Abstract
The Escherichia coli rhaSR operon encodes two AraC family transcription activator proteins, RhaS and RhaR, which regulate expression of the l-rhamnose catabolic regulon in response to l-rhamnose availability. RhaR positively regulates rhaSR in response to l-rhamnose, and RhaR activation can be enhanced by the cyclic AMP (cAMP) receptor protein (CRP) protein. CRP is a well-studied global transcription regulator that binds to DNA as a dimer and activates transcription in the presence of cAMP. We investigated the mechanism of CRP activation at rhaSR both alone and in combination with RhaR in vivo and in vitro. Base pair substitutions at potential CRP binding sites in the rhaSR-rhaBAD intergenic region demonstrate that CRP site 3, centered at position −111.5 relative to the rhaSR transcription start site, is required for the majority of the CRP-dependent activation of rhaSR. DNase I footprinting confirms that CRP binds to site 3; CRP binding to the other potential CRP sites at rhaSR was not detected. We show that, at least in vitro, CRP is capable of both RhaR-dependent and RhaR-independent activation of rhaSR from a total of three transcription start sites. In vitro transcription assays indicate that the carboxy-terminal domain of the alpha subunit (α-CTD) of RNA polymerase is at least partially dispensable for RhaR-dependent activation but that the α-CTD is required for CRP activation of rhaSR. Although CRP requires the presence of RhaR for efficient in vivo activation of rhaSR, DNase I footprinting assays indicated that cooperative binding between RhaR and CRP does not make a significant contribution to the mechanism of CRP activation at rhaSR. It therefore appears that CRP activates transcription from rhaSR as it would at simple class I promoters, albeit from a relatively distant position.
The l-rhamnose regulon of Escherichia coli consists of the rhaT, rhaSR, and rhaBAD operons. The rhaSR operon encodes the l-rhamnose-responsive transcription activators RhaS and RhaR, both of which are members of the AraC/XylS family of transcription regulators (13), while the rhaBAD and rhaT operons encode the l-rhamnose catabolic enzymes and the l-rhamnose transport protein, respectively (2, 23). RhaS activates transcription of the rhaBAD and rhaT operons in the presence of l-rhamnose by binding to DNA sites that overlap the −35 hexamers by four base pairs and extend upstream to −81 at rhaBAD and −82 at rhaT (11, 32). Part of the mechanism of RhaS activation involves contact with the σ70 subunit of RNA polymerase (RNAP), specifically, RhaS residues D241 and D250 make contact with σ70 residues R599 and K593, respectively (7, 34). The other l-rhamnose-responsive activator protein, RhaR, activates transcription of the rhaSR operon (31). Full activation by RhaR requires contact with σ70 (RhaR D276 contacts σ70 R599), and a rhaSR promoter containing a RhaR binding site, but not a cyclic AMP (cAMP) receptor protein (CRP) binding site, requires the carboxy-terminal domain of the alpha subunit (α-CTD) of RNAP for full activation (15, 34).
Full activation of each of the three rha operons also requires the cyclic AMP receptor protein (CRP, also known as the catabolite activator protein or CAP) in addition to either RhaS or RhaR (12, 15, 32). CRP binds as a dimer to specific DNA sites and induces a DNA bend of approximately 90 degrees in the presence of its ligand, cAMP (21). The mechanism of CRP activation varies depending on where CRP binds relative to the promoter. At simple class I CRP-dependent promoters, CRP is the only activator and binds upstream but not adjacent to the −35 hexamer of the promoter. In this case, activating region 1 (AR1) of the downstream monomer of CRP contacts the RNAP α-CTD to recruit RNAP to the promoter and activate transcription (reviewed in reference 9). At class II CRP-dependent promoters, CRP binds at a site partially overlapping the −35 hexamer, thereby allowing activating region 2 (AR2) of the downstream CRP monomer to interact with the N-terminal domain of the α subunit (α-NTD) of RNAP. Additionally, AR1 of the upstream monomer of CRP interacts with α-CTD at class II promoters (reviewed in reference 9).
CRP also activates many promoters in combination with a second activator. At a subset of such class III promoters, such as araBAD, ansB, and some artificial promoters, the mechanism of CRP activation appears to involve the same interactions with RNAP as those used at class I and class II promoters (3, 17, 26, 37). At other class III promoters, CRP apparently regulates gene expression indirectly by influencing the binding affinity of other regulators. For example, at the E. coli melAB promoter, CRP binds to DNA cooperatively with the second activator, MelR, to increase MelR binding to the site that is required for transcription activation (4, 33). Another mechanism by which CRP can activate transcription at class III promoters involves DNA bending. At the malKp promoter of E. coli, CRP and MalT are both required for maximal transcription activation (10). In the absence of CRP, MalT binds to three sites from which it cannot activate transcription. When present, CRP binds to three sites over 100 base pairs upstream of the malKp transcription start site. CRP binding to these sites bends the DNA and causes MalT to shift 3 bp downstream to binding sites from which MalT can activate transcription (24).
All three promoters in the l-rhamnose regulon in E. coli are class III CRP-dependent promoters. CRP contributes approximately 50- to 100-fold activation to each of the rha promoters in vivo; however, the mechanism of CRP activation has not been identified for any of these promoters. At the rhaBAD promoter, CRP can activate transcription only when RhaS is bound adjacent to RNAP (12; unpublished results). At the rhaSR promoter, CRP alone can weakly activate transcription, but binding of the other l-rhamnose-responsive activator, RhaR, is required for maximal CRP-dependent activation in vivo (15; unpublished results).
In this study we set out to identify the mechanism of CRP activation at the rhaSR promoter. We tested four potential CRP binding sites located between the divergent rhaBAD and rhaSR promoters for contributions to rhaBAD and rhaSR expression by creating triple base pair substitutions in each site. Our in vivo transcription assays indicated that CRP site 3 is required for CRP activation of rhaSR expression and confirmed that CRP site 1 is the only site required for CRP activation at the rhaBAD promoter. The importance of CRP site 3 is also supported by our in vitro transcription results. Both in vivo and in vitro transcription assays, as well as DNase I footprinting results, suggest that CRP sites 2 and 4 do not play a major role at either promoter. In vitro transcription results also indicated that CRP activation at rhaSR requires the α-CTD of RNAP. In addition, our results indicate that neither CRP-induced DNA bending nor cooperative binding between CRP and RhaR make significant contributions to CRP activation at rhaSR. Thus, CRP activation of rhaSR transcription requires CRP binding to site 3 (at −111.5) and direct contact with RNAP through a mechanism similar to the mechanism of CRP activation at simple class I CRP-dependent promoters.
MATERIALS AND METHODS
Culture media and general methods.
E. coli cultures for β-galactosidase assays were grown in morpholinepropanesulfonic acid (MOPS) buffered medium (19). Tryptone-yeast extract (TY) liquid medium (0.8% tryptone, 0.5% yeast extract, and 0.5% NaCl) was used to grow cells for overexpression of RhaR for purification. Antibiotics were used as indicated at the following concentrations: ampicillin, 200 μg/ml; carbenicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; and tetracycline, 20 μg/ml. The Expand High Fidelity PCR system (Roche, Indianapolis, IN) was used to amplify DNA fragments for cloning. The QIAquick PCR purification kit (QIAGEN, Chatsworth, CA) was used to clean up PCR products. The DNA sequence of both strands was determined for the entire cloned region of all cloned and/or mutagenized DNA fragments.
Strains, plasmids, and phages.
