Abstract
WT1, a transcription factor implicated in both normal kidney differentiation and tumorigenesis, is also expressed in differentiating hematopoietic progenitors. Most human acute leukemias contain high levels of the wild-type transcript, while a minority have point mutations, raising the possibility that this tumor suppressor might have a paradoxical oncogenic effect in some hematopoietic cells. Using high titer retroviral infection, we demonstrate that WT1 triggers rapid growth arrest and lineage-specific differentiation in primary hematopoietic progenitors and differentiation-competent leukemia cell lines, while it induces cellular quiescence in a primitive subset of primary precursors. Growth arrest by WT1 is associated with induction of p21CIP1, but expression of this cyclin-dependent kinase inhibitor alone is insufficient for either cellular differentiation or primitive cell preservation. The effects of WT1 are enhanced by co-expression of its naturally occurring isoforms, and are correlated with the physiological expression pattern of WT1 in vivo. Our observations suggest a role for WT1 in the differentiation of human hematopoietic cells, and provide a functional model that supports its capacity as a tumor suppressor in human acute leukemia.
Keywords: hematopoiesis/leukemia/tumor suppressor/WT1
Introduction
The WT1 tumor suppressor gene encodes a Cys–His zinc finger transcription factor that exemplifies the link between normal organ development and cancer (reviewed in Hastie, 1994). Loss of one germ-line allele is associated with genitourinary malformations, attributed to reduced gene dosage during critical stages in development, and it confers a high risk for Wilms tumor, resulting from somatic loss of the second WT1 allele. Approximately 10% of sporadic Wilms tumors also have inactivating mutations in WT1, although the majority express high levels of the wild-type transcript (Little et al., 1992). Inactivation of WT1 in the mouse leads to an embryonic lethal phenotype, characterized by renal agenesis and aberrant development of mesothelial structures (Kreidberg et al., 1993). The failure of renal differentiation in WT1-null mice is attributable to widespread apoptosis of the blastemal cells that comprise the renal stem cell population, an observation that suggests a role for WT1 in the survival of these undifferentiated cells. At the later stages of renal differentiation, which are not achieved in wt1-null mice, WT1 expression peaks in specific precursors of the glomerulus, suggesting a distinct role in the differentiation of these highly specialized structures. Thus, the physiological function of WT1 at different stages of renal differentiation, and the consequences of its inactivation in the mouse, appear complex, and have been difficult to reconcile with its presumed function as a tumor suppressor.
A contribution by WT1 to blood cell development has been suggested by its expression in early hematopoietic precursors and its rapid downregulation following differentiation in primary blood cells and leukemia-derived cell lines (Sekiya et al., 1994; Baird and Simmons, 1997; Maurer et al., 1997; Menssen et al., 1997). However, the role of WT1 in this process also appears paradoxical. Most human acute myeloid and lymphoid leukemias express high levels of wild-type WT1 (Miwa et al., 1992; Menssen et al., 1995), which have been correlated in some studies with the development of more primitive and refractory forms of disease (Inoue et al., 1994; Bergmann et al., 1997). Yet, mutations have been reported in ∼10% of cases, suggesting a role for disruption of WT1 function in leukemogenesis that is comparable to its role in Wilms tumor (King-Underwood et al., 1996; Miyagawa et al., 1999). Attempts at defining the consequences of WT1 expression in leukemia-derived cell lines have also produced conflicting results, reporting enhanced cellular proliferation in some systems but induction of growth arrest in others, and proposing both mediation as well as inhibition of cellular differentiation (Algar et al., 1996; Inoue et al., 1998; Smith et al., 1998, 2000; Svedberg et al., 1998; Deuel et al., 1999). These studies, some of which have raised the possibility of an oncogenic role for WT1 in leukemogenesis, have relied on antisense strategies or on the selection of stably transfected clones, which may fail to detect immediate effects on cellular proliferation associated with the expression of a tumor suppressor gene. No prior studies have examined the consequences of WT1 expression in normal hematopoietic cells.
WT1 is encoded by distinct isoforms resulting from alternative pre-mRNA splicing, of which the most functionally significant is the insertion of three amino acids, leucine–threonine–serine (or KTS), that disrupt the critical spacing between zinc fingers 3 and 4 in the DNA binding domain (Haber et al., 1991). WT1 proteins lacking this insertion, WT1(–KTS), bind to a 5′-GCGTGGGAGT-3′ sequence and demonstrate potent transcriptional activation of target genes including the cyclin-dependent kinase inhibitor p21CIP1 and the epidermal growth factor family member amphiregulin (Nakagama et al., 1995; Englert et al., 1997; English and Licht, 1999; Lee et al., 1999). In contrast, the more abundant WT1(+KTS) isoform does not bind to a known DNA sequence, and while it may physically associate with WT1(–KTS), it does not appear to mediate transcriptional regulation directly (Englert et al., 1995b; Moffett et al., 1995; Reddy et al., 1995). The localization of WT1(+KTS) within subnuclear speckles, in association with factors involved in pre-RNA splicing, has also suggested a role in RNA processing (Larsson et al., 1995; Davies et al., 1998). Expression of both (–KTS) and (+KTS) isoforms appears to be critical for normal WT1 function. Frasier syndrome, characterized by glomerulopathy and gonadal dysgenesis (Barbaux et al., 1997), results from an inherited mutation within the KTS splice donor site that disrupts the normally constant ratio between these splicing variants. Although these observations point to important roles for both (–KTS) and (+KTS) isoforms of WT1, it is unclear whether their functions are complementary or antagonistic.
To determine the relevance of WT1 expression in normal and transformed hematopoietic cells, we used pseudotyped retroviruses to introduce the different alternative splicing variants of WT1 at high efficiency into human primary hematopoietic progenitors and leukemia-derived cell lines. In both primary progenitors and differentiation-competent leukemic cells, WT1 expression was sufficient to induce G1 arrest and to trigger differentiation along the myelo-monocytic lineage. In a subpopulation of early, stem cell-enriched progenitors, WT1 induced cellular quiescence, leading to the preservation of phenotypically primitive cells. Co-expression of the naturally occurring isoforms of WT1 enhanced its transcriptional and physiological effects. The expression pattern of WT1 during blood cell differentiation in vivo correlated with these effects: present first in the quiescent stem cell compartment, and subsequently during lineage-specific differentiation. Taken together, these observations imply a role for WT1 in the differentiation of normal human hematopoietic cells; they argue against an oncogenic role for WT1 in leukemogenesis and support the functional relevance of WT1 mutations in human acute leukemias.
Results
WT1 induces myelo-monocytic differentiation in human leukemia-derived cell lines
To examine the functional properties of WT1 in a well established model of cellular differentiation, we first expressed WT1 in two human myeloid leukemia-derived cell lines: HL60 and U937. These lines retain the capacity for differentiation following induction with all-trans retinoic acid (ATRA) and tetrahydro-phorbol-ester acid (TPA) (Breitman et al., 1980; Bjare et al., 1988), and they differ in their expression of WT1: wild-type WT1 is expressed in HL60 cells, but absent in U937 cells (Sekiya et al., 1994; Svedberg et al., 1998). To avoid the possibility of selection bias associated with the isolation of rare clones stably expressing WT1, we transduced cells with the retroviral vector MSCV-GFP (where MSCV is murine stem cell virus), which drives expression of both WT1 and green fluorescent protein (GFP) from a single bicistronic message (Hawley et al., 1992), and analyzed unselected pools of GFP-expressing cells (Figure 1A). For each experiment we conducted multiple independent transductions and analyzed results within 3–5 days following retroviral infection. Approximately 20–40% of HL60 cells and 40–80% of U937 cells expressed GFP following infection with retroviruses encoding either the (–KTS) or (+KTS) isoforms of WT1 (Figure 2A). Levels of ectopic protein expression were comparable to those of the endogenous WT1 in HL60 and other leukemic cells (Figure 1B).
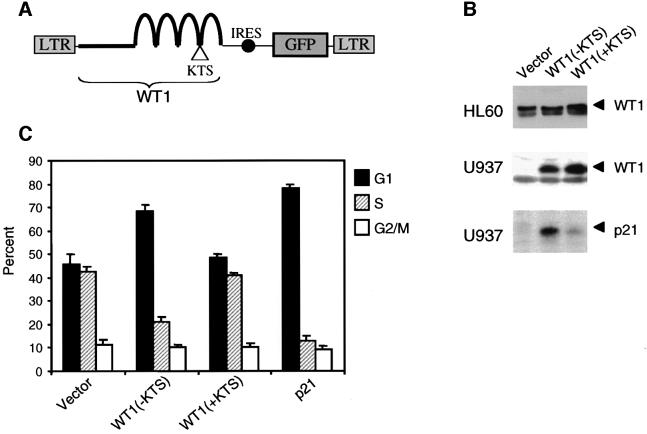
Fig. 1. G1 arrest and induction of p21cip1 in U937 cells following WT1 expression. (A) Schematic representation of retroviral constructs, encoding the (–KTS) and (+KTS) isoforms of WT1, linked by an internal ribosomal entry site (IRES) to a cDNA encoding GFP. The LTRs are derived from MSCV, which allows high levels of expression in hematopoietic precursors. (B) Western blots showing levels of WT1 protein in retrovirally transduced HL60 and U937 cells, and endogenous p21cip1 in U937 cells. Cell lysates were prepared 48 h following retroviral infection and analyzed on immunoblots probed with polyclonal anti-WT1 and anti- p21cip1 antibodies. Endogenous WT1 expression is not detectable in U937 cells. In HL60 cells, the lower band in the doublet is endogenous WT1 lacking alternative splice I (17 amino acids); the upper band contains both endogenous and transduced WT1 encoding this isoform. (C) Effect of WT1 expression on cell cycle kinetics of transduced U937 cells. Cells were harvested 48 h after retroviral infection, stained with PI and analyzed by flow cytometry gating on the GFP-positive population. Results are derived from three independent experiments, with SE. The difference in the G1 fraction between vector and WT1(–KTS)-expressing cells is significant (P = 0.0048), and is similar to that between cells expressing the vector or a retrovirus encoding p21CIP1(right-most bars).
