Abstract
The physiological patterns, the sequence polymorphisms of the internal transcriber spacer (ITS), and intergenic spacer regions (IGS) of the rRNA genes and the antifungal susceptibility profile were evaluated for their ability to identify Trichosporon spp. and their specificity for the identification of 49 clinical isolates of Trichosporon spp. Morphological and biochemical methodologies were unable to differentiate among the Trichosporon species. ITS sequencing was also unable to differentiate several species. However, IGS1 sequencing unambiguously identified all Trichosporon isolates. Following the results of DNA-based identification, Trichosporon asahii was the species most frequently isolated from deep sites (15 of 25 strains; 60%). In the main, other Trichosporon species were recovered from cutaneous samples. The majority of T. asahii, T. faecale, and T. coremiiforme clinical isolates exhibited resistance in vitro to amphotericin B, with geometric mean (GM) MICs >4 μg/ml. The other species of Trichosporon did not show high MICs of amphotericin B, and GM MICs were <1 μg/ml. Azole agents were active in vitro against the majority of clinical strains. The most potent compound in vitro was voriconazole, with a GM MIC ≤0.14 μg/ml. The sequencing of IGS correctly identified Trichosporon isolates; however, this technique is not available in many clinical laboratories, and strains should be dispatched to reference centers where these complex methods are available. Therefore, it seems to be more practical to perform antifungal susceptibility testing of all isolates belonging to Trichosporon spp., since correct identification could take several weeks, delaying the indication of an antifungal agent which exhibits activity against the infectious strain.
The number of species of yeasts pathogenic for humans reported in the literature has been greatly augmented in recent decades. The increase in the number of species of yeasts able to infect humans is a consequence of the rise of hosts presenting with factors which predispose them to fungal infections, such as cytotoxic chemotherapy, neutropenia, broad-spectrum antibiotic treatment, steroid use, and invasive catheterization. In these situations almost all yeasts are potential pathogens (10, 32).
Trichosporon species are the causative agents of cutaneous infections and are involved in systemic, localized, or disseminated mycoses, particularly in patients with underlying hematological malignancies, AIDS, burns, and solid tumors (3, 12, 21). Likewise, nonimmunosuppressed patients have suffered from Trichosporon infections associated with ophthalmologic surgery, infections of prosthetic devices, intravenous drug abuse, and peritoneal dialysis (16, 20).
It was thought that there was only one species of Trichosporon and that it was most appropriately called Trichosporon beigelii. However, the genus Trichosporon has recently undergone extensive taxonomic reevaluation, and a rearrangement of the genus has been proposed. Several morphological and biochemical patterns were recognized among clinical and environmental isolates; and ultrastructural and DNA studies confirmed those findings, with Trichosporon beigelii being split into a number of distinct species (13, 14, 17). So far, the genus Trichosporon includes seven species associated with human infections: Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon jirovecii, Trichosporon mucoides, and Trichosporon ovoides. Trichosporon asahii appears to be much more common in cases of systemic mycosis, while other Trichosporon species are involved in superficial skin lesions (2). In addition, differences in the antifungal susceptibility profiles of isolates within species have been reported as well (23).
The identification of these fungal pathogens by conventional methods is often difficult and is frequently inconclusive. DNA-based molecular procedures can provide alternative and more useful methods for the characterization and identification of Trichosporon spp. (26, 28, 30). In this study, the morphological and biochemical patterns, the sequence polymorphisms of the internal transcriber spacer 1 and 2 regions (ITS1 and ITS2, respectively), intergenic spacer 1 (IGS1) of the rRNA gene, and the antifungal susceptibility profile were evaluated for their ability to identify Trichosporon spp. and their specificity in the identification of a collection of 49 clinical isolates of Trichosporon spp.
MATERIALS AND METHODS
Fungi.
Clinical strains. A collection of 49 clinical isolates was included in the study. All strains were recovered from 42 Spanish and Argentinean hospitals over a period of 10 years, from 1993 to 2002. The isolates were obtained from a variety of clinical sources, as displayed in Table 1. Twenty-four strains were isolated from superficial sites; and 25 strains were isolated from deep sites, including 8 from blood cultures, 5 from respiratory tract samples, and 3 from urine samples. Each isolate was obtained from a different patient. The isolates were sent to the Mycology Reference Laboratory of National Center for Microbiology of Spain for identification and susceptibility testing.
TABLE 1.
