Abstract
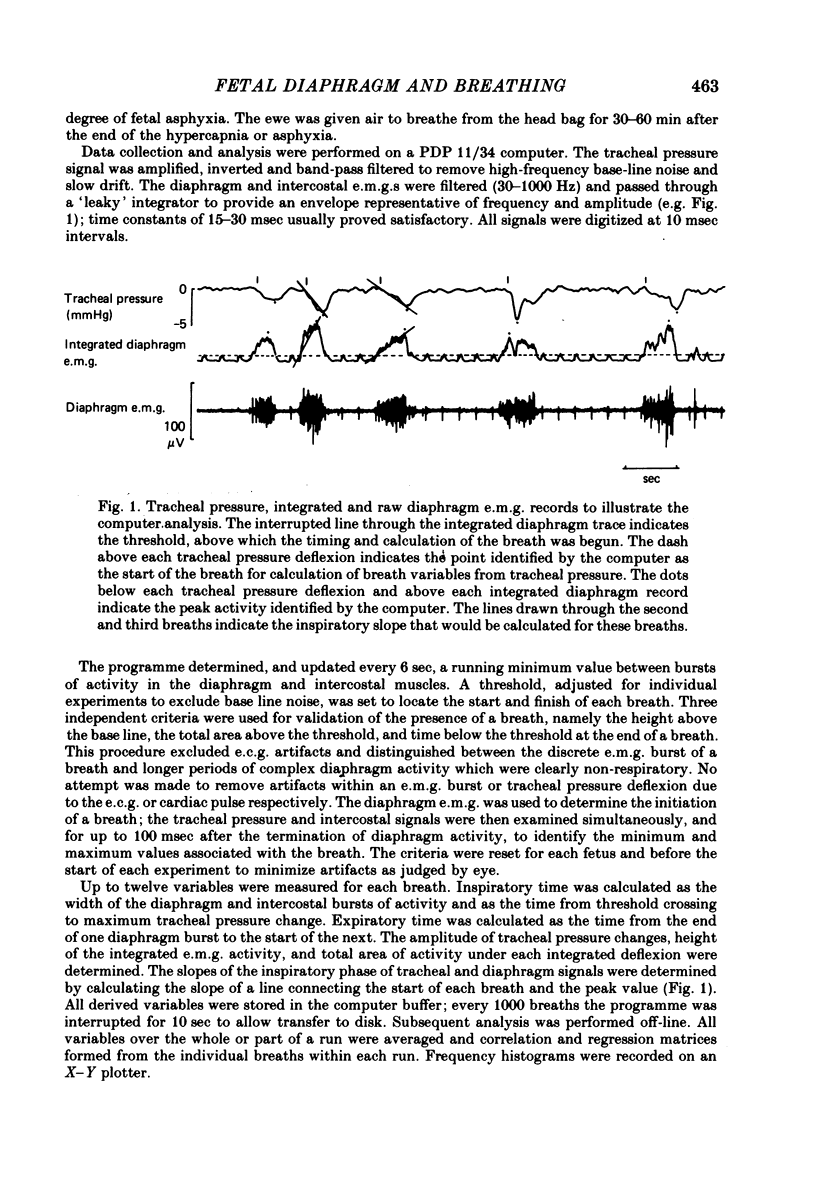
1. The electromyographic (e.m.g.) activity of the diaphragm and intercostal muscles has been recorded during breathing movements of unanaesthetized lambs in utero (109-135 days), and compared with the changes of tracheal pressure.
