Abstract
The stress-activated signalling cascade leading to phosphorylation of the p38 family of kinases plays a crucial role during development and in the cellular response to a wide variety of stress-inducing agents. Although alterations in gene expression characteristic of the stress response require the regulation of key transcription factors by the p38 family, few downstream targets for this signalling pathway have been identified. By examining the ability of pigment cells to respond to UV irradiation as part of the UV-induced tanning response, we show that while the microphthalmia-associated transcription factor Mitf regulates basal Tyrosinase expression, it is the ubiquitous basic helix–loop–helix-leucine zipper transcription factor Usf-1 that is required for the UV activation of the Tyrosinase promoter. Consistent with this we demonstrate that Usf-1 is phosphorylated and activated by the stress-responsive p38 kinase. The results suggest that activation of Usf-1 by p38 at a wide variety of viral and cellular promoters will provide a link between stimuli as diverse as UV irradiation, glucose, viral infection and pro-inflammatory cytokines, and the changes in gene expression associated with the stress response.
Keywords: melanocytes/p38 kinase/stress response/tyrosinase promoter/Usf-1
Introduction
The cellular response to environmental cues, including mitogens, cytokines and a wide variety of stress-inducing agents such as UV or osmotic shock, is mediated by the activation of specific kinase cascades (for reviews see Cano and Mahadevan, 1995; Cohen, 1997). With >10 members of the mitogen-activated protein kinase (MAPK) and stress-activated protein kinase (SAPK) families being identified in mammalian cells, the identification of physiological substrates for these kinases represents a major challenge.
A major environmental stress encountered by humans is solar UV light, which can cause skin inflammation, the induction of pro-inflammatory cytokines and skin ageing, as well as skin cancer, including the highly aggressive and increasingly common malignant melanoma (Elwood, 1996; Armstrong et al., 1997; Park and Gilchrest, 1999). The serious adverse effect of UV light on the skin has meant that humans have evolved an effective defence mechanism. In response to low levels of UV irradiation epidermal melanocytes increase the production of the pigment melanin in specialized organelles termed melanosomes (Jimbow et al., 1991). The melanosomes are transferred into surrounding keratinocytes where they act to protect against UV-induced DNA damage. Although the tanning response is one of the most obvious manifestations of the effects of UV irradiation on mammalian cells, the signal transduction pathways operating to bring about UV-induced hyper-pigmentation are not well understood.
The Tyrosinase gene encodes the rate-limiting enzyme for the production of melanin and is absolutely required for pigmentation; the absence of a functional tyrosinase enzyme results in an albino phenotype. Although much of the tanning response comprises a post-translational activation of the melanosome, transcription of the Tyrosinase gene is UV responsive (Hara et al., 1994; Sturm et al., 1994; Imokawa et al., 1995, 1997; Ota et al., 1998). However, analysis of the Tyrosinase promoter (Bentley et al., 1994; Ganss et al., 1994) failed to reveal any classical UV or stress-response element. The human Tyrosinase promoter comprises an SP1 site and two E box motifs, one at the initiator, and a second, termed the M box (Lowings et al., 1992), located at –100 with respect to the transcription initiation site (Bentley et al., 1994). The E box motifs are essential for Tyrosinase promoter activity and are highly evolutionarily conserved. Numerous studies (Bentley et al., 1994; Ganss et al., 1994; Hemesath et al., 1994; Yasumoto et al., 1994; Yavuzer et al., 1995; Krylov et al., 1997) have demonstrated that the Tyrosinase initiator E box and M box elements are targets for the microphthalmia-associated basic helix–loop–helix-leucine zipper (bHLH-LZ) transcription factor Mitf (Hodgkinson et al., 1993; Hughes et al., 1993). In addition to its role in regulating pigmentation genes, Mitf is also critically required for the development of the melanocyte (Steingrímsson et al., 1994; Opdecamp et al., 1997). However, there is no evidence to suggest that Mitf is UV responsive. How the Tyrosinase promoter may be regulated in response to UV light remains unknown.
We show here that the UV response is mediated by the ubiquitous bHLH-LZ transcription factor Usf-1, which, like Mitf, binds the conserved E box elements in the Tyrosinase promoter. The ability of Usf-1 to activate transcription is regulated by a signal transduction pathway that culminates in phosphorylation and activation of Usf-1 by the p38 stress-activated kinase.
Results
UV-inducibility of the Tyrosinase promoter is inhibited by dominant-negative USF
The ability of Tyrosinase gene expression to be induced in response to UV irradiation is shown in Figure 1A. The level of Tyrosinase mRNA in either the melan-a mouse melanocyte cell line or the 501mel human melanoma cell line following UV irradiation was compared with that observed in mock-irradiated cells. While no significant difference was observed in the levels of control G3PDH expression, Tyrosinase expression was substantially increased following UV treatment in the melanocyte or 501mel cells. Similar results (not shown) were also obtained in B16 mouse melanoma cells. The results shown in Figure 1B indicate that the Tyrosinase promoter is UV responsive, with 5-fold activation of a Tyrosinase promoter-luciferase reporter being observed in B16 melanoma cells and up to 10-fold activation in the 501mel cells following UV treatment of cells. Melan-a cells were not used in this experiment owing to their poor transfectability.