Clinical sources and DNA-based and biochemical identities of Trichosporon isolates analyzed
| Strain no. | Strain code | Clinical source | Identity by IGS analysis | ITS clade | Identity by biochemical method |
|---|---|---|---|---|---|
| 1 | CL-4551 | Catheter | T. faecale | Clade III | T. asahii |
| 2 | CL-4327 | Skin | T. faecale | Clade III | T. mucoides |
| 3 | CL-4293 | Nails | T. faecale | Clade III | T. asahii |
| 4 | CL-4294 | Nails | T. faecale | Clade III | T. asahii |
| 5 | CL-0382 | Subcutaneous abscess | T. coremiiforme | Clade III | T. asahii |
| 6 | CL-2148 | Urine | T. coremiiforme | Clade III | T. asahii |
| 7 | CL-4323 | Blood culture | T. asahii | Clade III | T. asahii |
| 8 | CL-2457 | Bronchial aspirate | T. asahii | Clade III | T. mucoidesa |
| 9 | CL-4322 | Blood culture | T. asahii | Clade III | T. asahii |
| 10 | CL-4296 | Catheter | T. asahii | Clade III | T. mucoides |
| 11 | CL-4099 | Pleural fluid | T. asahii | Clade III | T. asahiia |
| 12 | CL-3553 | Blood culture | T. asahii | Clade III | T. ovoidesa |
| 13 | CL-3876 | Sputum | T. asahii | Clade III | T. asahii |
| 14 | CL-3562 | Blood culture | T. asahii | Clade III | T. asahiia |
| 15 | CL-4295 | Subcutaneous abscess | T. asahii | Clade III | T. asahii |
| 16 | CL-2324 | Urine | T. asahii | Clade III | T. mucoides |
| 17 | CL-4032 | Bronchial aspirate | T. asahii | Clade III | T. asahii |
| 18 | CL-3316 | Blood culture | T. asahii | Clade III | T. mucoides |
| 19 | CL-2846 | Blood culture | T. asahii | Clade III | T. mucoides |
| 20 | CL-2847 | Blood culture | T. asahii | Clade III | T. mucoidesa |
| 21 | CL-2848 | Blood culture | T. asahii | Clade III | T. asahii |
| 22 | CL-0225 | Bone Biopsy | T. inkin | Clade III | T. inkin |
| 23 | CL-4290 | Skin | T. inkin | Clade III | T. inkin |
| 24 | CL-4291 | Skin | T. inkin | Clade III | T. inkin |
| 25 | CL-4292 | Urethral exudate | T. inkin | Clade III | T. inkin |
| 26 | CL-4320 | Skin | T. inkin | Clade III | T. inkin |
| 27 | CL-4319 | Subcutaneous abscess | T. inkin | Clade III | T. inkin |
| 28 | CL-4324 | Corneal exudate | T. inkin | Clade III | T. ovoides |
| 29 | CL-3925 | Nails | T. ovoides | Clade III | T. ovoides |
| 30 | CL-4220 | Skin | T. ovoides | Clade III | T. cutaneum |
| 31 | CL-0696 | Skin | T. ovoides | Clade III | T. inkina |
| 32 | CL-3915 | Peritoneal fluid | T. ovoides | Clade III | T. inkina |
| 33 | CL-4157 | Nails | T. ovoides | Clade III | T. inkin |
| 34 | CL-4232 | Nails | T. japonicum | Clade III | T. mucoidesa |
| 35 | CL-4298 | Pleural fluid | T. japonicum | Clade III | T. asahii |
| 36 | CL-3740 | Skin | T. jirovecii | Clade I | T. cutaneuma |
| 37 | CL-4074 | Urine | T. jirovecii | Clade I | T. inkina |
| 38 | CL-3727 | Skin | T. dermatis | Clade I | T. cutaneuma |
| 39 | CL-3092 | Nails | T. dermatis | Clade I | T. mucoides |
| 40 | CL-3736 | Nails | T. dermatis | Clade I | T. mucoides |
| 41 | CL-4399 | Nails | T. dermatis | Clade I | T. mucoides |
| 42 | CL-4326 | Subcutaneous abscess | T. dermatis | Clade I | T. mucoides |
| 43 | CL-4262 | Nails | T. dermatis | Clade I | T. mucoides |
| 44 | CL-5651 | Nails | T. dermatis | Clade I | T. mucoides |
| 45 | CL-5672 | Blood culture | T. dermatis | Clade I | T. mucoides |
| 46 | CL-0066 | Skin | T. cutaneum | Clade I | T. asahiia |
| 47 | CL-0160 | Nails | T. cutaneum | Clade I | T. cutaneum |
| 48 | CL-4289 | Skin | T. montevideense | Clade II | T. mucoides |
| 49 | CL-3916 | Skin | T. domesticum | Clade II | T. inkin |
Inconclusive results by biochemical and morphological identification. Based on the results, the isolate was identified as the species to which it was more closely related, although the percentages of reliability were low.
Reference strains and sequences obtained from the GenBank database.
Table 2 lists the Trichosporon strains used for comparative analysis of the ITS and IGS sequences.
TABLE 2.
Trichosporon reference strains used for comparison of ITS and IGS sequences and their GenBank accession numbers
| Trichosporon species | Strain identification | GenBank accession no.
|
Strain acquired from CBS for this study | |
|---|---|---|---|---|
| ITS | IGS | |||
| T. asahii | CBS 2530 | AB018014 | AB066397 | |
| CBS 2479 | AB018013 | AB066386 | ||
| T. faecale | CBS 4828 | AB018016 | AB066413 | |
| T. coremiiforme | CBS 2482 | AB018015 | AB066406 | |
| T. asteroides | CBS 2481 | AF444416 | AB081515 | |
| T. jirovecii | CBS 6864 | AF444437 | AB066427 | |
| CBS 6950 | CL 5675 | |||
| T. mucoides | CBS 7625 | AF444423 | AB066433 | |
| T. cutaneum | CBS 2466 | AF444325 | AB066415 | |
| T. ovoides | CBS 5580 | CL 5685 | ||
| T. inkin | CBS 5585 | AF444420 | AB066424 | |
| CBS 7629 | AB018024 | AB066425 | ||
| T. dermatis | CBS 2043 | AB035581 | AB066412 | |
| T. montevideense | CBS 6721 | AF444422 | AB066431 | |
| CBS 8261 | CL 5777 | |||
| T. domesticum | CBS 8111 | CL 5676 | ||
| CBS 8280 | CL 5684 | |||
| T. japonicum | JCMa 8357 | AJ319760 | AB066426 | |
| CBS 8641 | CL 5680 | |||
| T. aquatile | CBS 5988 | AB066404 | ||
| CBS 5973 | AB066403 | |||
| T. dulcitum | CBS 8257 | AF444428 | AB066419 | |
| CBS 5785 | AB018022 | AB066420 | ||
| CBS 8193 | AB066422 | |||
| T. moniliforme | CBS 2467 | AF444415 | AB066429 | |
| T. veenhuisii | CBS 7136 | CL 5678 | ||
| T. gracile | CBS 8189 | AB018023 | AB066421 | |
| T. sporotrichoides | CBS 8246 | AF444470 | AB066438 | |
| T. loubieri | CBS 7065 | AB018027 | AB066428 | |
| T. brassicae | CBS 6382 | AF444436 | AB066414 | |
JCM, Journal Clinical Microbiology.