Fig. 2. Induction of spontaneous differentiation in U937 cells following WT1 expression. (A) Two-color FACS analysis for GFP and CD11b on transduced U937 cells in a representative experiment. Cells were harvested 48 h after retroviral infection and stained with a PE-conjugated anti-CD11b antibody. Dot plots represent fluorescence intensity for GFP (y-axis) and CD11b–PE (x-axis), with the upper right quadrant representing infected cells expressing the differentiation marker. Differences in the percentage of GFP-positive cells are consistent, and reflect different packaging efficiencies for individual retroviral constructs as well as the growth-suppressive effect of WT1(–KTS). In the right panel, vector-transduced cells cultured for 24 h in the presence of 3 nM TPA are shown as a positive control for differentiation. (B) Quantitation of three independent experiments showing the mean expression of CD11b among GFP-positive transduced U937 or HL60 cells. (C) Morphological and functional differentiation of U937 cells expressing WT1(–KTS). Unsorted transduced cells were stained with Wright–Giemsa (upper panel, 250× magnification) or with 1 mg/ml NBT (lower panel, 100×), and analyzed by light microscopy. Representative fields are shown. Among WT1(–KTS)-transduced populations, fluorescence microscopy indicated that >90% of morphologically differentiated and NBT-positive cells were GFP positive (not shown).
Despite the difference in expression of endogenous WT1, the consequences of ectopic WT1 expression in HL60 and U937 cells were virtually identical, and hence are shown for U937 cells (Figure 1). Within 48 h following retroviral infection, cells expressing WT1(–KTS) demonstrated a dramatic increase in the G1-phase fraction and a corresponding reduction in S phase compared with the control vector (68% versus 46%, P = 0.006). This was demonstrated by both analysis of propidium iodide (PI)-stained cells (Figure 1C) and bromodeoxyuridine (BrdU) labeling (data not shown). Little or no increase in the G1-phase fraction was observed in parallel cultures infected with WT1(+KTS). The G1 arrest mediated by WT1(–KTS) was accompanied by marked induction of the cyclin-dependent kinase inhibitor p21CIP1, a known WT1 transcriptional target gene (Figure 1B) (Englert et al., 1997; English and Licht, 1999). In HL60 cells, these effects were observed despite the high levels of endogenous WT1 expression and the relatively modest levels of ectopic WT1(–KTS) expression. These cells may be sensitive to subtle alterations in the relative ratio of WT1 isoforms. Alternatively, endogenous WT1 may reside in non-functional pools in HL60 cells.
Differentiation of U937 and HL60 cells induced by either retinoids or by the phorbol ester TPA is first evident by expression of characteristic cell surface markers, such as CD11b and CD14, followed by gross morphological changes (Hickstein et al., 1989; Prieto et al., 1994). Within 48 h following retroviral infection, 30–50% of HL60 and U937 cells expressing WT1(–KTS) showed spontaneous expression of CD11b in the absence of differentiation-inducing agents (Figure 2A and B). In contrast, <2% of cells infected with WT1(+KTS) or control vector expressed this marker. Spontaneous morphological changes accompanied CD11b expression in U937 cells transduced by WT1(–KTS), which adhered to the tissue culture dish and acquired the characteristic appearance of differentiated U937 cells (Figure 2C). To confirm the functional properties of these differentiated cells, we tested their ability to reduce the substrate dye nitroblue tetrazolium (NBT), a characteristic that correlates directly with myeloid differentiation (Breitman et al., 1980). In multiple experiments, 40–80% of U937 cells infected with WT1(–KTS) were positive for NBT, while <5% of WT1(+KTS)- or vector-infected populations were NBT positive (Figure 2C). Taken together, these observations indicate that expression of WT1(–KTS), but not WT1 (+KTS), in U937 and HL60 cells results in spontaneous differentiation within 48 h.
The mechanism by which WT1 triggers hematopoietic differentiation is likely to involve transcriptional regulation of differentiation-associated target genes. One of these, p21CIP1, is induced by WT1(–KTS) as well as other agents that trigger hematopoietic differentiation, and is thought to mediate a cell cycle arrest that contributes to activation of a cellular differentiation program (Zeng and el-Deiry, 1996; Steinman et al., 1998). To determine whether WT1-mediated blood cell differentiation is simply the indirect result of a p21CIP1-induced G1 arrest, we infected U937 cells with a bicistronic MSCV-GFP retrovirus encoding p21CIP1. p21CIP1-expressing cells exhibited prompt G1 arrest (Figure 1C), but little or no spontaneous expression of cell surface differentiation markers or morphological changes (Figure 2A and B). The triggering of hematopoietic differentiation by WT1 is, therefore, likely to involve transcriptional regulation of additional target genes. Of note, induction of p21CIP1 by WT1 may contribute to this effect, since we did observe that retrovirus-mediated expression of p21CIP1 accelerated the time course of differentiation induced by the phorbol ester TPA in U937 cells, even though it was not sufficient to cause spontaneous differentiation (data not shown).
WT1(+KTS) enhances differentiation mediated by the (–KTS) isoform
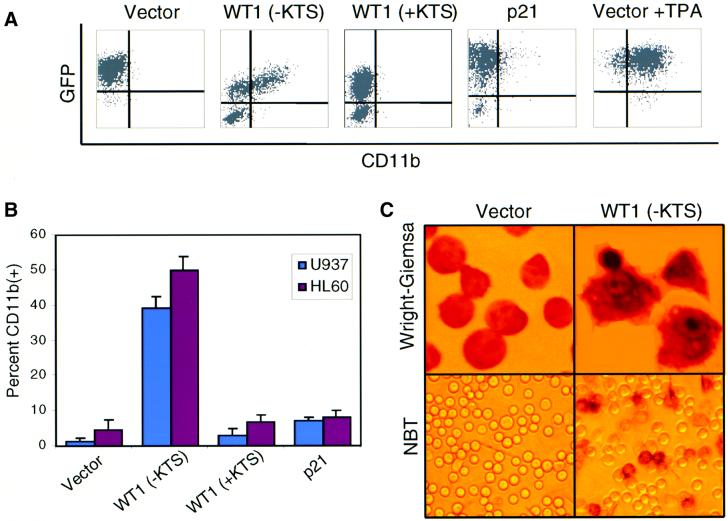
WT1(+KTS) encodes the most abundant isoform of WT1, but it has no known functional properties. While WT1(+KTS) was inactive in mediating cellular differentiation by itself, the observation that the WT1 isoforms are normally co-expressed in a fixed ratio (Haber et al., 1991) prompted us to examine whether simultaneous ectopic expression of these two isoforms might have functional consequences. The high retroviral infection rate achieved in U937 cells made it possible to co-infect a significant subset of cells (13%) with two different bicistronic constructs, one encoding WT1(–KTS) and GFP, and the other encoding WT1(+KTS) and the cell surface marker placental alkaline phosphatase (PLAP) (Figure 3A) (Jacob and Baltimore, 1999). In multiple experiments, fluorescence-activated cell sorting (FACS) analysis of U937 cells expressing both WT1(–KTS) and WT1(+KTS) showed a 30–50% relative increase in the number of cells positive for the differentiation marker CD11b, compared with cells co-expressing WT1(–KTS) and the control PLAP vector (Figure 3B). Cells singly infected with the control PLAP vector or the bicistronic WT1(+)KTS/PLAP construct showed little or no spontaneous CD11b expression. We also examined expression of a second differentiation marker, CD14, which is not spontaneously induced by the expression of WT1(–KTS), but is strongly induced during TPA-mediated differentiation in WT1(–KTS)-expressing populations. Co-expression of both WT1(+KTS) and (–KTS) isoforms markedly enhanced the induction of CD14 following TPA-stimulated differentiation, compared with expression of WT1(–KTS) plus the control vector (Figure 3C). Moreover, the subset of co-infected cells with the highest levels of PLAP, the WT1(+KTS) surrogate marker, showed the largest increases in CD11b and CD14 staining (data not shown). Taken together, these observations indicate that WT1(+KTS) is not sufficient to trigger differentiation alone, but exhibits a potent cooperative effect in enhancing WT1(–KTS)-mediated differentiation.
