Abstract
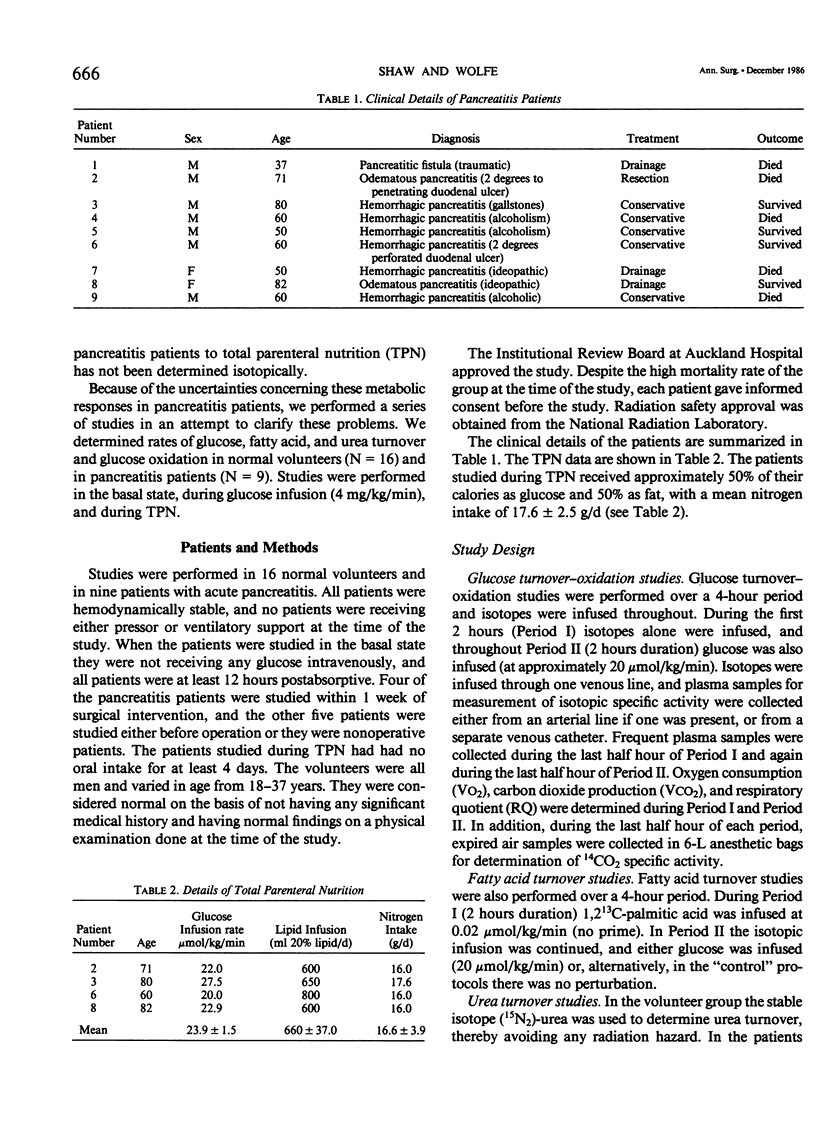
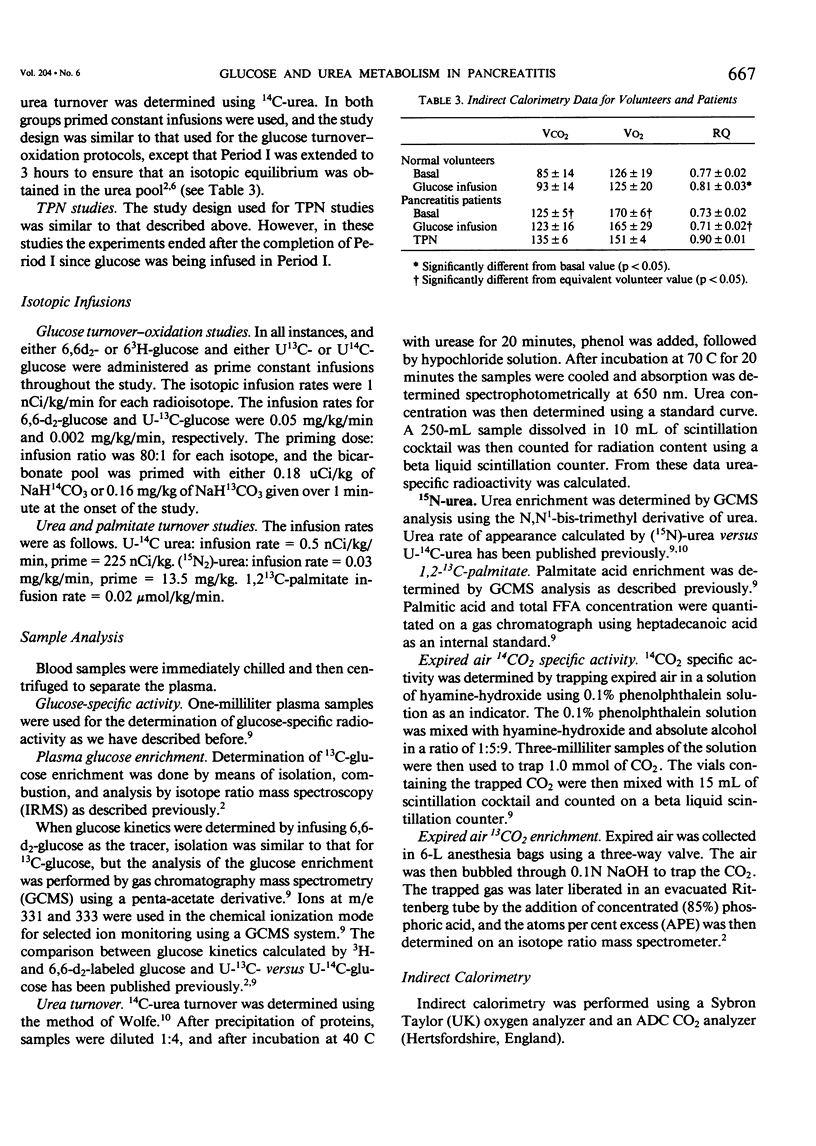
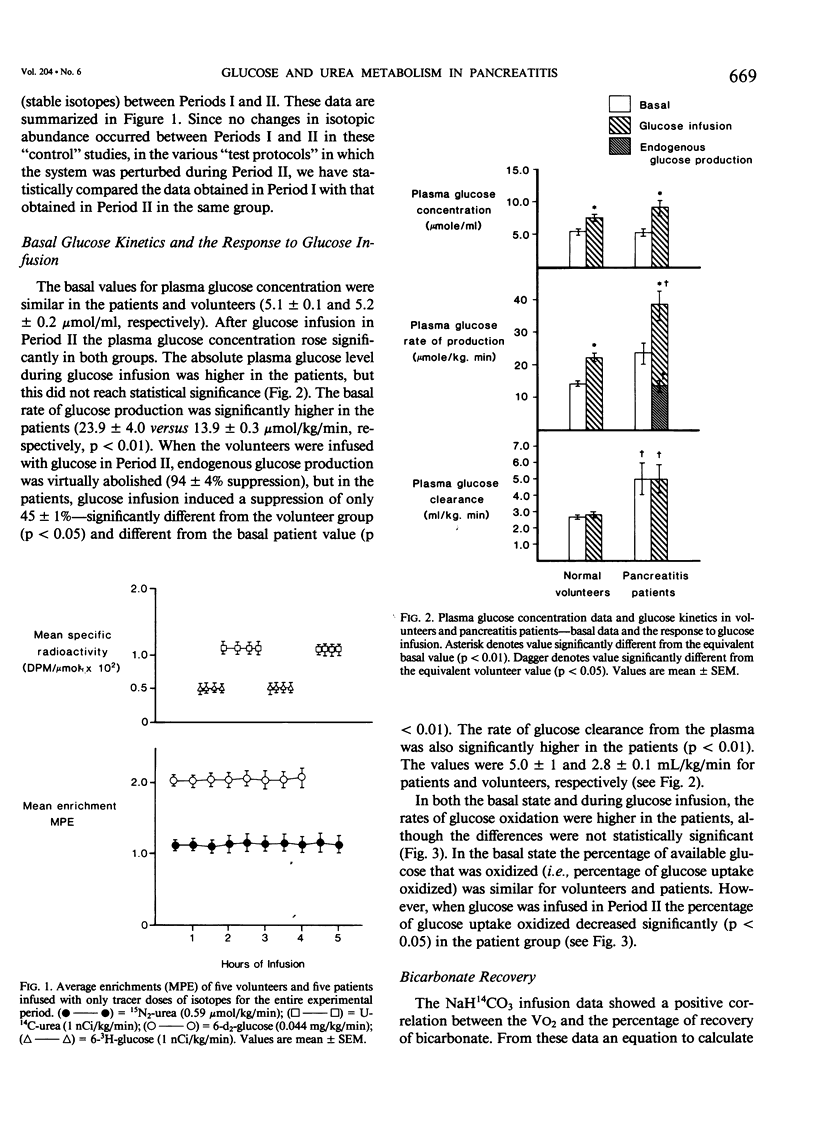
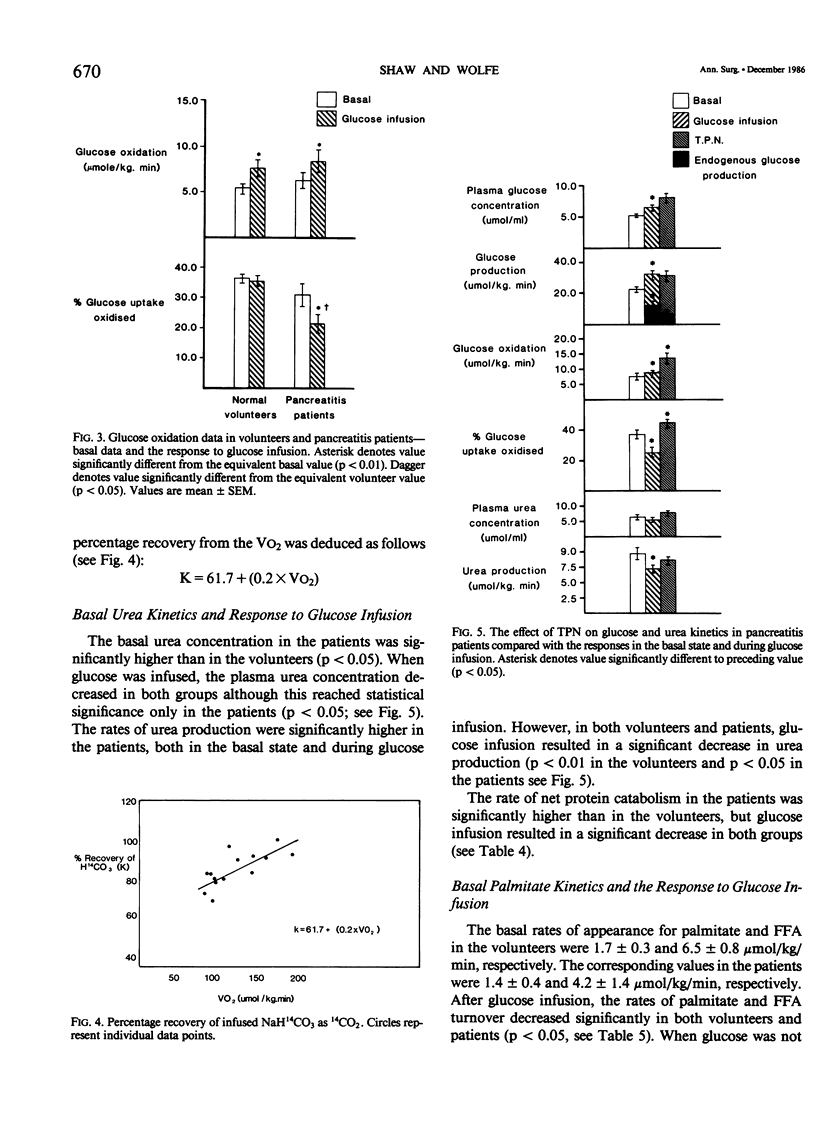
Rates of glucose turnover and oxidation in normal volunteers (N = 16) and in severely ill patients with pancreatitis (N = 9) were isotopically determined. Glucose turnover was determined using primed constant infusions of either 6-3H-glucose or 6-d2-glucose, and glucose oxidation with either U-14C-glucose or U-13C-glucose after appropriate priming of the bicarbonate pool. Urea kinetics were determined using primed constant infusions of either (15N2)-urea or U-14C-urea, whereas free fatty acid (FFA) kinetics were determined by the constant infusion of 1,2-13C palmitate. Basal rates of glucose production and plasma glucose clearance were significantly higher in the patients than in the volunteers. During glucose infusion (4 mg/kg/min) endogenous glucose production was virtually totally suppressed in the volunteers (94 +/- 4%). There was significantly less suppression in the patients, however (44 +/- 1%). In addition, the percentage of