Outgroup strains.
The ITS and IGS sequences of Cryptococcus neoformans (CBS 131; GenBank accession no. AJ300916) and C. neoformans var. gattii (CBS 6956; GenBank accession no. AJ300920) were used as outgroups.
Morphological and biochemical identification.
The clinical strains were subcultured on 4% malt extract-0.5% yeast extract agar. After 10 days at 30°C, macroscopic and microscopic examinations were done. The isolates were identified by classical physiological tests: fermentation of and growth on carbon sources, growth on nitrogen sources, growth at various temperatures, and ability to hydrolyze urea (2, 7, 14). Table 3 displays the distinctive characteristics of each species of Trichosporon. Biochemical and morphological characterizations were done as described elsewhere (2, 7, 14). For T. dermatis, the biochemical characteristics were obtained from reference 31.
TABLE 3.
Distinctive morphological and biochemical characteristics of each Trichosporon species pathogenic for humansa
| Species | Assimilation of:
|
Growth
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rha | Mel | Raff | Rib | Xyl | l-Arab | Galac | Ino | 0.1% | 37°C | App | |
| T. asahii | + | − | − | V | V | + | − | − | + | + | − |
| T. asteroides | V | − | − | − | V | V | − | + | V | V | V |
| T. coremiiforme | + | − | − | − | + | + | − | + | V | + | − |
| T. cutaneum | + | + | + | + | + | + | − | + | − | − | − |
| T. dermatisb | + | + | + | + | + | + | V | + | + | + | − |
| T. faecale | + | − | − | + | V | + | − | + | + | + | − |
| T. inkin | − | − | − | − | − | − | − | + | V | + | + |
| T. japonicum | − | − | − | V | V | V | V | − | NA | + | − |
| T. jirovecii | + | + | + | V | V | + | + | + | V | − | − |
| T. montevideense | V | − | − | V | V | V | V | + | V | V | V |
| T. mucoides | + | + | + | + | + | + | + | + | + | + | − |
| T. ovoides | + | − | V | − | V | − | − | − | + | V | + |
Abbreviations: Rha, rhamnose; Mel, melibiose; Raff, raffinose; Rib, ribitol; Xyl, xylitol; l-Arab, l-arabinitol; Galac, galactitol; Ino, inositol; 0.1%, growth on medium with 0.1% cycloheximide; 37°C, growth at 37°C; App, appresoria; V, strain variation; NA, not available.
Results obtained from reference 31.
PCR and DNA sequencing of ITS and IGS regions.
Yeasts were cultured in YEPD medium (1% yeast extract, 2% peptone, 2% dextrose [Oxoid, Madrid, Spain]) by centrifugation at 150 rpm for 24 to 48 h at 30°C. One milliliter of the medium was centrifuged at 13,000 rpm for 10 min. The pellet was suspended in 1 ml of cold SE buffer (20 mM citrate-phosphate buffer, pH 5.6, 50 mM EDTA, 0.9 M sorbitol) and centrifuged under the same conditions. This process was repeated twice. A small quantity of glass pearls was added together with 50 μl of lysis buffer (50 mM Tris- HCl, pH 7.2, 50 mM EDTA, 3% sodium dodecyl sulfate). The mixture was vortexed at low speed for at least 15 s. Lysis buffer (400 μl) and 4 μl of beta-mercaptoethanol (final concentration, 1%; Sigma-Aldrich Química S.A., Madrid, Spain) were added, and the mixture was incubated at 65°C for 2 h. The blend was gently mixed every 30 min. DNA was then purified by repeated phenol-chloroform-isoamyl (25:24:1) extractions, ethanol precipitation, and RNase treatment. Finally, the DNA concentration was estimated by comparing the bands obtained from each sample with the bands of known amounts of lambda phage DNA in a 0.8% agarose gel (Pronadisa, Madrid, Spain). DNA was purified by using Chroma Spin + TE 200 columns (Clontech Laboratories, Inc., Becton Dickinson, Madrid, Spain).
DNA segments comprising the ITS1 and ITS2 regions were amplified with primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (34). DNA segments comprising the IGS1 region were amplified with primers 26SF (IGS1-26SS) (5′-ATCCTTTGCAGACGACTTGA-3′) and 5SR (IGS2-58S) (5′-AGCTTGACTTCGCAGATCGG-3′) (26). The reaction mixtures contained 0.5 μM of each primer, 0.2 mM of each deoxynucleoside triphosphate, 5 μl of PCR 10× buffer (Applied Biosystems, Madrid, Spain), 2.5 U Taq DNA polymerase (Amplitaq; Applied Biosystems), and 25 ng of DNA in a final volume of 50 μl. The samples were amplified in a GeneAmp PCR System 9700 (Applied Biosystems) by using the following cycling parameters: one initial cycle of 2 min at 94°C, followed by 35 cycles of 30 s at 94°C, 45 s at 56°C, and 2 min at 72°C, with one final cycle of 5 min at 72°C. The reaction products were analyzed in a 0.8% agarose gel.
Sequencing reactions were done with 4 μl of a sequencing kit (BigDye Terminator cycle sequencing ready reaction; Applied Biosystems), 1 μM of the primers (ITS1, ITS4, 26SF, and 5SR), and 3 μl of the PCR product in a final volume of 10 μl. Sequences were assembled and edited by using the SeqMan II and EditsEq software packages (Lasergene; DNAstar, Inc., Madison, Wis.). Sequence analysis was performed by comparison of the nucleotide sequences obtained with the nucleotide sequences of Trichosporon reference isolates obtained from the GenBank database (http://www.ncbi.nih.gov/GenBank/) and from the sequences of reference strains from the Centraalbureau voor Schimmelcultures (CBS; Delft, The Netherlands). Those strains are displayed in Table 2.
