposure to high calcium, (40 mM for 2 s, or 10 mM for 30 s). The maximum amplitudes of the hyperpolarization caused by adrenaline or ATP after exposure to high calcium (40 mM or 10 mM) were similar, while the maximum hyperpolarization after application of 2·5 mM-calcium was smaller.
4. The area of the maximal response evoked by adrenaline or ATP was independent of the exposure time to calcium-free solution after removal of the extracellular calcium (20 min). The sum of the areas of a first submaximal response, obtained by applying adrenaline for less than 5 min to the calcium-free solution (20 min), and of the second response (5 min application) elicited after continuing in calcium-free medium for another 8 min, was constant.
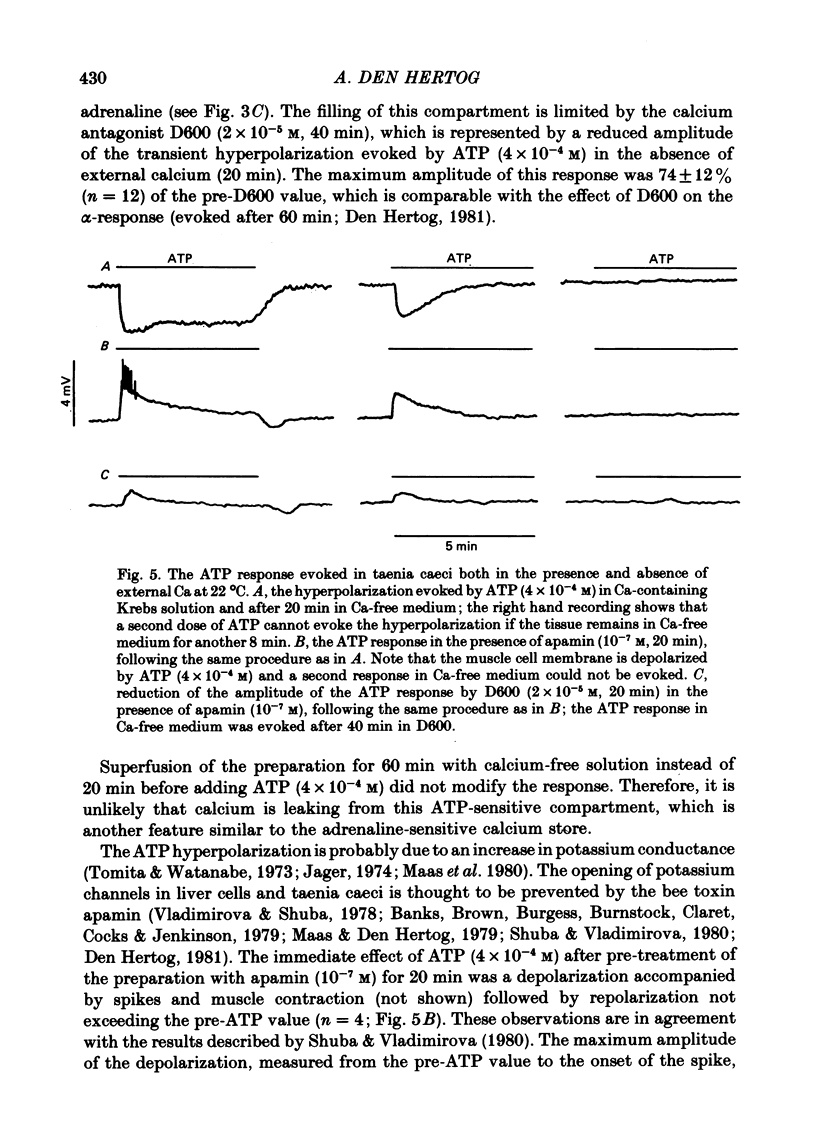
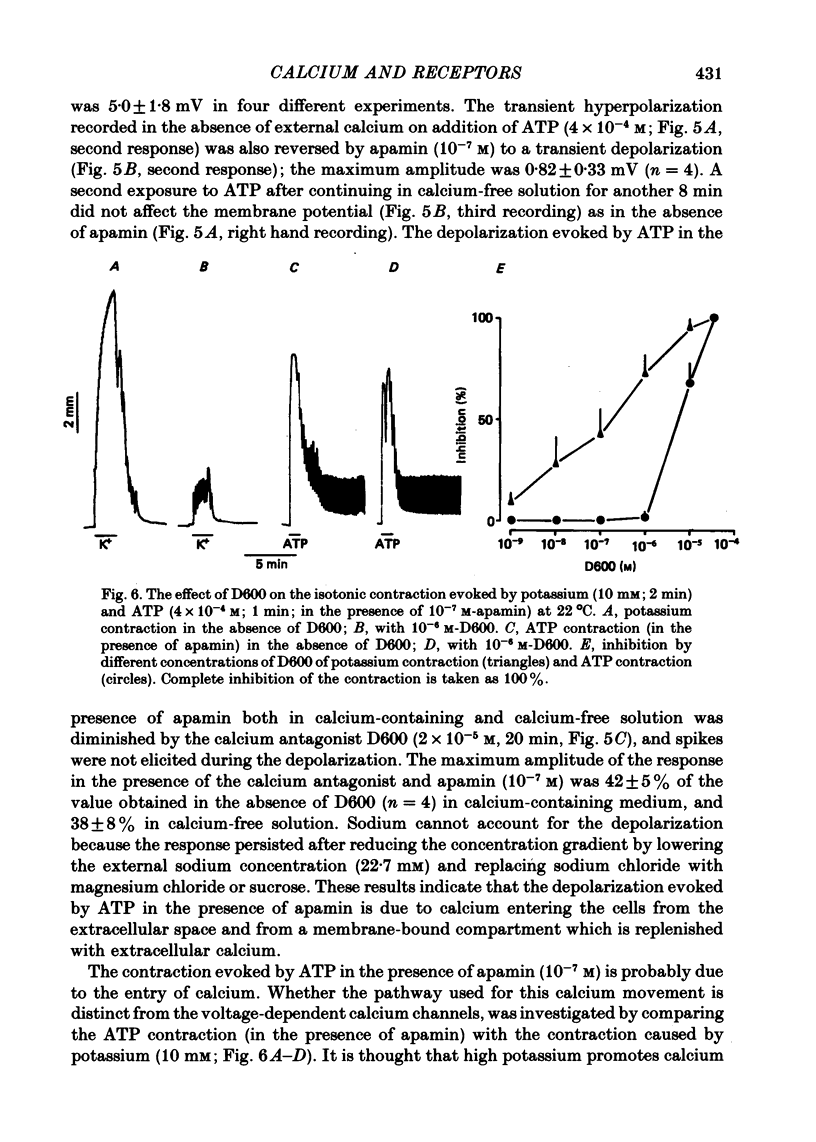
5. In the presence of the bee toxin apamin (10-7 M), addition of ATP (4 × 10-4 M) caused depolarization of the membrane both in the presence and absence of external calcium. These responses were not blocked in low sodium solution (22·7 mM) but were reduced by the calcium antagonist D600 (2 × 10-5 M).