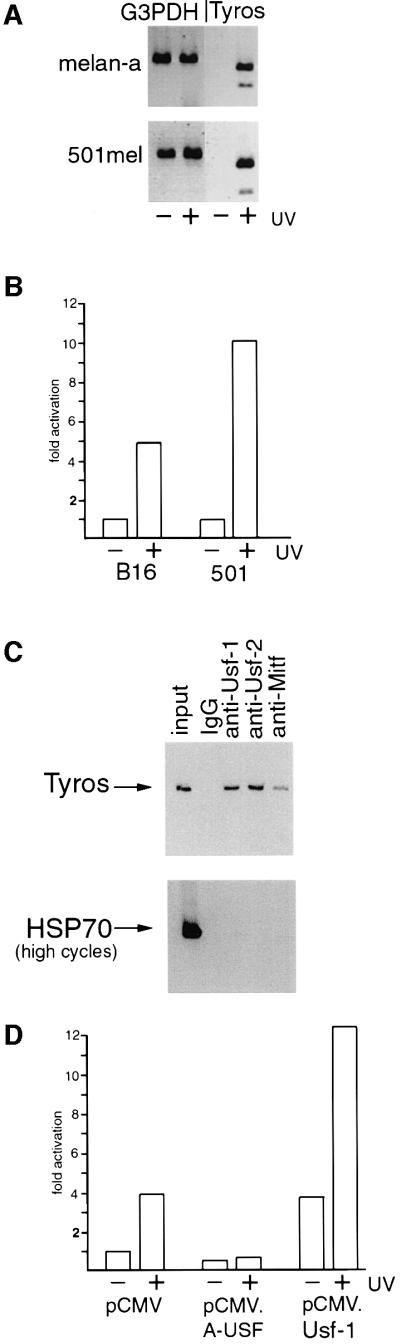
Fig. 1. UV-inducibility of the Tyrosinase promoter is USF dependent. (A) The Tyrosinase gene is UV inducible. RT–PCR using mRNA derived from mouse melanocytes (melan-a) or a human melanoma cell line (501mel) treated or untreated with UV (254 nm, 40 J/m2). Primers were specific for the Tyrosinase gene and G3PDH was used as a control. RNA was prepared 3 h post-irradiation. (B) The Tyrosinase promoter is UV responsive. A Tyrosinase promoter-luciferase reporter (100 ng) extending from –300 to +80 was transfected into either mouse (B16) or human (501mel) melanoma, and luciferase activity determined. UV irradiation was performed 24 h post-transfection where indicated. (C) Usf-1 and Usf-2 bind the Tyrosinase promoter in vivo. Chromatin immunoprecipitations were performed on 501mel cells using the indicated antibodies or control non-specific IgG. Recovered DNA was subject to PCR using primers specific for either Tyrosinase or the HSP70 promoter. Controls (not shown) confirmed that the PCR reactions were in the logarithmic phase. (D) UV-inducibility of the Tyrosinase promoter is inhibited by dominant-negative USF. The Tyrosinase promoter-luciferase reporter (100 ng) was co-transfected into B16 melanoma cells together with either a Usf-1 expression vector (pCMV.Usf-1, 500 ng), a dominant-negative USF expression vector (pCMV.A-USF, 500 ng), or a control vector (pCMV), and luciferase activity determined. Where indicated, cells were treated with UV.
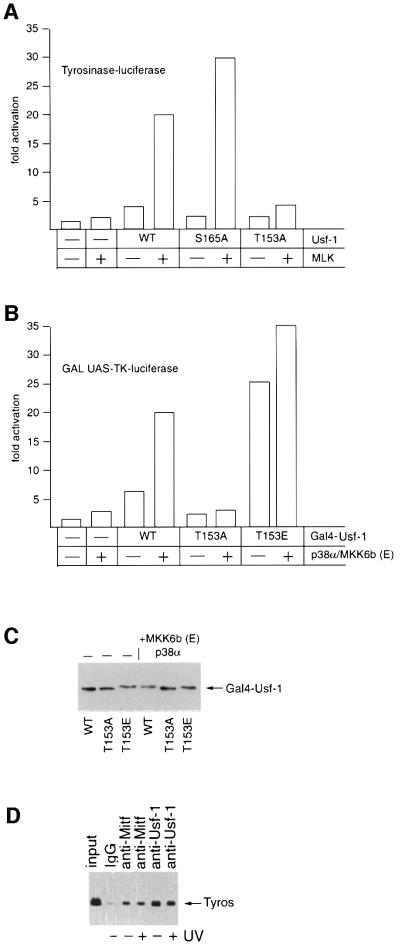
Although constitutive Tyrosinase expression is dependent on the bHLH-LZ factor Mitf (Krylov et al., 1997), Mitf is not apparently regulated in response to UV irradiation (our unpublished observations). However, the elements recognized by Mitf are also targets in vitro for USF (Bentley et al., 1994; Hemesath et al., 1994; Aksan and Goding, 1998), originally described as binding to the adenovirus major late promoter (Gregor et al., 1990). USF comprises a combination of related ubiquitous bHLH-LZ transcription factors encoded by the Usf-1 and Usf-2 genes (Gregor et al., 1990; Sirito et al., 1992, 1994). Usf-1 and Usf-2 can form either homo- or heterodimers (Sirito et al., 1992; Viollet et al., 1996), and both are present in melanocytes and melanoma cell lines (data not shown). To provide direct evidence that Usf-1 recognizes the Tyrosinase promoter in vivo, we used a chromatin immunoprecipitation assay (Braunstein et al., 1993). 501mel cells were cross-linked using formaldehyde, and DNA fragments of between 200 and 1000 bp were generated by extensive sonication of a crude nuclear lysate. The cross-linked DNA fragments were then immunoprecipitated using specific anti-Mitf, anti-Usf-1 or anti-Usf-2 antibodies, and after reversing the cross-linking and recovery of the DNA, the presence of Tyrosinase promoter DNA in the immunoprecipitated material was detected by PCR. Under the conditions used for the immunoprecipitation, Tyrosinase promoter DNA was detected using either the anti-Mitf, anti-Usf-1or anti-Usf-2 antibodies (Figure 1C). No Tyrosinase DNA was recovered if non-specific IgG was used. Note that although more Tyrosinase DNA is recovered using the anti-Usf-1 or Usf-2 antibodies compared with that obtained using the anti-Mitf antibody, this might reflect the reduced efficiency of the anti-Mitf antibody to immunoprecipitate Mitf compared with the immunoprecipitation of Usf-1 or Usf-2. Nevertheless, it is evident that in vivo within the population of cells assayed the Tyrosinase promoter can be bound by Usf-1 and Usf-2 as well as by Mitf. Similar results were obtained using B16 melanoma cells both before and after UV irradiation (see Figure 5D).