Fig. 3. Enhancement of WT1(–KTS)-mediated differentiation by WT1(+KTS). (A) Two-color FACS analysis for the surrogate markers GFP [linked to WT1(–KTS)] and PLAP [vector alone or linked to WT1(+KTS)] on transduced U937 cells (upper panel). Cells were harvested 48 h after retroviral infection and stained for PLAP and CD11b or CD14. Boxes denote cell populations with comparable expression of GFP and PLAP that were selected for CD11b or CD14 analysis (see below). FACS analysis using control IgG was used to define the appropriate threshold for PLAP expression (lower panel). (B) Quantitative analysis of two independent experiments showing spontaneous expression of CD11b among U937 cells transduced with vector, WT1(–KTS), WT1(+KTS) or both isoforms [see cell populations selected in (A)]. CD11b expression was determined by two- and three-color FACS analysis. (C) Time course of CD14 expression in U937 cells transduced with either or both WT1 isoforms [see (A)], following TPA-induced differentiation. Cells were treated with 3 nM TPA 24 h after retroviral infection (time 0), harvested and stained at the indicated time points, then analyzed by two- and three-color FACS analysis. A representative experiment is shown.
WT1 enhances myelo-monocytic differentiation in primary hematopoietic progenitors
While leukemia-derived cell lines provide an important model for human hematopoietic differentiation, they are derived from cells arrested at a single stage of hematopoiesis and capable of differentiation along a limited lineage. We therefore examined the effect of ectopic WT1 expression on differentiation of primary CD34(+) hematopoietic progenitor cells isolated from human umbilical cord blood. CD34(+) cells represent a heterogeneous population of undifferentiated cells, including both lineage-committed progenitors and rare uncommitted, multipotent stem cells (Civin and Small, 1995; Krause et al., 1996). WT1 expression gave rise to distinct phenotypes in these two populations: enhanced differentiation of the more mature progenitors, and quiescence of a rare, primitive subset of CD34(+) cells.
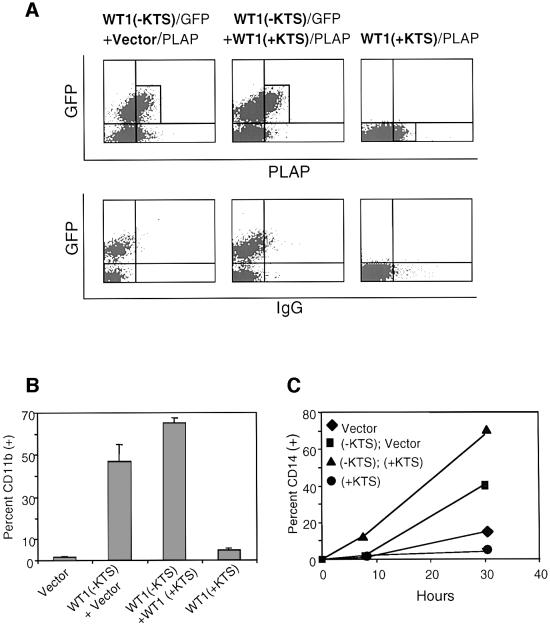
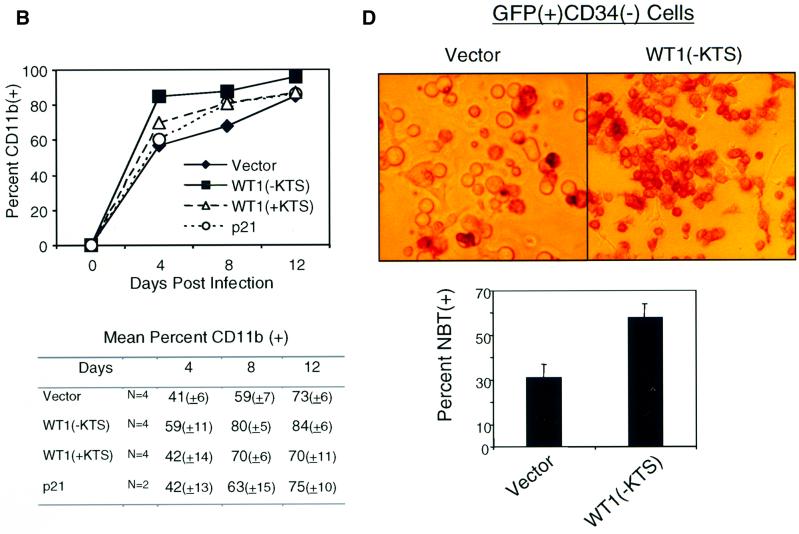
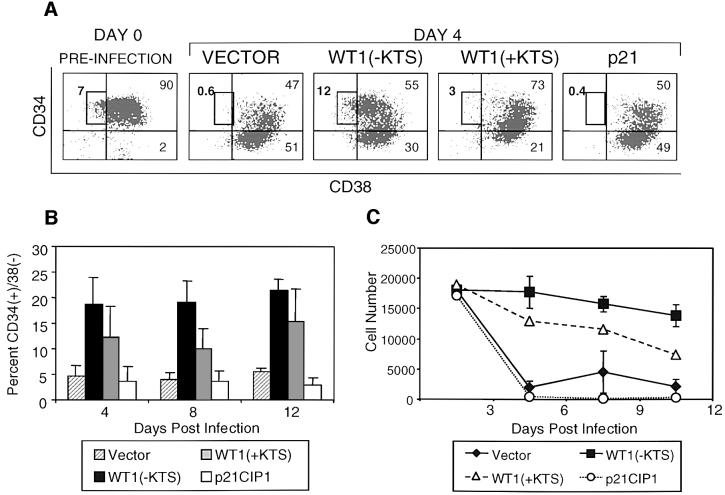
Retroviral titers and infection conditions were optimized, allowing routine infection of 20–50% of CD34(+) cells with bicistronic constructs encoding WT1 and GFP (Figure 4A, upper panel). Infected cells were cultured in the presence of the cytokines c-kit ligand (KL), FLT-3 ligand (FLT-3 L), thrombopoietin (TPO) and interleukin-3 (IL-3), and were harvested at different time points for immunophenotypic and functional analysis. Primary CD34(+) cells normally proliferate and differentiate rapidly once placed in culture, giving rise to cells that lose expression of this marker, gain lineage-specific markers and eventually cease to proliferate (Civin and Small, 1995; Traycoff et al., 1996). These effects were significantly enhanced among cells expressing WT1 (–KTS), and were reproduced in multiple independent experiments. Cells expressing WT1(–KTS) consistently showed more frequent expression of the differentiation marker CD11b compared with vector or WT1(+KTS)-infected cells (Figure 4). Figure 4A (lower panel) shows expression of CD11b and CD34 on transduced GFP(+) cells at day 7 following retroviral infection in a representative experiment. The increased fraction of WT1(–KTS)-transduced cells expressing CD11b was most prominent in the population that had lost the CD34 surface marker: 79% [51/(51 + 13)] of CD34(–) cells were CD11b positive among the WT1(–KTS)-expressing population versus 51% [38/(38 + 36)] for the vector control population. On average, we observed a 40–50% relative increase in the proportion of CD34(–) cells expressing CD11b in WT1(–KTS)-infected versus vector-infected populations (Figure 4B; P = 0.001). This effect persisted for ∼12 days of in vitro culture, after which time most cells in all infected populations expressed differentiation markers. Of note, the intensity of CD11b staining correlated with the level of WT1(–KTS) expression, as assessed by the surrogate marker GFP (Figure 4C). Increased functional differentiation of primary WT1(–KTS)-expressing cells was confirmed by NBT staining (Figure 4D). Taken together, these data indicate that WT1(–KTS) enhances the differentiation of primary blood cell progenitors, in particular the more mature subset identified by the rapid loss of CD34. WT1(+KTS) had a less pronounced but consistent effect on in vitro differentiation (Figure 4).
Fig. 4. Enhanced differentiation of primary hematopoietic progenitors by WT1. Purified human cord blood CD34(+) cells were transduced with GFP-linked vector, WT1(–KTS), WT1(+KTS) or p21Cip1, cultured in cytokine-supplemented medium, and harvested at days 4, 8 and 12 following retroviral transduction, for immunophenotypic and functional analysis. (A) FACS analysis of transduced cells, showing GFP staining as a marker for retroviral infection, and forward scatter (FSC), an indicator of cell size (upper panel). The percentage of infected cells (boxed) is quantitated. Analysis using anti-CD34–PE and anti-CD11b–APC on the gated GFP-positive population is shown in the lower panel. The percentage of cells in each quadrant is indicated. A representative experiment is shown for one time point (day 8). (B) Time course of CD11b expression during in vitro culture of transduced primary cell populations. A representative experiment is plotted, showing the percentage of CD11b-expressing cells within the GFP(+)CD34(–) population. Results from six independent experiments are shown in the table, indicating the mean percentage of CD11b-expressing cells (± SE). Differences in marker expression between vector and WT1(–KTS)-expressing cells at days 4, 8 and 12 were analyzed using the Wilcoxon matched-pairs signed ranks test (P <0.001). (C) Correlation between the intensity of the WT1(–KTS)-linked marker GFP and that of CD11b in transduced cord blood cells. GFP expression was quantitated by FACS analysis (day 8) for cells stained as in (A), and subpopulations were selected as GFP-negative, dim and bright (boxed in upper panel). Expression of CD11b was measured in these subpopulations (shown as a histogram in the lower panel). (D) Functional differentiation of primary hematopoietic progenitors. NBT staining of FACS-sorted GFP(+)CD34(–) cells, following infection with vector or WT1(–KTS)-encoding retroviral constructs (day 7). Representative fields are shown by light microscopy (100×) (upper panel). Quantitation of the mean percent NBT-positive cells with SE is shown in the lower panel. The differences are statistically significant (P <0.001).
