Abstract
1. The intracellular Cl- activity (aCli) of isolated crayfish stretch receptor neurones was measured using liquid ion exchanger Cl--selective micro-electrodes. The potential developed due to the difference between the normal extracellular Cl- activity (aClo) and aCli (VCl) was compared with the simultaneously measured reversal potential of the inhibitory post-synaptic potential (Ei.p.s.p.) to further clarify the ionic basis of the i.p.s.p..
2. In normal Ringer solution, VCl (63·3 ± 2·3 mV) was found to be close to the resting membrane potential (Em, 62·6 ± 3·9 mV) while Ei.p.s.p. (74·5 ± 1·9 mV) was more negative than either. The VCl value corresponds to an apparent aCli of 12·7 ± 1·3 mM, which is about 4 mM more than required for a Cl- governed Ei.p.s.p. of 74·5 mV.
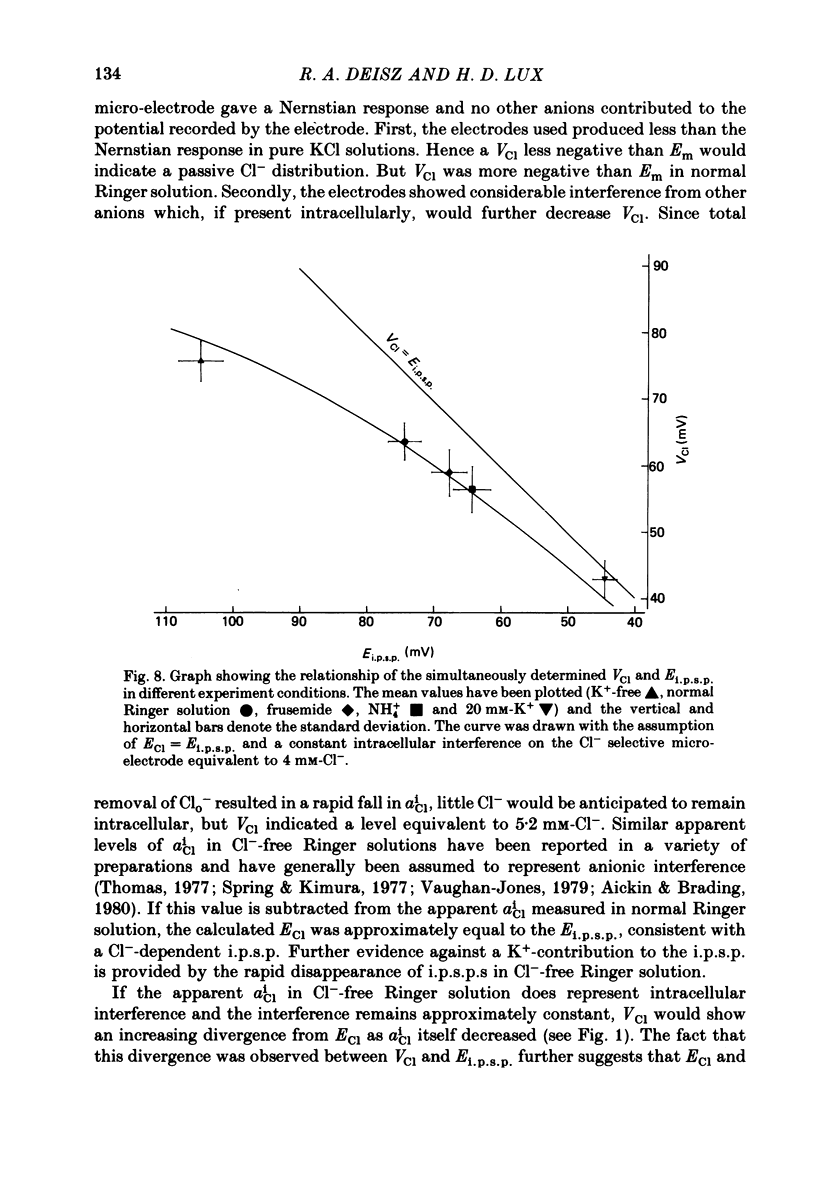
3. Reducing aClo caused smaller changes in VCl than predicted for passive Cl- re-distributions. On complete removal of extracellular Cl- (Clo-), VCl increased to 84·6 ± 2·7 mV, equivalent to an apparent aCli of about 5 mM-Cl-. This value can be used as an estimate of the level of intracellular interference on the Cl--selective micro-electrode.
