Abstract
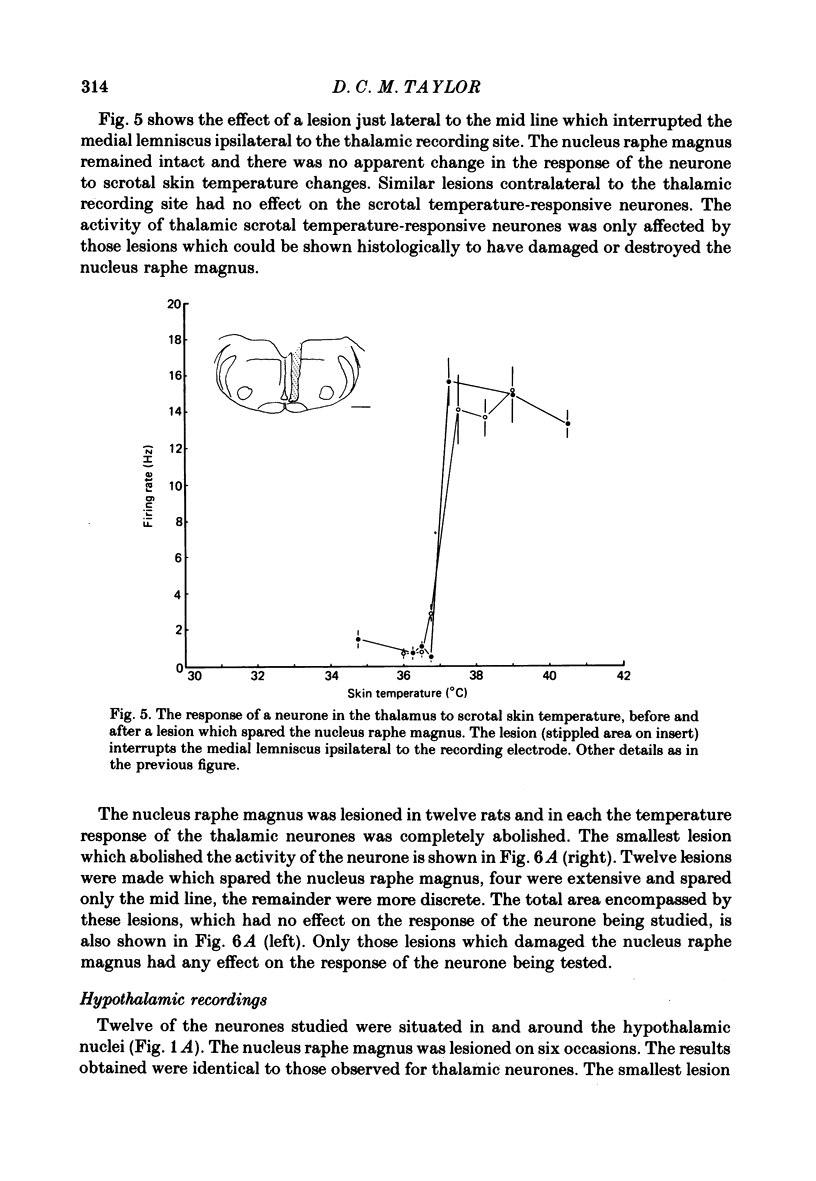
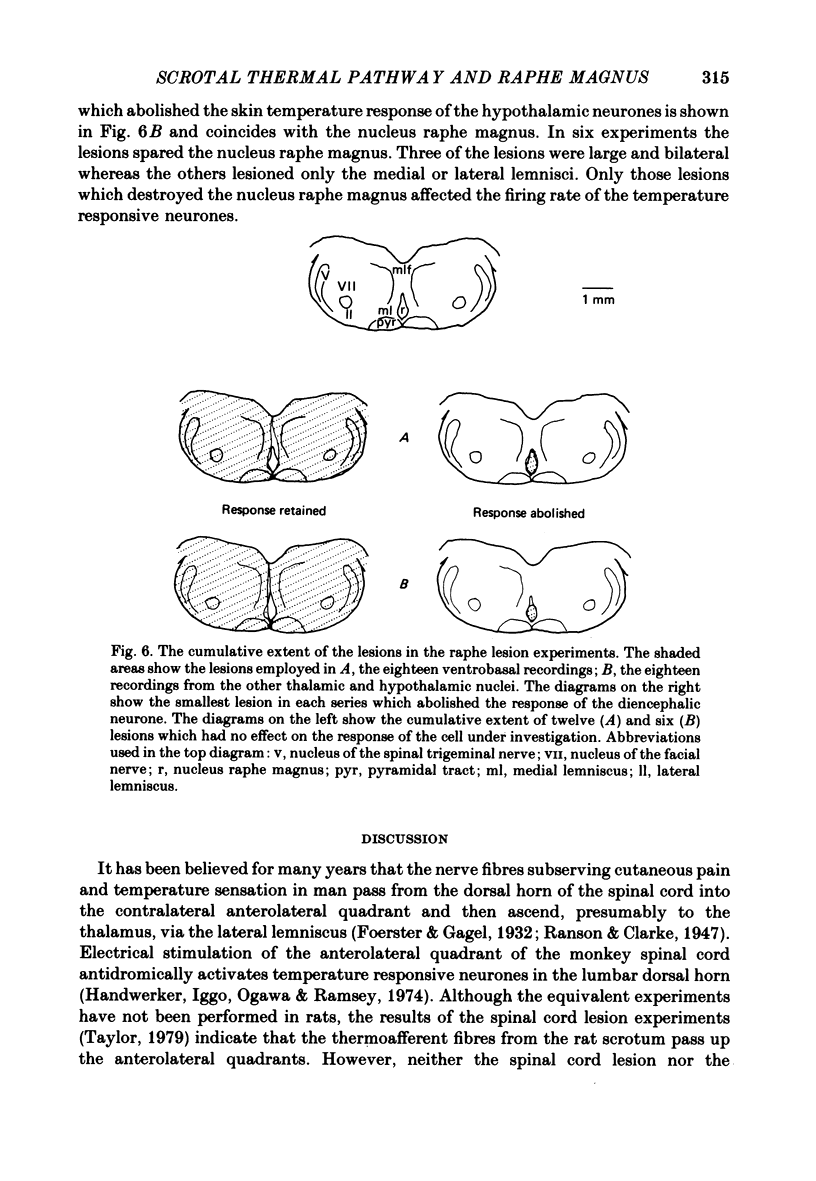
1. In the thalamus and hypothalamus of rats, anaesthetized with Urethane, single unit recordings have been made from cells which respond to small innocuous changes in scrotal skin temperature applied with a water-perfused brass thermode. 2. Once a scrotal temperature-sensitive neurone had been isolated the brain stem was electrolytically lesioned through implanted tungsten electrodes to determine whether the input from the scrotal skin temperature sensors ascends through the brain stem lemniscal pathways or the mid-line raphe nuclei. All recordings sites and lesions were identified histologically. 3. Thirty-six neurones have been studied of which half were located in the ventrobasal thalamus, six were located in the anterior thalamic nuclei and the remainder were in the medial hypothalamus. 4. The nucleus raphe magnus was lesioned on eighteen separate occasions; in each case the temperature-responsive activity of the thalamic or hypothalamic neurone was abolished. 5. Extensive brain-stem lesions which spared only the mid-line nucleus raphe magnus had no discernible effect on the responses of the thalamic or hypothalamic neurones to scrotal skin temperature. 6. The ascending pathway from the thermal sensors of the rat scrotal skin must pass through, or relay in, the nucleus raphe magnus.