Fig. 5. Thr153 and active p38α are required for efficient transcription activation by Usf-1. (A) The Tyrosinase promoter-luciferase reporter (300 ng) was transfected into B16 melanoma cells either alone or together with the MLK and Usf-1 expression vectors (50 ng) in the indicated combinations and luciferase activity determined. (B) A GAL UAS-luciferase reporter (200 ng) was transfected into COS7 cells either alone or together with vectors expressing WT or mutant Usf-1 fused to the Gal4 DNA-binding domain in the presence or absence of co-expressed p38α/MKK6b(E) kinases as indicated. (C) Western blot of the indicated Gal4–Usf-1 chimeric proteins expressed in the transfected cells using anti-Gal4 DNA-binding domain antibody (Clontech). (D) Chromatin immunoprecipitation assay at the Tyrosinase promoter using the indicated antibodies before or after UV irradiation (254 nm, 40 J/m2) of B16 melanoma cells.
To examine any possible role for Usf-1 in UV-regulated expression of the Tyrosinase promoter we used a potent, dominant-negative version of Usf-1 termed A-USF (Qyang et al., 1999) comprising the HLH-LZ dimerization domain of Usf-1 linked to an acidic stretch of amino acids (EEEDDEEELEELE) in place of the basic region which would normally contact the DNA. The presence of the acidic stretch increases the stability of the heterodimer between A-USF and endogenous Usf-1 or Usf-2 molecules, but the heterodimer formed is unable to bind DNA. Figure 1D shows that in the B16 melanoma cell line, expression of A-USF failed to affect significantly the basal level of Tyrosinase promoter activity, consistent with previous work which demonstrated that constitutive expression of Tyrosinase was dependent on Mitf (Krylov et al., 1997). In contrast, induction of Tyrosinase promoter activity by UV irradiation was abolished by A-USF. As a control we also over-expressed WT Usf-1. In this case, the basal activity of the promoter was elevated, but the promoter retained its ability to respond to UV irradiation. Mutation of the USF/Mitf binding sites in the Tyrosinase promoter extinguishes its basal activity (Bentley et al., 1994) and as such we are not able to determine whether either or both E box motifs are required for the UV inducibility. Nevertheless, taken together the results obtained implicate USF in the UV responsiveness of the Tyrosinase promoter.
Stress-induced phosphorylation of Usf-1 mediated by a p38 stress-activated kinase
We have shown previously that Usf-1 isolated from nuclear extracts is present in two forms of 44 and 45 kDa which can be separated by SDS–PAGE, the 45 kDa band reflecting phosphorylation of Usf-1 (Galibert et al., 1997) by an unidentified kinase.
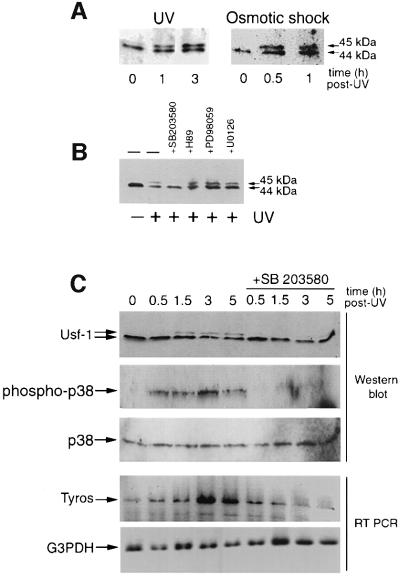
To investigate the possibility that Usf-1 was a target for a UV-responsive kinase, we UV irradiated the B16 melanoma cell line and examined Usf-1 by SDS–PAGE and western blotting 1 and 3 h post irradiation. The results (Figure 2A) indicate that UV treatment of cells (UVC, 254 nm, 40 J/m2) induced the appearance of the 45 kDa band corresponding to the phosphorylated form of Usf-1, which persisted for at least 3 h following UV treatment. A similar effect was observed using an average wavelength of 312 nm (60% UVB/40% UVA) at doses of 2000 J/m2 or above (not shown). Phosphorylation of Usf-1 was also observed if B16 (Figure 2A) or NIH 3T3 cells (not shown) were subjected to osmotic shock, which, like UV, can induce stress-activated signal transduction pathways.
Fig. 2. Stress-induced phosphorylation of Usf-1. (A) Western blotting analysis using anti-Usf-1 Ab (Santa Cruz) of B16 melanoma cells, before and after UV irradiation (254 nm, 40 J/m2) or osmotic shock (0.5 M sorbitol, 20 min), at indicated time post-treatment. The 45 kDa band corresponds to the phosphorylated form of Usf-1 described previously (Galibert et al., 1997). (B) Stress-induced phosphorylation of Usf-1 is abolished when B16 melanoma cells are pre-treated with the SB 203580 compound (10 µM, 20 min prior to UVC stimulation), a highly specific inhibitor of the p38 family kinases, but not with the MEK inhibitors PD98059 (50 µM) or U0126 (10 µM) or the H89 (10 µM) inhibitor of MSK1. (C) A time course of induction of Tyrosinase expression in 501mel cells in response to UV irradiation in the presence or absence of the SB 203580 compound. Tyrosinase mRNA was detected by RT–PCR and compared with the levels of a G3PDH control. Protein extracts from the corresponding time points were western blotted and probed for Usf-1, total p38 or double-phospho-p38. Similar results have been obtained using B16 cells.