Table 1 lists the oligonucleotides, and Table 2 lists the strains, phages, and plasmids used in this study. All CRP site mutations were constructed by site-directed mutagenesis on pSE213 (CRP site 2 and 3 down mutants were previously described [15]). The wild-type and mutant rha promoter regions were then PCR amplified with primers 744 and 896 (Table 1) and subcloned to pRS414 in each of the two possible orientations to construct Φ(rhaB-lacZ)Δ320 (pSE258) and Φ(rhaS-lacZ)Δ312 (pSE218) and their mutant derivatives (“Φ” stands for fusion, and the upstream endpoint of each fusion relative to the respective transcription start site [for example, −320] is given after the “Δ”). Each fusion was then recombined onto λRS45 (27) and integrated into the chromosome as a single-copy λ lysogen. Single-copy lysogens were confirmed with either the Ter test or by a PCR-based assay (14, 22). P1 phage-mediated generalized transduction was used to move Δcrp zhc-511::Tn10 (from strain SME1853) and/or recA::cat into the desired strains selecting for tetracycline or chloramphenicol resistance, respectively. Plasmid pSE261, which overexpresses RhaR protein, was constructed by first amplifying rhaR with oligonucleotides 2249 and 2251 (Table 1), digesting with NdeI and SapI, and ligating with similarly digested pTYB1.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′)a | Use |
|---|---|---|
| 744 | CGCGGATCCCCACTGGATGCGCCGAGATCG | Amplify rha promoter region for subcloning |
| 896 | CGCGGATCCTCTATCGCCACGGACGCGTT | Amplify pSR or rha promoter region for subcloning |
| 1170 | CCGGAATTCTTGTGGTGATGTGATGCTCAC | Amplify pSR for subcloning |
| 2168 | AGCAAATTCACAACATCATCAC | CRP site 1− mutagenic oligonucleotide |
| 2184 | CGCCAGGGTTTTCCCAGTCACGA | Sequencing lacZ fusions (IRD41 labeled) |
| 2198 | TTCAGGAAATGCGGTGAGCATCACAATGCCACAATTCAGC | CRP site 4− mutagenic oligonucleotide |
| 2239 | ACAGCGTGAATTTTCAGGAAATGTGATGAGCATCACATC | CRP site 3 up mutagenic oligonucleotide |
| 2240 | GCAAATTGTGAACATCATCACATTCATCTTTC | CRP site 1 up mutagenic oligonucleotide |
| 2249 | GCGCCGCGCATATGGCTTTCTGCAATAACG | Downstream oligonucleotide to amplify rhaR for cloning into pTYB1 |
| 2251 | CTCGTCGCTCTTCGGCAGGCATCTTTCTGCGAATTGAG | Upstream oligonucleotide to amplify rhaR for cloning into pTYB1 |
| 2371 | CGCGGATCCCCACTGGATGCGCCGAGATCG | PCR for DNase footprinting template |
| 2409 | GGTAAGATCTAAAAAAATCCACACTATGTAATACGGTCAT | PCR for DNase footprinting template |
Regions of oligonucleotides not complementary to wild-type DNA sequence (encode restriction endonuclease sites and flanking DNA or mutations) are underlined.
TABLE 2.
Strains, phages, and plasmids used in this study
| Strain, phage, or plasmid | Genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| ECL116 | F− ΔlacU169 endA hsdR thi | 1 |
| ER2566 | F− λ−fhuA2 [lon] ompT lacZ::T7 gene1 gal sulA11 Δ(mcrC-mrr)114::IS10 R(mcr-73::mini-Tn10-TetS)2 R(zgb-210::Tn10)(TetS) endA1 [dcm] | New England Biolabs |
| SME1853 | ECL116 Φ(rhaB-lacZ)Δ226 Δcrp zhc-511::Tn10 | 16 |
| SME2510 | ECL116 λSME115 | This study |
| SME2511 | ECL116 λSME116 | This study |
| SME2517 | SME2510 recA::cat | This study |
| SME2518 | SME2511 recA::cat | This study |
| SME2562 | ECL116 λ(SME115 CRP site 1−) recA::cat | This study |
| SME2563 | ECL116 λ(SME116 CRP site 1−) recA::cat | This study |
| SME2564 | ECL116 λ(SME115 CRP site 2−) recA::cat | This study |
| SME2565 | ECL116 λ(SME116 CRP site 2−) recA::cat | This study |
| SME2566 | ECL116 λ(SME115 CRP site 3−) recA::cat | This study |
| SME2567 | ECL116 λ(SME116 CRP site 3−) recA::cat | This study |
| SME2568 | ECL116 λ(SME115 CRP site 4−) recA::cat | This study |
| SME2569 | ECL116 λ(SME116 CRP site 4−) recA::cat | This study |
| SME2590 | SME2510 Δcrp zhc-511::Tn10 | This study |
| SME2591 | SME2511 Δcrp zhc-511::Tn10 | This study |
| SME2576 | ECL116 λ(SME115 CRP site 1−) Δcrp zhc-511::Tn10 | This study |
| SME2577 | ECL116 λ(SME116 CRP site 1−) Δcrp zhc-511::Tn10 | This study |
| SME2578 | ECL116 λ(SME115 CRP site 2−) Δcrp zhc-511::Tn10 | This study |
| SME2579 | ECL116 λ(SME116 CRP site 2−) Δcrp zhc-511::Tn10 | This study |
| SME2580 | ECL116 λ(SME115 CRP site 3−) Δcrp zhc-511::Tn10 | This study |
| SME2581 | ECL116 λ(SME116 CRP site 3−) Δcrp zhc-511::Tn10 | This study |
| SME2582 | ECL116 λ(SME115 CRP site 4−) Δcrp zhc-511::Tn10 | This study |
| SME2583 | ECL116 λ(SME116 CRP site 4−) Δcrp zhc-511::Tn10 | This study |
| Phages | ||
| λRS45 | bla′-lacZscatt+imm21ind+ | 27 |
| λSME115 | λRS45Φ(rhaB-lacZ)Δ320 | This study |
| λSME116 | λRS45Φ(rhaS-lacZ)Δ312 | This study |
| Plasmids | ||
| pBluescript SK II | AprlacZα | Stratagene |
| pET15b | AprlacI | Novagen |
| pGEM | AprlacZα | Promega |
| pRS414 | Aprlac′ZYA | 27 |
| pSE101 | pTZ18R AprrhaSR + rhaBA′ | Laboratory collection |
| pSE213 | pGEM-11Zf(+) (rhaSR-rhaBAD) | 15 |
| pSE214 | pSE213 CRP site 2− | 15 |
| pSE215 | pSE213 CRP site 3− | 15 |
| pSE218 | pRS414 Φ(rhaS-lacZ)Δ312 | 15 |
| pSE233 | pET15b crp | 35 |
| pSE258 | pRS414 Φ(rhaB-lacZ)Δ320 | This study |
| pSE250 | pUC18 rhaSRT′ wild type | This study |
| pSE254 | pUC18 ΔrhaSR::cat-sac | This study |
| pSE259 | pSE213 CRP site 1 down mutant | This study |
| pSE260 | pSE213 CRP site 4 down mutant | This study |
| pSE261 | pTYB1 rhaR | New England Biolabs |
| pTS134 | pBluescript SK II pSR wild type | This study |
| pTS136 | pBluescript SK II pSR CRP site 2− | This study |
| pTS137 | pBluescript SK II pSR CRP site 3− | This study |
| pTS138 | pBluescript SK II pSR CRP site 4− | This study |
| pTYB1 | AprlacI | New England Biolabs |
| pUC18 | AprlacZα | 36 |
All plasmids used as templates for in vitro transcription reactions are derivatives of pBluescript II SK (Stratagene). pTS132 contains the rhaSR promoter (pSR) extending from −133 to +86, amplified from pSE101 using primers 896 and 1170 and cloned into pTS123. (Plasmid pTS123 is a derivative of pBluescript II SK that contains the control promoter p82 and the terminator t500.) The terminator trpoC was cloned downstream of the rhaSR promoter in pTS132 to make pTS134, which yields a 125-nucleotide (nt) transcript. Transcription initiated at the control promoter, p82, is divergent from pSR and terminates at the t500 intrinsic terminator to generate transcripts of 103, 104, and 105 nt that served as an internal control to normalize transcription. The down mutations in CRP binding sites 2, 3, and 4 were subcloned using primers 896 and 1170 from pSE214, pSE215, and pSE260 into pTS123 to make pTS136, pTS137, and pTS138, respectively.