Full text
PDF

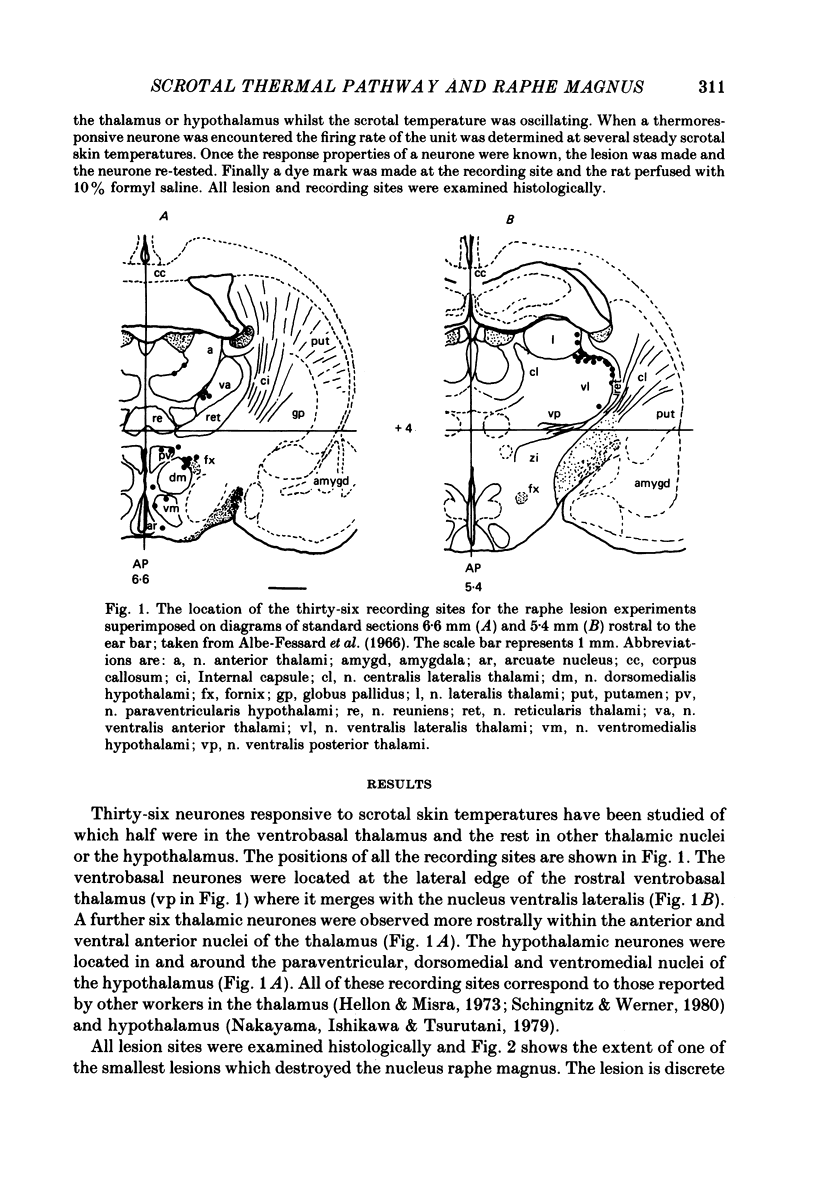
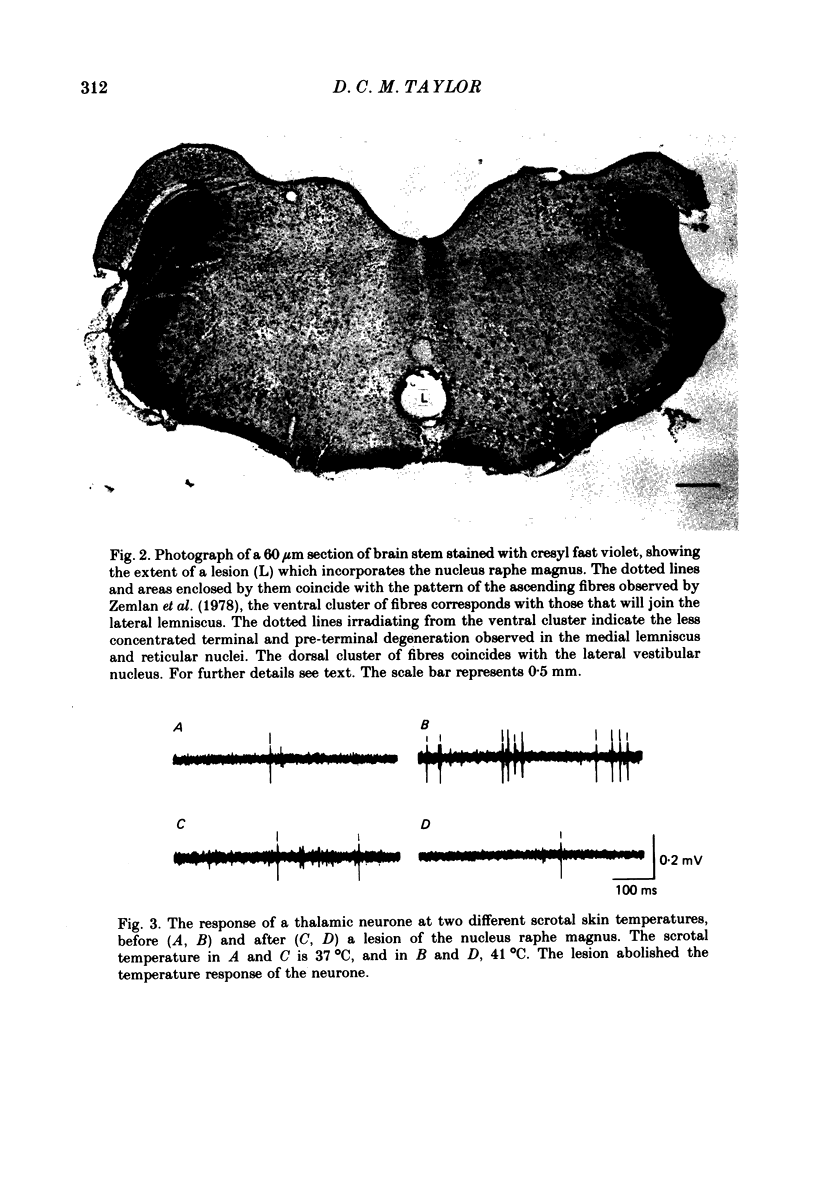
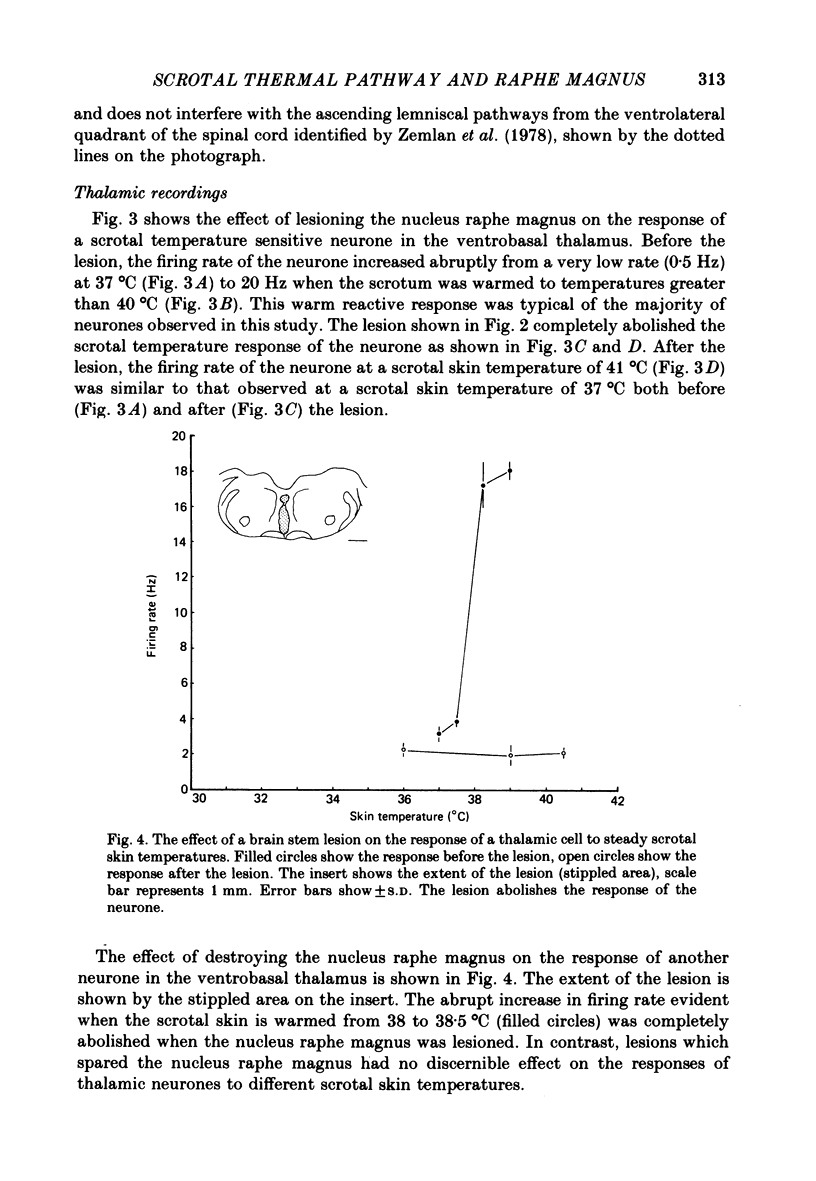



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobillier P., Seguin S., Petitjean F., Salvert D., Touret M., Jouvet M. The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976 Sep 3;113(3):449–486. doi: 10.1016/0006-8993(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Brück K., Hinckel P. Thermoregulatory noradrenergic and serotonergic pathways to hypothalamic units. J Physiol. 1980 Jul;304:193–202. doi: 10.1113/jphysiol.1980.sp013319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N. J., Dickenson A. H., Hellon R. F., Woolf C. J. Inhibitory controls on thermal neurones in the spinal trigeminal nucleus of cats and rats. Brain Res. 1981 Mar 30;209(2):440–445. doi: 10.1016/0006-8993(81)90167-0. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H. Specific responses of rat raphé neurones to skin temperature. J Physiol. 