We next considered the possibility that the appearance of the 45 kDa phosphorylated form of Usf-1 was mediated by either the JNK or the p38 pathway, which are both strongly activated by UV irradiation as well as by a variety of other stress stimuli. To facilitate the identification of the relevant pathway we used the SB 203580 compound (Goedert et al., 1997; Tong et al., 1997) at a concentration that results in a highly specific inhibition of p38α (Han et al., 1994) [also known as SAPK2a, RK (Rouse et al., 1994), p40 (Freshney et al., 1994) and CSBP (Lee et al., 1994)] and p38β (Jiang et al., 1996), while being ineffective against other members of the p38 family or JNK (Goedert et al., 1997).
As shown in Figure 2B, UV irradiation results in the appearance of the 45 kDa phosphorylated form of Usf-1. In contrast, the presence of 10 µM SB 203580 completely inhibits phosphorylation of Usf-1 in response to UV irradiation, implicating either p38α or p38β in phosphorylation of Usf-1. In agreement with this result, inhibition of the MAP kinase pathway using the PD 98059 or U0126 inhibitors failed to abolish the UV-induced appearance of the 45 kDa form of Usf-1. Similarly, H89, an inhibitor of the p38-activated kinase MSK1 (Thomson et al., 1999), was also ineffective in preventing the appearance of the 45 kDa form of Usf-1.
Further evidence that p38 signalling was essential for UV-mediated induction of Tyrosinase expression was provided by examining the kinetics of Tyrosinase induction and Usf-1 phosphorylation in response to UV irradiation in the presence and absence of the SB 203580 compound (Figure 2C). Upon UV irradiation, the appearance of the 45 kDa form of Usf-1 was apparent at ∼1.5 h post irradiation in this experiment. Longer exposure of the blot reveals some phosphorylation of Usf-1 occurring at 30 min post-irradiation (not shown), consistent with the appearance of phosphorylated and activated p38 at 30 min as detected using an antibody specific for the dual phosphorylated form of p38. The levels of active p38 peak at 3 h post-irradiation and then are reduced slightly by 5 h. No UV-dependent phosphorylation of p38 or Usf-1 was observed in the presence of the SB 203580 compound. Consistent with Usf-1 and p38 signalling being required for UV-induced activation of Tyrosinase transcription, Tyrosinase expression begins to increase by ∼1.5 h after UV irradiation, reaches a maximum at 3 h and is beginning to decline slightly by 5 h. No activation of Tyrosinase expression was observed in the presence of the SB 203580 p38 inhibitor, and if anything, basal expression of Tyrosinase was slightly reduced compared with the G3PDH control.
Requirements within Usf-1 for stress-induced phosphorylation
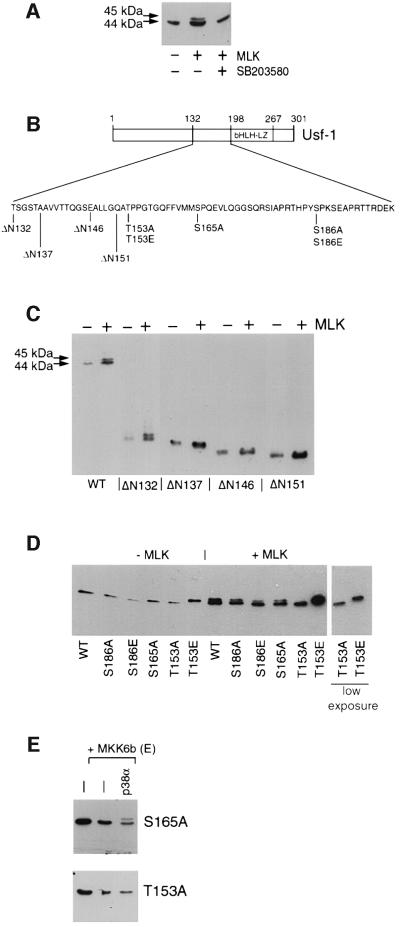
To facilitate the identification of the requirements within Usf-1 for phosphorylation in response to stress signalling, we used a mixed lineage kinase MLK (Katoh et al., 1995), which has the capacity to activate the p38 signal transduction pathway as well as the JNK pathway. When overexpressed in transfected cells, this kinase is sufficiently active to phosphorylate its downstream targets without the need for upstream activators. The appearance of the 45 kDa phosphorylated form of Usf-1 could be induced in the absence of any other stimulus by co-transfection of an MLK expression vector into B16 melanoma cells together with a vector expressing an N-terminal epitope-tagged Usf-1 (Figure 3A). As anticipated, the effect of MLK expression on Usf-1 was abolished by the SB 203580 compound, indicating that MLK signalling to Usf-1 was also mediated through a p38 kinase and not via JNK. We then used the MLK expression vector together with a series of vectors expressing epitope-tagged deletion mutants of Usf-1 to determine which region of Usf-1 was required for p38-mediated phosphorylation. A map of Usf-1 and the various mutants used is shown in Figure 3B. As shown in Figure 3C, the appearance of the 45 kDa phosphorylated form of Usf-1 could be induced by MLK using either WT Usf-1 or an N-terminal deletion mutant extending to amino acid 132. In contrast, a further N-terminal deletion to residue 137 abolished the MLK-induced appearance of the slower migrating band corresponding to the phosphorylated form of Usf-1. Similarly, no effect of MLK on Usf-1 was observed using additional N-terminal deletion mutants ΔN146 or ΔN151. Although we cannot absolutely rule out the possibility that some deletion mutants of Usf-1 may be constitutively phosphorylated in vivo and that their mobility in this assay might not necessarily reflect their phosphorylation status, the most likely explanation for these results is that residues N-terminal to amino acid 132 are dispensable for the phosphorylation of Usf-1 induced by MLK.