Phylogenetic analyses.
All phylogenetic analyses were conducted with Fingerprinting II Informatix software, version 3.0 (Bio-Rad Laboratories, Madrid, Spain). The methodology used was maximum parsimony clustering. Phylogram stability was assessed by parsimony bootstrapping with 1,000 simulations. Phylograms were rooted to outgroups comprising Cryptococcus neoformans (CBS 132) and C. neoformans var. gattii (CBS 6956).
Antifungal susceptibility testing.
The susceptibility testing strictly followed the recommendations proposed by the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing for the testing of fermentative yeasts (AFST-EUCAST, discussion document 7.1) (24). These recommendation are based on the CLSI (formerly NCCLS) procedure described in document M27-A2 (22), but some modifications were included in order to allow for automation of the susceptibility method and to permit the incubation period to be shortened from 48 to 24 h. Briefly, the susceptibility testing included RPMI supplemented with 2% glucose as the assay medium, an inoculum size of 105 CFU/ml, flat-bottomed trays, and spectrophotometric reading. In order to improve the growth, a minor modification was included (25). That is, all microplates were wrapped with film sealer to prevent the medium from evaporating; the plates were attached to an electrically driven wheel inside the incubator, agitated at 350 rpm, and incubated at 30°C for 48 h. Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality control strains.
The antifungal agents used in the study were as follows: amphotericin B (Sigma Aldrich Química), flucytosine (Sigma Aldrich Química), fluconazole (Pfizer S.A., Madrid, Spain), itraconazole (Janssen S.A., Madrid, Spain), and voriconazole (Pfizer S.A.). The MICs were determined at 24 and 48 h. MICs were obtained measuring the absorbance at 530 nm with an MRXII reader (Cultek; Dynatech, Madrid, Spain). For amphotericin B the MIC endpoints were defined as the lowest drug concentration that resulted in a reduction of growth of 90% or more compared with that of the control growth. For flucytosine and the azole drugs, the MIC endpoint was defined as 50% inhibition.
RESULTS
Morphological and biochemical identification.
Table 1 includes the identities of the clinical isolates tested as determined by morphological and biochemical methods. Table 3 shows the differential morphological and biochemical characteristics of Trichosporon species. Sixteen strains were identified as T. mucoides, 15 strains were identified as T. asahii, 11 strains were identified as T. inkin, 4 strains were identified as T. cutaneum, and 3 strains were identified as T. ovoides.
Molecular identification.
The genetic relatedness of 49 clinical strains (Table 1) together with reference strains and nucleotide sequence data obtained from GenBank included in Table 2 were analyzed. The aligned complete sequences of IGS1, ITS1, and ITS2 were analyzed phylogenetically by using two strains, C. neoformans CBS 132 and C. neoformans var. gattii CBS 6956, to root the trees.
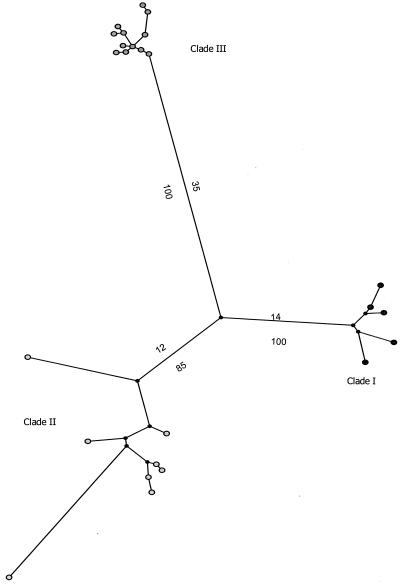
Maximum parsimony analysis of ITS sequences did not adequately resolve the Trichosporon species, as shown in Fig. 1. Three clades that can be seen in Table 1 were identified. Clade I had a maximum of eight base conversions, and clade III had a maximum of three base conversions. As an exception, in clade II, T. sporotrichoides, T. brasiccae, and T. veenhuisii were resolved. However, there was only one sequence for each species, and therefore, no conclusion can be made. The rest of the species included in this clade had a maximum of five base conversions.
FIG. 1.
Unrooted phylogenetic tree of Trichosporon species, based on confidently aligned ITS sequences, obtained by using maximum parsimony cluster analysis and 1,000 bootstrap simulations.