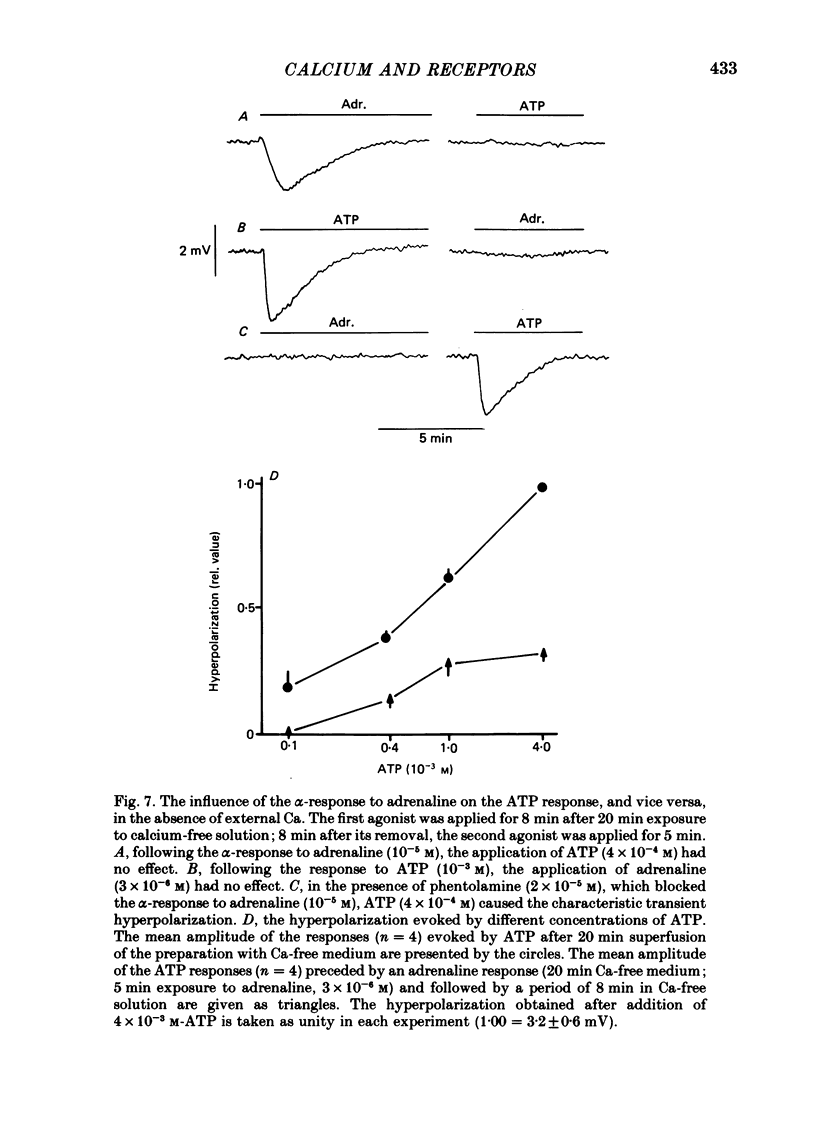
6. In calcium-free conditions the α-response to adrenaline was decreased by a preceding addition of ATP and vice versa. Abolition of the ATP response (4 × 10-4 M) by adrenaline (10-5 M) was prevented by blocking the α-receptors with phentolamine (2 × 10-5 M).
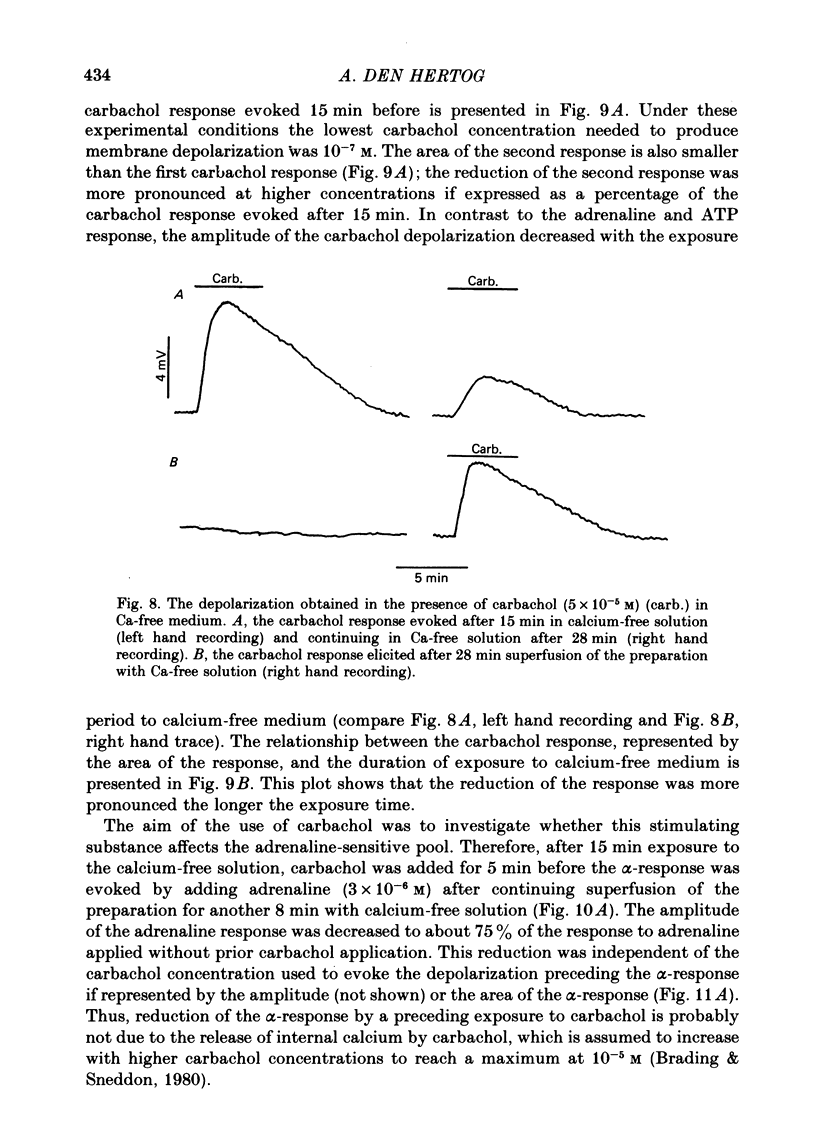