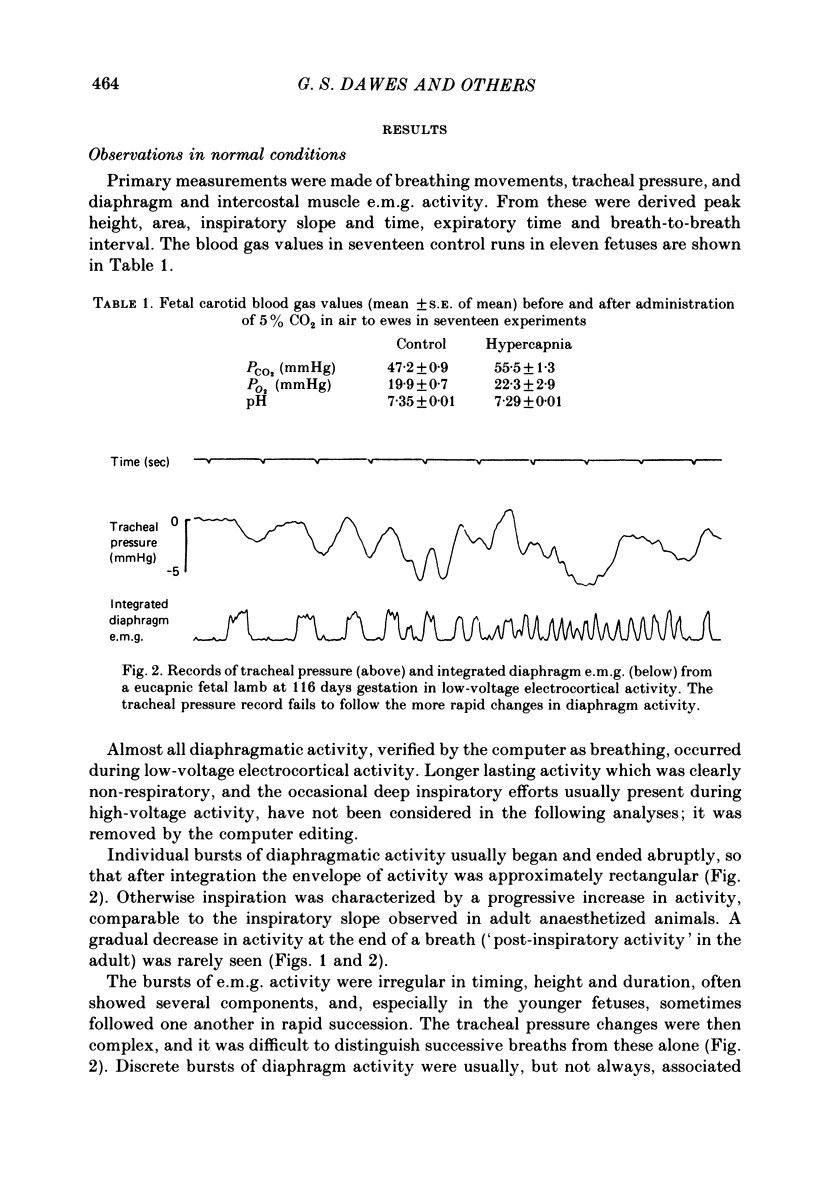
2. The diaphragm e.m.g. was irregular in size, shape and timing, with a variable rate of rise during inspiration, often with a flattening of integrated activity before the end of a breath and with little or no post-inspiratory activity.
3. The diaphragm e.m.g. gave the most reliable measurements of breath interval and incidence: in eucapnia mean TI was 0.45±0.02 (S.E. of mean) and TE was 0.74±0.05 sec, and 