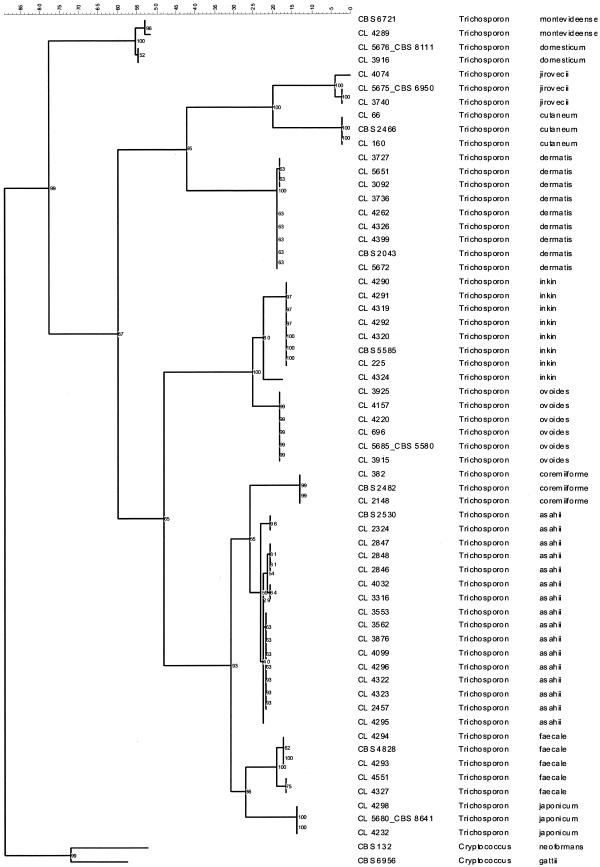
Maximum parsimony analysis of the IGS sequences adequately resolved all Trichosporon species. Figure 2 shows a rooted cladogram with all clinical isolates and the respective reference strains or sequences from GenBank included in this study. Those species without clinical isolates are not included in the cladogram, although they were unambiguously differentiated. Therefore, each clinical isolate was considered to belong to the species that the maximum parsimony analysis of IGS sequences had indicated. Table 1 contains the final identification of clinical strains. Thus, the 49 clinical isolates were identified as follows: 15 T. asahii isolates, 8 T. dermatis isolates, 7 T. inkin isolates, 5 T. ovoides isolates, 4 T. faecale isolates, 2 T. coremiiforme isolates, 2 T. cutaneum isolates, 2 T. jirovecii isolates, 2 T. japonicum isolates, 1 T. montevideense isolate, and 1 T. domesticum isolate. Notably, all T. asahii isolates were isolated from deep samples, while other species were more frequently associated with superficial sources (100% versus 29.4%; Table 1). As exceptions, one T. faecale isolate was from a catheter, two T. coremiiforme isolates were from urine and a subcutaneous abscess, two T. inkin isolates were from a bone biopsy specimen and a subcutaneous abscess, one T. ovoides isolate was from peritoneal fluid, one T. jirovecii isolate was from urine, one T. japonicum isolates was from pleural fluid, and two T. dermatis isolates were from a subcutaneous abscess and a blood culture. The last two species, T. japonicum and T. dermatis, have not been associated with human samples before; but in this study they were detected two and seven times, respectively.
FIG. 2.
Rooted phylogenetic tree of Trichosporon species, based on confidently aligned IGS1 sequences, obtained by using maximum parsimony cluster analysis and 1,000 bootstrap simulations.
Discrepancies among molecular identification and morphological and biochemical identification.
Table 4 shows the discrepancies among the results of molecular identification and those of morphological and biochemical identification. Morphological and biochemical methods were unable to identify the following species: T. dermatis, T. faecale, T. coremiiforme, T. jirovecii, T. japonicum, T. montevideense, and T. domesticum (Table 4). In addition, T. asahii, T. ovoides, and T. cutaneum were misidentified most of the time as other Trichosporon species (Table 4). As an exception, T. inkin was correctly identified by the classical methodology six of seven times (Table 4).
TABLE 4.
Isolates erroneously identified by morphological and biochemical methods
| IGS sequencing identification | No. of isolates by IGS sequencing identification | Biochemical identification | No. of isolates by biochemical identification |
|---|---|---|---|
| T. asahii | 15 | T. asahii | 8 |
| T. mucoides | 6 | ||
| T. ovoides | 1 | ||
| T. inkin | 7 | T. inkin | 6 |
| T. ovoides | 1 | ||
| T. dermatis | 6 | T. mucoides | 5 |
| T. cutaneum | 1 | ||
| T. ovoides | 5 | T. ovoides | 1 |
| T. inkin | 3 | ||
| T. cutaneum | 1 | ||
| T. faecale | 4 | T. asahii | 3 |
| T. mucoides | 1 | ||
| T. coremiiforme | 2 | T. asahii | 2 |
| T. cutaneum | 2 | T. cutaneum | 1 |
| T. asahii | 1 | ||
| T. jirovecii | 2 | T. cutaneum | 1 |
| T. inkin | 1 | ||
| T. japonicum | 2 | T. mucoides | 1 |
| T. asahii | 1 | ||
| T. montevideense | 1 | T. mucoides | 1 |
| T. domesticum | 1 | T. inkin | 1 |
Antifungal susceptibility testing.
Susceptibility results are showed in Table 5. Table 5 displays the geometric means (GMs) and ranges of the MICs of each antifungal agent grouped by Trichosporon spp. and identification procedure. According to DNA-based identification, it should be stressed that the majority of T. asahii, T. faecale, and T. coremiiforme clinical isolates exhibited resistance to amphotericin B in vitro, with GM MICs >4.0 μg/ml. Only one isolate of T. faecale had an amphotericin B MIC of 0.25 μg/ml, while the rest had MICs ≥2 μg/ml. The other species of Trichosporon did not show high MICs of amphotericin B, and GM MICs were <1.0 μg/ml. In addition, all 11 species exhibited high MICs of flucytosine, and most isolates were resistant in vitro, with MICs over 16.0 μg/ml. On the contrary, the azole agents were active in vitro against the majority of clinical strains, independent of the species of Trichosporon analyzed. The most potent compound in vitro was voriconazole, with a GM MIC ≤0.14 μg/ml.
TABLE 5.
Antifungal susceptibility results of clinical isolates of Trichosporon spp.
