Abstract
1. Intracellular responses were recorded from on-centre and off-centre ganglion cells in isolated eyecups of the mudpuppy, Necturus maculosus.
2. Current—voltage relations were measured in darkness, during illumination of the receptive field centre, and after chemically mediated synaptic inputs were blocked by 4 mM-cobalt chloride.
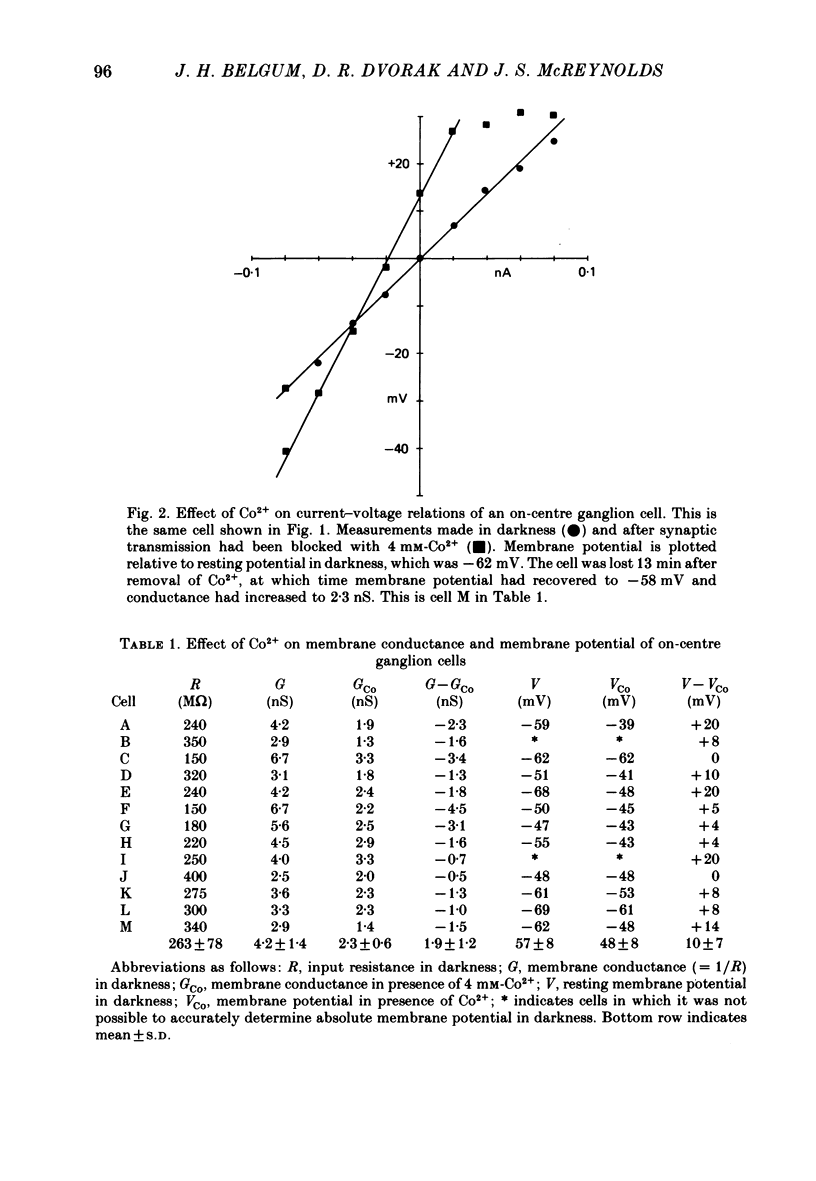
3. In on-centre cells the membrane potential in darkness was -56±6 mV (mean±S.D.). Addition of Co2+ resulted in an average depolarization of 10 mV and an average decrease in conductance of 2·1 nS. These results suggest that in darkness on-centre cells are tonically inhibited by synaptic input which increases conductance and has a reversal potential more negative than the dark membrane potential.