1977 Dec;273(1):277–293. doi: 10.1113/jphysiol.1977.sp012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker H. O., Iggo A., Ogawa H., Ramsey R. L. Input characteristics and rostral projection of dorsal horn neurones in the monkey. J Physiol. 1975 Jan;244(1):76P–77P. [PubMed] [Google Scholar]
- Hellon R. F., Taylor D. C. An analysis of a thermal afferent pathway in the rat. J Physiol. 1982 May;326:319–328. doi: 10.1113/jphysiol.1982.sp014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns R. Different projections of cutaneous thermal inputs to single units of the midbrain raphe nuclei. Brain Res. 1976 Jan 16;101(2):355–361. doi: 10.1016/0006-8993(76)90276-6. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Chern Y. F., Chern S. I. The effects of brain monoamine depletion on p-chlorophenyl-alanine-induced hypothermia. Experientia. 1978 Dec 15;34(12):1595–1596. doi: 10.1007/BF02034695. [DOI] [PubMed] [Google Scholar]
- Lister W. C., Woodget L. L. Precision stereotaxic equipment. J Physiol. 1972 Apr;222(2):130P–132P. [PubMed] [Google Scholar]
- Nakayama T., Ishikawa Y., Tsurutani T. Projection of scrotal thermal afferents to the preoptic and hypothalamic neurons in rats. Pflugers Arch. 1979 May 15;380(1):59–64. doi: 10.1007/BF00582613. [DOI] [PubMed] [Google Scholar]
- Norrsell U. Thermosensory defects after cervical spinal cord lesions in the cat. Exp Brain Res. 1979 May 2;35(3):479–494. doi: 10.1007/BF00236766. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Wurster R. D., Neya T., Yamasato T., Ulrich J. Generation and processing of peripheral temperature signals in mammals. Int J Biometeorol. 1980 Sep;24(3):243–252. doi: 10.1007/BF02249793. [DOI] [PubMed] [Google Scholar]
- Waites G. M. Temperature regulation and fertility in male and female mammals. Isr J Med Sci. 1976 Sep;12(9):982–993. [PubMed] [Google Scholar]
- Zemlan F. P., Leonard C. M., Kow L. M., Pfaff D. W. Ascending tracts of the lateral columns of the rat spinal cord: a study using the silver impregnation and horseradish peroxidase techniques. Exp Neurol. 1978 Nov;62(2):298–334. doi: 10.1016/0014-4886(78)90059-6. [DOI] [PubMed] [Google Scholar]