Fig. 3. Requirements within Usf-1 for stress-induced phosphorylation in vivo. (A) MLK-mediated phosphorylation of Usf-1. B16 melanoma cells were transfected with a vector expressing SV5 epitope-tagged Usf-1 (800 ng) either alone or together with 10 ng of an MLK expression vector (pCMV.MLK). Cells were pre-treated with the SB 203580 compound (10 µM) where indicated. (B) Schematic representation of the Usf-1 protein and various point and deletion mutations expressed in the co-transfection assays with MLK shown in (C) and (D). (C and D) Vectors expressing SV5 epitope-tagged WT or the indicated Usf-1 deletion or point mutants were transfected into COS7 cells either alone or together with the MLK expression vector. Cell extracts were assayed by SDS–PAGE and western blotting using the anti-SV5 antibody. Similar results were obtained in B16 melanoma cells. (E) SV5 epitope-tagged Usf-1 (pCMV.SV5-Usf-1) or the S165A or T153A mutants were expressed in B16 melanoma cells either alone or together with MKK6b(E) and p38α as indicated.
If p38 were directly involved in the phosphorylation of Usf-1, the phosphorylation site should be either an SP or TP motif. Three candidate sites present within the 132–198 region of Usf-1, namely Thr153, Ser165 and Ser186, were mutated to alanine in the context of full-length Usf-1 and used in the assay using the co-transfected MLK expression vector. The results obtained (Figure 3D) indicate that neither the S186A nor the S165A mutation prevented the appearance of the 45 kDa phosphorylated form of Usf-1 induced by MLK. In contrast, no phosphorylation was observed using the T153A mutation, suggesting that this threonine represented a candidate target for stress-induced signalling to Usf-1. In an attempt to mimic the phosphorylation event, we also mutated Thr153 to glutamic acid. Interestingly, this mutation induced a mobility shift of Usf-1 even in the absence of the MLK expression, again consistent with T153 being the target for stress-activated signalling. No similar mobility shift was observed using an S186E mutant in the absence of MLK. Moreover, as shown in Figure 4E, phosphorylation of Usf-1 in response to expression of a constitutively active upstream kinase MKK6b (Han et al., 1996) and p38α was unaffected by the S165A mutation, but was completely blocked by substitution of Thr153 by alanine. No phosphorylation of Usf-1 was observed by coexpressing a constitutively active MKK7 and JNK (not shown).
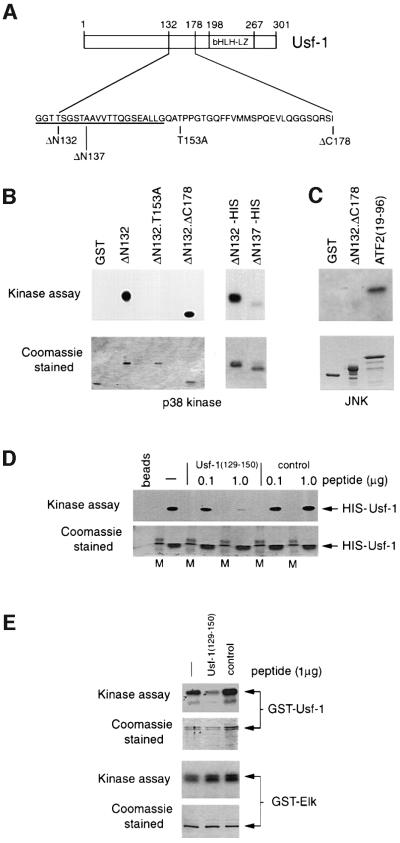
Fig. 4. Requirements within Usf-1 for stress-induced phosphorylation in vitro. (A) Schematic of Usf-1 proteins used in the in vitro kinase assays. (B) Usf-1 is phosphorylated by p38α in vitro. In vitro kinase assays were performed using either purified GST–Usf-1 fusion protein or His6-tagged Usf-1 proteins in the presence of recombinant activated p38α kinase [γ-32P]ATP. Kinase assays using the indicated Usf-1 derivatives are shown in the upper panel after SDS–PAGE and autoradiography. The lower panel shows the same gel stained with Coomassie Blue indicating that equivalent amounts of protein were used in each assay. (C) JNK does not efficiently phosphorylate Usf-1. The indicated GST fusion proteins were incubated with purified active JNK and [γ-32P]ATP. Efficient phosphorylation of ATF2 was observed but not of either GST alone or the GST-Usf-1 fusion protein. (D) A competitor peptide can inhibit the phosphorylation of His-tagged Usf-1 by p38α in vitro. Active recombinant p38α kinase was pre-incubated with either Usf-1 competitor peptide corresponding to Usf-1 amino acids 129–150, or a control peptide (0.1–1 µg) derived from the Pho4 activation domain, for 30 min prior to the kinase reaction in the presence of His-tagged Usf-1 recombinant protein as indicated. M indicates a molecular weight marker. (E) The Usf-1 peptide inhibits phosphorylation of Usf-1 but not Elk by p38. Kinase assays were performed as in (D) using the indicated GST fusion proteins in the presence or absence of the Usf-1 peptide or the control Pho4 peptide.