4. Increasing extracellular K+ (K0+) decreased both VCl and Ei.p.s.p.. Decreasing Ko+ had the converse effect. The time course of the changes in VCl and Ei.p.s.p. was much the same. The difference between VCl and Ei.p.s.p. decreased to about 3 mV in high Ko+, and increased to about 30 mV in low Ko+. This variation in the difference between Ei.p.s.p. and VCl is consistent with the assumption that anions other than Cl- contribute to the recorded VCl rather than another ion contributes to the inhibitory current.
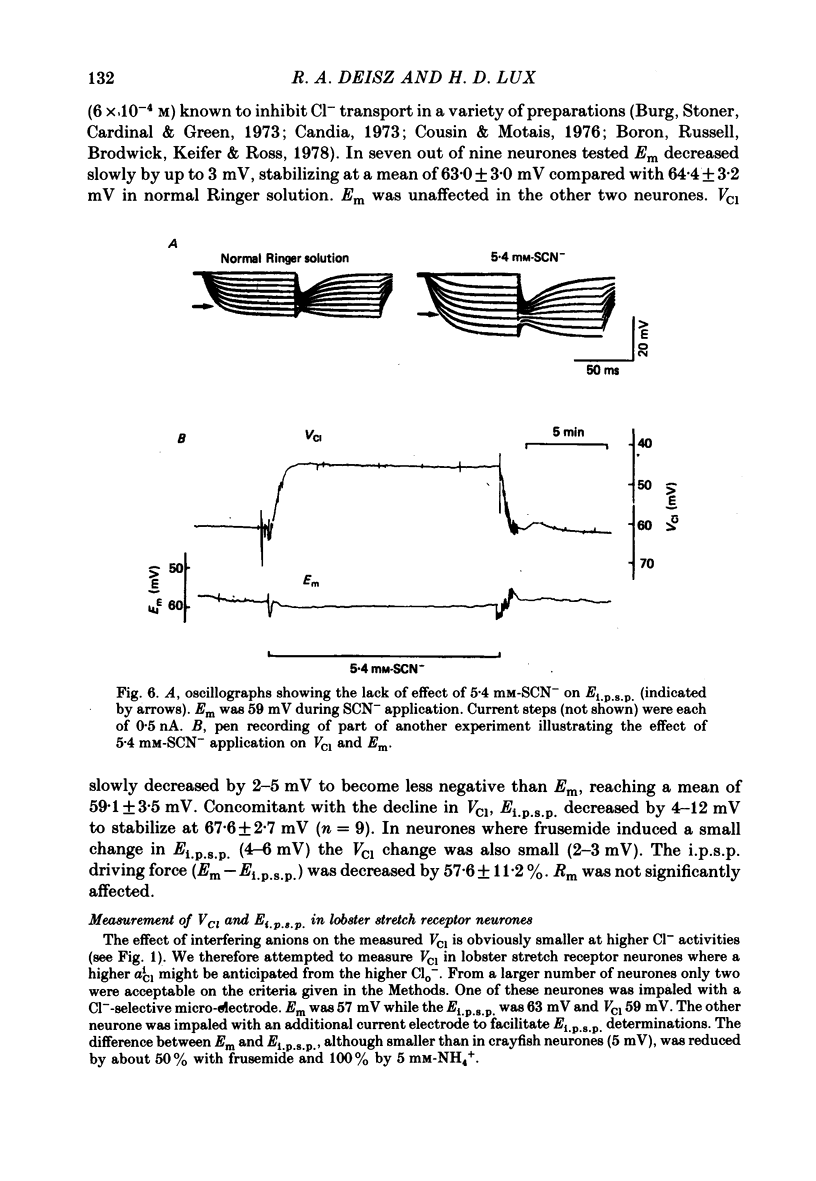
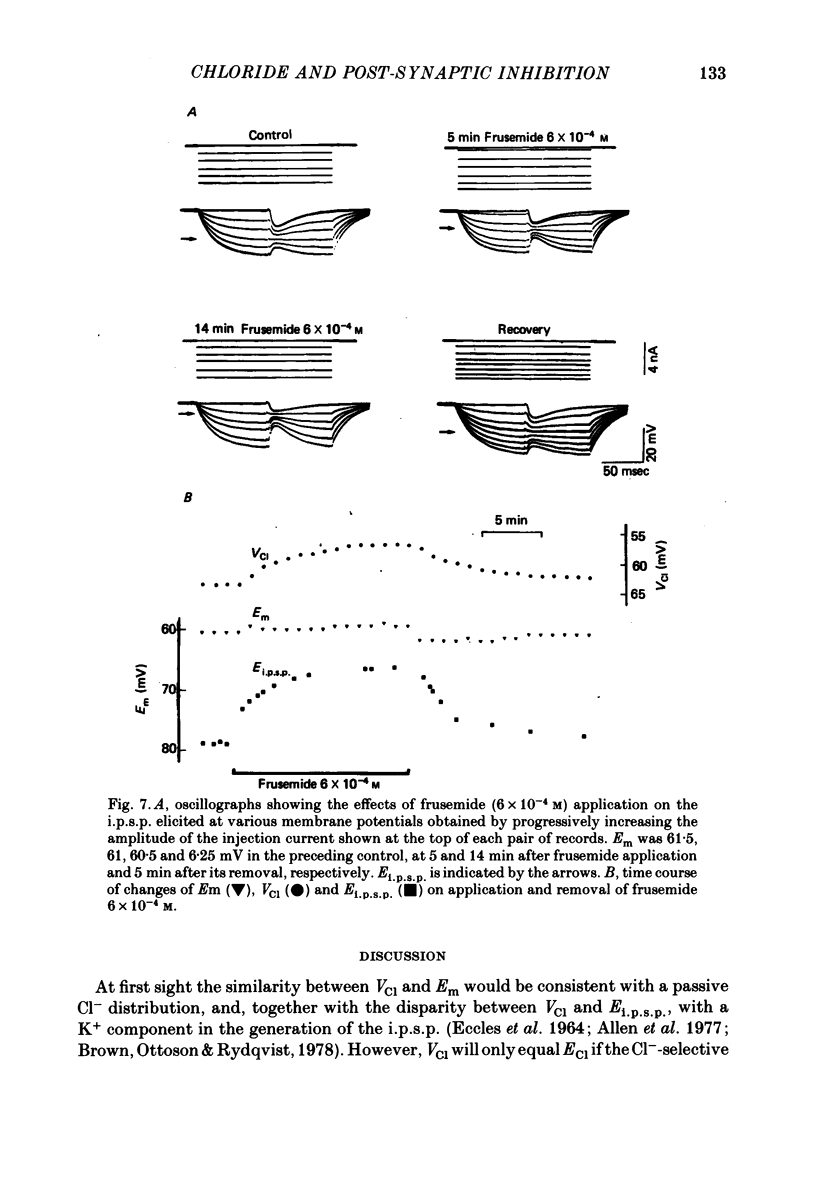
5. Application of 5 mM-NH4+ or of frusemide (6 × 10-4 M) decreased VCl and Ei.p.s.p.. The difference between VCl and Ei.p.s.p. was also decreased.
6. We conclude that aCli is lower than predicted from a passive distribution and thus the chloride equilibrium potential (ECl) is more negative than Em. If a constant intracellular interference equivalent to about 4 mM-Cl- is assumed to contribute to the recorded VCl, ECl was approximately equal to Ei.p.s.p. in all the experimental conditions. Therefore we suggest that the i.p.s.p. is solely generated by Cl- ions.