β-Galactosidase assay.
β-Galactosidase assays were performed as previously described (6). Briefly, the final cultures for assays were grown in MOPS buffered minimal medium with glycerol as the carbon source and l-rhamnose added as an inducer. Specific activities were averaged from at least three independent assays with two replicates in each assay.
Protein purification.
RhaR was overexpressed as a fusion protein with an intein domain and a chitin-binding domain (CBD) at the C terminus of RhaR. For overexpression, E. coli ER2566 cells containing the RhaR-intein-CBD-expressing plasmid, pSE261, were grown in TY plus carbenicillin to an optical density at 600 nm of 0.7. The cells were then induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and then maintained at a temperature of 15°C until the following morning. All subsequent steps in RhaR purification were carried out at 4°C using degassed buffers. The cell pellet was resuspended in column buffer [500 mM NaCl, 20 mM HEPES, 1 mM EDTA, 0.5 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), 0.05% NP-40, 0.2% l-rhamnose; pH 8.0], sonicated, and then centrifuged at 12,000 × g for 20 min. Ammonium sulfate was added to the supernatant fraction to 30%, stirred for 2 h, and then centrifuged at 17,000 × g for 20 min. The supernatant fraction was dialyzed against column buffer to remove ammonium sulfate and loaded onto a chitin column (New England Biolabs), and the column was washed with 20 column volumes of column buffer. Cleavage buffer (column buffer plus 50 mM dithiothreitol [DTT]) was added, and the column was removed from the chromatography system and gently agitated (by rocking) for 2 days to promote the intein-mediated self-cleavage of RhaR from the fusion protein. The released RhaR protein was eluted in 4 column volumes of column buffer and dialyzed into storage buffer (10 mM Tris, 1 mM KEDTA, 50 mM KCl, 0.5 mM TCEP, 5% glycerol, 50 mM l-rhamnose). For storage at −80°C, the glycerol concentration was increased to 50%.
Native (untagged) CRP was overexpressed and purified by nickel affinity chromatography as previously described (35). E. coli RNAP holoenzyme was purified as described previously (25). To assess the role of α-CTD, RNAP containing either truncated alpha subunits (α-Δ235) or full-length alpha subunits (α-WT) was reconstituted from individual RNAP subunits that were each purified under denaturing conditions (28). Reconstituted core RNAP (α-WT and α-Δ235) was supplemented with a 2 M excess of N-terminally His6-tagged σ70 purified as described previously (18).
In vitro transcription.
Typical in vitro transcription reaction mixtures contained 25 mM Tris-HCl, pH 8.0, 50 mM KCl, 0.1 mM EDTA, 1.0 mM DTT, 100 μg/ml acetylated bovine serum albumin, 2 nM supercoiled plasmid template, and 4 mM MgCl2 in the presence or absence of 50 mM l-rhamnose and 100 μM cAMP. When present, both RhaR and CRP were added 10 min prior to RNAP addition and incubated with the DNA template at 37°C. RNAP was then added to a final concentration of 20 nM, and open complexes were allowed to form by incubating the mixtures at 37°C for an additional 5 min. Transcription was initiated with the addition of a mixture of all four nucleoside triphosphates (NTPs) and heparin to limit transcription to a single round. The final concentrations of NTPs were as follows: 200 μM ATP, CTP, and GTP; 50 μM UTP; and 1.0 μC/μl [α-32P]UTP. Heparin was added to a final concentration of 100 μg/ml. Reaction mixtures were incubated for 5 min at 37°C; the reactions were stopped, and the reaction products were purified, precipitated, resolved on denaturing polyacrylamide gels, and analyzed using a STORM model 860 phosphorimager as previously described (25).
Initial in vitro transcription experiments confirmed that RhaR activation of the rhaSR promoter (pSR) required supercoiled templates (31; unpublished results). Altering the concentration of RNAP and KCl used in transcription experiments or the incubation period before initiating transcription did not increase transcription from any of the promoters (data not shown). Transcription from the control promoter p82 was not affected by the addition of RhaR, CRP, cAMP, or l-rhamnose and was equivalent with either wild-type RNA polymerase or RNA polymerase reconstituted with both alpha subunits lacking the carboxy-terminal domain (data not shown).
Primer extension mapping of CRP-dependent upstream transcription start sites.
In vitro transcription was used to produce non-32P-labeled transcripts. Reactions were stopped by the addition of 5 volumes TRIZOL (Invitrogen) and immediately vortexed. Glycogen was added as a carrier, and reaction products were extracted with an equal volume of chloroform. The aqueous phase was then precipitated by the addition of isopropanol (0.7 volume). Dried RNA pellets were resuspended in hybridization buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2) containing 1 pmol of 32P-labeled primer 896, heated to 65°C for 5 min, and then quickly cooled on ice. Reaction mixtures were supplemented with 1 mM of all four dNTPs, 10 mM DTT, and 200 U SuperScript reverse transcriptase (Invitrogen) and incubated at 42°C for 1 h. Reaction products were extracted with phenol:chloroform:isoamyl alcohol (25:24:1), and the aqueous phase was precipitated with 10 mM MgCl2 and 5 volumes 100% ethanol. Reaction products were resolved as described above for the in vitro transcription products. Dideoxy-sequencing reactions (USB Thermosequenase cycle sequencing kit) were generated using identical primers and were run alongside primer extension products to determine transcription start sites.
DNase I footprinting.
The wild-type or mutant rhaSR-rhaBAD promoter regions for DNase I footprinting were PCR amplified using 32P-labeled primer 2371 and unlabeled primer 2409, and footprinting was performed as previously described (11). The gels were imaged and analyzed using a Bio-Rad phosphorimager FX.
RESULTS
In vivo analysis of CRP binding site mutations at the rhaBAD and rhaSR promoters.
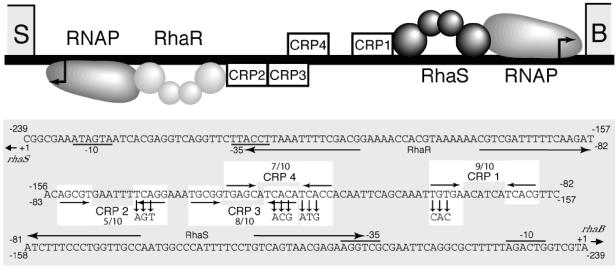
CRP is required for full transcription activation of both the rhaBAD and rhaSR promoters in vivo (12, 15). Four potential CRP binding sites located between the two promoters were initially predicted on the basis of position and similarity with the consensus CRP binding site (5) (Fig. 1). Egan and Schleif (12) previously used truncations of the rhaBAD promoter to determine that CRP site 1 is required for full rhaBAD expression; however, point mutations in the CRP sites have not been previously tested at rhaBAD. We predict that CRP bound at site 4 would be on the same “face” of the DNA as CRP at site 1 and RhaS and RNAP at rhaBAD, while CRPs bound at sites 2 and 3 are predicted to be on the same “face” of the DNA as RhaR and RNAP at rhaSR. Further, it appears that all of the proteins at rhaSR are on the opposite face of the DNA relative to the proteins at rhaBAD, as depicted in Fig. 1.
FIG. 1.
Potential CRP sites in the rhaSR-rhaBAD promoter regions. (Top) Schematic representation of the rhaSR-rhaBAD intergenic region. Transcription start sites are shown as bent arrows. RNAP, RhaS, and RhaR proteins are shown at their binding sites, and potential CRP binding sites are shown as open boxes. RhaS and RhaR are shown as dimers with two spheres per monomer to represent the N-terminal domain and C-terminal domain. (Bottom) DNA sequence of the divergent rhaSR and rhaBAD promoter region. The DNA sequences of the potential CRP binding sites are highlighted by white boxes with the down arrows indicating the base pair substitutions in each of the down mutants used in this study. Also, the number of base pair matches out of 10 with the consensus CRP binding site is indicated.