available glucose oxidized (i.e., percentage of uptake oxidized) was significantly less in the patients than in the volunteers. The basal rate of urea production was significantly higher in the patients; however, in both patients and volunteers, glucose infusion resulted in a significant decrease. The rate of FFA turnover was similar in the patients and volunteers, and the patients and volunteers were equally sensitive to the suppressive effects of glucose infusion. When the patients were studied during total parenteral nutrition (TPN), there was no further suppression of endogenous glucose turnover than that seen during 2 hours of glucose infusion, and the mean rate of urea turnover measured during TPN (7.0 +/- 1.9 mumol/kg/min) was also not significantly different than the value determined during glucose infusion (8.9 +/- 1.8 mumol/kg/min). It was concluded from these studies that patients with pancreatitis are metabolically similar to septic patients, have an impairment in their ability to oxidize infused glucose when compared with normal volunteers, have an elevated rate of net protein catabolism, and have FFA kinetics similar to those seen in normal humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Border J. R., Chenier R., McManamy R. H., La Duca J., Seibel R., Birkhahn R., Yu L. Multiple systems organ failure: muscle fuel deficit with visceral protein malnutrition. Surg Clin North Am. 1976 Oct;56(5):1147–1167. doi: 10.1016/s0039-6109(16)41035-2. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, O'Donnell T. F., Blackburn G. L., Maki T. N. Energy metabolism and proteolysis in traumatized and septic man. Surg Clin North Am. 1976 Oct;56(5):1169–1184. doi: 10.1016/s0039-6109(16)41036-4. [DOI] [PubMed] [Google Scholar]
- Kirby D. F., Craig R. M. The value of intensive nutritional support in pancreatitis. JPEN J Parenter Enteral Nutr. 1985 May-Jun;9(3):353–357. doi: 10.1177/0148607185009003353. [DOI] [PubMed] [Google Scholar]
- Kuttner R. E., Spitzer J. J. Gluconeogenesis from alanine in endotoxin-treated dogs. J Surg Res. 1978 Aug;25(2):166–173. doi: 10.1016/0022-4804(78)90072-0. [DOI] [PubMed] [Google Scholar]
- Long C. L. Energy balance and carbohydrate metabolism in infection and sepsis. Am J Clin Nutr. 1977 Aug;30(8):1301–1310. doi: 10.1093/ajcn/30.8.1301. [DOI] [PubMed] [Google Scholar]
- Long C. L., Kinney J. M., Geiger J. W. Nonsuppressability of gluconeogenesis by glucose in septic patients. Metabolism. 1976 Feb;25(2):193–201. doi: 10.1016/0026-0495(76)90049-4. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Klein S., Wolfe R. R. Assessment of alanine, urea, and glucose interrelationships in normal subjects and in patients with sepsis with stable isotopic tracers. Surgery. 1985 May;97(5):557–568. [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Response to glucose and lipid infusions in sepsis: a kinetic analysis. Metabolism. 1985 May;34(5):442–449. doi: 10.1016/0026-0495(85)90210-0. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R. Measurement of urea kinetics in vivo by means of a constant tracer infusion of di-15N-urea. Am J Physiol. 1981 Apr;240(4):E428–E434. doi: 10.1152/ajpendo.1981.240.4.E428. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Durkot M. J. Energy metabolism in trauma and sepsis: the role of fat. Prog Clin Biol Res. 1983;111:89–109. [PubMed] [Google Scholar]