Full text
PDF


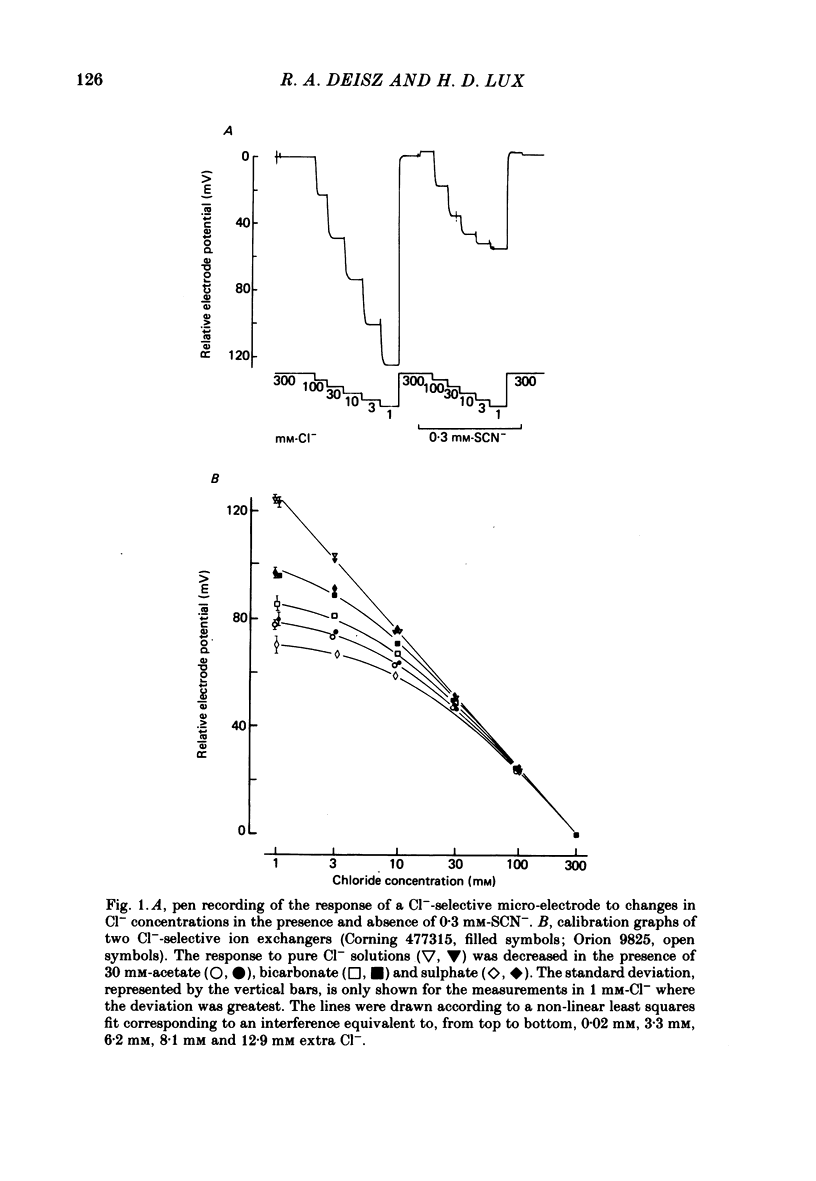
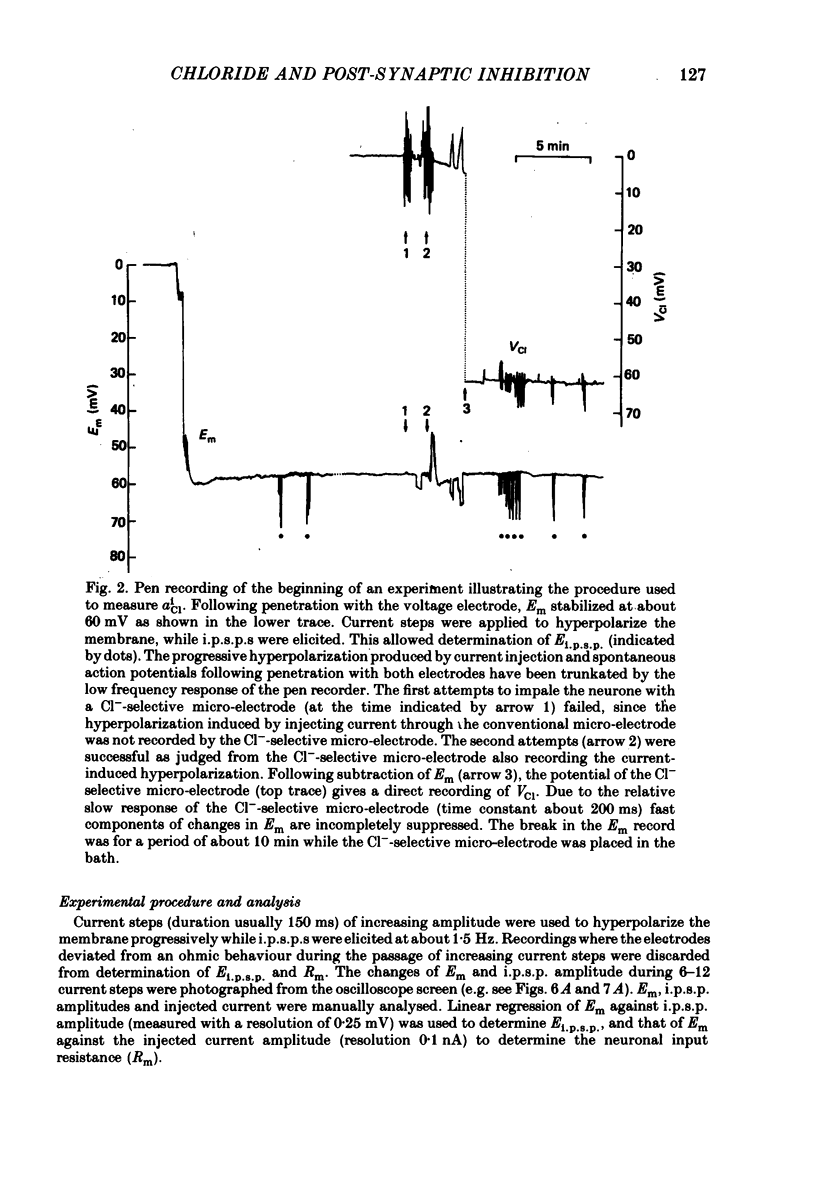

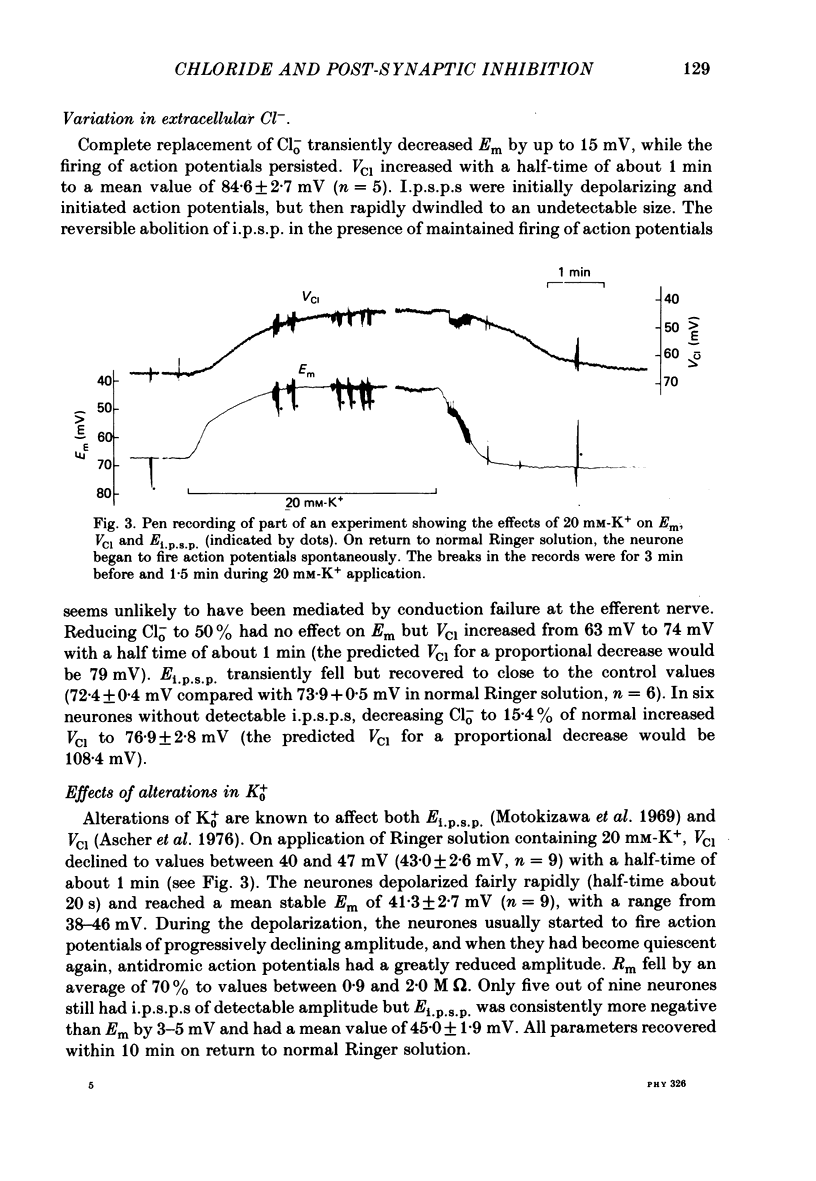
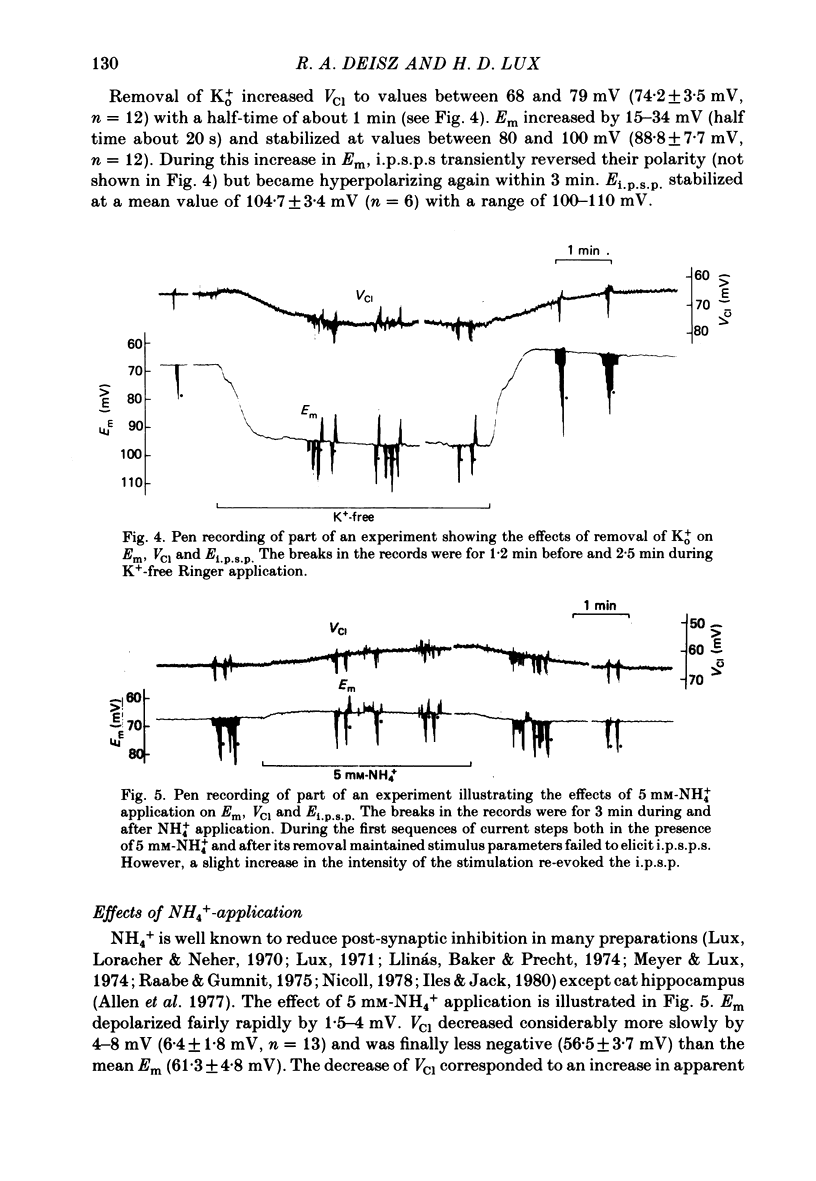





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., ITO M., OSCARSSON O. Anion permeability of the synaptic and non-synaptic motoneurone membrane. J Physiol. 1961 Dec;159:410–435. doi: 10.1113/jphysiol.1961.sp006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A., Lux H. D. On the action of the anticonvulsant 5,5-diphenylhydantoin and the convulsant picrotoxin in crayfish stretch receptor. J Physiol. 1981 Jun;315:157–173. doi: 10.1113/jphysiol.1981.sp013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. I., Eccles J., Nicoll R. A., Oshima T., Rubia F. J. The ionic mechanisms concerned in generating the i.p.s.ps of hippocampal pyramidal cells. Proc R Soc Lond B Biol Sci. 1977 Sep 19;198(1133):363–384. doi: 10.1098/rspb.1977.0103. [DOI] [PubMed] [Google Scholar]
- Ascher P., Kunze D., Neild T. O. Chloride distribution in Aplysia neurones. J Physiol. 1976 Apr;256(2):441–464. doi: 10.1113/jphysiol.1976.sp011332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOISTEL J., FATT P. Membrane permeability change during inhibitory transmitter action in crustacean muscle. J Physiol. 1958 Nov 10;144(1):176–191. doi: 10.1113/jphysiol.1958.sp006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M., Brodwick M. S., Keifer D. W., Roos A. Influence of cyclic AMP on intracellular pH regulation and chloride fluxes in barnacle muscle fibers. Nature. 