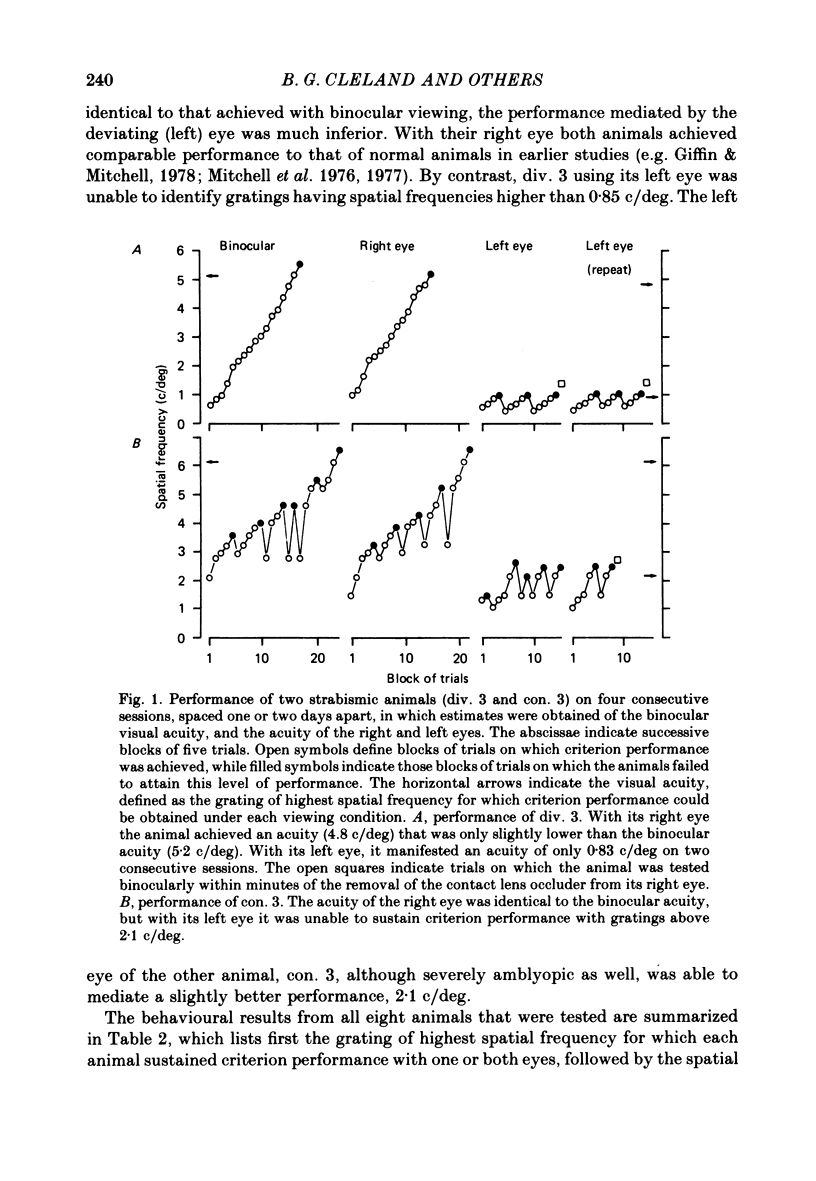
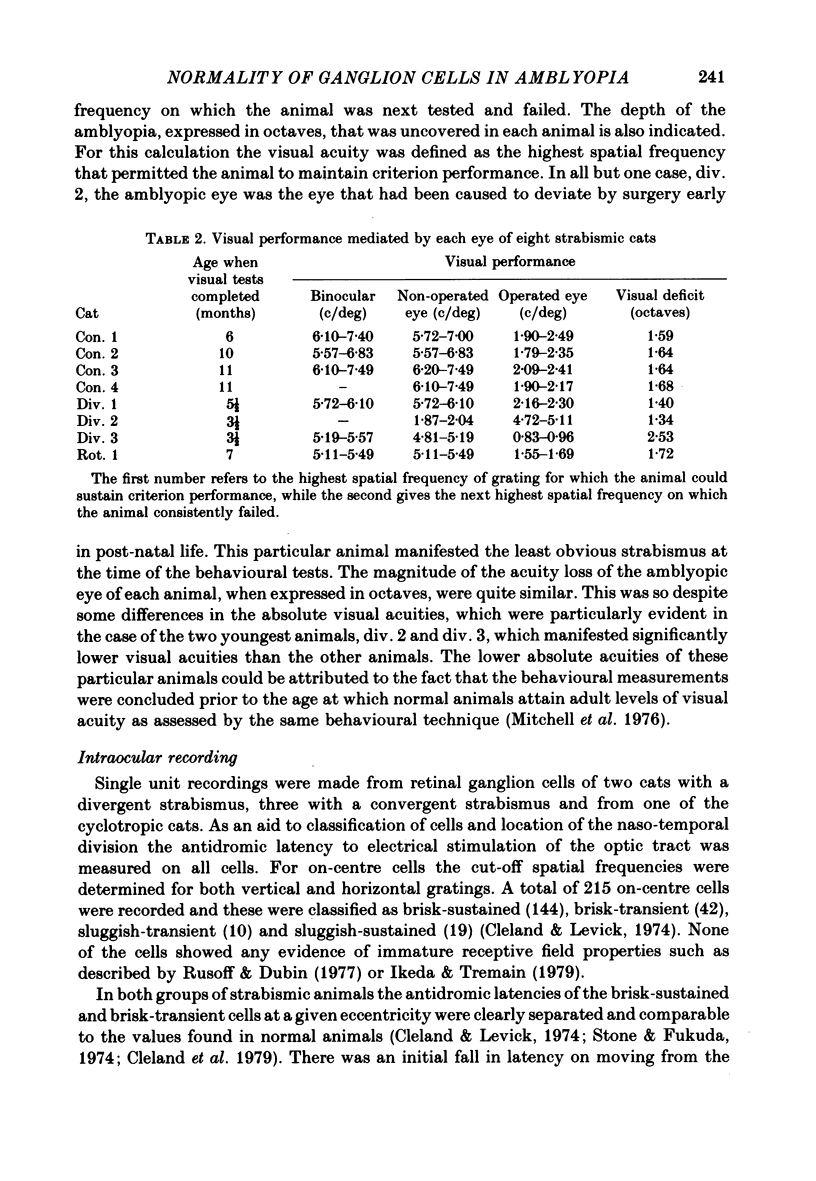
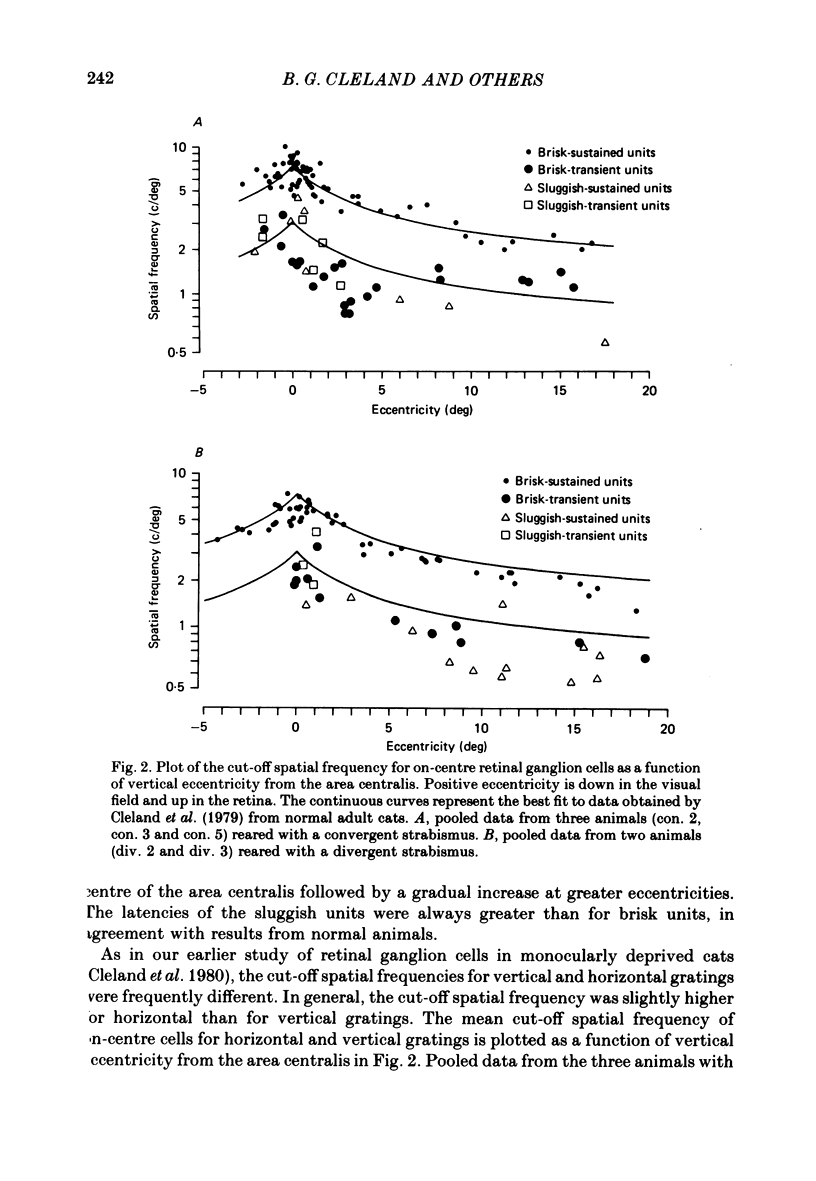
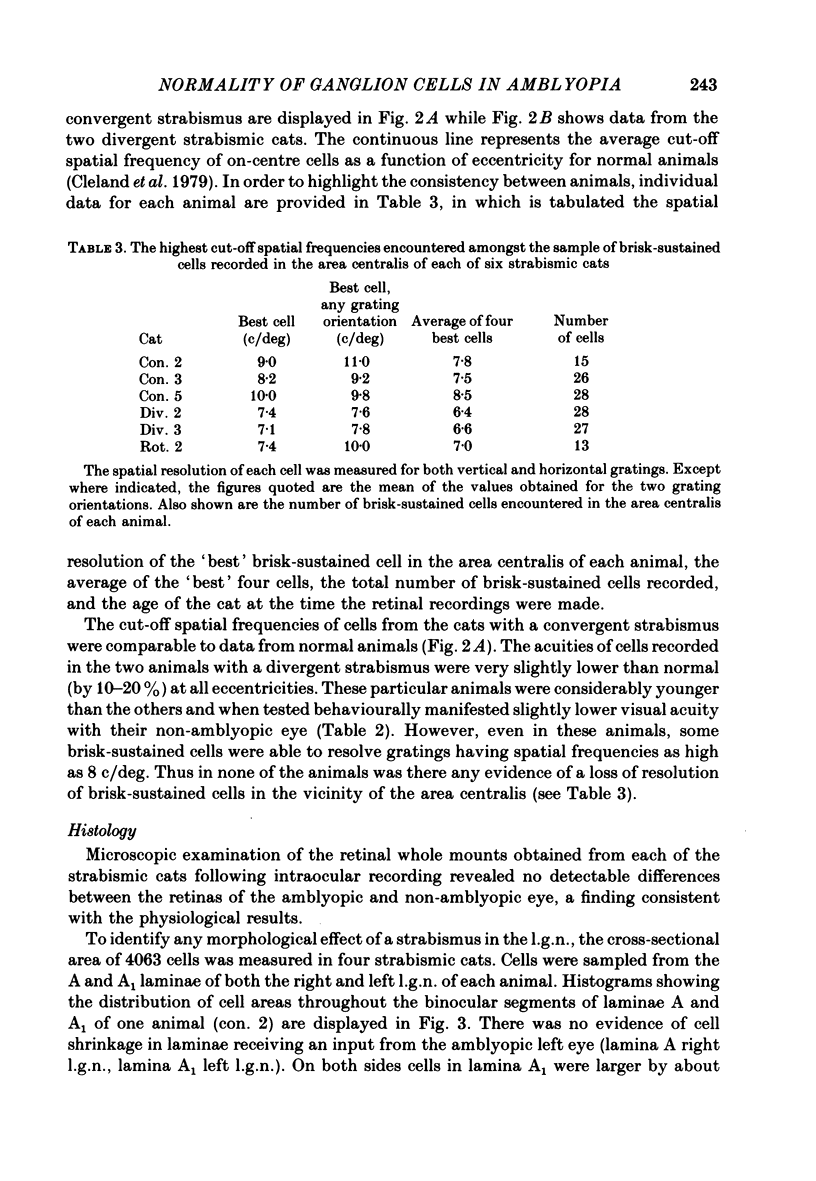
Abstract
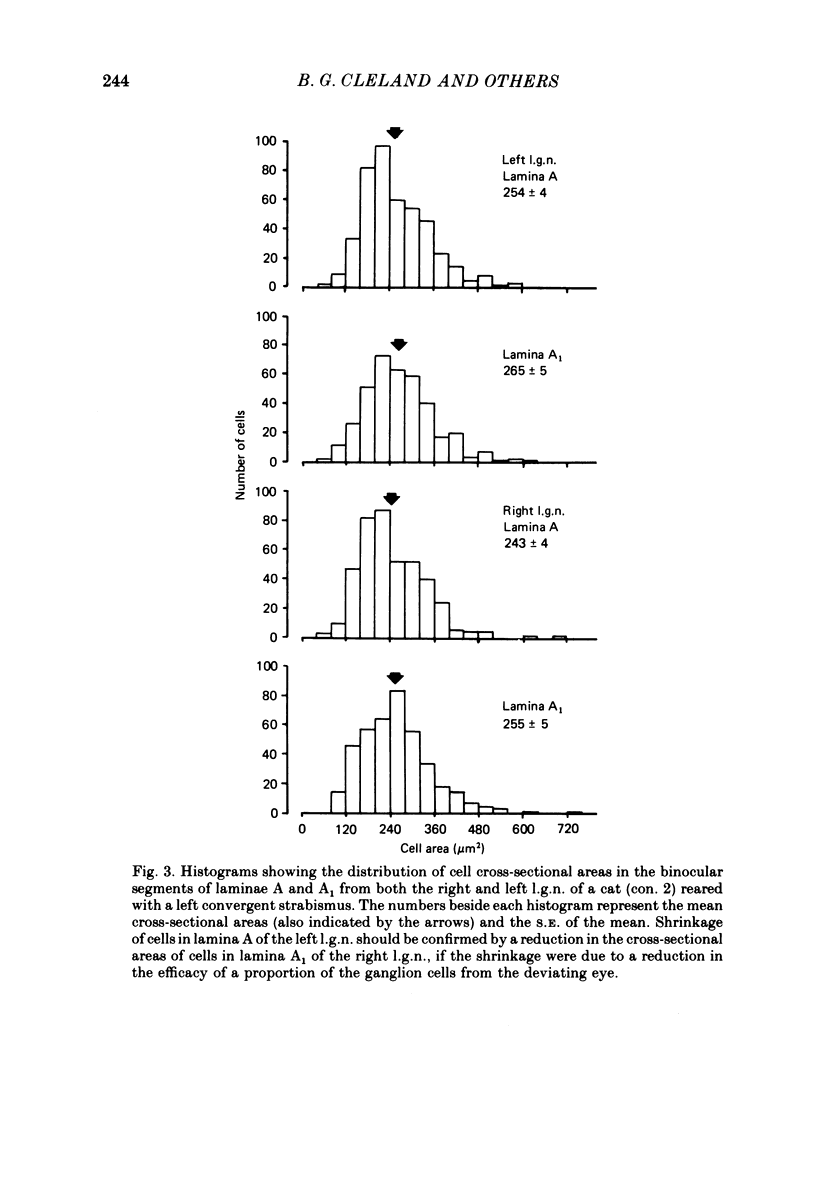
1. A convergent or divergent strabismus was induced surgically in eight kittens and a cyclotropia of about 90 deg in two additional kittens. 2. Behavioural measurements were made of the visual acuity of each eye for square-wave gratings. All eight animals that were so tested displayed a reduction of acuity in one eye relative to the other of 1.3-2.5 octaves. 3. The activity of retinal ganglion cells was recorded within the amblyopic eye of six cats, three with a convergent strabismus, two with a divergent strabismus and one with a cyclotropia. Measurements were made of the spatial resolution with 215 on-centre cells for horizontal and vertical gratings. 4. In contrast to other reports, we found the spatial resolution of ganglion cells in the amblyopic eye of the strabismic animals to be comparable to those of normal cats at all retinal eccentricities. In particular there was no evidence for a loss of resolution in the vicinity of the area centralis. 