Mutations that were predicted to decrease the strength of CRP binding were introduced to each of the four CRP sites in the rhaSR-rhaBAD intergenic region. Each of these CRP site “down” mutations consisted of three simultaneous base pair substitutions that changed consensus base pairs to nonconsensus base pairs (5) (Fig. 1). Additionally, “up” mutations were made in CRP sites 1 and 3 such that these sites perfectly matched the consensus CRP binding site sequence at the 10 most conserved positions (5). The mutations in the CRP binding sites were constructed in DNA fragments that extended from the beginning of the rhaSR open reading frame at one end to the beginning of the rhaBAD open reading frame at the other end. Translational fusions were constructed by fusing each end of the DNA fragment carrying each mutation with the lacZ gene to make Φ(rhaS-lacZ)Δ312 and Φ(rhaB-lacZ)Δ320, thereby allowing us to test each mutation in the context of identical DNA fragments for possible effects on both rhaSR and rhaBAD expression. All assays were performed with the lacZ fusions integrated into the chromosome as single-copy λ lysogens (27). We previously reported the results of assays of strains with the CRP site 2 down mutation and the CRP site 3 down mutation at (rhaS-lacZ)Δ312 in multicopy, and only the strain with the CRP site 3 down mutation had a significant defect in that context (15).
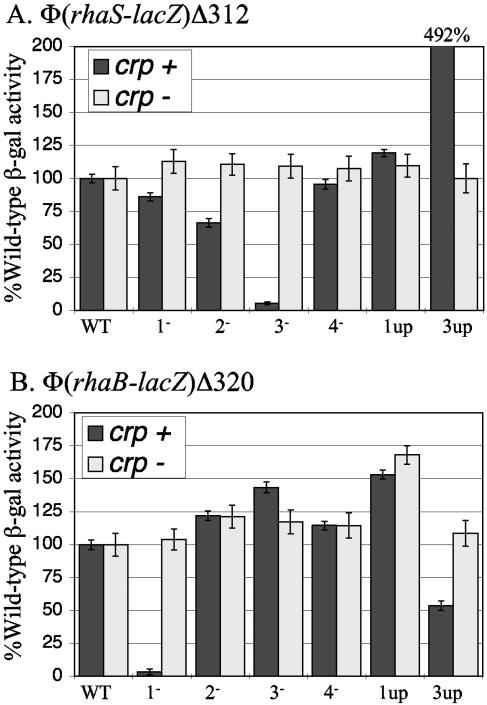
Figure 2A shows the results of β-galactosidase assays to measure the effect of the CRP binding site mutations in single copy at Φ(rhaS-lacZ)Δ312. Substitutions in CRP site 3 had the strongest effects; the down mutation retained only 5% of wild-type activity, whereas the up mutation showed nearly 500% of wild-type activity. CRP site 2, which has the same relative position at rhaSR as CRP site 1 at rhaBAD, had at most a minor role in rhaSR activation on the basis of the small defect of the CRP site 2 down mutation. Mutations in CRP sites 1 and 4 did not significantly alter expression from the rhaSR promoter. None of the substitutions in the CRP sites had significant effects on reporter gene expression in crp deletion strains, demonstrating that these DNA sequence changes did not alter expression in the absence of CRP. CRP site 3 therefore emerges as the critical CRP site for rhaSR transcription activation, consistent with our previous, less comprehensive, results obtained using multicopy fusions (15).
FIG. 2.
Substitutions at CRP site 1, 2, 3, or 4 analyzed at Φ(rhaS-lacZ)Δ312 (A) and Φ(rhaB-lacZ)Δ320 (B). Each mutant was analyzed in the presence (crp+) and absence (Δcrp) of CRP to distinguish the effects on CRP binding from other effects of the DNA sequence changes. In each panel, the activity of the wild-type promoter in each strain (crp+ and crp mutant) was set at 100% [wild-type β-galactosidase (β-gal) activities (in Miller units) were 83 in the Φ(rhaS-lacZ)Δ312 crp+ strain, 1.8 in the Φ(rhaS-lacZ)Δ312 crp mutant, 440 in the Φ(rhaB-lacZ)Δ320 crp+ strain, and 0.053 in the Φ(rhaB-lacZ)Δ320 crp mutant]. WT, wild type.
When the same CRP binding site mutations were assayed for their role at the rhaBAD promoter, the strain with the CRP site 1 down mutation showed by far the greatest effect, retaining only 3% of wild-type activity (Fig. 2B), confirming the importance of CRP site 1 in rhaBAD activation (12). While the substitutions changing CRP site 1 to the consensus sequence at the 10 most conserved positions resulted in a small increase in rhaBAD expression, a similar increase in expression was observed in the absence of CRP, suggesting that the increase in expression may be due to the DNA sequence change and not a change in CRP occupancy of the site. These results suggest that the degree of CRP occupancy of the wild-type site 1, which matches the consensus sequence at 9 out of 10 positions, does not limit rhaBAD expression, whereas the degree of occupancy of CRP site 3 does normally limit rhaSR expression. Interestingly, the mutations creating a consensus sequence at CRP site 3 had a twofold negative effect on rhaBAD expression, while the CRP site 3 down mutation had a small positive effect on rhaBAD expression. This suggests that either transcription from the rhaSR promoter or protein binding to the rhaSR promoter region can influence rhaBAD expression, although the mechanism of this influence is not currently understood. Our results further showed no significant role for CRP site 2 or 4 in the regulation of rhaBAD transcription.
RhaR and CRP synergistically activate transcription of rhaSR in vitro.
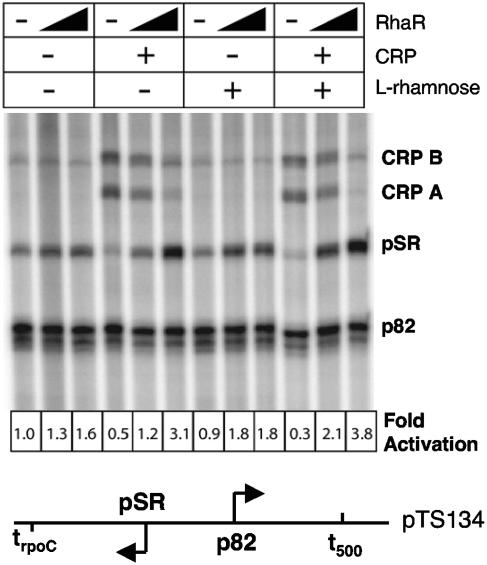
Tobin and Schleif first demonstrated RhaR activation of rhaSR transcription in vitro (31); however, at that time it was not known that CRP also regulates rhaSR expression. We constructed plasmid templates containing the rhaSR promoter (pSR) in the context of natural upstream and downstream DNA sequences to determine the roles of RhaR and CRP during in vitro transcription reactions with purified components. We first confirmed that RhaR activated transcription only when transcription was initiated from supercoiled templates (data not shown) and that this activation was stimulated by the addition of l-rhamnose (Fig. 3). Activation by RhaR alone was modest, approximately twofold, and the addition of l-rhamnose had a small but reproducible effect that was more significant at lower RhaR concentrations (Fig. 3). Addition of higher concentrations of RhaR and/or l-rhamnose did not stimulate transcription of pSR to a greater extent (data not shown). When CRP and cAMP were added in combination with RhaR and l-rhamnose, an approximately fourfold synergistic activation of transcription from pSR was seen (Fig. 3). Surprisingly, the addition of CRP in the absence of RhaR reduced transcription from the expected transcription start site at pSR and stimulated the production of two longer transcripts, CRP A and CRP B. The reduction in transcription from the normal pSR start site by CRP in the absence of RhaR was likely the result of CRP-dependent alternative promoters that overlapped and were mutually exclusive with the RhaR-dependent rhaSR promoter (see below and Fig. 4 and 5). Removal of the trpoC intrinsic terminator downstream of the pSR promoter resulted in the loss of all discrete transcripts from pSR, CRP A, and CRP B, as would be expected if the CRP A and CRP B transcripts initiated at or upstream of pSR (data not shown).