1978 Nov 30;276(5687):511–513. doi: 10.1038/276511a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Sutton R. B., Walker J. L., Jr Increased chloride conductance as the proximate cause of hydrogen ion concentration effects in Aplysia neurons. J Gen Physiol. 1970 Nov;56(5):559–582. doi: 10.1085/jgp.56.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Ottoson D., Rydqvist B. Crayfish stretch receptor: an investigation with voltage-clamp and ion-sensitive electrodes. J Physiol. 1978 Nov;284:155–179. doi: 10.1113/jphysiol.1978.sp012533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia O. A. Short-circuit current related to active transport of chloride in frog cornea: effects of furosemide and ethacrynic acid. Biochim Biophys Acta. 1973 Apr 16;298(4):1011–1014. doi: 10.1016/0005-2736(73)90407-0. [DOI] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976 Mar;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Aickin C. C., Lux H. D. Decrease of inhibitory driving force in crayfish stretch reception: a mechanism of the convulsant action of penicillin. Neurosci Lett. 1979 Mar;11(3):347–352. doi: 10.1016/0304-3940(79)90020-x. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. Intracellular chloride concentration and postsynaptic inhibition in crayfish stretch receptor neurons [proceedings]. Arzneimittelforschung. 1978;28(5):870–871. [PubMed] [Google Scholar]
- ECCLES J., ECCLES R. M., ITO M. EFFECTS PRODUCED ON INHIBITORY POSTSYNAPTIC POTENTIALS BY THE COUPLED INJECTIONS OF CATIONS AND ANIONS INTO MOTONEURONS. Proc R Soc Lond B Biol Sci. 1964 May 19;160:197–210. doi: 10.1098/rspb.1964.0036. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Maetz J., de Renzis G. Active transport of chloride by the teleost gill: inhibition by thiocyanate. Am J Physiol. 1973 Jun;224(6):1295–1299. doi: 10.1152/ajplegacy.1973.224.6.1295. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino N. Action of ammonium ions on the resting membrane of crayfish stretch receptor neuron. Jpn J Physiol. 1979;29(1):99–102. doi: 10.2170/jjphysiol.29.99. [DOI] [PubMed] [Google Scholar]
- Iles J. F., Jack J. J. Ammonia: assessment of its action on postsynaptic inhibition as a cause of convulsions. Brain. 1980 Sep;103(3):555–578. doi: 10.1093/brain/103.3.555. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Florey E. Inhibitory miniature potentials in the stretch receptor neurons of crayfish. J Gen Physiol. 1969 May;53(5):666–682. doi: 10.1085/jgp.53.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Llinas R., Baker R., Precht W. Blockage of inhibition by ammonium acetate action on chloride pump in cat trochlear motoneurons. J Neurophysiol. 1974 May;37(3):522–532. doi: 10.1152/jn.1974.37.3.522. [DOI] [PubMed] [Google Scholar]
- Lux H. D. Fast recording ion specific microelectrodes: their use in pharmacological studies in the CNS. Neuropharmacology. 1974 Jun;13(6):509–517. doi: 10.1016/0028-3908(74)90140-3. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Loracher C., Neher E. The action of ammonium on postsynaptic inhibition of cat spinal motoneurons. Exp Brain Res. 1970;11(5):431–447. doi: 10.1007/BF00233967. [DOI] [PubMed] [Google Scholar]