| Trichosporon spp. | Identification procedurea (no. of isolates) | MIC (mg/liter)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B
|
Flucytosine
|
Fluconazole
|
Itraconazole
|
Voriconazole
|
|||||||
| GM | Range | GM | Range | GM | Range | GM | Range | GM | Range | ||
| T. asahii | Conventional (15) | 4.8 | 0.5-16.0 | 12.6 | 2.0-128.0 | 4.3 | 0.12-64.0 | 0.57 | 0.03-8.0 | 0.18 | 0.015-8.0 |
| DNA based (15) | 5.2 | 2.0-16.0 | 13.2 | 4.0-64.0 | 7.6 | 0.5-64.0 | 0.43 | 0.03-8.0 | 0.28 | 0.03-8.0 | |
| T. faecale | Conventional (0) | ||||||||||
| DNA based (4) | 3.3 | 0.25-16.0 | 19 | 8.0-32.0 | 4.7 | 4.0-8.0 | 0.69 | 0.06-4.0 | 0.17 | 0.06-1.0 | |
| T. coremiiforme | Conventional (0) | ||||||||||
| DNA based (2) | 4.0-4.0 | 4.0-128.0 | 2.0-2.0 | 0.25-1.0 | 0.06-0.12 | ||||||
| T. inkin | Conventional (11) | 0.21 | 0.03-1.0 | 41.1 | 4.0-128.0 | 2 | 0.5-4.0 | 0.129 | 0.06-0.25 | 0.12 | 0.03-2.0 |
| DNA based (7) | 0.14 | 0.03-1.0 | 57.9 | 4.0-128.0 | 1.8 | 0.5-4.0 | 0.13 | 0.06-0.25 | 0.12 | 0.015-2.0 | |
| T. ovoides | Conventional (3) | 1.0 | 0.5-4.0 | 40.3 | 8.0-128.0 | 1.5 | 0.5-8.0 | 0.24 | 0.12-1.0 | 0.04 | 0.015-0.25 |
| DNA based (5) | 0.37 | 0.25-0.5 | 48.5 | 16.0-128.0 | 1.74 | 1.0-8.0 | 0.1 | 0.06-0.25 | 0.05 | 0.03-0.12 | |
| T. japonicum | Conventional (0) | ||||||||||
| DNA based (2) | 0.25-1.0 | 4.0-32.0 | 0.12-1.0 | 0.5-0.5 | 0.015-0.03 | ||||||
| T. domesticum | Conventional (0) | ||||||||||
| DNA based (1) | 0.25 | 128 | 2.0 | 0.25 | 0.12 | ||||||
| T. montevideense | Conventional (0) | ||||||||||
| DNA based (1) | 0.12 | 128.0 | 2.0 | 0.12 | 0.06 | ||||||
| T. dermatis | Conventional (0) | ||||||||||
| DNA based (8) | 0.24 | 0.015-16.0 | 58.6 | 16.0-128.0 | 12.3 | 1.0-128.0 | 0.58 | 0.06-8.0 | 0.48 | 0.06-16.0 | |
| T. cutaneum | Conventional (4) | 0.29 | 0.12-1.0 | 32.0 | 4.0-128.0 | 0.84 | 0.25-8.0 | 0.42 | 0.015-0.12 | 0.02 | 0.07-0.06 |
| DNA based (2) | 0.25-0.5 | 2.0-4.0 | 0.25-0.25 | 0.015-0.03 | 0.07-0.015 | ||||||
| T. jirovecii | Conventional (0) | ||||||||||
| DNA based (2) | 1.0-1.0 | 4.0-32.0 | 0.25-4.0 | 0.03-0.12 | 0.07-0.12 | ||||||
| T. mucoides | Conventional (16) | 0.69 | 0.015-16 | 30.6 | 4.0-128.0 | 7.0 | 0.5-128.0 | 0.38 | 0.06-8.0 | 0.25 | 0.015-16.0 |
| DNA based (0) | |||||||||||
Conventional, morphological and biochemical identification; DNA based, identification by IGS region sequencing.
Four strains of T. asahii (4 of 15; 26.6%) and three isolates of T. dermatis (3 of 8; 37.5%) exhibited MICs of fluconazole as high as 32.0 to 64.0 μg/ml. Five of those strains (two T. asahii strains and all T. dermatis strains) showed higher MICs of itraconazole (≥2.0 μg/ml) and voriconazole (≥8.0 μg/ml) as well.
Differences in antifungal susceptibility were found when MICs were analyzed by isolation site (deep versus superficial isolates). Thus, the strains isolated form deep samples had higher MICs to all antifungals except flucytosine. The most significant difference was found among the MICs of amphotericin B. Hence, the GM MIC of amphotericin B of strains isolated from deep samples was 2.7 μg/ml, and that of strains isolated from superficial sources was 0.3 μg/ml.
DISCUSSION
The identification of yeasts is largely based on physiological and biochemical characteristics. The tests most used for routine identification purposes are fermentation of and growth on carbon sources, growth on nitrogen sources, requirements for vitamins, growth at various temperatures, hydrolysis of urea, and resistance to antibiotics (2, 14). The combined use of biochemical characteristics and direct observation of fungal structures under a light microscope permits the identification of species causing the majority of human infections. However, the number of new species of opportunistic yeasts causing mycoses has increased during the past two or three decades, making the identification by conventional methods more difficult, even for those with expertise in mycology (19). In addition, conventional identification by the evaluation of morphological and biochemical characteristics can be laborious, leading to inconclusive or presumptive identifications, particularly for infrequent pathogenic species.
In order to overcome these drawbacks, several groups have designed molecular procedures for the identification of yeasts. One of the main advantages of molecular methods is their sensitivity and specificity, being fully discriminative even for closely related species (7). The majority of molecular methods are PCR-based techniques and use either specific probes or universal fungal primers that are normally directed to conserved regions of the rRNA gene, particularly to the ITS regions (4-6). The identification by using ITS regions must include a subsequent analysis such as sequencing or restriction fragment length polymorphism analysis. This approach has been put into practice in clinical laboratories for the rapid detection and identification of yeasts found in positive blood cultures and is being evaluated as a promising diagnostic tool (11, 18).
Sugita et al. have developed procedures based on ITS regions for identification of Trichosporon spp. These procedures are capable of characterizing the majority of species of Trichosporon, including those pathogenic for humans (26, 28, 30). The results obtained with these techniques have led to the reclassification of both clinical and environmental isolates of these species, the design of a nested PCR assay for the detection of DNA in sera for the diagnosis of deep-seated trichosporonosis, description of the first bloodstream infection due T. asteroides, and the association of T. ovoides with hypersensitivity pneumonitis (15, 27-29). Recently, DNA-based procedures for the identification of Trichosporon have been stepped up by the sequence analysis of the rRNA IGS1, which provides a more powerful and alternative method for distinguishing between phylogenetically closely related species (26).