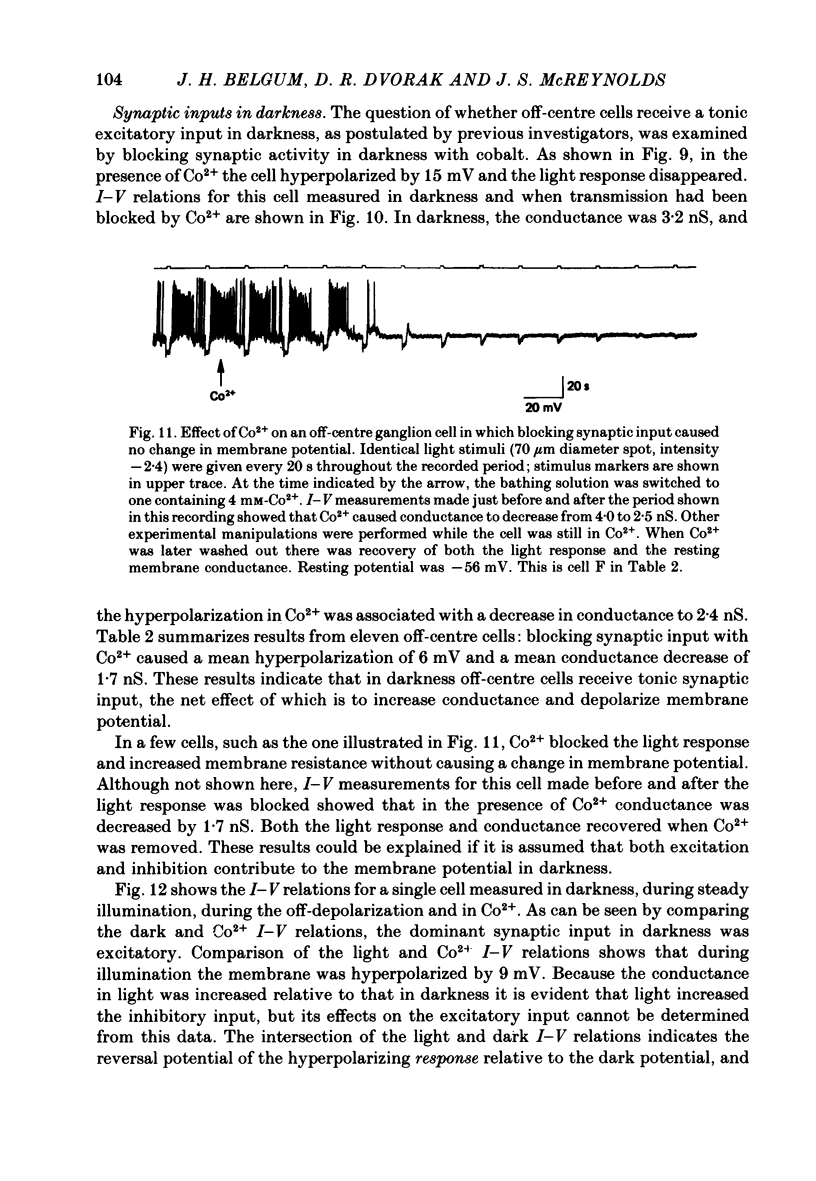
In off-centre cells the membrane potential in darkness was -46±5 mV. Addition of Co2+ caused an average hyperpolarization of 6 mV and an average decrease in conductance of 1·5 nS. These results suggest that in darkness off-centre cells receive a tonic excitatory input which increases conductance and has a reversal potential more positive than the dark membrane potential.
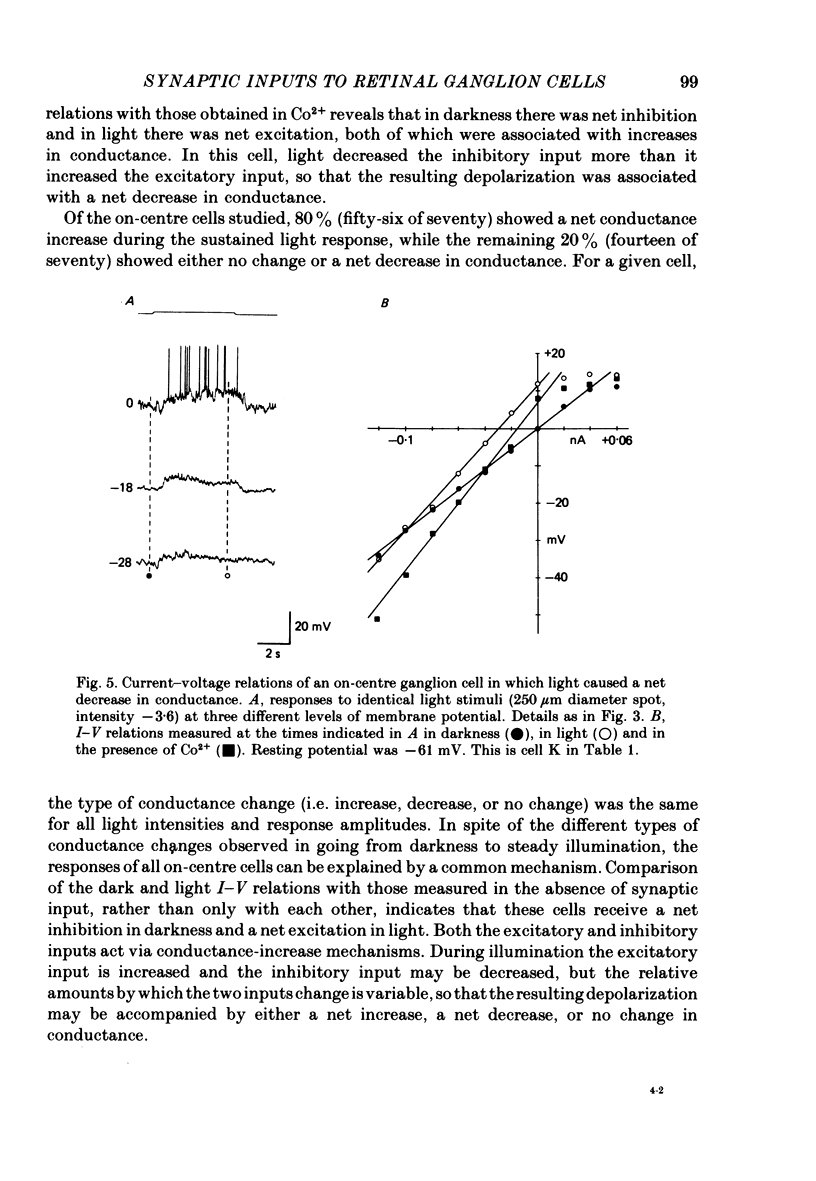
4. In on-centre cells light causes a sustained depolarization. This response involves an increase in a tonic excitatory input which increases conductance and has a reversal potential more positive than the dark membrane potential.