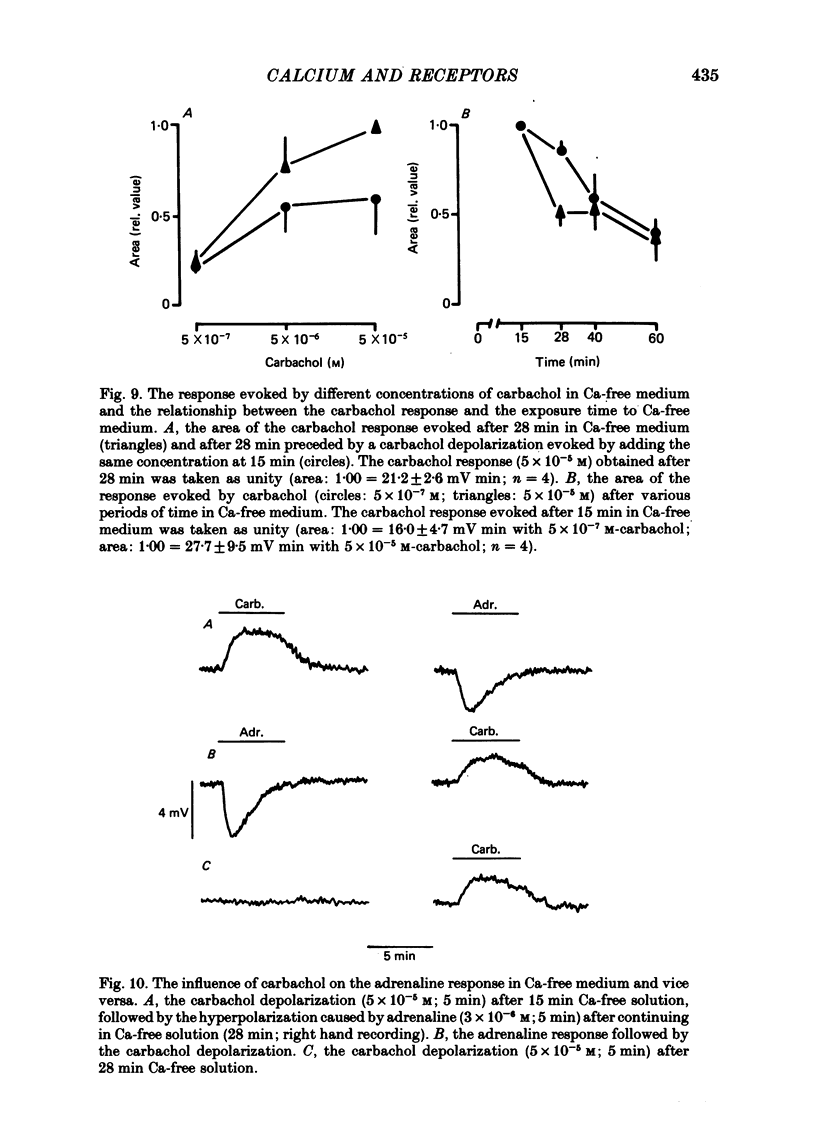
7. Carbachol (5 × 10-7-5 × 10-5 M) depolarized the muscle cells in calcium-free medium; a second addition of carbachol also caused depolarization, the amplitude being lower. The carbachol depolarization was dependent on the exposure time to calcium-free solution.