FIG. 3.
In vitro transcription from the rhaSR promoter in the presence (+) and absence (−) of CRP, RhaR, and l-rhamnose. The final concentration of CRP, when present, was 800 nM. The final concentration of RhaR, when present, was 40 nM or 160 nM, as indicated by the thickness of the black triangle. Transcripts of defined length were generated downstream of the rhaSR promoter by termination at the strong intrinsic terminator trpoC. Transcription levels were normalized to the divergent p82 promoter, which does not respond to RhaR, CRP, l-rhamnose, or cAMP. The fold activation value is the ratio of pSR transcripts to p82 transcripts with the ratio in the leftmost lane (no RhaR and no CRP) set at 1.0. The map at the bottom of the figure shows the relative positions of pSR and p82 and their terminators. The pSR transcript is 125 bp long, while three transcripts (103, 104, and 105 bp) are generated from the p82 promoter.
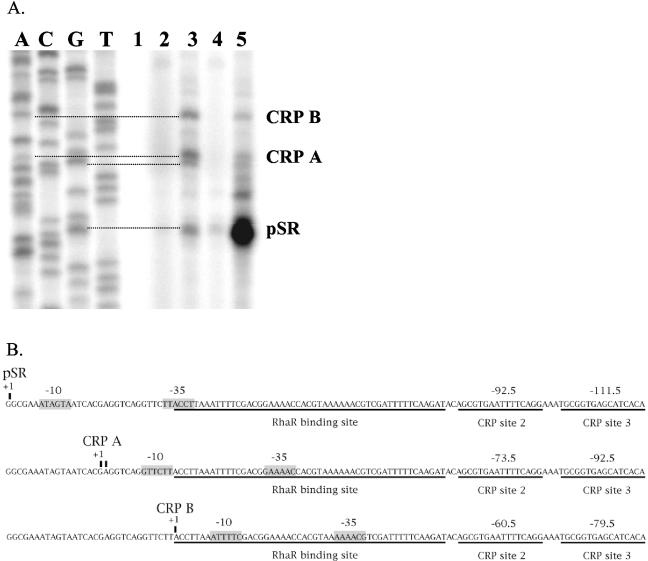
FIG. 4.
Reverse transcription with radioactively labeled primers complementary to RNA transcripts from in vitro transcription reactions (A). Lanes A, C, G, and T are sequencing reactions generated from the same primer used for the reverse transcription reactions. Lane 1 was a control transcription reaction without NTPs. The transcripts used as a template for lanes 2 through 5 were generated in the presence of the following activators: none (lane 2), CRP (lane 3), RhaR(lane 4), and RhaR and CRP (lane 5). Panel B shows the positions of the RNAP binding sites (gray boxes labeled −10 and −35) for pSR compared to the putative RNAP binding sites used to generate the CRP A and CRP B transcripts. The RhaR and CRP binding sites are underlined, and the positions of the CRP sites relative to each transcription start site are shown.
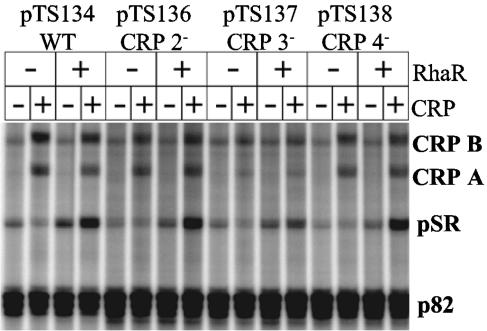
FIG. 5.
In vitro transcription from the rhaSR promoter with wild-type (WT) CRP sites or with down mutations in CRP site 2, 3, or 4 (CRP 2−, CRP 3−, or CRP 4−, respectively). The wild-type template was the same as that used in Fig. 3. The remaining templates were identical except for point mutations in CRP site 2, 3, or 4. The positions of the pSR transcript, the two CRP-dependent transcripts (CRP A and CRP B), as well as the control p82 transcript are labeled. Reactions were performed in the presence (+) or absence (−) of 800 nM CRP and 75 nM RhaR as indicated. cAMP and l-rhamnose were present in all reaction mixtures.
To further establish that the CRP A and CRP B transcripts initiated upstream of the pSR transcription start site, we used primer extension to map the transcription start sites of nonradioactive in vitro-transcribed mRNA. Our results (Fig. 4A) demonstrated that both the CRP A and CRP B transcripts result from initiation upstream of the normal pSR start site and that CRP A transcription initiated at either position −19 or −18 with respect to the normal pSR transcription start site and CRP B transcription initiated at position −32. Weak promoters that overlap the RhaR binding site and that could generate transcripts from these positions were identified (Fig. 4B). It is likely that binding of RNAP to these alternate promoters is mutually exclusive with RNAP binding to the RhaR-dependent pSR promoter (Fig. 4B), explaining why basal pSR transcription is reduced when CRP is the only activator protein present in the in vitro transcription reaction mixtures. It is unclear what role, if any, these alternative promoters may play in vivo; previous primer extension experiments utilizing cellular RNA to map the in vivo pSR transcription start site, although not performed under the analogous conditions (ΔrhaR), did not detect these transcripts (12).
CRP site 3 is critical for in vitro CRP activation of the rhaSR promoter.
To further determine the roles of CRP sites 2, 3, and 4 in transcription activation of the rhaSR promoter, we constructed plasmid templates containing the CRP site down mutations for in vitro transcription reactions. Our results demonstrated that CRP site 3 was most critical for CRP activation of pSR (Fig. 5), in agreement with the in vivo results. Down mutations in CRP site 3 reduced transcription levels of pSR to approximately 50% of the wild-type levels in the presence of CRP alone as well as in the presence of CRP and RhaR. Some CRP activation of pSR was seen with the template carrying the CRP down mutations, suggesting that there may be some residual CRP binding to the weakened CRP site 3. In addition to decreasing CRP activation of pSR, mutation of CRP site 3 also decreased the amount of the CRP A and CRP B transcripts. Mutation of CRP site 2 and CRP site 4 had little or no effect on transcription initiation at the pSR start site in vitro.
CRP binding at the rhaSR and rhaBAD promoters.