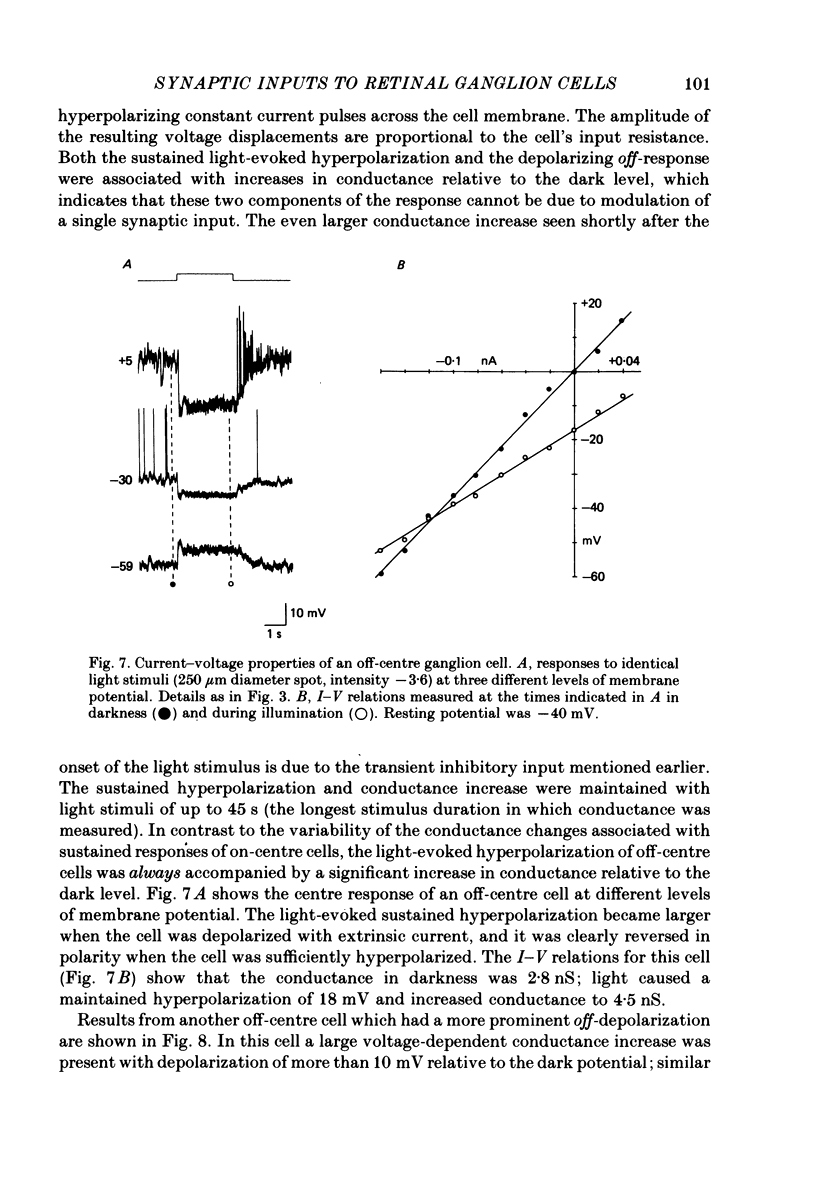
5. In off-centre cells, light causes a sustained hyperpolarization. This response involves an increase in a sustained inhibitory input which increases conductance and has a reversal potential more negative than the dark membrane potential.