To confirm that Thr153 could be a direct target for p38α, we expressed either the ΔN132 mutant of Usf-1 or a derivative bearing the T153A mutation as GST fusion proteins, and asked whether they could be phosphorylated in vitro using p38α kinase. As an additional control we used either GST alone or GST fused to a region of Usf-1 comprising residues 132–178. A map of the proteins used is shown in Figure 4A and the result of the kinase assay in Figure 4B. Using equivalent amounts of the GST fusion proteins as substrates, as visualized by Coomassie staining, both the ΔN132 and ΔN132.ΔC178 mutants were efficiently phosphorylated. In contrast, significant levels of phosphorylation were not observed using either the GST protein alone, or the GST–ΔN132 protein in which Thr153 had been mutated to alanine, consistent with Thr153 being a target for p38-mediated phosphorylation of Usf-1. Consistent with p38 and not JNK being responsible for phosphorylating Usf-1, Usf-1 is poorly phosphorylated by JNK in vitro compared with ATF2, a known JNK substrate (Figure 4C)
The in vivo assays suggested that the region of Usf-1 lying N-terminal to Thr153 between residues 132 and 137 played a critical role in phosphorylation by p38α. This was confirmed in vitro where a His-tagged ΔN132 mutant, but not a ΔN137 mutant, was efficiently phosphorylated by p38α. Although other explanations are possible, one role for the residues lying between 132 and 137 may be in providing an interface for docking by the p38 kinase. Consistent with this, the ability of Usf-1 to be phosphorylated in vitro by p38α is reduced if the kinase is pre-incubated with an excess of peptide corresponding to Usf-1 residues 129–150, which span the putative docking site, but do not include the Thr153 phosphorylation site (Figure 4D). The effect of this peptide is specific, as no effect was observed using a control peptide derived from the Pho4 transcription factor activation domain and no effect of the Usf-1 peptide was observed on phosphorylation of GST–Elk by p38 (Figure 4E). Taken together, the results suggest that Usf-1 is a direct target for p38 rather than a substrate for a p38-activated kinase.
Phosphorylation of Usf-1 is required for transcription activation
To investigate the possibility that stress signalling potentiates the ability of Usf-1 to activate transcription, we transfected B16 cells with a Tyrosinase promoter-luciferase reporter and asked whether we could regulate its activity by coexpressing MLK and WT or mutant forms of Usf-1. The results are shown in Figure 5A. Expression of MLK alone gave rise to a low level of activation of the Tyrosinase promoter, most likely arising through regulation of endogenous Usf-1. Similarly, co-transfection with a Usf-1 expression vector in the absence of MLK resulted in an ∼4-fold activation of the promoter. However, up to 20-fold activation of the Tyrosinase promoter was achieved if Usf-1 and MLK were coexpressed. This strongly synergistic effect was reproduced using the Usf-1 S165A mutant, which does not affect phosphorylation of Usf-1 by p38α. In contrast, the ability of the T153A Usf-1 mutant to activate the Tyrosinase promoter in response to coexpressed MLK was largely abolished. The low level of activation observed in the presence of Usf-1 (T153A) and MLK may reflect in part the activation of endogenous Usf-1 by MLK.
To determine whether the effects of p38α signalling to Usf-1 affected its ability to activate transcription as opposed to its capacity to bind DNA, we fused WT Usf-1 and the T153A and T153E mutants to the Gal4 DNA-binding domain and measured the ability of the Gal4–Usf-1 chimeras to activate transcription from a GAL UAS reporter in the presence or absence of the combination of p38α and constitutively active MKK6b. The results (Figure 5B) provide a further illustration that Usf-1 is a p38-responsive transcription factor. Thus, expression of MKK6b(E) together with p38α failed to activate the GAL reporter significantly in the absence of coexpressed Gal4–Usf-1. An ∼5-fold activation was achieved by expressing Gal4–Usf-1, and this was elevated a further 4-fold by coexpression of MKK6b(E) and p38α. In contrast, the T153A mutant, which is not efficiently phosphorylated by p38α, was largely inactive irrespective of whether MKK6b(E) and p38α were coexpressed. Strikingly, the T153E mutant, which mimics constitutive phosphorylation, was constitutively active, increasing transcription from the GAL reporter by ∼25-fold even in the absence of MKK6b(E) and p38α. The effects on the ability of Usf-1 to activate transcription in response to MKK6b(E) and p38α were not a consequence of differential protein expression as western blotting using anti-Gal4 antibody revealed that similar levels of each Gal4 fusion protein were expressed in the presence or absence of MKK6b(E) and p38α (Figure 5C). Furthermore, we did not detect any increase in binding by Usf-1 (or Mitf) to the Tyrosinase promoter in vivo in response to UV irradiation as measured using the chromatin immunoprecipitation assay (Figure 5D). We conclude, therefore, that it is most likely the ability of Usf-1 to activate transcription that is increased in response to phosphorylation by p38α, and that phosphorylation can be effectively mimicked using the T153E substitution.
Discussion
Although the p38 signalling cascade plays a major role in the cellular response to stress or pro-inflammatory cytokines (Freshney et al., 1994; Rouse et al., 1994; Martin-Blanco, 2000) and in cellular differentiation and development (Nebreda and Porras, 2000), few downstream targets have been identified to date (Cohen, 1997). In this study we identify the ubiquitous transcription factor Usf-1 as an in vivo target for the stress-responsive p38 kinase.