58-100% of the diaphragm bursts were associated with identifiable and appropriate changes of tracheal pressure.
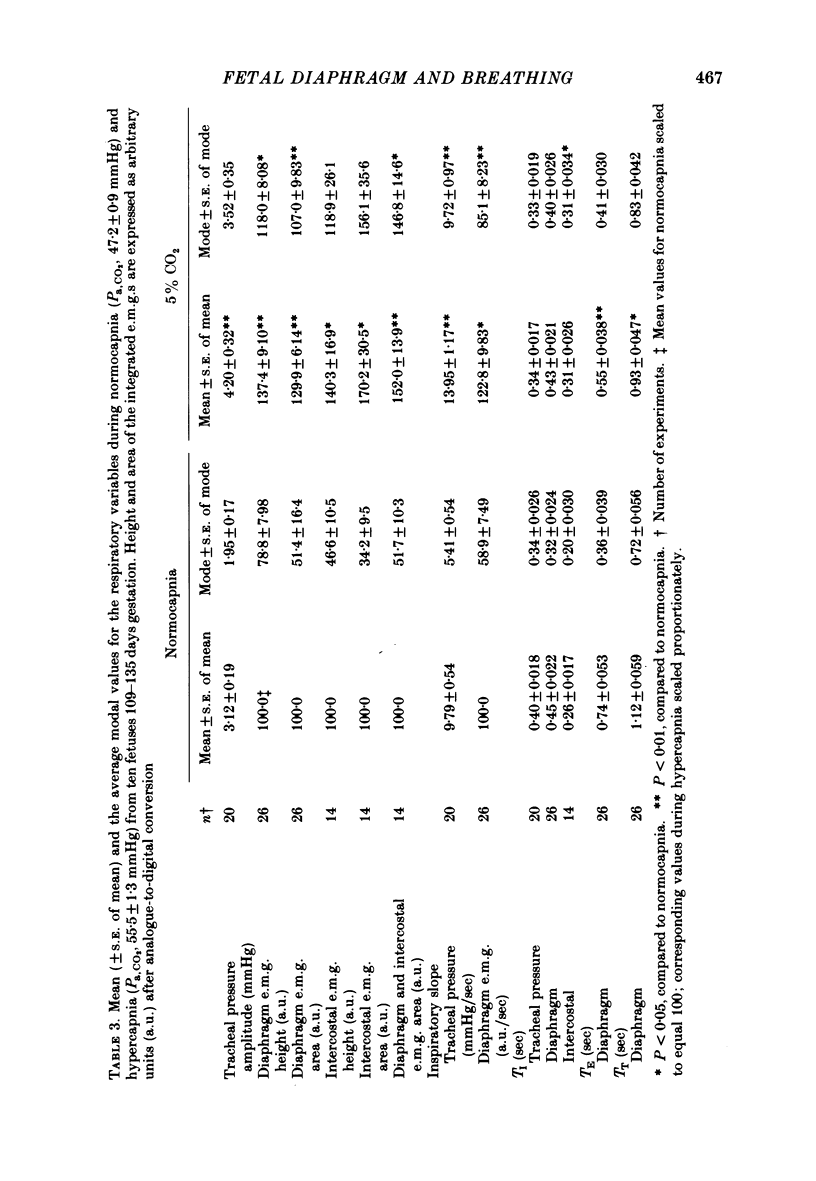
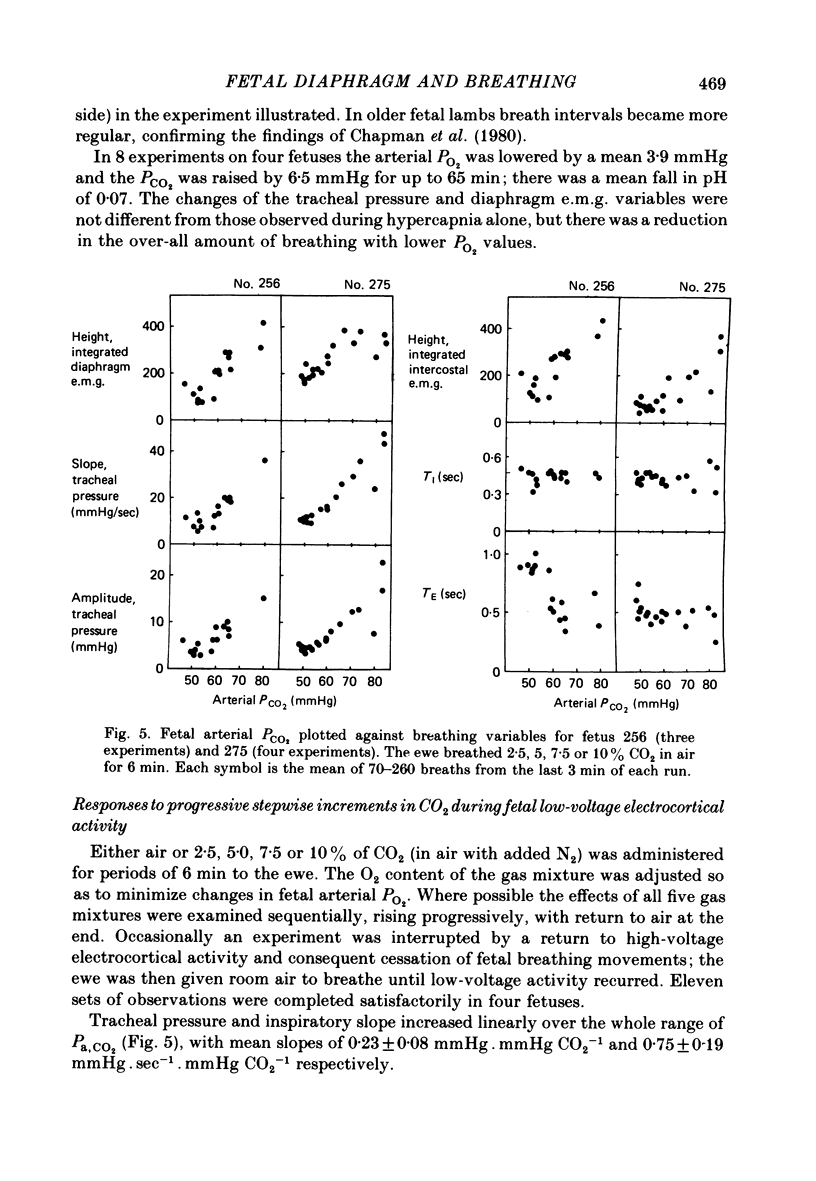
4. During fetal hypercapnia, produced by increasing the maternal inspired CO2 in a single change or series of step changes, tracheal pressure amplitude and its rate of change during inspiration increased progressively over the Pa, CO2 range of 37-87 mmHg.