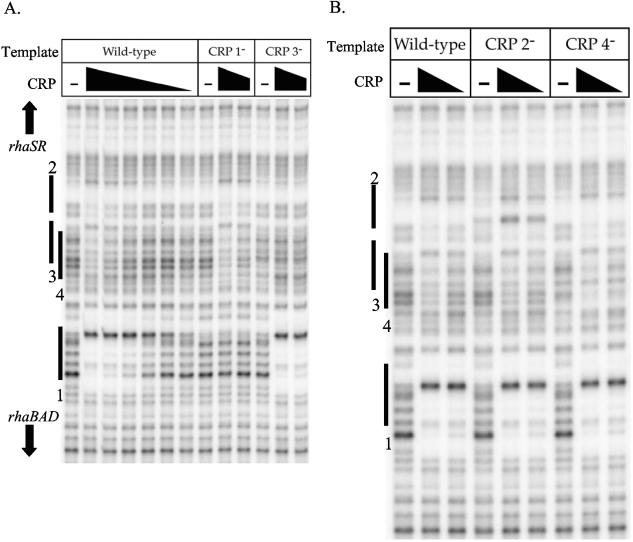
As discussed above, the four CRP binding sites in the rhaSR-rhaBAD intergenic region were predicted on the basis of their DNA sequence and position. Electrophoretic mobility shift assays previously confirmed CRP binding to DNA fragments containing either CRP site 1 alone or sites 2, 3, and 4 together (12, 15); however, it was not clear to which site(s) CRP was binding on the fragment that carried sites 2, 3, and 4. To test CRP binding to the four predicted CRP sites and to determine whether CRP could bind to any other potential sites, we used DNase I footprinting assays to monitor occupancy of CRP on 32P-labeled DNA fragments. The footprint obtained with the wild-type template showed two protected regions (Fig. 6A), one corresponding to CRP site 1 and a second weaker footprint in the region of CRP sites 2, 3, and 4. No additional protected regions were detected outside the area of the gel that is shown. Given the overlap between CRP sites 3 and 4 and the relative lack of DNase I cleavage sites on the free DNA in the region of CRP site 2, it was not possible to distinguish exactly which CRP site(s) was bound from footprints of the wild-type promoter region. In order to identify which CRP sites were capable of binding CRP in vitro, we next performed DNase I footprinting of templates carrying mutations in each of the CRP sites. The CRP site 1 down mutation resulted in a loss of detectable binding to CRP site 1, but no change in binding to the region of CRP sites 2, 3, and 4. The CRP site 3 down mutation resulted in the complete loss of the footprint in the region of sites 2, 3, and 4, suggesting that the footprint seen with the wild-type template required CRP binding at site 3. The results in Fig. 6B show no change in the footprint in the region of CRP sites 2, 3, and 4 using the CRP site 2 or CRP site 4 down mutation templates (with the exception of DNA sequence-dependent changes in the DNase I cleavage pattern), indicating that the observed footprint in the region of CRP sites 2, 3, and 4 was probably due to CRP binding only to site 3. Higher concentrations of CRP inhibited DNase activity in these footprinting reactions, so we cannot rule out the possibility that CRP is capable of binding to sites 2 and/or 4 at higher protein concentrations. However, given that neither the in vivo nor the in vitro transcription assays indicated a significant role for CRP binding sites 2 and 4, we conclude that they play little or no role in regulation of either rhaSR or rhaBAD, at least under the conditions of our assays.
FIG. 6.
DNase I footprinting assays of down mutations in the CRP sites in the rhaSR and rhaBAD promoters. At the wild-type promoter in panel A, the following concentrations of CRP (for the lanes from left to right) were used: 0 nM (−), 500 nM, 167 nM, 56 nM, 19 nM, 6 nM, and 2 nM. For other templates in panel A (with down mutations in CRP sites 1 and 3 [CRP 1− and CRP 3−, respectively) and all templates in panel B (wild type and with down mutations in CRP sites 2 and 4 [CRP 2− and CRP 4−, respectively]), the following concentrations of CRP (for the lanes from left to right) were present: 0 nM (−), 500 nM, and 167 nM. The positions of the CRP sites 1 through 4 marked on the side of the gel are based on a sequencing ladder that is not shown.
CRP activation of rhaSR expression is primarily due to neither cooperative binding with RhaR nor DNA bending.
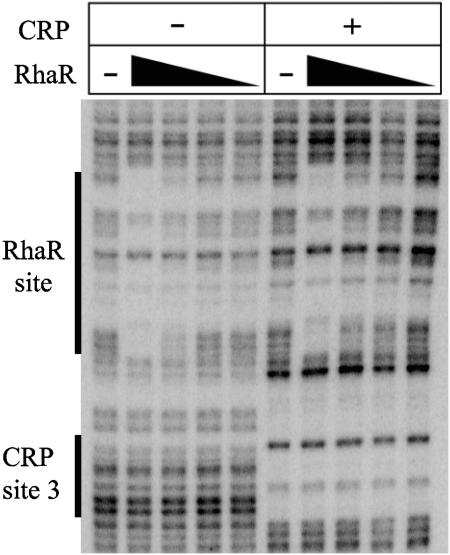
The above results identify CRP site 3 as the most important site for activation of rhaSR transcription, but they do not provide information about the mechanism of activation by CRP bound at site 3. Previous results by Holcroft and Egan did not provide conclusive evidence for or against an interaction between CRP and α-CTD at the rhaSR promoter (15), so we first tested other possible mechanisms of CRP activation. One possibility was that CRP binds to DNA cooperatively with RhaR to improve the ability of RhaR to activate transcription, similar to the cooperative binding of MelR and CRP at the melAB promoter (4). There are 21 base pairs between CRP site 3 and the RhaR binding site, which may be too far to allow cooperative binding; however, RhaR binding has been estimated to bend DNA by 160 degrees, and CRP can bend DNA by 87 degrees (21, 30). Given this large degree of DNA bending, it is possible that RhaR and CRP could each cooperatively influence the binding of the other. Another possible way that CRP and RhaR could bind cooperatively would be if CRP could bind to site 2 but only cooperatively as part of a complex with RhaR and CRP at site 3. If this were the case, it would explain the absence of CRP binding to site 2 when footprinting CRP alone. DNase I footprinting was used to test for cooperative binding by CRP and RhaR, and the results are shown in Fig. 7. The footprint of RhaR alone corresponds with the previously identified RhaR binding site (29) and shows that the extent of RhaR binding was proportional to the RhaR concentration. When RhaR was footprinted in the presence of CRP, there was no significant change in the strength of the RhaR footprint, suggesting that RhaR and CRP do not bind cooperatively (Fig. 7). Additionally, the boundaries of the RhaR and CRP footprints did not change when both proteins were present compared with their individual footprints, which argues against the possibility that CRP binds to site 2 in the presence of RhaR.
FIG. 7.
DNase I footprinting assay of RhaR in the presence (+) and absence (−) of CRP at the rhaSR promoter. RhaR was used at the following concentrations (for the lanes from left to right): 0 nM (−), 20 nM, 7 nM, 2 nM, and 0.7 nM. These same concentrations of RhaR were used in the presence of 400 nM CRP. The positions of the CRP site 3 and the RhaR binding site marked on the side of the gel are based on a sequencing ladder that is not shown.
We also tested whether DNA bending by CRP influenced rhaSR expression. Richet and Sogaard-Andersen (24) found that replacing the CRP binding site at the malKp promoter with a site for integration host factor (IHF), a DNA-bending protein, resulted in nearly equivalent promoter activity, suggesting that DNA bending by CRP allows MalT to activate transcription. We therefore tested whether DNA bending contributes to CRP-dependent activation at rhaSR in vivo. Replacement of CRP site 3 at the rhaSR promoter with an IHF binding site resulted in at most a twofold stimulation of transcription, despite centering the IHF binding site at 10 of the 11 possible positions from −122.5 and −132.5 relative to the rhaSR transcription start site (data not shown). This twofold activation (which was lost in a strain carrying an ihfB deletion [8]) suggests that DNA bending may make a small contribution to CRP activation of rhaSR transcription, but it is at most a small part of the approximately 100-fold activation by CRP at this promoter.
CRP activation of rhaSR transcription is dependent on α-CTD in vitro.
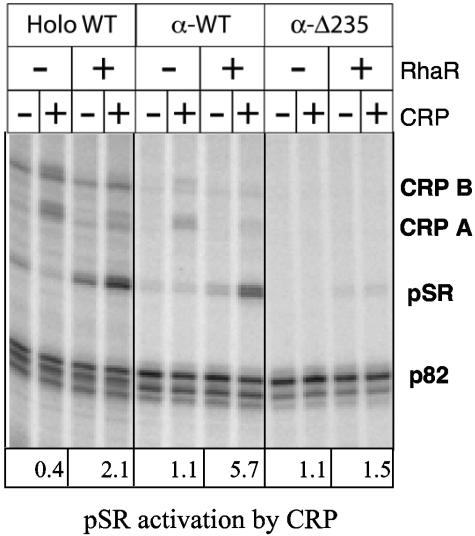
There are many examples of transcription regulators that interact with the α-CTD of RNAP to activate transcription, and interactions between α-CTD and CRP have been particularly well studied (reviewed in reference 8). We therefore investigated the importance of the α-CTD for CRP and RhaR activation of rhaSR transcription. As mentioned above, previous in vivo experiments from this lab did not provide conclusive evidence for or against an interaction between CRP and α-CTD; therefore, we used in vitro transcription assays to more directly measure the role of α-CTD in transcription activation at the rhaSR promoter.