In melanocytes, the response to UV irradiation is characterized by increased expression of Tyrosinase. From the chromatin immunoprecipitation experiments it is clear that in vivo, the Tyrosinase promoter is bound by Mitf as well as by Usf-1 and Usf-2. Usf-1 and Usf-2 can bind DNA as homodimers, but can also heterodimerize and can bind the same elements in the Tyrosinase promoter as Mitf (Lowings et al., 1992; Bentley et al., 1994; Yasumoto et al., 1994; Aksan and Goding, 1998), namely the initiator E box and the M box motifs. Which bHLH-LZ factor will be bound to the Tyrosinase promoter at any given time will be determined by multiple factors, including the relative abundance of Usf-1, Usf-2 and Mitf. In this respect, it is already known that the amount of Mitf present in a cell is tightly regulated (Bertolotto et al., 1998; Wu et al., 2000; Xu et al., 2000), and indeed, consistent with a role for USF in regulating Tyrosinase expression in the skin, Mitf appears not to be expressed in epidermal melanocytes, at least in mice (Nakayama et al., 1998).
The use of a dominant-negative Mitf revealed that Mitf is required for the constitutive expression of Tyrosinase (Krylov et al., 1997). Using a similar approach, we show that the UV-induced increase in Tyrosinase promoter activity is abrogated by the expression of the dominant-negative A-USF. The involvement of Usf-1 in the UV response was further substantiated by the observation that Usf-1 is phosphorylated by p38 SAP kinase. Thus, phosphorylation in vivo was inhibited by the SB 203580 compound, which at the concentration used is highly specific for the closely related kinases p38α and p38β, and could be reproduced both in vitro using purified p38α and by co-transfection of vectors expressing a constitutively active form of MKK6b and p38α. Moreover, p38-mediated phosphorylation of Usf-1 appears to act primarily at the level of its ability to activate transcription, as the capacity of Gal4–Usf-1 chimeras to activate transcription from a GAL UAS could be enhanced by stress signalling or by the introduction of a T to E substitution at residue 153 of Usf-1 to mimic phosphorylation. The fact that Usf-1 can form heterodimers with Usf-2 might imply that both Usf-1 homodimers and heterodimers would be stress-responsive and, although we have not investigated this directly, it is possible that Usf-2 is also stress-regulated; Usf-2 has been implicated in glucose responsiveness at the liver pyruvate kinase promoter (Vallet et al., 1997) and glucose is known to signal via p38 SAP kinase (Igarashi et al., 1999), and indeed Usf-2 can be efficiently phosphorylated by p38α in vitro (our unpublished results). We imagine, therefore, that UV irradiation will lead to activation of promoter-bound Usf-1 (and Usf-2) via phosphorylation by p38 SAP kinase, and thus consequently to the increased Tyrosinase expression characteristic of the tanning response.
Although UV can penetrate the skin and directly target melanocytes, much of the impact of UV on epidermal melanocytes may be mediated by the action of signalling molecules generated by the surrounding keratinocytes such as endothelin-1 and melanocyte-stimulating hormone (α-MSH; Imokawa et al., 1995, 1997; Thody and Graham, 1998; Abdel-Malek et al., 1999). Importantly, although both molecules can activate other signalling pathways, endothelin-1 (Aquilla et al., 1996; Clerk et al., 1998) and MSH (Scott and Cassidy 1998; Smalley and Eisen 2000) are known to signal via the p38 pathway. It seems likely, therefore, that in the skin, one consequence of UV irradiation will be increased USF-mediated transcription and Tyrosinase expression in melanocytes in response to endothelin-1 and MSH signalling. Consistent with a critical role for p38 in tanning, treatment of human skin with the SB 203580 compound immediately after irradiation completely abrogated UV-induced increased pigmentation (T.Zervos, personal communication).
Although in this study we have focused on the ability of Usf-1 to respond to stress by activating Tyrosinase expression in melanocytes, Usf-1 is ubiquitously expressed and it seems likely that Usf-1 will act as a key link between stress signalling and the transcriptional response of a variety of cellular and viral promoters. For example, USF has been implicated in activated, but not basal transcription of the mouse metallothionein promoter in response to cadmium (Li et al., 1998), as well as in the expression of the major histocompatibility class I genes (Howcroft et al., 1999), the C4 complement gene (Galibert et al., 1997), and the p53 promoter (Reisman and Rotter, 1993). Moreover, activation by cytokines of the HIV LTR that contains a USF-binding site (Lu et al., 1990; Giacca et al., 1992; Sieweke et al., 1998) requires p38 SAP kinase (Kumar et al., 1996). Similarly, activation of the p38 stress signalling pathway by herpes simplex virus (HSV) (Zachos et al., 1999) may lead to more efficient activation of the virus latency-associated promoter via a USF site (Soares et al., 1996; Kenny et al., 1997) prior to the virus going latent, while stress-dependent reactivation of a latent HSV is characterized by induction of the USF-dependent VP16 promoter (Greaves and O’Hare, 1991). Whether other viruses induce a stress response on infection is not known, although we note that the adenovirus major late promoter is regulated by USF (Gregor et al., 1990).
For these and many other genes the presence of USF binding sites within their promoters may allow a degree of stress responsiveness. However, the location of the binding site within the promoter and the ability of USF to cooperate with other factors in regulating gene expression may dictate the relative contribution of USF to any stress response. Moreover, the capacity of Usf-1 to respond to stress signalling may also depend strongly on cell type and the level of constitutive signalling via the p38 pathway.
The identification of Usf-1 as a stress-responsive transcription factor is an important step not only towards understanding the process of UV-induced pigmentation, but also towards achieving a better understanding of the global transcriptional response to cellular stress.