As in leukemia-derived cell lines, WT1-mediated differentiation in primary hematopoietic precursors was associated with a cell cycle arrest. Within 48–72 h following retroviral infection, PI staining showed a 35% relative increase in Go–G1-phase cells compared with vector control or WT1(+KTS) (data not shown). Infection of primary hematopoietic precursors with a retrovirus encoding p21CIP1 also led to growth arrest but did not reproduce the effect of WT1(–KTS) on cellular differentiation. Only a small increase in the number of CD11b(+) cells was observed after 7–12 days of ectopic p21CIP1 expression (Figure 4B). WT1 therefore induces a distinct program of differentiation in hematopoietic precursors in addition to its effect on cell cycle progression.
WT1 expression promotes quiescence of the CD34(+)CD38(–) stem cell population
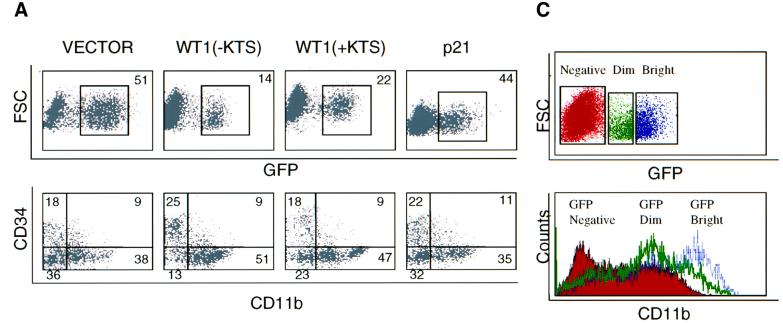
Coincident with the enhanced differentiation of CD34(–) cells in WT(–KTS)-transduced populations, we also observed a dramatic and consistent increase in the proportion of very early progenitors expressing high levels of CD34 (‘CD34 bright’) and lacking differentiation markers such as CD11b (Figure 4A and data not shown) (DiGiusto et al., 1994). Further analysis showed that these cells also lacked surface expression of CD38, a marker of lineage-committed progenitors. CD38(–) cells normally comprise only 1–5% of CD34(+) cells in cord blood, and represent a subset that is highly enriched for hematopoietic stem cells (Terstappen et al., 1991; Conneally et al., 1997). Remarkably, within 4 days following infection with WT1(–KTS), CD34(+)CD38(–) cells accounted for ∼20% of all GFP-positive cells (Figure 5A and B). In contrast, vector- and p21CIP1-infected populations showed only the expected small fraction (mean 3–5%) of cells with this phenotype (P = 0.002). The consistent increase in the fraction of cells within this subset persisted throughout the 12 day time course of in vitro culture. Quantitative analysis showed no expansion in the absolute number of CD34(+)CD38(–) cells, but rather a relative increase due to their slower decline and the rapid differentiation of committed progenitors in the WT1(–KTS)-transduced population (Figure 5C). Detailed phenotypic analysis of these WT1-expressing CD34(+)CD38(–) cells was consistent with uncommitted progenitor cells (Dorrell et al., 2000), including absent staining for the cell surface markers CD11b, CD13, CD15 and CD33 (data not shown). While WT1(–KTS) had the strongest effect, expression of the WT1(+KTS) isoform also led to an increase in the fraction of cells within this hematopoietic progenitor compartment (Figure 5B and C).
Fig. 5. Preservation of the CD34(+)CD38(–) stem cell population by WT1. (A) Expression of CD34 and CD38 markers on GFP-positive cord blood cells, on day 4 following transduction with GFP-linked constructs encoding vector, WT1(–KTS), WT1(+KTS) or p21CIP1. Cells were stained with anti-CD34–PE and anti-CD38–APC and analyzed by three-color FACS analysis. Boxes denote the CD34(+)CD38(–) cell subset within the total CD34(+) population at day 0, and within gated GFP-positive transduced cells at day 4. Numbers indicate the percentage of cells in each subset. A representative experiment is shown. (B) Quantitation of percent CD34(+)CD38(–) cells in the GFP-positive population as a function of days in culture, shown as the mean of six independent experiments with SE. Differences between vector- and WT1(–KTS)-transduced cells were calculated to include observations at days 4, 8 and 12 by the Wilcoxon matched-pairs signed ranks test (P = 0.002). (C) Absolute number of CD34(+)CD38(–)CD11b(–) cells following transduction of primary progenitors with vector, WT1(–KTS), WT1(+KTS) or p21CIP1. For each time point, cells were counted and stained with anti-CD34–PerCP, anti-CD38–APC and anti-CD11b–PE, and the CD34(+)CD38(–)CD11b(–) fraction of GFP-positive cells was determined by four-color FACS analysis. Values indicate cells per milliliter of culture and represent the mean of two experiments for WT1(–KTS) and vector, and one experiment for WT1(+KTS) and p21CIP1.
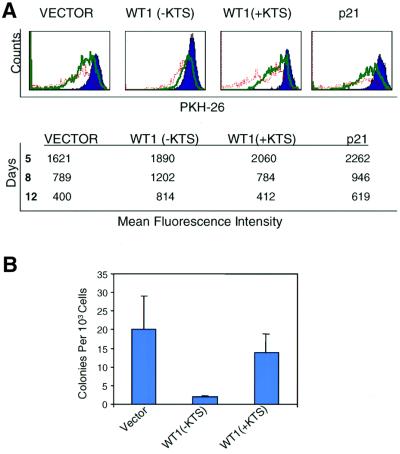
The persistence of CD34(+)CD38(–) cells in WT1 (–KTS)-infected cultures could represent either quiescence associated with reduced differentiation, or alternatively, enhanced proliferation coupled with self-renewal or apoptosis. To examine the proliferative activity of WT1-infected CD34(+)CD38(–) cells, we stained cells using the vital fluorescent membrane dye PKH26, whose progressive dilution serves to monitor cell division (Lansdorp, 1996; Ladd et al., 1997). Using three- and four-color cytometric analysis, we determined the change in PKH26 fluorescence intensity over time in each phenotypic subset of infected cells, including total CD34(+) and CD34(+) CD38(–) populations. WT1(–KTS)-expressing CD34(+) CD38(–) cells demonstrated a high level of dye retention, indicating profound growth arrest compared with vector-infected cells (Figure 6A). The inhibition of proliferation in this stem cell-enriched population by WT1(–KTS) was considerably more pronounced than that mediated by p21CIP1, whose ectopic expression only modestly and transiently decreased proliferation of CD34(+) cells. Despite their profound unresponsiveness to growth and differentiation signals, the viability of this cell population was confirmed, and no differences in apoptosis were observed among WT1(–KTS), WT1(+KTS), p21CIP1 or control vector-transduced cells (data not shown).
Fig. 6. Quiescence of uncommitted progenitors induced by WT1(–KTS). (A) Reduced proliferation of WT1(–KTS)-expressing primary hematopoietic progenitors, demonstrated by retention of the dye PKH26. GFP-positive transduced cells were purified by FACS on day 4 following retroviral infection, stained with PKH26, then cultured and harvested on days 5, 8 and 12. PKH26 expression was quantitated in CD34(+) cells and is shown as a histogram [upper panel; day 5 (blue), day 8 (green) and day 12 (red)]. For each transduced construct, the table shows the absolute mean fluorescence intensity at each time point in a representative experiment. Immediately after labeling at day 4, the mean fluorescence intensity of PKH26 in all four samples was comparable (∼2000). (B) Effects of WT1 on colony-forming ability of primary hematopoietic progenitors. CD34(+) cells expressing GFP-linked vector, WT1(–KTS) or WT1(+KTS) were purified by FACS after 8–10 days of liquid culture. Sorted cells were plated in triplicate, at a density of 1000 cells/ml in methylcellulose supplemented with IL-3, KL and EPO. Colony counts at day 14 are shown as the average of three independent experiments with SE. The difference between WT1(–KTS)- and vector-transduced populations is statistically significant (P <0.003).
The profound cellular quiescence induced by constitutive WT1 expression in this stem cell-enriched population was further supported by reduced activity in short-term colony-forming (CFU) assays and in long-term culture with primary bone marrow stroma (Ploemacher et al., 1989; Sutherland et al., 1990). Sorted GFP(+)CD34(+) cells expressing WT1(–KTS) cells showed a 10-fold decrease in clonogenic efficiency compared with control vector and WT1(+KTS) (P = 0.027; Figure 6B), and very low or undectable proliferative activity at weeks 1–5 following plating onto stroma, despite preserved viability (data not shown).