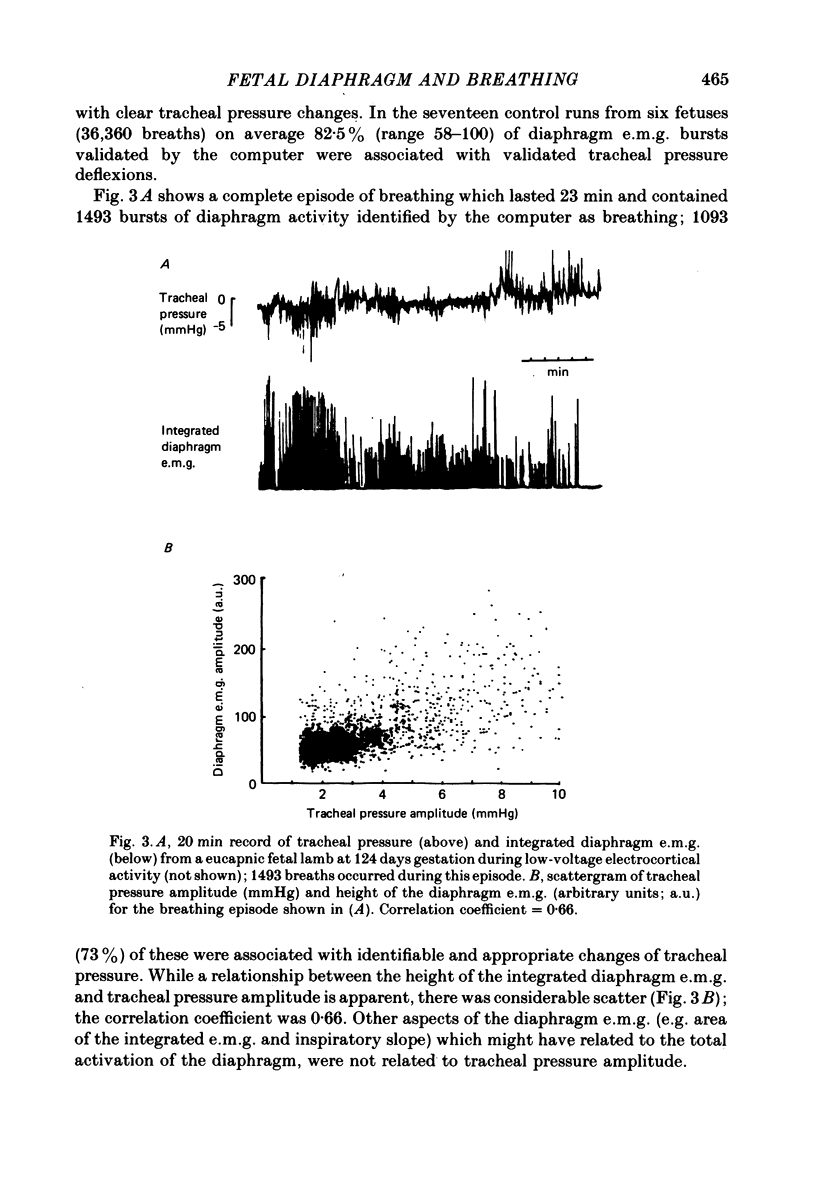
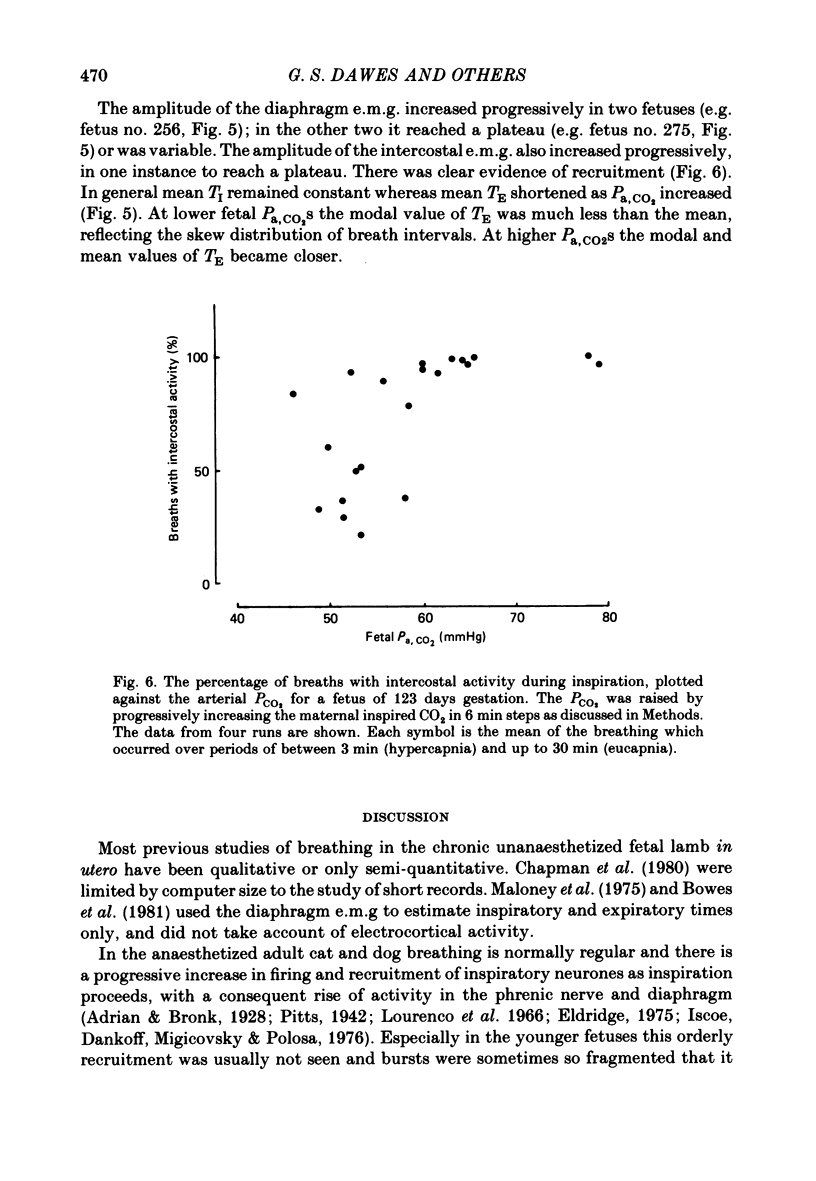
5. In eucapnia the area, amplitude and inspiratory slope of the integrated diaphragm e.m.g. were not always correlated with tracheal pressure amplitude, and in hypercapnia they increased only in the lower part of the Pa, CO2 range. Inspiratory intercostal activity increased progressively as the Pa, CO2 was raised.
