Abstract
1. Brisk joint displacements and tendon taps were applied to voluntarily contracting wrist flexor muscles in subjects who did not attempt to react to them. Different types of mechanotransducers, some of them attached to an intramuscular needle, were used to detect mechanical oscillations in the wrist flexors, resulting from the imposed impacts. The transducer responses to the perturbations were compared with simultaneously recorded reflex electromyogram (e.m.g.) responses. Experiments were also carried out on a rubber band model, exposed to similar mechanical stimuli.
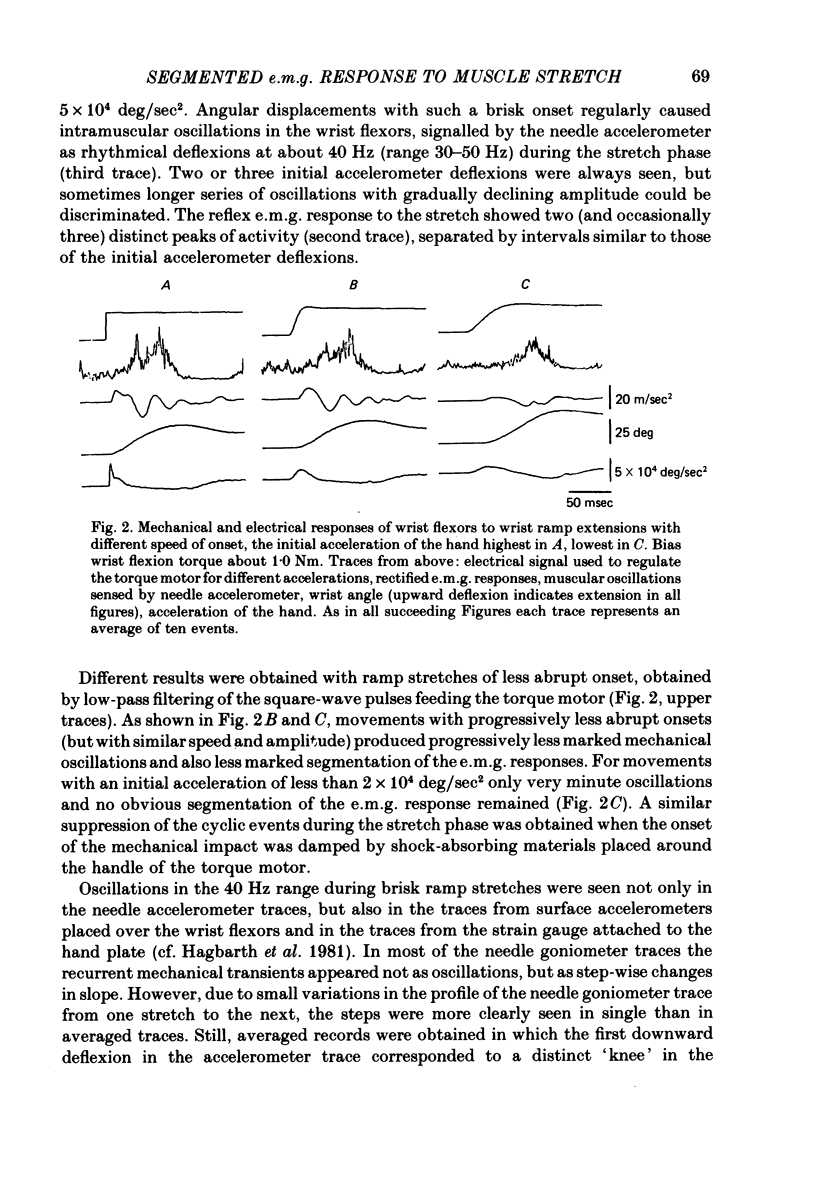
2. During brisk ramp wrist extensions the transducers signalled damped muscular oscillations at 30-50 Hz. The oscillations grew in amplitude with increasing speed of onset of the stretch movement and at angular accelerations exceeding about 2 × 104 deg/sec2 the e.m.g. response changed from a non-segmented to a progressively more pronounced segmented pattern. Peak intervals in the segmented reflex e.m.g. responses were similar to those of the mechanical oscillations and did not change significantly with small or moderate variations in background contraction force. Latencies from successive deflexions in the accelerometer records to corresponding deflexions in the e.m.g. were 20-25 msec.