Biphasic in vivo expression of WT1 during human hematopoiesis
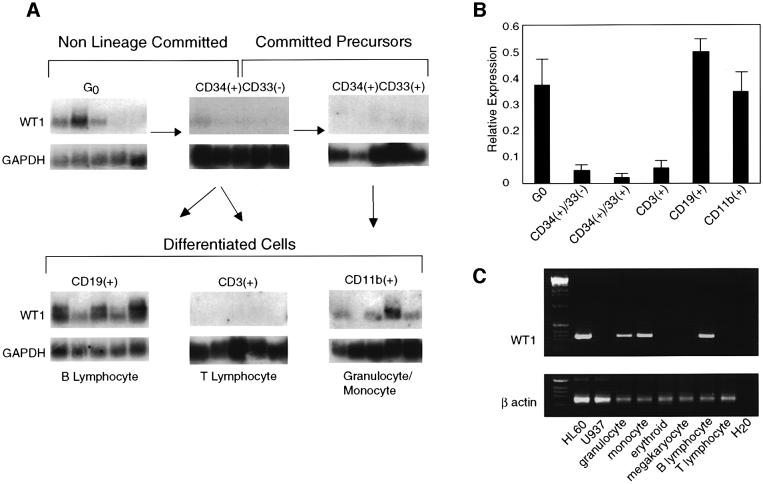
The distinct effects of retrovirally mediated WT1 expression on hematopoietic precursors suggest that it may have different functions at early and late stages of blood cell differentiation. To correlate these stage-specific effects with endogenous WT1 expression during in vivo hematopoiesis, we quantified WT1 expression in individual cells representing different stages of hematopoietic differentiation, isolated from FACS-sorted populations of human hematopoietic cells. Previous studies using direct RT– PCR analysis had indicated variable expression of WT1 in CD34(+) populations (Menssen et al., 1995; Maurer et al., 1997). To determine the expression pattern of WT1 in different subsets of CD34(+) cells, we used a quantitative method involving representational amplification of all polyA(+) RNA from single cells, followed by blot hybridization using a WT1 probe and controls. This approach, performed in multiple replicates, has proved to be highly accurate in measuring gene expression from rare populations of hematopoietic precursors (Cheng et al., 1996). Because this technique examines expression in individual cells within heterogeneous populations, expression levels vary from cell to cell (each lane in Figure 7A represents cDNA from a single cell), and these are therefore averaged for each population studied (Figure 7B). WT1 expression in human bone marrow and peripheral blood cells showed a biphasic pattern. WT1 was present in some quiescent primitive progenitors, absent in lineage-committed precursors, then present again in a subset of the more differentiated populations (Figure 7A and B). The early progenitors expressing WT1 comprised the rare population of non-lineage-committed precursors that are in G0, isolated by treatment of CD34(+) cells with the anti-metabolite 5-fluorouracil (5-FU) to eliminate cycling cells. This cell population represents a subset of CD34(+)CD38(–) cells that is highly enriched for functional stem cells capable of producing both myeloid and lymphoid progeny (Berardi et al., 1995; Gothot et al., 1998). In contrast, WT1 mRNA was below detection using this assay in the bulk population of both lineage-committed and uncommitted CD34(+) precursors. WT1 expression reappeared at late stages of hematopoietic differentiation, in a pattern consistent with the lineage-specific differentiation induced by WT1 in primary hematopoietic precursors. In the more abundant differentiated populations, similar results were obtained using either the single-cell technique or direct RT–PCR amplification of the WT1 transcript, a more commonly used but less quantitative approach (Figure 7C). Among myeloid cells, WT1 expression was restricted to cells expressing the granulocytic/monocytic marker CD11b, and was absent in cells expressing erythroid as well as megakaryocytic markers. WT1 was also present in B lymphocytes expressing the CD19 antigen. The correlation between WT1 expression in vivo and the effects of its ectopic expression, both in primitive uncommitted progenitors and in lineage-committed cells, underscores the physiological relevance of these effects.
Fig. 7. Biphasic expression of WT1 during human hematopoiesis in vivo. (A) Quantitation of WT1 expression in individual hematopoietic precursor cells. Single-cell RT–PCR was used to amplify total cellular mRNA from individual cells isolated by FACS or micromanipulation from human bone marrow and peripheral blood as described in Cheng et al. (1996). G0, stem cell-enriched quiescent cells were obtained by treatment of CD34(+) cells with the anti-metabolite 5-FU to eliminate cycling cells (Berardi et al., 1995). Southern blots of amplified cDNA were then hybridized with WT1 or GAPDH cDNA probes and quantified by densitometry. Representative blots are shown, and the phenotype of each cell type examined and their relationship to each other are indicated. The CD34(+)CD33(–) population contains both lineage-committed and uncommitted precursors. Each lane represents cDNA from a single cell; thus, differences in expression between cells in each sorted population reflect the heterogeneity of each population. The size distribution of the bands detected is that of the total pool of amplified WT1 or GAPDH cDNA, and therefore does not correspond to a precise message size. (B) Quantitation of WT1 expression levels for each cell population examined in (A). Results are expressed as the mean ratio of WT1/GAPDH expression for at least 15 lanes examined from each population, with SE. (C) Expression of WT1 in differentiated hematopoietic populations. Ethidium bromide-stained gel showing products of direct RT–PCR amplification of the WT1 and β actin transcripts from leukemia cell lines and FACS-isolated populations of cord blood cells. Populations were isolated by two-color cell sorting using the following surface markers: CD11b(+)/CD14(–), granulocyte; CD11b(+)/CD14(+), monocyte; glycophorin A (GPA)(+)/CD41(–), erythroid; GPA(–)/CD41(+), megakaryocyte; CD19(+)/CD3(–), B lymphocyte; CD19(–)/CD3(+), T lymphocyte. The 284 bp WT1 PCR fragment shown corresponds to a portion of exons 1–4.
Discussion
WT1 has a well defined role in kidney development and malignancy, but its potential function in human hematopoiesis remains controversial. Prior studies of cloned leukemic cell lines stably transfected with WT1 constructs have typically shown defects in the response to differentiation agents, suggesting that inhibition of differentiation by wild-type WT1 may in fact contribute to leukemogenesis (Inoue et al., 1998; Svedberg et al., 1998; Deuel et al., 1999). Other studies have argued for a pro-differentiation effect of the WT1(+KTS) isoform in hematopoietic cells (Smith et al., 1998). Here, introduction of WT1 by high titer retroviral infection has allowed us to examine unselected populations of hematopoietic cells at early time points following WT1 expression, and to examine the fate of cells belonging to different lineages and developmental stages. In primary hematopoietic precursors and in differentiation-competent leukemic cell lines, WT1 expression is associated with growth arrest and spontaneous differentiation. Given the rapid and potent effect of WT1 on cellular differentiation, prior conflicting results are likely to be explained by the selection of individual clones whose resistance to differentiation signals may allow for stable expression of WT1.
Phenotypes resulting from ectopic expression of WT1 warrant cautious interpretation, but several points support the physiological relevance of our observations. First, the levels of retrovirally expressed WT1 protein are similar to those in leukemic cells. Secondly, the lineage-specific differentiation induced by WT1 in primary hematopoietic precursors correlates with its lineage-specific expression among myeloid cells in vivo. Thirdly, although relatively few bona fide transcriptional target genes of WT1 have been identified, one of these, p21CIP1, is induced by WT1 in our assays and appears to mediate a portion of the observed effects. Nonetheless, additional studies will be required to conclusively demonstrate a role for WT1 in normal hematopoiesis. Inactivation of WT1 in the mouse is not associated with gross hematological deficits, although embryonic lethality at day 13–14 precludes evaluation of adult hematopoiesis. Species-specific differences may also need to be considered, since WT1 inactivation in the mouse has not been linked to renal tumorigenesis, and murine leukemia cells do not commonly express endogenous WT1, unlike their human counterparts.
Like sporadic Wilms tumor, only 10–15% of human acute leukemias harbor mutations in WT1, while the majority express high levels of the wild-type transcript (Miwa et al., 1992; Menssen et al., 1995; King-Underwood et al., 1996; Miyagawa et al., 1999). Some clinical studies have suggested a correlation between WT1 expression in acute leukemias and a more primitive histology associated with an adverse prognosis (Inoue et al., 1994; Bergmann et al., 1997), while others have not found such an association (Gaiger et al., 1998, 1999). Our demonstration that WT1 induces growth arrest and differentiation in hematopoietic cells argues against an oncogenic role for WT1 in human leukemia, and instead suggests a parallel with sporadic Wilms tumor. Thus, disrupted WT1 function may contribute to malignancy in a subset of leukemias, while the majority of cases have acquired other mutations that may render them insensitive to the effects of wild-type WT1. This is consistent with our finding that ectopic WT1 expression fails to induce differentiation in common myeloid leukemia-derived cell lines, such as KG1 and SR-91, that do not respond to standard differentiation agents (data not shown).
Early studies of ectopic WT1 expression in immortalized cell lines revealed its ability to induce a cell cycle arrest as well as trigger apoptosis (Englert et al., 1995a; Kudoh et al., 1995). However, the cell fates that can be studied in most cancer-derived cell lines are limited. Our analysis of primary hematopoietic cells has allowed us to demonstrate a direct effect of WT1 on cellular differentiation, consistent with its presumed role in renal organogenesis. Using this in vitro system we have demonstrated cooperative roles in differentiation for two WT1 isoforms. In contrast to WT1(–KTS), the more abundant WT1(+KTS) isoform does not possess sequence-specific DNA binding activity (Rauscher et al., 1990). WT1(+KTS) has appeared to be inactive when expressed in most cell lines, and variable effects have been noted in other cell types that do not tolerate expression of WT1(–KTS) (Murata et al., 1997; Smith et al., 1998). Nonetheless, the physiological importance of the WT1(+KTS) isoform is underscored by the severe developmental abnormalities that result from its decreased expression in patients with Frasier syndrome (Barbaux et al., 1997). Indeed, we did observe a subtle but reproducible effect of WT1(+KTS) alone on the differentiation of primary hematopoietic progenitors (Figure 4C), and some enhancement of induced differentiation of leukemic cells (Figure 3C). These effects are consistent with an ability of WT1(+KTS) to modulate but not initiate differentiation. The precise mechanism by which the WT1(+KTS) and (–KTS) isoforms cooperate to induce differentiation remains to be determined, and a complete understanding of WT1 function is likely to require analysis of cooperation among all four expressed isoforms.