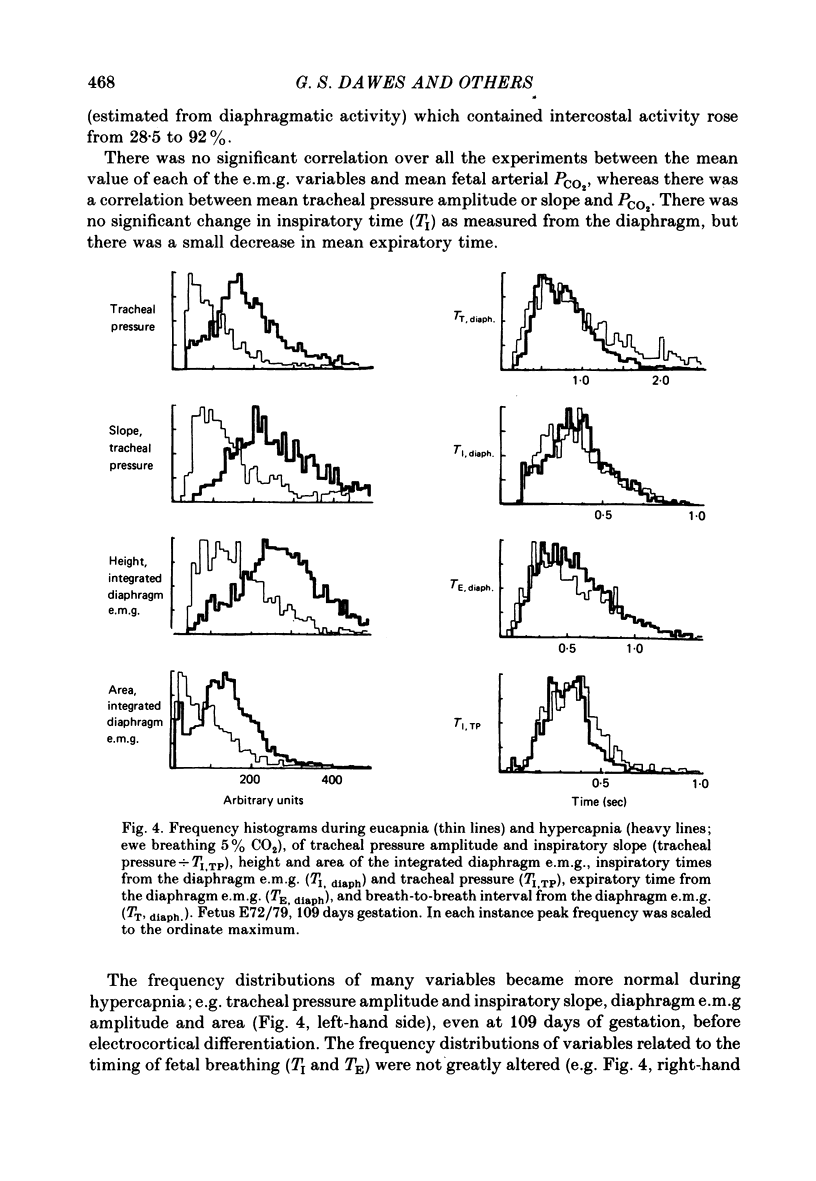
6. The frequency histograms of variables derived from the tracheal pressure, diaphragm and intercostal e.m.g.s were skewed to the left in eucapnia but became normalized during hypercapnia. The rate and depth of breathing became regular.