3. Damped muscular oscillations in the 30-50 Hz range were also initiated by sudden halts of voluntary wrist movements, by electrically induced twitches, and by voluntary brisk contractions. In these instances too, the mechanical oscillations were reflected in the shape of the succeeding e.m.g. response.
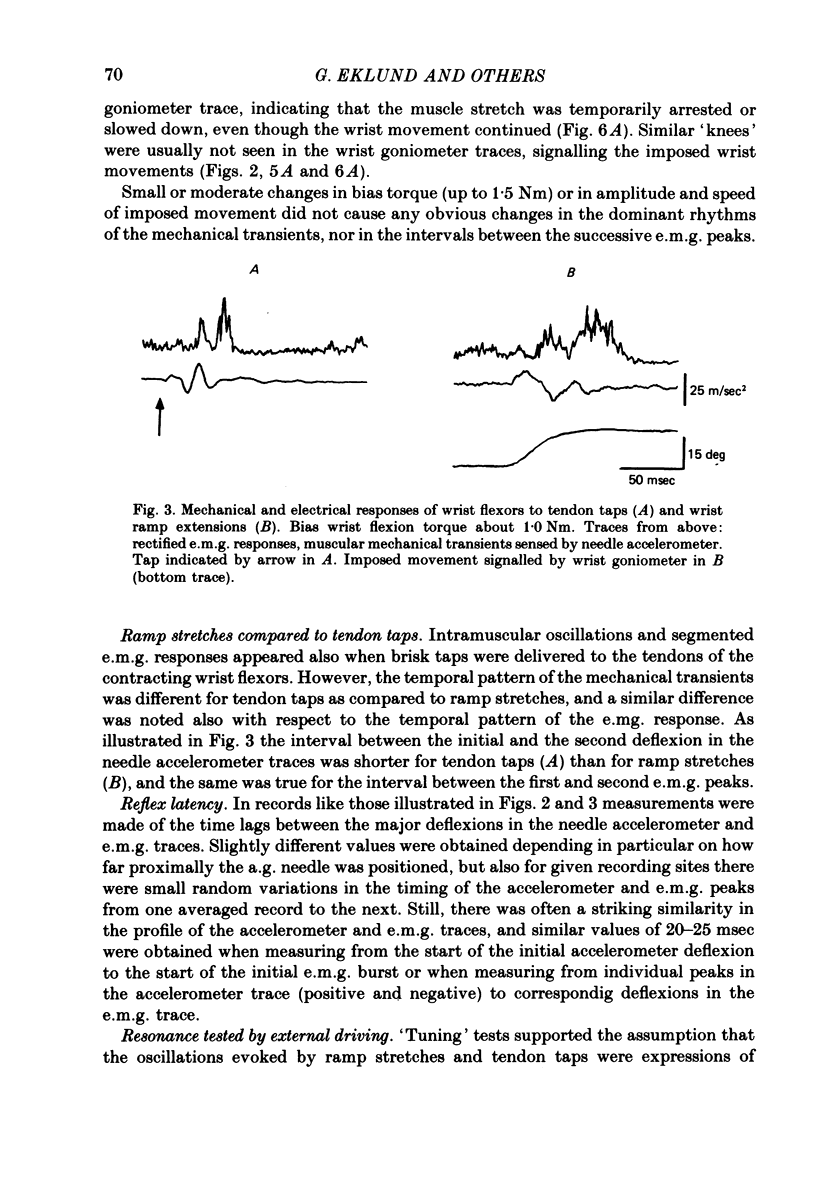
4. The interval between the two initial peaks in the accelerometer records was always shorter with tendon taps than in ramp stretch experiments. A corresponding difference was noted in the intervals between the following two peaks in the reflex e.m.g. response.