To test whether the α-CTD was required for full activation by CRP or RhaR, we reconstituted RNAP from its individual subunits in order to obtain RNAP preparations in which both copies of the α-CTD were deleted for all residues after amino acid 235 (α-Δ235). A completely wild-type RNAP preparation (α-WT) was reconstituted in the same manner to serve as a control. The α-WT RNAP showed levels of activation by CRP and RhaR that were comparable to those obtained with RNAP purified as a holoenzyme (Fig. 8). Comparison of transcripts synthesized by the α-Δ235 and α-WT enzymes in the absence and presence of CRP demonstrated that nearly all of the CRP-dependent activation of pSR depended on α-CTD in the presence of RhaR. We also found that transcription of the longer CRP A and CRP B transcripts was completely dependent on the α-CTD. These results are consistent with the hypothesis that activation of the previously identified rhaSR promoter as well as the alternative promoters that result in the CRP A and CRP B transcripts are all due to interactions between CRP and α-CTD. Our finding that several point mutations in α-CTD also decreased CRP-dependent in vitro activation of the rhaSR promoter further supports our conclusion that CRP activation of rhaSR requires interaction with α-CTD (unpublished results).
FIG. 8.
In vitro transcription from the rhaSR promoter in the presence of RNAP reconstituted with wild-type α (α-WT) or α-Δ235 compared to purified holoenzyme RNAP (Holo WT). Reactions were carried out in the presence (+) or absence (−) of 75 nM RhaR and 800 nM CRP. pSR activation by CRP was determined by dividing the intensity in the presence of CRP by the intensity in the absence of CRP for each pair of lanes (intensity of pSR was corrected on the basis of the intensity for control p82 transcripts).
DISCUSSION
CRP site 3 is critical for CRP activation of rhaSR transcription.
We investigated the roles of three sites in the rhaSR-rhaBAD intergenic region predicted by Holcroft and Egan (15) to possibly function as CRP binding sites, as well as CRP site 1, which was previously shown to be required for full rhaBAD activation (12). In this study, both in vivo and in vitro transcription assay methods demonstrated that CRP site 3 was required for significant transcription activation of rhaSR by CRP (Fig. 2A and 5). In addition to transcription assays, DNase I footprinting of the rhaSR-rhaBAD intergenic region provided evidence of CRP binding to site 3, but not to site 2 or 4, supporting the hypothesis that site 3 is the most important site for CRP activation of rhaSR. Given that CRP site 3 is predicted to be the second strongest CRP site in the rhaSR-rhaBAD promoter region (after CRP site 1 at rhaBAD), these results are not surprising. However, the position of CRP site 3 at rhaSR (−111.5) is quite different than the position of CRP site 1 at rhaBAD (−92.5), suggesting possible differences in the details of synergistic CRP activation with RhaS versus RhaR.
The CRP binding site mutations assayed in this study were constructed in the context of DNA fragments that could be fused at either one end or the other with lacZ in order to determine whether expression from the rhaBAD and rhaSR operons influenced each other. While it might be logical for rhaBAD expression to negatively influence rhaSR expression, we found no significant influence of the mutations in CRP site 1 on rhaSR expression. The position of CRP site 4 on the same face of the DNA as the rhaBAD activators and overlapping CRP site 3 looks like it might provide a mechanism for rhaBAD expression to influence rhaSR expression, but, at least under the conditions of our assays, there is no evidence that such a mechanism occurs. However, our results do suggest that CRP binding to site 3 has a small negative influence on rhaBAD expression, suggesting that rhaSR expression may slightly influence rhaBAD expression.
The in vivo and in vitro transcription assays and the DNase footprinting assays show little or no role for CRP site 2 in rhaSR activation. CRP site 2 is a very weak match to the CRP consensus sequence that we would not have considered had it not been positioned symmetrically with CRP site 1 at rhaBAD. While the CRP site 2 down mutation had a small effect on rhaSR expression in vivo (66% of wild-type activity [Fig. 2A]) that was dependent on CRP, we predict that this defect is not due to decreased CRP binding to site 2 but more likely is due to an indirect effect on CRP bound at site 3, such as a change in DNA bending or a change in the ability of α-CTD to contact CRP at site 3. Some of our reasons for this conclusion follow. (i) CRP protein binding to site 2 was not detected. (ii) There are multiple other sequences with five out of ten matches to the CRP consensus sequence in the rhaSR-rhaBAD intergenic region. (iii) There is not a potential CRP site at the position of the CRP site 2 in Salmonella enterica serovar Typhimurium (20) (although there are CRP sites at the positions of sites 1 and 3, each with the same number of matches to the CRP consensus sequence). (iv) There are only 19 base pairs between the centers of CRP sites 2 and 3, and there are not currently any known cases of two CRP dimers binding that close to each other.
CRP activation of rhaSR transcription requires α-CTD and RhaR.
Previous in vitro transcription studies of the rhaSR promoter demonstrated that the RhaR protein and the sugar l-rhamnose each stimulated rhaSR expression and that supercoiled DNA templates were required for this stimulation of transcription (31). Here we have recapitulated each of those findings with RhaR purified using a new method. In addition, we have demonstrated that CRP can further stimulate rhaSR expression in vitro and have obtained evidence that CRP activation at rhaSR requires contact with α-CTD. In contrast, our previous in vivo experiments showed that overexpression of α-Δ235 resulted in only a two- to threefold defect at promoters that included a CRP binding site, although the total activation by CRP is normally 100-fold (15). One possible explanation for the apparent discrepancy between these in vitro results and our previous in vivo results is that RNAP with a single α-CTD is sufficient for full rhaSR expression. In vivo expression of α-Δ235 in a strain that also expresses α-WT is expected to result in a mixture of RNAPs carrying zero, one, or two α-CTDs. If one α-CTD were sufficient for full rhaSR expression, then only the fraction of the RNAP pool with zero α-CTDs would contribute to the in vivo defect, and a relatively small defect would be expected. Since 100% of the RNAP used in the in vitro experiments should contain zero α-CTDs, a larger in vitro defect would be expected. Since our results also indicate that CRP activation at rhaSR involves little or no contribution from cooperative binding with RhaR or DNA bending, we conclude that the majority (at least) of the mechanism of CRP activation at rhaSR involves contact with α-CTD. Whether the details of that contact are similar to those of simple CRP-dependent promoters remains to be determined.
We have also found that in the absence of saturating concentrations of RhaR protein, two CRP-dependent transcripts that were longer than the “normal” RhaR-dependent rhaSR transcript were produced (CRP A and CRP B). Our prediction that the core promoter for each of these transcripts (on the basis of mapping their start sites) was partially or fully within the RhaR binding site likely explains their inverse relationship with RhaR concentration. We also found that each of these transcripts was dependent on α-CTD and at least partially dependent on CRP site 3. Although we have not previously tested the appropriate conditions to detect these transcripts using in vivo-purified RNA (12), we do routinely detect some level of RhaR-independent transcription from the rhaSR promoter in lacZ assays that could be due to these alternative promoters (15; unpublished data).
Our in vitro transcription assay results indicate that CRP can activate transcription only from the “normal” pSR transcription start point in the presence of RhaR (Fig. 3). The mechanism by which RhaR increases CRP activation is not known, but it does not appear to be through cooperative binding on the basis of our DNase footprinting results (Fig. 7). The requirement of both RhaR and α-CTD for significant CRP activation of pSR suggests a possible mechanism by which RhaR bends DNA to allow CRP to interact with α-CTD. RhaR was found to bend DNA by 160 degrees in vitro (30), and this bending may be required to bring CRP bound at −111.5 close enough to interact with α-CTD. The observation that the RhaR binding site contains four phased A-tracts supports the hypothesis that DNA bending is important at rhaSR.