6. The depolarizing off-response of off-centre cells is associated with an increase in an excitatory input which increases conductance and has a reversal potential more positive than the dark membrane potential. This response may be due to a temporary increase in the excitatory input which is tonically active in darkness or may reflect an additional excitatory input.
7. It is suggested that in both on- and off-centre ganglion cells the balance of sustained excitatory and inhibitory synaptic inputs determines the resting potential in darkness. Centre illumination alters the balance of these inputs, by increasing one and decreasing the other, to produce the characteristic sustained light responses.
8. The possible presynaptic sources of the sustained excitatory and inhibitory inputs are discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fettiplace R. Transmission from photoreceptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):391–424. doi: 10.1113/jphysiol.1977.sp012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Chan R. Y., Naka K. The amacrine cell. Vision Res. 1976;16(10):1119–1129. doi: 10.1016/0042-6989(76)90252-2. [DOI] [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Frumkes T. E., Miller R. F. Pathways and polarities of synaptic interactions in the inner retina of the mudpuppy: I. Synaptic blocking studies. Brain Res. 1979 Jan 26;161(1):1–12. doi: 10.1016/0006-8993(79)90191-4. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Miller R. F. Photoreceptor-bipolar cell transmission in the perfused retina eyecup of the mudpuppy. Science. 1976 Mar 5;191(4230):963–964. doi: 10.1126/science.175443. [DOI] [PubMed] [Google Scholar]
- Davis G. W., Naka K. Spatial organization of catfish retinal neurons. I. Single- and random-bar stimulation. J Neurophysiol. 1980 Mar;43(3):807–831. doi: 10.1152/jn.1980.43.3.807. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Pure central responses from off-centre cells and pure surround responses from on-centre cells. J Physiol. 1972 Jan;220(2):441–464. doi: 10.1113/jphysiol.1972.sp009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr Functional architecture of cone bipolar cells in mammalian retina. Vision Res. 1981;21(11):1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kaneko A., Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977 Dec 23;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J Physiol. 1975 Nov;252(2):509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwoski C. J., Brukhardt D. A. Ganglion cell responses of the mudpuppy retina to flashing and moving stimuli. Vision Res. 1976;16(12):1483–1495. doi: 10.1016/0042-6989(76)90169-3. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. Variability in ganglion cell firing patterns; implications for separate "on" and "off" processes. Vision Res. 1977;17(7):765–776. doi: 10.1016/0042-6989(77)90118-3. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Lam D. M. Glycinergic pathways in the goldfish retina. J Neurosci. 1981 Feb;1(2):152–165. doi: 10.1523/JNEUROSCI.01-02-00152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. An autoradiographic study of the cells accumulating 3H gamma-aminobutyric acid in the isolated retinas of pigeons and chickens. Invest Ophthalmol. 1974 Aug;13(8):602–607. [PubMed] [Google Scholar]
- Marshall L. M., Werblin F. S. Synaptic transmission to the horizontal cells in the retina of the larval tiger salamander. J Physiol. 1978 Jun;279:321–346. doi: 10.1113/jphysiol.1978.sp012347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. II. Chloride-dependent ganglion cell mechanisms. J Gen Physiol. 1976 Jun;67(6):661–678. doi: 10.1085/jgp.67.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. III. A model of ganglion cell receptive field organization based on chloride-free experiments. J Gen Physiol. 1976 Jun;67(6):679–690. doi: 10.1085/jgp.67.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y. Identification of amacrine and ganglion cells in the carp retina. J Physiol. 1977 Jan;264(3):801–818. doi: 10.1113/jphysiol.1977.sp011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. Functional organization of catfish retina. J Neurophysiol. 1977 Jan;40(1):26–43. doi: 10.1152/jn.1977.40.1.26. [DOI] [PubMed] [Google Scholar]
- Naka K., Otsuka T. Morphological and functional identifications of catfish retinal neurons. II. Morphological identification. J Neurophysiol. 1975 Jan;38(1):72–91. doi: 10.1152/jn.1975.38.1.72. [DOI] [PubMed] [Google Scholar]
- Nelson R., Famiglietti E. V., Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978 Mar;41(2):472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. R. Comparison of the responses of Necturus retinal ganglion cells to stationary and moving stimuli. Vision Res. 1977;17(7):777–786. doi: 10.1016/0042-6989(77)90119-5. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R., Marchiafava P. L. Physiological and morphological study of the inner plexiform layer in the turtle retina. Vision Res. 1981;21(11):1635–1638. doi: 10.1016/0042-6989(81)90047-x. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wu S. M., Dowling J. E. L-aspartate: evidence for a role in cone photoreceptor synaptic transmission in the carp retina. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5205–5209. doi: 10.1073/pnas.75.10.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunk D. F., Werblin F. S. Synaptic inputs to the ganglion cells in the tiger salamander retina. J Gen Physiol. 1979 Mar;73(3):265–286. doi: 10.1085/jgp.73.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]