5. Measurement of the cross-sectional area of cells in the lateral geniculate nucleus (l.g.n.) revealed no evidence of cell shrinkage in laminae receiving a projection from the amblyopic eye. 6. Together, these findings lead to the conclusion that the neural deficit responsible for the strabismic amblyopia in these animals did not lie in the retina but rather at more central levels of the visual pathway.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chino Y. M., Shansky M. S., Hamasaki D. I. Development of receptive field properties of retinal ganglion cells in kittens raised with a convergent squint. Exp Brain Res. 1980;39(3):313–320. doi: 10.1007/BF00237120. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Harding T. H., Tulunay-Keesey U. Visual resolution and receptive field size: examination of two kinds of cat retinal ganglion cell. Science. 1979 Sep 7;205(4410):1015–1017. doi: 10.1126/science.472720. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Mitchell D. E., Gillard-Crewther S., Crewther D. P. Visual resolution of retinal ganglion cells in monocularly-deprived cats. Brain Res. 1980 Jun 16;192(1):261–266. doi: 10.1016/0006-8993(80)91026-4. [DOI] [PubMed] [Google Scholar]
- Crawford M. L., von Noorden G. K. The effects of short-term experimental strabismus on the visual system in Macaca mulatta. Invest Ophthalmol Vis Sci. 1979 May;18(5):496–505. [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Giffin F., Mitchell D. E. The rate of recovery of vision after early monocular deprivation in kittens. J Physiol. 1978 Jan;274:511–537. doi: 10.1113/jphysiol.1978.sp012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. The effect of lid suture upon the growth of cells in the dorsal lateral geniculate nucleus of kittens. J Comp Neurol. 1973 Apr 15;148(4):417–422. doi: 10.1002/cne.901480402. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Ikeda H. Is amblyopia a peripheral defect? Trans Ophthalmol Soc U K. 1979;99(3):347–352. [PubMed] [Google Scholar]
- Ikeda H., Plant G. T., Tremain K. E. Nasal field loss in kittens reared with convergent squint: neurophysiological and morphological studies of the lateral geniculate nucleus. J Physiol. 1977 Sep;270(2):345–366. doi: 10.1113/jphysiol.1977.sp011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. Amblyopia occurs in retinal ganglion cells in cats reared with convergent squint without alternating fixation. Exp Brain Res. 1979 May 2;35(3):559–582. doi: 10.1007/BF00236772. [DOI] [PubMed] [Google Scholar]