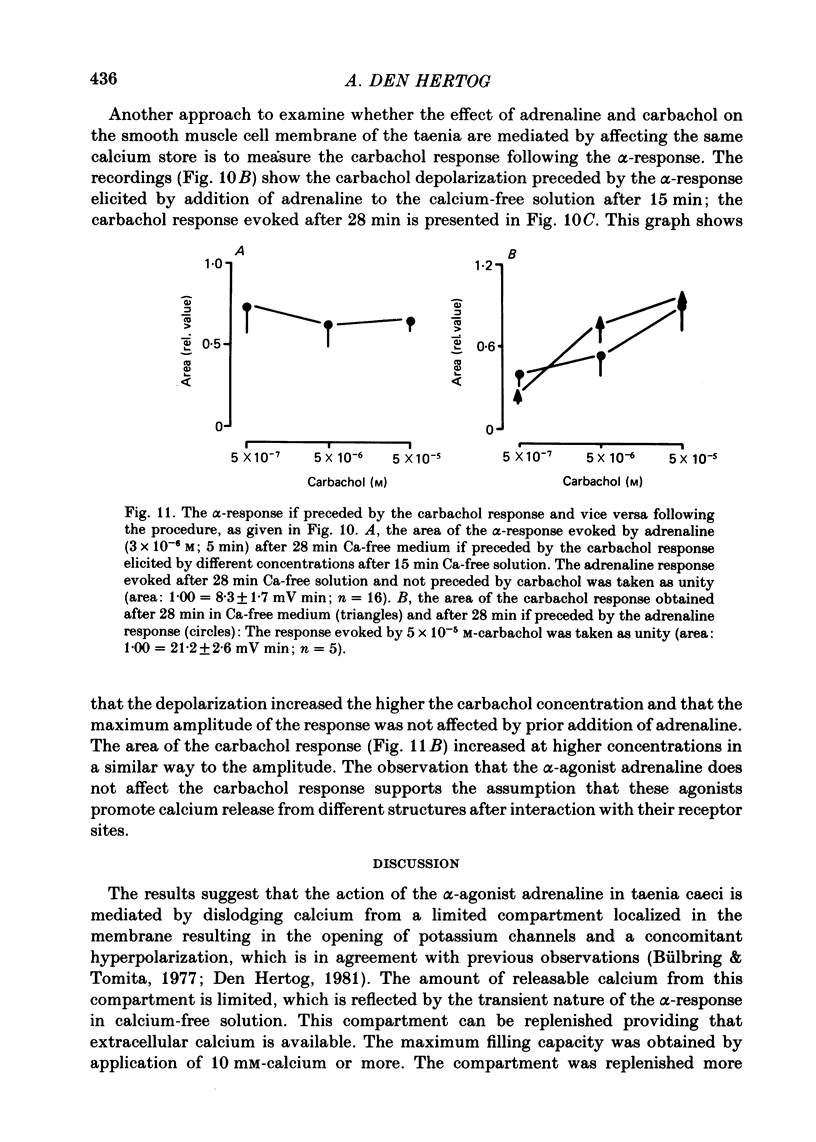
8. The adrenaline response was reduced by about 25% by carbachol if applied previously, independent of the carbachol concentration (5 × 10-7-5 × 10-5 M). The carbachol response, however, was not affected if preceded by the α-response.