The effect of WT1 on hematopoietic precursor subsets appears to be stage specific: cellular differentiation is induced in lineage-committed precursors, while cellular quiescence is enhanced in more primitive cells. These effects may provide insight into the potentially analogous role of WT1 in kidney development, a complex process that is not as readily analyzed in vitro. The biphasic expression of WT1 during hematopoiesis is remarkably similar to that in the developing kidney, where low levels of the transcript are present in undifferentiated blastemal stem cells and very high levels appear in subsequent stages of differentiation, as epithelial cells give rise to the podocytes of glomeruli (Pritchard-Jones et al., 1990). WT1 has been postulated to be essential for the survival of blastemal cells, based on widespread apoptosis of these cells in wt1-null mice (Kreidberg et al., 1993), a phenomenon that is consistent with the preservation of the hematopoietic stem cell compartment by WT1. We have demonstrated recently that p21Cip1 is required for the long-term maintenance of hematopoietic stem cells in the mouse (Cheng et al., 2000), raising the possibility that induction of p21Cip1 by WT1 itself may contribute to this effect. A subsequent role for WT1 in the differentiation of glomerular podocytes may also be analogous to its ability to induce myelo-monocytic differentiation in more mature hematopoietic precursors. Other genes are also known to have dual roles in blood and kidney development. For instance, treatment of cultured primary metanephric mesenchyme with leukemia inhibitory factor (LIF)/IL-6 triggers early stages of renal differentiation in vitro (Barasch et al., 1999), and GATA-2-null mice display severe genitourinary defects following rescue of their hematopoietic deficits (Zhou et al., 1998). Thus, WT1 may demonstrate a role within convergent hematopoietic and renal differentiation pathways, consistent with its mutational inactivation in both Wilms tumor and acute leukemia.
Materials and methods
Retroviral expression constructs
cDNAs encoding (+KTS) and (–KTS) isoforms of WT1 have been described elsewhere (Englert et al., 1995a), and the human p21CIP1 cDNA was generated by PCR amplification of cDNA from primary human fibroblasts using coding region-specific primers. cDNA fragments were subcloned into the multicloning site of the retroviral vector MSCV-GFP (Hawley et al., 1992) (Figure 1A), or into MSCVpuro IRES PLAP (kindly provided by J.Jacob) (Jacob and Baltimore, 1999). Both of these vectors drive expression from the 5′ long terminal repeat (LTR) of a single biscistronic mRNA encoding the protein of interest and either an enhanced GFP (Clontech Laboratories, Palo Alto, CA), or a catalytically inactive mutant PLAP, linked by an IRES.
Cells and cell culture
The human myeloid cell lines HL-60 and U937 were obtained from the American Type Culture Collection. Parental and transduced cell lines were maintained in RPMI medium supplemented with 10% fetal calf serum (FCS; Sigma) (R10). To induce cellular differentiation, 3 nM TPA (Sigma) was added to HL-60 or U937 cells in R10. Human embryonic kidney-derived 293T cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FCS. All cells were cultured at 37°C under 5% CO2. Cord blood samples were obtained from the Pediatric Research Institute, University of St Louis, MO, according to the guidelines established by the Human Investigation Committee. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation, and CD34(+) cells were purified by immunomagnetic bead selection (Milteny Biotec, Auburn, CA), according to the manufacturer’s instructions. Transduced CD34(+) cells were grown in Iscove’s modified Dulbecco medium (IMDM) (Mediatech Inc., Harndon, VT) containing 10% FCS (IMDM10) and supplemented with KL (20 ng/ml), TPO (20 ng/ml), FLT-3 L (20 ng/ml) and IL-3 (20 ng/ml) (R & D, Minneapolis, MN). Cells were initially cultured in 1 ml final volume at a density of 0.5–1 × 106/ml in 24-well plates. Fresh media and cytokines were added every 3–4 days. Clonogenic progenitor assays were performed with cells harvested from the liquid cultures. Harvested cells were cultured in methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with IL-3 (50 ng/ml), KL (50 ng/ml) and erythropoietin (EPO) (Amgen, Thousand Oaks, CA) according to the manufacturer’s recommendations. Colonies were evaluated by phase microscopy and scored according to standard morphological criteria at day 14.
Retroviral generation and transduction
Retroviral generation and transduction were performed essentially as described (Carlesso et al., 1999). Vectors were co-transfected with pKat, an amphotropic packaging plasmid (kindly provided by M.Finer, Cell Genesys Inc., Foster City, CA), and pCMV-VSV-G, a plasmid encoding the vesicular stomatitis virus G-glycoprotein (kindly provided by T.Friedmann, University of San Diego, CA), into 293T cells using calcium phosphate precipitation (CalPhos reagent; Clontech). Supernatants containing pseudotyped retrovirus were collected at 36 h and used to infect primary CD34(+) cells, HL-60 and U937 cell lines. CD34(+) progenitors were cultured in IMDM10 supplemented with KL (50 ng/ml), TPO (20 ng/ml), FLT-3 L (20 ng/ml) and IL-3 (20 ng/ml) (R & D) for 24 h prior to infection, in order to induce cells to enter into cell cycle. HL-60, U937 or stimulated CD34(+) cells were washed in phosphate-buffered saline (PBS; Sigma) and resuspended at 1–2 × 105 cells/ml in 1 ml of 50% retroviral supernatant and 50% fresh complete medium plus Polybrene (final concentration 8 µg/ml; Sigma). Cells in suspension were placed in a 48-well plate (Becton Dickinson Inc., Franklin Lakes, NJ), spinoculated at 650 g for 50 min, then incubated at 37°C under 5% CO2 for an additional 8–12 h, washed, and resuspended in fresh medium overnight. A second and a third infection were conducted on the following days using an identical procedure. Infection efficiency was evaluated by GFP expression 4 days after the first infection.
Antibodies, cell staining, FACS analysis and cell sorting
Western blots were carried out using commercial polyclonal antibodies against WT1 (sc-192) and p21CIP1 (sc-397) (Santa Cruz). Fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC) or peridinin chlorophyll protein (PerCP)-coupled control immunoglobulins or specific monoclonal antibodies directed to CD34, CD38, CD33, CD13, CD11b, CD14 and CD15 (Becton Dickinson, San Jose, CA) were used in three- or four-color labeling to determine hematopoietic cell subsets in conjunction with GFP. Cells were incubated with 0.5% human IgG (Sigma) in PBS for 20 min at 4°C to block the Fc receptor prior to staining with antigen-specific antibodies. For PLAP staining, cells were incubated with polyclonal rabbit anti-PLAP or control Ig (Zymed, San Francisco, CA), then washed and incubated with biotin-conjugated polyclonal goat anti-rabbit (PharMingen), and then washed and incubated with APC-conjugated streptavidin (PharMingen) plus anti-CD11b–PE or anti-CD14–PE. Flow cytometric analysis was performed using the FACScalibur instrument (Becton Dickinson, San Jose, CA) and Cell Quest program (Becton Dickinson). A minimum of 70 000 events were analyzed in each sample. For cell sorting, GFP(+) or GFP(+)CD34(+) fractions were purified from cord blood CD34(+) transduced cells at day 4 or 7 of culture by FACS on FACS Vantage (Becton Dickinson). For each sorting, 4 × 105–2 × 106 harvested cells were used. GFP(+), GFP(+)CD34(+) and GFP(+)CD34(–) cells were isolated at 90–95% purity. Sorted populations were then used for PKH26 labeling, colony assay, long-term culture and NBT staining.
Cell cycle analysis
Cell cycle analysis was performed using two-color FACS analysis for GFP and the DNA binding dye PI (Molecular Probes, Eugene, OR). Cells were fixed in PBS/1% formaldehyde for 30 min, permeabilized in 0.1% Triton X-100 (Sigma) for 30 min at room temperature, washed and resuspended in PBS/1 mg/ml RNase A (Sigma) and 10 µg/ml PI for 30 min at 37°C prior to FACS analysis. S phase fractions were analyzed by BrdU (Sigma) incorporation in asyncronous populations. BrdU-pulsed cells were fixed in 70% ethanol at –20°C overnight and denatured by 2 N HCl, 0.5% Triton X-100 for 30 min at room temperature, followed by neutralization with borate buffer pH 8.5. Treated cells were subjected to dual color staining with a monoclonal anti-BrdU antibody (Becton Dickinson) followed by goat anti-mouse FITC and 10 µg/ml PI. Analyses were carried out using the FACScalibur flow cytometer, Cell Quest and Modefit software (Becton Dickinson).
PKH26 and NBT staining
Total transduced or sorted GFP(+) cells were stained with PKH26 (Sigma ImmunoChemicals) between days 4 and 7 of liquid culture following the manufacturer’s instructions and as previously described (Ladd et al., 1997). Briefly, cells were labeled in 2 µM PKH26 in diluent for 10 min at room temperature. After FBS blocking and extensive washing, cells were resuspended and cultured in IMDM10 supplemented with cytokines at a density of 2.5 × 105/ml. Samples collected immediately after labeling and at different time points of liquid culture were then stained with anti-CD34–APC or anti-CD34–PerCP and anti-CD38–APC. Labeled cells were fixed in 1% paraformaldehyde and analyzed by three- or four-color FACS analysis for determination of the PKH26 red fluorescence intensity on GFP(+)CD34(+) and GFP(+)CD34(+)CD38(–) subsets. For NBT staining, transduced U937 cells were aliquoted into 24-well plates at 2 × 105 cells/well, and treated with 1 mg/ml NBT and 3 nM TPA in complete medium for 60 min at 37°C. Transduced cord blood cells were stained with anti-CD34–PE after 7 days in culture, and GFP(+)CD34(–) cells were sorted and plated in growth factor-supplemented medium for an additional 2 days prior to staining. Percent NBT positivity was scored by light microscopy, and quantitated by counting at least three representative fields of 200 or more cells.