The data presented in this work suggest that the morphological and biochemical methodology is not a useful and reliable method for Trichosporon identification. This methodology was unable to identify seven species, and others were frequently misidentified (Table 4). On the other hand, sequencing of DNA fragments seems to be a better approach for identification to the species level of isolates of this genus. However, ITS sequencing was also unable to differentiate several species, as shown in the unrooted cladogram in Fig. 1. Therefore, three clades were established. Clade I comprised T. cutaneum, T. dermatis, T. jirovecii, T. moniliforme, and T. mucoides; clade II comprised T. brasiccae, T. domesticum, T. dulcitum, T. gracile, T. laibachii, T. loubieri, T. montevideense, T. sporotrichoides, and T. veenhuisii; and clade III comprised T. asahii, T. asteroides, T. coremiiforme, T. faecale, T. inkin, T. japonicum, and T. ovoides. Furthermore, by looking at the sequences (data not shown), there are very few differences among the ITS sequences of each clade, and therefore, it is not reliable to assume the identification to the species level based on analyses with this DNA fragment.
IGS sequences unambiguously identified all Trichosporon isolates, as shown in Fig. 2. For some species, such as T. jirovecii, T. dermatis, T. inkin, T. faecale, and T. asahii, polymorphisms were detected in IGS sequences. Further experiments with more strains are required to ascertain if those polymorphisms can be used for genotyping. However, there was preliminary evidence of the existence of five genotypes when IGS sequences of T. asahii were analyzed (26). In this work, T. asahii is also divided into five types, supporting the results obtained by Sugita et al. (26) (Fig. 2).
A second area for consideration is the antifungal susceptibility testing of Trichosporon spp. A 1990 study defined T. beigelii as an emerging pathogen resistant to amphotericin B (33). After the taxonomic reevaluation, it has been proven that T. asahii is more resistant in vitro to amphotericin B than to triazole compounds (1, 23). These data obtained from in vitro studies have been corroborated by the results of studies performed in vivo, and successful outcomes with treatments with voriconazole have been documented in some cases of deep infections due to this species that did not respond to amphotericin B therapy (8, 9, 35). On the contrary, the non-T. asahii species appear to be more resistant in vitro to triazole agents than amphotericin B (23), although these data have not been proven in vivo. Our results confirmed that T. asahii is resistant in vitro to amphotericin B because all isolates exhibited amphotericin B MICs ≥2.0 μg/ml. In addition, T. faecale and T. coremiiforme also seem to be resistant to amphotericin B in vitro because all except one of the isolates included in this study had MICs >2.0 μg/ml. These two species are considered by some authors to be varieties of T. asahii (14). The other Trichosporon species were susceptible to the polyene in vitro.
With regard to azole agents, resistance to fluconazole in vitro was found in a minority of T. asahii and T. dermatis isolates, which were the remaining isolates susceptible in vitro, although a number of fluconazole MICs were 4.0 to 8.0 μg/ml. Itraconazole and voriconazole were the most potent agents in vitro against all Trichosporon spp., particularly voriconazole, with GM MICs less than 0.14 μg/ml, confirming that these compounds can be useful for the treatment of these infections.
This work shows that biochemical and morphological identification and ITS sequencing are not reliable techniques for the identification of Trichosporon spp., as they were unable to distinguish between species. Correct characterization of these species can be significant at the therapeutic level in view of their distinct antifungal susceptibility profiles, particularly those of T. asahii, which is highly resistant to amphotericin B in vitro. The sequencing of IGS correctly identified Trichosporon isolates; however, this technique is not available in many clinical laboratories, and strains should be dispatched to reference centers where these complex methods are available. Therefore, it seems to be more practical to perform antifungal susceptibility testing with all isolates belonging to Trichosporon spp. since correct identification could take several weeks, delaying the identification of the antifungal agent which exhibits activity against the infectious strain.
REFERENCES
- 1.Arikan, S., and G. Hascelik. 2002. Comparison of NCCLS microdilution method and Etest in antifungal susceptibility testing of clinical Trichosporon asahii isolates. Diagn. Microbiol. Infect. Dis. 43:107-111. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, J. A., R. W. Payne, and D. Yarrow. 2000. Yeasts: characteristics and identification, 3rd ed. Cambridge University Press, Cambridge, United Kingdom.
- 3.Cawley, M. J., G. R. Braxton, L. R. Haith, K. J. Reilly, R. E. Guilday, and M. L. Patton. 2000. Trichosporon beigelii infection: experience in a regional burn center. Burns 26:483-486. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y. C., J. D. Eisner, M. M. Kattar, S. L. Rassoulian-Barrett, K. Lafe, U. Bui, A. P. Limaye, and B. T. Cookson. 2001. Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J. Clin. Microbiol. 39:4042-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y. C., J. D. Eisner, M. M. Kattar, S. L. Rassoulian-Barrett, K. Lafe, S. L. Yarfitz, A. P. Limaye, and B. T. Cookson. 2000. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 38:2302-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Baere, T., G. Claeys, D. Swinne, G. Verschraegen, A. Muylaert, C. Massonet, and M. Vaneechoutte. 2002. Identification of cultured isolates of clinically important yeast species using fluorescent fragment length analysis of the amplified internally transcribed rRNA spacer 2 region (ITS2). BMC Microbiol. 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueres. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures Utrecht, The Netherlands, and Universitat Rovira i Virgili, Reus, Italy.