- Meyer H., Lux H. D. Action of ammonium on a chloride pump. Removal of hyperpolarizing inhibition in an isolated neuron. Pflugers Arch. 1974;350(2):185–195. doi: 10.1007/BF00586236. [DOI] [PubMed] [Google Scholar]
- Motokizawa F., Reuben J. P., Grundfest H. Ionic permeability of the inhibitory postsynaptic membrane of lobster muscle fibers. J Gen Physiol. 1969 Oct;54(4):437–461. doi: 10.1085/jgp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Thomas R. C. Intracellular chloride activity and the effects of acetylcholine in snail neurones. J Physiol. 1974 Oct;242(2):453–470. doi: 10.1113/jphysiol.1974.sp010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Effect of acetazolamide on sodium and chloride transport by in vitro rabbit ileum. Am J Physiol. 1975 Jun;228(6):1808–1814. doi: 10.1152/ajplegacy.1975.228.6.1808. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The blockade of GABA mediated responses in the frog spinal cord by ammonium ions and furosemide. J Physiol. 1978 Oct;283:121–132. doi: 10.1113/jphysiol.1978.sp012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Tsuda K. Membrane permeability change during inhibitory transmitter action in crayfish stretch receptor cell. J Neurophysiol. 1973 Sep;36(5):805–816. doi: 10.1152/jn.1973.36.5.805. [DOI] [PubMed] [Google Scholar]
- Ozeki M., Freeman A. R., Grundfest H. The membrane components of crustacean neuromuscular systems. II. Analysis of interactions among the electrogenic components. J Gen Physiol. 1966 Jul;49(6):1335–1349. doi: 10.1085/jgp.0491335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. P., PEPE F. The fine structure of inhibitory synapses in the crayfish. J Biophys Biochem Cytol. 