Determination of endogenous WT1 expression
For single-cell expression studies, FACS-isolated cell populations from human bone marrow [CD34(+)CD33(–), CD34(+)CD33(+)] or peripheral blood [CD11b(+), CD19(+), CD3(+)] were plated by micromanipulator selection into 96-well plates. G0 or ‘cytokine non-responsive’ stem cells were obtained by culturing purified CD34(+) cells in IMDM10 supplemented with 200 mg/ml 5-FU (Pharmacia Inc., Kalamazoo, MI), KL (100 ng/ml) and IL-3 (100 ng/ml) for 7 days, then sorting by FACS based on Annexin V (Caltag Laboratories, San Francisco, CA) and PI or 7-amino actinomycin D (7-AAD) (Calbiochem, La Jolla, CA) staining. RT–PCR and Southern blotting were performed as described previously (Cheng et al., 1996). Briefly, single cells were lysed, subjected to reverse transcription with murine Moloney reverse transcriptase (Gibco-BRL) and avian reverse transcriptase (Boehringer Mannheim), and then poly(A)-tailed with terminal transferase (Boehringer Mannheim). PCR was performed using a (dT)24 primer and AmpliTaq polymerase (Perkin-Elmer). Cell- and RT-free samples were used as negative controls. Samples were amplified for a total of 50 cycles (42°C annealing temperature). The PCR product was electrophoresed through 1.2% agarose, transferred to a nylon membrane with vacuum blotter (Pharmacia), immobilized by UV (Stratagene) and hybridized to the indicated 32P-radiolabeled cDNA probes in Express Hybridization Solution (Clontech). Washed blots were analyzed by autoradiography and Molecular Dynamics Computing Densitometer and Image Quant Software v3.22. At least three separate blots were hybridized for each cell population analyzed. For direct RT–PCR analysis, total RNA was prepared and reverse transcribed from 3 × 104 FACS-isolated cord blood cells, and 10% of the cDNA was subjected to PCR analysis using a nested strategy. A portion of WT1 exons 1–4 was amplified using forward 5′-CCTACCTGCCCAGCTGCCTC-3′ and reverse 5′-CTCCTAAGTTCATCTGATTCC-3′ primers for 20 cycles (56°C annealing temperature), followed by nested forward 5′-AGAGCCAGCCCGCTATTCG-3′ and reverse 5′-GGTCATGCATTCAAGCTGG-3′ primers for 30 cycles (58°C annealing temperature). For β-actin quantitation, identical samples were amplified for 35 cycles with actin-specific forward and reverse primers.
Statistical analysis
Equality of distributions for matched pairs of observations was tested using the Wilcoxon matched-pairs signed ranks test and the two-tailed t-test. The analyses were performed using the software STATA (r) (Stata Corporation, College Station, TX).
Acknowledgments
Acknowledgements
We thank Richard Van Etten for critical review of the manuscript and Kate Ramsayer for technical assistance. This work was supported by National Institutes of Health grants CA82831 (L.W.E.), DK02761 (T.C.), HL44851 and DK50234 (D.T.S.), and CA58596 (D.A.H.), the National Foundation for Cancer Research (D.A.H.), and the American Medical Association Education and Research Foundation and Partners Investigator (Nesson) Award (N.C.).
References
- Algar E.M., Khromykh,T., Smith,S.I., Blackburn,D.M., Bryson,G.J. and Smith,P.J. (1996) A WT1 antisense oligonucleotide inhibits proliferation and induces apoptosis in myeloid leukemia cell lines. Oncogene, 12, 1005–1014. [PubMed] [Google Scholar]
- Baird P.N. and Simmons,P.J. (1997) Expression of the Wilms’ tumor gene (WT1) in normal hemopoiesis. Exp. Hematol., 25, 312–320. [PubMed] [Google Scholar]
- Barasch J. et al. (1999) Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell, 99, 377–386. [DOI] [PubMed] [Google Scholar]
- Barbaux S. et al. (1997) Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nature Genet., 17, 467–470. [DOI] [PubMed] [Google Scholar]
- Berardi A.C., Wang,A., Levine,J.D., Lopez,P. and Scadden,D.T. (1995) Functional isolation and characterization of human hematopoietic stem cells. Science, 267, 104–108. [DOI] [PubMed] [Google Scholar]
- Bergmann L., Miething,C., Maurer,U., Brieger,J., Karakas,T., Weidmann,E. and Hoelzer,D. (1997) High levels of Wilm’s tumor gene (WT1) mRNA in acute myeloid leukemia are associated with a worse long-term outcome. Blood, 90, 1217–1225. [PubMed] [Google Scholar]
- Bjare U., Lundblad,G., Ivhed,I. and Nilsson,K. (1988) Patterns of glycosidases in the histiocytic cell line U-937. Effects of agents inducing cell differentiation. Int. J. Biochem., 20, 1291–1298. [DOI] [PubMed] [Google Scholar]
- Breitman T.R., Selonick,S.E. and Collins,S.J. (1980) Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl Acad. Sci. USA, 77, 2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesso N., Aster,J.C., Sklar,J. and Scadden,D.T. (1999) Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood, 93, 838–848. [PubMed] [Google Scholar]
- Cheng T., Shen,H., Giokas,D., Gere,J., Tenen,D.G. and Scadden,D.T. (1996) Temporal mapping of gene expression levels during the differentiation of individual primary hematopoietic cells. Proc. Natl Acad. Sci. USA, 93, 13158–13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Rodrigues,N., Shen,H., Yang,Y., Dombkowski,D., Sykes,M. and Scadden,D. (2000) Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science, 287, 1804–1808. [DOI] [PubMed] [Google Scholar]
- Civin C.I. and Small,D. (1995) Purification and expansion of human hematopoietic stem/progenitor cells. Ann. N. Y. Acad. Sci., 770, 91–98. [DOI] [PubMed] [Google Scholar]
- Conneally E., Cashman,J., Petzer,A. and Eaves,C. (1997) Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in non-obese diabetic-scid/scid mice. Proc. Natl Acad. Sci. USA, 94, 9836–9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R.C., Calvio,C., Bratt,E., Larsson,S.H., Lamond,A.I. and Hastie,N.D. (1998) WT1 interacts with the splicing factor U2AF65 in an isoform-dependent manner and can be incorporated into spliceosomes. Genes Dev., 12, 3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T.F., Guan,L.S. and Wang,Z.Y. (1999) Wilms’ tumor gene product WT1 arrests macrophage differentiation of HL-60 cells through its zinc-finger domain. Biochem. Biophys. Res. Commun., 254, 192–196. [DOI] [PubMed] [Google Scholar]
- DiGiusto D., Chen,S., Combs,J., Webb,S., Namikawa,R., Tsukamoto,A., Chen,B.P. and Galy,A.H.M. (1994) Human fetal bone marrow early progenitors for T, B and myeloid cells are found exclusively in the population expressing high levels of CD34. Blood, 84, 421–432. [PubMed] [Google Scholar]
- Dorrell C., Gan,O.I., Pereira,D.S., Hawley,R.G. and Dick,J.E. (2000) Expansion of human cord blood CD34+CD38– cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood, 95, 102–110. [PubMed] [Google Scholar]
- Englert C., Hou,X., Maheswaran,S., Bennett,P., Ngwu,C., Re,G.G., Garvin,A.J., Rosner,M.R. and Haber,D.A. (1995a) WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J., 14, 4662–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C., Vidal,M., Maheswaran,S., Ge,Y., Ezzell,R.M., Isselbacher,K.J. and Haber,D.A. (1995b) Truncated WT1 mutants alter the subnuclear localization of the wild-type protein. Proc. Natl Acad. Sci. USA, 92, 11960–11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C., Maheswaran,S., Garvin,A.J., Kreidberg,J. and Haber,D.A. (1997) Induction of p21 by the Wilms’ tumor suppressor gene WT1. Cancer Res., 57, 1429–1434. [PubMed] [Google Scholar]
- English M.A. and Licht,J.D. (1999) Tumor-associated WT1 missense mutants indicate that transcriptional activation by WT1 is critical for growth control. J. Biol. Chem., 274, 13258–13263. [DOI] [PubMed] [Google Scholar]
- Gaiger A., Schmid,D., Heinze,G., Linnerth,B., Greinix,H., Mannhalter,C., Haas,O.A., Lechner,K. and Jager,U. (1998) Detection of the WT1 transcript by RT–PCR in complete remission has no prognostic relevance in de novo acute myeloid leukemia. Leukemia, 12, 1886–1894. [DOI] [PubMed] [Google Scholar]
- Gaiger A., Linnerth,B., Mann,G., Schmid,D., Heinze,G., Tisljar,K., Haas,O.A., Gadner,H. and Lion,T. (1999) Wilms’ tumor gene (wt1) expression at diagnosis has no prognostic relevance in childhood acute leukemia treated by an intensive chemotherapy program. Eur. J. Haematol., 63, 86–93. [DOI] [PubMed] [Google Scholar]