- 8.Falk, R., D. G. Wolf, M. Shapiro, and I. Polacheck. 2003. Multidrug-resistant Trichosporon asahii isolates are susceptible to voriconazole. J. Clin. Microbiol. 41:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier, S., W. Pavageau, M. Feuillhade, S. Deplus, A. M. Zagdanski, O. Verola, H. Dombret, and J. M. Molina. 2002. Use of voriconazole to successfully treat disseminated Trichosporon asahii infection in a patient with acute myeloid leukaemia. Eur. J. Clin. Microbiol. Infect. Dis. 21:892-896. [DOI] [PubMed] [Google Scholar]
- 10.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita, S. I., Y. Senda, S. Nakaguchi, and T. Hashimoto. 2001. Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeast strains. J. Clin. Microbiol. 39:3617-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gueho, E., L. Improvisi, G. S. de Hoog, and B. Dupont. 1994. Trichosporon on humans: a practical account. Mycoses 37:3-10. [DOI] [PubMed] [Google Scholar]
- 13.Kemker, B. J., P. F. Lehmann, J. W. Lee, and T. J. Walsh. 1991. Distinction of deep versus superficial clinical and nonclinical isolates of Trichosporon beigelii by isoenzymes and restriction fragment length polymorphisms of rDNA generated by polymerase chain reaction. J. Clin. Microbiol. 29:1677-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzman, C. P., and J. W. Fell. 1998. The yeasts. A taxonomic study. Elsevier, Amsterdam, The Netherlands.
- 15.Kustimur, S., A. Kalkanci, K. Caglar, M. Dizbay, F. Aktas, and T. Sugita. 2002. Nosocomial fungemia due to Trichosporon asteroides: firstly described bloodstream infection. Diagn. Microbiol. Infect. Dis. 43:167-170. [DOI] [PubMed] [Google Scholar]
- 16.Kwon-Chung, K. J., and J. E. Bennett. 1992. Infections due to Trichosporon and other miscellaneous yeast-like fungi, p. 768-782. In K. J. Kwon-Chung and J. E. Bennett (ed.), Medical mycology. Lea & Febiger, Malvern, Pa.
- 17.Lee, J. W., G. A. Melcher, M. G. Rinaldi, P. A. Pizzo, and T. J. Walsh. 1990. Patterns of morphologic variation among isolates of Trichosporon beigelii. J. Clin. Microbiol. 28:2823-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Y. L., S. N. Leaw, J. H. Chen, H. C. Chang, and T. C. Chang. 2003. Rapid identification of yeasts commonly found in positive blood cultures by amplification of the internal transcribed spacer regions 1 and 2. Eur. J. Clin. Microbiol. Infect. Dis. 22:693-696. [DOI] [PubMed] [Google Scholar]
- 19.Middelhoven, W. J. 2003. Identification of clinically relevant Trichosporon species. Mycoses 46:7-11. [DOI] [PubMed] [Google Scholar]
- 20.Mooty, M. Y., S. S. Kanj, M. Y. Obeid, G. Y. Hassan, and G. F. Araj. 2001. A case of Trichosporon beigelii endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 20:139-142. [DOI] [PubMed] [Google Scholar]
- 21.Moretti-Branch, M. L., K. Fukushima, A. Z. Schreiber, K. Nishimura, P. M. Papaiordanou, P. Trabasso, R. Tanaka, and M. Miyaji. 2001. Trichosporon species infection in bone marrow transplanted patients. Diagn. Microbiol. Infect. Dis. 39:161-164. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 46:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Tudela, J. L., F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, D. Denning, J. P. Donnelly, B. Dupont, W. Fegeler, C. Moore, M. Richardson, P. E. Verweij, and Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2003. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect 9:I-VIII. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Tudela, J. L., Martin-Diez, F., M. Cuenca-Estrella, L. Rodero, Y. Carpintero, and B. Gorgojo. 2000. Influence of shaking on antifungal susceptibility testing of Cryptococcus neoformans: a comparison of the NCCLS standard M27A medium, buffered yeast nitrogen base, and RPMI-2% glucose. Antimicrob. Agents Chemother. 44:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugita, T., M. Nakajima, R. Ikeda, T. Matsushima, and T. Shinoda. 2002. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J. Clin. Microbiol. 40:1826-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugita, T., M. Nakajima, R. Ikeda, Y. Niki, T. Matsushima, and T. Shinoda. 2001. A nested PCR assay to detect DNA in sera for the diagnosis of deep-seated trichosporonosis. Microbiol. Immunol. 45:143-148. [DOI] [PubMed] [Google Scholar]
- 28.Sugita, T., A. Nishikawa, R. Ikeda, and T. Shinoda. 1999. Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J. Clin. Microbiol. 37:1985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugita, T., A. Nishikawa, R. Ikeda, T. Shinoda, H. Sakashita, Y. Sakai, and Y. Yoshizawa. 1998. First report of Trichosporon ovoides isolated from the home of a summer-type hypersensitivity pneumonitis patient. Microbiol. Immunol. 42:475-478. [DOI] [PubMed] [Google Scholar]
- 30.Sugita, T., A. Nishikawa, and T. Shinoda. 1998. Identification of Trichosporon asahii by PCR based on sequences of the internal transcribed spacer regions. J. Clin. Microbiol. 36:2742-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugita, T., M. Takashima, T. Nakase, T. Ichikawa, R. Ikeda, and T. Shinoda. 2001. Two new yeasts, Trichosporon debeurmannianum sp. nov. and Trichosporon dermatis sp. nov., transferred from the Cryptococcus humicola complex. Int J. Syst. Evol Microbiol. 51:1221-1228. [DOI] [PubMed] [Google Scholar]
- 32.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transplant. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 33.Walsh, T. J., G. P. Melcher, M. G. Rinaldi, J. Lecciones, D. A. McGough, P. Kelly, J. Lee, D. Callender, M. Rubin, and P. A. Pizzo. 1990. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-324. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. A guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 35.Wolf, D. G., R. Falk, M. Hacham, B. Theelen, T. Boekhout, G. Scorzetti, M. Shapiro, C. Block, I. F. Salkin, and I. Polacheck. 2001. Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J. Clin. Microbiol. 39:4420-4425. [DOI] [PMC free article] [PubMed] [Google Scholar]