5. The initial peak in the accelerometer records could be ascribed to a wave propagated at about 40 m/sec in the wrist flexor muscles. Inconclusive results were obtained in attempts to determine whether the subsequent oscillations represented reflexions of the propagated wave at the ends of the muscle.
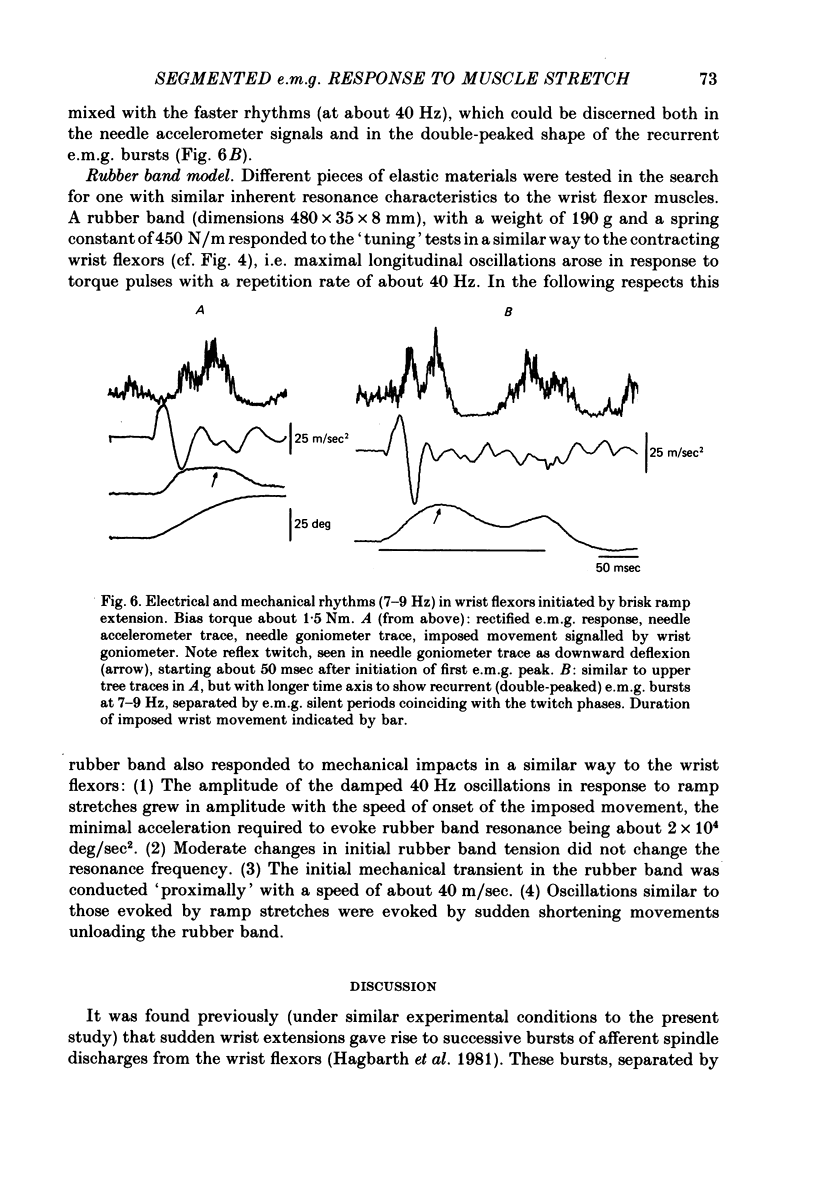
6. The muscles were also exposed to recurrent stretch stimuli (torque pulses) with a repetition rate varying between 15 and 100 Hz. At 30-50 Hz the intramuscular oscillations reached their maximal amplitude, and such repetition rates were also most efficient in producing synchronized e.m.g. bursts, time-locked to the oscillations. The mechanical responses of the wrist flexors to single or recurrent perturbations were to a large extent mimicked by a rubber band model with a longitudinal resonance frequency of about 40 Hz.