7. The response to mild asphyxia was a combination of the responses to hypercapnia and hypoxia.
8. The interpretation of the tracheal pressure and diaphragm e.m.g. as measures of the `depth' of breathing and respiratory `drive' in the fetal lamb is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Bronk D. W. The discharge of impulses in motor nerve fibres: Part I. Impulses in single fibres of the phrenic nerve. J Physiol. 1928 Sep 18;66(1):81–101. doi: 10.1113/jphysiol.1928.sp002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S., Fisher R., Pinter S., Robinson J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974 Dec;243(3):599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G. W., von Euler C., Marttila I., Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975 Aug 8;19(2):105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- CROSS K. W., WARNER P. The effect of inhalation of high and low oxygen concentrations on the respiration of the newborn infant. J Physiol. 1951 Jul;114(3):283–295. doi: 10.1113/jphysiol.1951.sp004620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. L., Dawes G. S., Rurak D. W., Wilds P. L. Breathing movements in fetal lambs and the effect of hypercapnia. J Physiol. 1980 May;302:19–29. doi: 10.1113/jphysiol.1980.sp013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. L., Dawes G. S., Rurak D. W., Wilds P. L. Intermittent breathing before death in fetal lambs. Am J Obstet Gynecol. 1978 Aug 15;131(8):894–898. doi: 10.1016/s0002-9378(16)33138-6. [DOI] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Fox H. E., Leduc B. M., Liggins G. C., Richards R. T. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972 Jan;220(1):119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P. Central respiratory effects of carbon dioxide, and carotid sinus nerve and muscle afferents. J Physiol. 1980 Mar;300:75–87. doi: 10.1113/jphysiol.1980.sp013152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975 Oct;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Gardner W. N. The relation between tidal volume and inspiratory and expiratory times during steady-state carbon dioxide inhalation in man. J Physiol. 1977 Nov;272(3):591–611. doi: 10.1113/jphysiol.1977.sp012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H., Remmers J. E., Bartlett D., Jr Control of the duration of expiration. Respir Physiol. 1973 Jul;18(2):205–221. doi: 10.1016/0034-5687(73)90051-0. [DOI] [PubMed] [Google Scholar]
- Grassino A. E., Whitelaw W. A., Milic-Emili J. Influence of lung volume and electrode position on electromyography of the diaphragm. J Appl Physiol. 1976 Jun;40(6):971–975. doi: 10.1152/jappl.1976.40.6.971. [DOI] [PubMed] [Google Scholar]
- Harding R. State-related and developmental changes in laryngeal function. Sleep. 1980;3(3-4):307–322. doi: 10.1093/sleep/3.3-4.307. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart D. J., Johnson P., McClelland M. E. Asynchronous activity of the diaphragm during breathing in lambs [proceedings]. J Physiol. 1979 Nov;296:22P–23P. [PubMed] [Google Scholar]
- Iscoe S., Dankoff J., Migicovsky R., Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol. 1976 Feb;26(1):113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- KATZ R. L., FINK B. R., NGAISH Relationship between electrical activity of the diaphragm and ventilation. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:792–794. doi: 10.3181/00379727-110-27652. [DOI] [PubMed] [Google Scholar]
- Lopata M., Evanich M. J., Lourenço R. V. Quantification of diaphragmatic EMG response to CO2 rebreathing in humans. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):262–270. doi: 10.1152/jappl.1977.43.2.262. [DOI] [PubMed] [Google Scholar]
- Lourenço R. V., Cherniack N. S., Malm J. R., Fishman A. P. Nervous output from the respiratory center during obstructed breathing. J Appl Physiol. 1966 Mar;21(2):527–533. doi: 10.1152/jappl.1966.21.2.527. [DOI] [PubMed] [Google Scholar]
- Maloney J. E., Adamson T. M., Brodecky A. V., Cranage S., Lambert T. F., Ritchie B. C. Diaphragmatic activity and lung liquid flow in the unanesthetized fetal sheep. J Appl Physiol. 1975 Sep;39(3):423–428. doi: 10.1152/jappl.1975.39.3.423. [DOI] [PubMed] [Google Scholar]
- Moss I. R., Scarpelli E. M. Generation and regulation of breathing in utero: fetal CO2 response test. J Appl Physiol Respir Environ Exerc Physiol. 1979 Sep;47(3):527–531. doi: 10.1152/jappl.1979.47.3.527. [DOI] [PubMed] [Google Scholar]
- PRIBAN I. P. An analysis of some short-term patterns of breathing in man at rest. J Physiol. 1963 May;166:425–434. doi: 10.1113/jphysiol.1963.sp007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore E. R., Walker D. W. Chest wall movements during fetal breathing in the sheep. J Physiol. 1980 Apr;301:307–315. doi: 10.1113/jphysiol.1980.sp013207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. S., Widdicombe J. G. The role of the vagus nerves in the ventilatory responses to hypercapnia and hypoxia in anaesthetized and unanaesthetized rabbits. Respir Physiol. 1969 Jun;7(1):122–135. doi: 10.1016/0034-5687(69)90073-5. [DOI] [PubMed] [Google Scholar]
- von Euler C., Marttila I., Remmers J. E., Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand. 1976 Mar;96(3):324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]
- von Euler C., Wexler I., Herrero F. Control mechanisms determining rate and depth of respiratory movements. Respir Physiol. 1970 Jul;10(1):93–108. doi: 10.1016/0034-5687(70)90030-7. [DOI] [PubMed] [Google Scholar]