Acknowledgments
We thank Carolyn Holcroft and Jennifer Jeffress for contributions to the mutagenesis and recombination of CRP binding site mutants, Niels Holten-Andersen and Visnja Jevtic for purification of RhaR, Fugen Yu for performing preliminary RhaR-CRP cooperativity experiments, and Evelyne Richet for a plasmid containing an IHF binding site. A strain carrying the ihfB deletion was obtained from D. Biek via V. Stewart.
This work was supported by NIH grant GM55099 from the National Institute of General Medical Sciences and NIH grant RR-P20 RR17708 from the Institutional Development Award (IDeA) Program of the National Center for Research Resources (both to S.M.E.) and NIH grant GM21941 to Jeffrey W. Roberts.
REFERENCES
- 1.Backman, K., Y.-M. Chen, and B. Magasanik. 1981. Physical and genetic characterization of the glnA-glnG region of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA 78:3743-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldoma, L., J. Badia, G. Sweet, and J. Aguilar. 1990. Cloning, mapping and gene product identification of rhaT from Escherichia coli K12. FEMS Microbiol. Lett. 60:103-107. [DOI] [PubMed] [Google Scholar]
- 3.Belyaeva, T. A., V. A. Rhodius, C. L. Webster, and S. J. W. Busby. 1998. Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organisation of the RNA polymerase α subunits. J. Mol. Biol. 277:789-804. [DOI] [PubMed] [Google Scholar]
- 4.Belyaeva, T. A., J. T. Wade, C. L. Webster, V. J. Howard, M. S. Thomas, E. I. Hyde, and S. J. Busby. 2000. Transcription activation at the Escherichia coli melAB promoter: the role of MelR and the cyclic AMP receptor protein. Mol. Microbiol. 36:211-222. [DOI] [PubMed] [Google Scholar]
- 5.Berg, O. G., and P. H. von Hippel. 1988. Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J. Mol. Biol. 200:709-723. [DOI] [PubMed] [Google Scholar]
- 6.Bhende, P. M., and S. M. Egan. 1999. Amino acid-DNA contacts by RhaS: an AraC family transcription activator. J. Bacteriol. 181:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhende, P. M., and S. M. Egan. 2000. Genetic evidence that transcription activation by RhaS involves specific amino acid contacts with sigma 70. J. Bacteriol. 182:4959-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biek, D. P., and S. N. Cohen. 1989. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: characterization of pSC101 mutants that replicate in the absence of IHF. J. Bacteriol. 171:2056-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 10.Chapon, C. 1982. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J. Bacteriol. 150:722-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, S. M., and R. F. Schleif. 1994. DNA-dependent renaturation of an insoluble DNA binding protein. Identification of the RhaS binding site at rhaBAD. J. Mol. Biol. 243:821-829. [DOI] [PubMed] [Google Scholar]
- 12.Egan, S. M., and R. F. Schleif. 1993. A regulatory cascade in the induction of rhaBAD. J. Mol. Biol. 234:87-98. [DOI] [PubMed] [Google Scholar]
- 13.Gallegos, M.-T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman, M. E., and M. B. Yarmolinsky. 1968. The integration and excision of the bacteriophage lambda genome. Cold Spring Harbor Symp. Quant. Biol. 33:735-747. [DOI] [PubMed] [Google Scholar]
- 15.Holcroft, C. C., and S. M. Egan. 2000. Interdependence of activation at rhaSR by cyclic AMP receptor protein, the RNA polymerase alpha subunit C-terminal domain, and RhaR. J. Bacteriol. 182:6774-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcroft, C. C., and S. M. Egan. 2000. Roles of cyclic AMP receptor protein and the carboxyl-terminal domain of the α subunit in transcription activation of the Escherichia coli rhaBAD operon. J. Bacteriol. 182:3529-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joung, J. K., D. M. Koepp, and A. Hochschild. 1994. Synergistic activation of transcription by bacteriophage λ cI protein and E. coli cAMP receptor protein. Science 265:1863-1866. [DOI] [PubMed] [Google Scholar]
- 18.Marr, M. T., and J. W. Roberts. 1997. Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science 276:1258-1260. [DOI] [PubMed] [Google Scholar]
- 19.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishitani, J., and G. Wilcox. 1991. Cloning and characterization of the l-rhamnose regulon in Salmonella typhimurium LT2. Gene 105:37-42. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson, G., C. Wilson, A. Gunasekera, Y. W. Ebright, R. E. Ebright, and H. M. Berman. 1996. Structure of the CAP-DNA complex at 2.5 angstroms resolution: a complete picture of the protein-DNA interface. J. Mol. Biol. 260:395-408. [DOI] [PubMed] [Google Scholar]
- 22.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough, Jr. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power, J. 1967. The L-rhamnose genetic system in Escherichia coli K-12. Genetics 55:557-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richet, E., and L. Sogaard-Andersen. 1994. CRP induces the repositioning of MalT at the Escherichia coli malKp promoter primarily through DNA bending. EMBO J. 13:4558-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santangelo, T. J., and J. W. Roberts. 2004. Forward translocation is the natural pathway of RNA release at an intrinsic terminator. Mol. Cell 14:117-126. [DOI] [PubMed] [Google Scholar]
- 26.Scott, S., S. Busby, and I. Beacham. 1995. Transcriptional co-activation at the ansB promoters: involvement of the activating regions of CRP and FNR when bound in tandem. Mol. Microbiol. 18:521-531. [DOI] [PubMed] [Google Scholar]
- 27.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 28.Tang, H., Y. Kim, K. Severinov, A. Goldfarb, and R. H. Ebright. 1996. Escherichia coli RNA polymerase holoenzyme: rapid reconstitution from recombinant alpha, beta, beta′, and sigma subunits. Methods Enzymol. 273:130-134. [DOI] [PubMed] [Google Scholar]
- 29.Tobin, J. F., and R. F. Schleif. 1987. Positive regulation of the Escherichia coli L-rhamnose operon is mediated by the products of tandemly repeated regulatory genes. J. Mol. Biol. 196:789-799. [DOI] [PubMed] [Google Scholar]
- 30.Tobin, J. F., and R. F. Schleif. 1990. Purification and properties of RhaR, the positive regulator of the L-rhamnose operons of Escherichia coli. J. Mol. Biol. 211:75-89. [DOI] [PubMed] [Google Scholar]
- 31.Tobin, J. F., and R. F. Schleif. 1990. Transcription from the rha operon psr promoter. J. Mol. Biol. 211:1-4. [DOI] [PubMed] [Google Scholar]
- 32.Via, P., J. Badia, L. Baldoma, N. Obradors, and J. Aguilar. 1996. Transcriptional regulation of the Escherichia coli rhaT gene. Microbiology 142:1833-1840. [DOI] [PubMed] [Google Scholar]
- 33.Wade, J. T., T. A. Belyaeva, E. I. Hyde, and S. J. Busby. 2001. A simple mechanism for co-dependence on two activators at an Escherichia coli promoter. EMBO J. 20:7160-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickstrum, J. R., and S. M. Egan. 2004. Amino acid contacts between sigma 70 domain 4 and the transcription activators RhaS and RhaR. J. Bacteriol. 186:6277-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickstrum, J. R., and S. M. Egan. 2002. Ni+-affinity purification of untagged cyclic AMP receptor protein. BioTechniques 33:728-730. [DOI] [PubMed] [Google Scholar]
- 36.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, X., and R. Schleif. 1998. Catabolite gene activator protein mutations affecting activity of the araBAD promoter. J. Bacteriol. 180:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]