7. It is concluded that segmentation of reflex e.m.g. responses to sudden joint displacements and other types of brisk muscle perturbations to a large extent depends on the inherent resonance characteristics of musculo-tendinous structures. Primary spindle endings with their high vibration sensitivity and their segmental projections to α-motoneurones are believed to be the receptors primarily responsible for reflex entrainment of the motor impulses.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawa P., Tatton W. G. Motor unit responses in muscles stretched by imposed displacements of the monkey wrist. Exp Brain Res. 1979;37(3):417–437. doi: 10.1007/BF00236815. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976 Oct;261(3):673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Schiller H. H. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J Neurol Neurosurg Psychiatry. 1976 Aug;39(8):729–741. doi: 10.1136/jnnp.39.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Godaux E. Vibration-induced discharge patterns of single motor units in the masseter muscle in man. J Physiol. 1975 Dec;253(2):429–442. doi: 10.1113/jphysiol.1975.sp011198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. The 'late' reflex responses to muscle stretch: the 'resonance hypothesis' versus the 'long-loop hypothesis'. J Physiol. 1982 May;326:79–90. doi: 10.1113/jphysiol.1982.sp014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C., Shinoda Y. Spinal mechanisms of the functional stretch reflex. Exp Brain Res. 1978 May 12;32(1):55–68. doi: 10.1007/BF00237390. [DOI] [PubMed] [Google Scholar]
- HAMMOND P. H. Involuntary activity in biceps following the sudden application of velocity to the abducted forearm. J Physiol. 1955 Feb 28;127(2):23–5P. [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Young R. R., Hägglund J. V., Wallin E. U. Segmentation of human spindle and EMG responses to sudden muscle stretch. Neurosci Lett. 1980 Sep;19(2):213–217. doi: 10.1016/0304-3940(80)90197-4. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Young R. R. Participation of the stretch reflex in human physiological tremor. Brain. 1979 Sep;102(3):509–526. doi: 10.1093/brain/102.3.509. [DOI] [PubMed] [Google Scholar]
- Homma S. Frequency characteristics of the impulse decoding ratio between the spinal afferents and efferents in the stretch reflex. Prog Brain Res. 1976;44:15–30. doi: 10.1016/S0079-6123(08)60720-7. [DOI] [PubMed] [Google Scholar]
- Homma S., Kanda K., Watanabe S. Preferred spike intervals in the vibration reflex. Jpn J Physiol. 1972 Aug;22(4):421–432. doi: 10.2170/jjphysiol.22.421. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. Isotonic lengthening and shortening movements of cat soleus muscle. J Physiol. 1969 Oct;204(2):475–491. doi: 10.1113/jphysiol.1969.sp008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. The effects of load and force on tremor at the normal human elbow joint. J Physiol. 1974 Jul;240(2):375–396. doi: 10.1113/jphysiol.1974.sp010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juricskay S. Mechanical characteristics of resting and contracting muscle. Acta Biochim Biophys Acad Sci Hung. 1974;9(1-2):151–158. [PubMed] [Google Scholar]
- LANCE J. W., DEGAIL P. SPREAD OF PHASIC MUSCLE REFLEXES IN NORMAL AND SPASTIC SUBJECTS. J Neurol Neurosurg Psychiatry. 1965 Aug;28:328–334. doi: 10.1136/jnnp.28.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippold O. C. Oscillation in the stretch reflex arc and the origin of the rhythmical, 8-12 C-S component of physiological tremor. J Physiol. 1970 Feb;206(2):359–382. doi: 10.1113/jphysiol.1970.sp009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomić A., Rosenfalck A., Buchthal F. Electrical and mechanical responses of normal and myasthenic muscle. Brain Res. 1968 Aug 5;10(1):1–78. doi: 10.1016/0006-8993(68)90227-8. [DOI] [PubMed] [Google Scholar]
- Tracey D. J., Walmsley B., Brinkman J. 'Long-loop' reflexes can be obtained in spinal monkeys. Neurosci Lett. 1980 May 15;18(1):59–65. doi: 10.1016/0304-3940(80)90213-x. [DOI] [PubMed] [Google Scholar]