9. It is concluded that ATP and the α-agonist, after binding to their receptor sites, activate the same mechanism, which is mobilization of calcium from the same membrane compartment to open potassium channels, causing hyperpolarization of the muscle cell membrane; the hyperpolarization is transient or sustained in nature depending on the availability of external calcium to replenish the calcium compartment localized in the membrane. This adrenaline and ATP-sensitive calcium compartment is distinct from that which is sensitive to carbachol.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson J., Holmberg B. The effects of extracellularly applied ATP and related compounds on electrical and mechanical activity of the smooth muscle taenia coli from the guinea-pig. Acta Physiol Scand. 1969 Jan-Feb;75(1):149–156. doi: 10.1111/j.1748-1716.1969.tb04366.x. [DOI] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueding E., Bülbring E., Gercken G., Hawkins J. T., Kuriyama H. The effect of adrenaline on the adenosine otriphosphate and creatine phosphate content of intestinal smooth muscle. J Physiol. 1967 Nov;193(1):187–212. doi: 10.1113/jphysiol.1967.sp008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Calcium requirement for the alpha-action of catecholamines on guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):271–284. doi: 10.1098/rspb.1977.0070. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Bülbring E., den Hertog A. The action of isoprenaline on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1980 Jul;304:277–296. doi: 10.1113/jphysiol.1980.sp013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the alpha-action of catecholamines on guinea-pig taenia caeci. J Physiol. 1981 Jul;316:109–125. doi: 10.1113/jphysiol.1981.sp013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G. Effects of sympathomimetic amines on 45Ca efflux from liver slices. Br J Pharmacol. 1976 May;57(1):158–160. doi: 10.1111/j.1476-5381.1976.tb07668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager L. P. The effect of catecholamines and ATP on the smooth muscle cell membrane of the guinea-pig taenia coli. Eur J Pharmacol. 1974 Mar;25(3):372–382. doi: 10.1016/0014-2999(74)90267-2. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Mishima K., Suzuki H. Some differences in contractile responses of isolated longitudinal and circular muscle from the guinea-pig stomach. J Physiol. 1975 Oct;251(2):317–331. doi: 10.1113/jphysiol.1975.sp011095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A., Ras R., Van den Akker J. The action of apamin on guinea-pig taenia caeci. Eur J Pharmacol. 1980 Oct 17;67(2-3):265–274. doi: 10.1016/0014-2999(80)90507-5. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi H., Takewaki T., Okada T. Calcium and the contractile effect of carbachol in the depolarized guinea-pig taenia caecum. Jpn J Pharmacol. 1974 Aug;24(4):601–611. doi: 10.1254/jjp.24.601. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba M. F., Vladimirova I. A. Effect of apamin on the electrical responses of smooth muscle to adenosine 5'-triphosphate and to non-adrenergic, non-cholinergic nerve stimulation. Neuroscience. 1980;5(5):853–859. doi: 10.1016/0306-4522(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. A comparison of the effects of adenosine triphosphate with noradrenaline and with the inhibitory potential of the guinea-pig taenia coli. J Physiol. 1973 May;231(1):167–177. doi: 10.1113/jphysiol.1973.sp010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirova I. A., Shuba M. F. Vliianie strikhnina, gidrastina i apamina na sinapticheskuiu peredachu v gladkomyshechnykh kletkakh. Neirofiziologiia. 1978;10(3):295–299. [PubMed] [Google Scholar]
- Whittam R. Control of membrane permeability to potassium in red blood cells. Nature. 1968 Aug 10;219(5154):610–610. doi: 10.1038/219610a0. [DOI] [PubMed] [Google Scholar]