- Ikeda H. Visual acuity, its development and amblyopia. J R Soc Med. 1980 Aug;73(8):546–555. doi: 10.1177/014107688007300803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Is amblyopia due to inappropriate stimulation of the "sustained" pathway during development? Br J Ophthalmol. 1974 Mar;58(3):165–175. doi: 10.1136/bjo.58.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S. G., Ikeda H. Behavioural studies of spatial vision in cats reared with convergent squint: is amblyopia due to arrest of development? Exp Brain Res. 1979 Jan 2;34(1):11–26. doi: 10.1007/BF00238338. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Mangel S. C., Lehmkuhle S., Sherman M. Retinal X- and Y-cells in monocularly lid-sutured cats: normality of spatial and temporal properties. Brain Res. 1979 Aug 31;172(3):545–551. doi: 10.1016/0006-8993(79)90586-9. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Giffin F., Timney B. A behavioural technique for the rapid assessment of the visual capabilities of kittens. Perception. 1977;6(2):181–193. doi: 10.1068/p060181. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Giffin F., Wilkinson F., Anderson P., Smith M. L. Visual resolution in young kittens. Vision Res. 1976;16(4):363–366. doi: 10.1016/0042-6989(76)90197-8. [DOI] [PubMed] [Google Scholar]
- Rusoff A. C., Dubin M. W. Development of receptive-field properties of retinal ganglion cells in kittens. J Neurophysiol. 1977 Sep;40(5):1188–1198. doi: 10.1152/jn.1977.40.5.1188. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Stone J. Physiological normality of the retinal in visually deprived cats. Brain Res. 1973 Sep 28;60(1):224–230. doi: 10.1016/0006-8993(73)90861-5. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Timney B., Peck C. K. Visual acuity in cats following surgically induced cyclotropia. Behav Brain Res. 1981 Nov;3(3):289–302. doi: 10.1016/0166-4328(81)90001-2. [DOI] [PubMed] [Google Scholar]
- von Grünau M. W., Singer W. Functional amblyopia in kittens with unilateral exotropia. II. Correspondence between behavioural and electrophysiological assessment. Exp Brain Res. 1980;40(3):305–310. doi: 10.1007/BF00237795. [DOI] [PubMed] [Google Scholar]