1961 Oct;11:157–169. doi: 10.1083/jcb.11.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe W., Gumnit R. J. Disinhibition in cat motor cortex by ammonia. J Neurophysiol. 1975 Mar;38(2):347–355. doi: 10.1152/jn.1975.38.2.347. [DOI] [PubMed] [Google Scholar]
- Russell J. M. Effects of ammonium and bicarbonate-CO2 on intracellular chloride levels in Aplysia neurons. Biophys J. 1978 Apr;22(1):131–137. doi: 10.1016/S0006-3495(78)85478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. H., Brown H. M. Liquid and solid-state Cl- -sensitive microelectrodes. Characteristics and application to intracellular Cl- activity in Balanus photoreceptor. J Gen Physiol. 1977 Oct;70(4):507–530. doi: 10.1085/jgp.70.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring K. R., Kimura G. Chloride reabsorption by renal proximal tubules of Necturus. J Membr Biol. 1978 Jan 18;38(3):233–254. doi: 10.1007/BF01871924. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol. 1979 Oct;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watlington C. O., Jessee S. D., Baldwin G. Ouabain, acetazolamide, and Cl-flux in isolated frog skin: evidence for two distinct active Cl-transport mechanisms. Am J Physiol. 1977 Jun;232(6):F550–F558. doi: 10.1152/ajprenal.1977.232.6.F550. [DOI] [PubMed] [Google Scholar]
- ZADUNAISKY J. A., CANDIA O. A., CHIARANDINI D. J. THE ORIGIN OF THE SHORT-CIRCUIT CURRENT IN THE ISOLATED SKIN OF THE SOUTH AMERICAN FROG LEPTODACTYLUS OCELLATUS. J Gen Physiol. 1963 Nov;47:393–402. doi: 10.1085/jgp.47.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