- Gothot A., ven der Loo,J.C., Clapp,D.W. and Srour,E.F. (1998) Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood, 92, 2641–2649. [PubMed] [Google Scholar]
- Haber D.A., Sohn,R.L., Buckler,A.J., Pelletier,J., Call,K.M. and Housman,D.E. (1991) Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc. Natl Acad. Sci. USA, 88, 9618–9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N.D. (1994) The genetics of Wilms’ tumor—a case of disrupted development. Annu. Rev. Genet., 28, 523–558. [DOI] [PubMed] [Google Scholar]
- Hawley R.G., Fong,A.Z., Burns,B.F. and Hawley,T.S. (1992) Transplantable myeloproliferative disease induced in mice by an interleukin 6 retrovirus. J. Exp. Med., 176, 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickstein D.D., Back,A.L. and Collins,S.J. (1989) Regulation of expression of the CD11b and CD18 subunits of the neutrophil adherence receptor during human myeloid differentiation. J. Biol. Chem., 264, 21812–21817. [PubMed] [Google Scholar]
- Inoue K. et al. (1994) WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood, 84, 3071–3079. [PubMed] [Google Scholar]
- Inoue K. et al. (1998) Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood, 91, 2969–2976. [PubMed] [Google Scholar]
- Jacob J. and Baltimore,D. (1999) Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature, 399, 593–597. [DOI] [PubMed] [Google Scholar]
- King-Underwood L., Renshaw,J. and Pritchard-Jones,K. (1996) Mutations in the Wilms’ tumor gene WT1 in leukemias. Blood, 87, 2171–2179. [PubMed] [Google Scholar]
- Krause D.S., Fackler,M.J., Civin,C.I. and May,W.S. (1996) CD34: structure, biology and clinical utility. Blood, 87, 1–13. [PubMed] [Google Scholar]
- Kreidberg J.A., Sariola,H., Loring,J.M., Maeda,M., Pelletier,J., Housman,D. and Jaenisch,R. (1993) WT1 is required for early kidney development. Cell, 74, 679–691. [DOI] [PubMed] [Google Scholar]
- Kudoh T., Ishidate,T., Moriyama,M., Toyoshima,K. and Akiyama,T. (1995) G1 phase arrest induced by Wilms tumor protein WT1 is abrogated by cyclin/CDK complexes. Proc. Natl Acad. Sci. USA, 92, 4517–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd A.C., Pyatt,R., Gothot,A., Rice,S., McMahel,J., Traycoff,C.M. and Srour,E.F. (1997) Orderly process of sequential cytokine stimulation is required for activation and maximal proliferation of primitive human bone marrow CD34(+) hematopoietic progenitor cells residing in G0. Blood, 90, 658–668. [PubMed] [Google Scholar]
- Lansdorp P.M. (1996) Retention of quiescent hematopoietic cells with high proliferative potential during ex vivo stem cell culture. Blood, 87, 545–556. [PubMed] [Google Scholar]
- Larsson S.H., Charlieu,J.P., Miyagawa,K., Engelkamp,D., Rassoulzadegan,M., Ross,A., Cuzin,F., van Heyningen,V. and Hastie,N.D. (1995) Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell, 81, 391–401. [DOI] [PubMed] [Google Scholar]
- Lee S.B. et al. (1999) The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell, 98, 663–673. [DOI] [PubMed] [Google Scholar]
- Little M.H., Prosser,J., Condie,A., Smith,P.J., van Heyningen,V. and Hastie,N.D. (1992) Zinc finger point mutations within the WT1 gene in Wilms tumor patients. Proc. Natl Acad. Sci. USA, 89, 4791–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U., Brieger,J., Weidmann,E., Mitrou,P.S., Hoelzer,D. and Bergmann,L. (1997) The Wilms’ tumor gene is expressed in a subset of CD34+ progenitors and downregulated early in the course of differentiation in vitro. Exp. Hematol., 25, 945–950. [PubMed] [Google Scholar]
- Menssen H.D., Renkl,H.J., Rodeck,U., Maurer,J., Notter,M., Schwartz,S., Reinhardt,R. and Thiel,E. (1995) Presence of Wilms’ tumor gene (wt1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia, 9, 1060–1067. [PubMed] [Google Scholar]
- Menssen H.D., Renkl,H.J., Entezami,M. and Thiel,E. (1997) Wilms’ tumor gene expression in human CD34+ hematopoietic progenitors during fetal development and early clonogenic growth. Blood, 89, 3486–3487. [PubMed] [Google Scholar]
- Miwa H., Beran,M. and Saunders,G.F. (1992) Expression of the Wilms’ tumor gene (WT1) in human leukemias. Leukemia, 6, 405–409. [PubMed] [Google Scholar]
- Miyagawa K., Hayashi,Y., Fukuda,T., Mitani,K., Hirai,H. and Kamiya,K. (1999) Mutations of the WT1 gene in childhood nonlymphoid hematological malignancies. Genes Chromosomes Cancer, 25, 176–183. [PubMed] [Google Scholar]
- Moffett P., Bruening,W., Nakagama,H., Bardeesy,N., Housman,D., Housman,D.E. and Pelletier,J. (1995) Antagonism of WT1 activity by protein self-association. Proc. Natl Acad. Sci. USA, 92, 11105–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Kudoh,T., Sugiyama,H., Toyoshima,K. and Akiyma,T. (1997) The Wilms tumor suppressor gene WT1 induces G1 arrest and apoptosis in myeloblastic leukemia cells. FEBS Lett., 409, 41–45. [DOI] [PubMed] [Google Scholar]
- Nakagama H., Heinrich,G., Pelletier,J. and Housman,D.E. (1995) Sequence and structural requirements for high-affinity binding by the WT1 gene product. Mol. Cell. Biol., 15, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemacher R.E., van der Sluijs,J.P., Voerman,J.S. and Brons,N.H. (1989) An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood, 74, 2755–2763. [PubMed] [Google Scholar]
- Prieto J., Eklund,A. and Patarroyo,M. (1994) Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell. Immunol., 156, 191–211. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K. et al. (1990) The candidate Wilms’ tumour gene is involved in genitourinary development. Nature, 346, 194–197. [DOI] [PubMed] [Google Scholar]
- Rauscher F.J.,III, Morris,J.F., Tournay,O.E., Cook,D.M. and Curran,T. (1990) Binding of the Wilms tumor locus zinc finger protein to the EGR-1 consensus sequence. Science, 250, 1259–1262. [DOI] [PubMed] [Google Scholar]
- Reddy J.C., Morris,J.C., Wang,J., English,M.A., Haber,D.A., Shi,Y. and Licht,J.D. (1995) WT1-mediated transcriptional activation is inhibited by dominant negative mutant proteins. J. Biol. Chem., 270, 10878–10884. [DOI] [PubMed] [Google Scholar]
- Sekiya M., Adachi,M., Hinoda,Y., Imai,K. and Yachi,A. (1994) Downregulation of the Wilms’ tumor gene (wt1) during myelomonocytic differentiation in HL60 cells. Blood, 83, 1876–1882. [PubMed] [Google Scholar]
- Smith S.I., Weil,D., Johnson,G.R., Boyd,A.W. and Li,C.L. (1998) Expression of the Wilms’ tumor suppressor gene, WT1, is upregulated by leukemia inhibitory factor and induces monocytic differentiation in M1 leukemic cells. Blood, 91, 764–773. [PubMed] [Google Scholar]
- Smith S.I., Down,M., Boyd,A.W. and Li,C.L. (2000) Expression of the Wilms tumor suppressor gene, WT1, reduces the tumorigenicity of the leukemic cell line MI in C.B-17 scid/scid mice. Cancer Res., 60, 808–814. [PubMed] [Google Scholar]
- Steinman R.A., Huang,J., Yaroslavskiy,B., Goff,J.P., Ball,E.D. and Nguyen,A. (1998) Regulation of p21WAF1 expression during normal myeloid differentiation. Blood, 91, 4531–4542. [PubMed] [Google Scholar]
- Sutherland H.J., Lansdorp,P.M., Henkelman,D.H., Eaves,A.C. and Eaves,C.J. (1990) Functional characteristics of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc. Natl Acad. Sci. USA, 87, 3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedberg H., Chylicki,K., Baldetorp,B., Rauscher,F.J.,III and Gullberg,U. (1998) Constitutive expression of the Wilms’ tumor gene (WT1) in the leukemic cell line U937 blocks part of the differentiation program. Oncogene, 16, 925–932. [DOI] [PubMed] [Google Scholar]
- Terstappen L.W., Huang,S., Safford,M., Lansdorp,P.M. and Loken,M.R. (1991) Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38– progenitor cells. Blood, 77, 1218–1227. [PubMed] [Google Scholar]
- Traycoff C.M., Cornetta,K., Yoder,M.C., Davidson,A. and Srour,E.F. (1996) Ex vivo expansion of murine hematopoietic progenitor cells generates classes of expanded cells possessing different levels of bone marrow repopulating potential. Exp. Hematol., 24, 299–306. [PubMed] [Google Scholar]
- Zeng Y.X. and el-Deiry,W.S. (1996) Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene, 12, 1557–1564. [PubMed] [Google Scholar]
- Zhou Y. et al. (1998) Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J., 17, 6689–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]