Materials and methods
Cell lines and transfection assays
COS7 and NIH 3T3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). Melanocyte and melanoma cell lines were grown in RPMI-1640 medium supplemented with 10% FCS (Gibco), and 200 nM tetradecanoyl phorbol acetate (TPA; Sigma) was added for the melanocytes. Transfections were performed using Fugene reagent (Roche), according to the manufacturer’s instructions using a total of 600 ng (24-well plate) or 1 µg (6-well plate) of DNA. Forty-eight hours post-transfection, cells were washed twice with phosphate-buffered saline (PBS), before harvesting using 100 µl of lysis buffer (100 mM potassium phosphate pH 7.8, 0.2% Triton X-100, 1 mM dithiothreitol) and 20 µl of cell extract used for the luciferase assay using the Promega luciferase assay system, according to the manufacturer’s instructions. Luciferase activities were detected using microplate luminometer apparatus (MicroLuminat Plus, EG&G Berthold).
Osmotic shocks were performed using 0.5 M sorbitol for 20 min at 37°C. UVC irradiation (254 nm, 40 J/m2) was performed using a UV Stratalinker 1800 apparatus (Stratagene). For both treatments, the medium was completely removed and replaced immediately after stimulation. Pretreatment with specific inhibitors was at 10 µM for SB203580 and 50 µM for PD98059 (Calbiochem) for 20 min prior to stimulation. On transfected cells, UVC irradiation was carried out 24 h post-transfection.
Plasmid constructs
The GAL UAS-TK-luciferase reporter was a gift from Dr Graham Goodwin. The pCMV.SV5 epitope vector contains sequences encoding the 13 amino acid SV5 epitope (Hanke et al., 1992). Usf-1 deletion or point mutants were cloned into this vector after PCR using appropriate primers. The mammalian Gal4 expression vector pSG424 (Sadowski and Ptashne, 1989), the Tyrosinase luciferase reporter (Galibert et al., 1999) and the pCMV–MLK expression plasmid (Katoh et al., 1995) have been described previously. Usf-1 WT and Usf-1 point mutants were cloned into the pSG424 polylinker in frame with the Gal4 DNA-binding domain, and Usf-1 deletion and point mutants were also cloned into the polylinkers 3′ to the GST or His tags in the pGEX2TK and pQE30 expression vectors, respectively. Details of all of the cloning strategies used are available on request. All other vectors for the expression of p38α or MKK6(E) signalling molecules were provided by Dr Jiahuai Han, and have been described previously by Alpert et al. (1999).
Chromatin immunoprecipitation assays
Chromatin immunoprecipitations were performed essentially as described (Braunstein et al., 1993) using 10 µl of antibody or non-specific IgG. The DNA recovered was analysed by quantitative PCR for 25 cycles taking care that the PCR reaction was in log phase.
The primers used for the PCR were as follows: 5′-GCTCTATTCCTGACACTACCTCTC-3′ and 5′-CAAGGTCTGCAGGAACTGGCTAATTG-3′ for the Tyrosinase promoter; and 5′-CCTCCAGTGAATCCCAGAAGACTCT-3′ and 5′-TGGGACAACGGGAGTCACTCTC-3′ for the Hsp70 promoter.
Western blotting analysis
Whole cell lysates were resolved by 10% SDS–PAGE, using a 200:1 acrylamide:bis-acrylamide ratio. After western blotting membranes were probed with appropriate primary antibodies and detected using peroxidase-conjugated anti-rabbit or anti-mouse Ab and visualized by ECL (Amersham).
The primary antibodies used were: the polyclonal anti-human Usf-1 Ab (USF-C20; Santa Cruz Biotechnology), the anti-Pk-Tag (SV5) Ab (MCA1360; Serotec), the anti-Gal4 monoclonal antibody (5399–1; Clontech), anti-p38 and anti-phospho38 (Biolabs).
Protein expression and purification
All GST fusion proteins were expressed in Escherichia coli BL21(DE3)pLysS, and His-tagged Usf-1 proteins in E.coli BLT21 strain. Isopropyl-β-d-thiogalactopyranoside induction was performed for 4 h at 37°C. Fusion proteins were purified using G–Sepharose-4B or NTA-agarose beads.
Protein kinase assays
Recombinant active p38α and JNK were obtained from Upstate Biotechnology. Kinase assays were performed in 30 µl of reaction volume (20 mM MOPS pH 7.2, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol) at 30°C for 30 min under agitation. Eighty nanograms of active p38 protein kinase were added to 2 µg of Usf-1-recombinant protein, in the presence of [γ-32P]ATP (10 µM Ci diluted with 9 µl of 500 µM unlabelled ATP, 75 mM MgCl2). Following the kinase reaction, beads were intensively washed (10 times with 1 ml of reaction buffer), and 30 µl of protein denaturing buffer were added.
Peptide competition was carried out as described above, except that pre-incubation with 100 ng–1 µg of the peptide competitors with active p38α –GST fusion protein was performed prior to the kinase reaction.
The Usf-1 competitor peptide was N-GGTTSGSTAAVVTTQGSEALLG, and the control Pho4 peptide N-SKATTVEAACRYIRHLQQN.
Acknowledgments
Acknowledgements
We would like to thank Jiahuai Han, Graham Goodwin, Richard Treisman and Alan Hall for providing various expression and reporter vectors, and Chuck Vinson for the A-USF expression vector. We also thank Tony Zervos for communicating results prior to publication. This work was supported by a European Union ‘Marie Curie’ fellowship to M.-D.G., by the Association for International Cancer Research and by Marie Curie Cancer Care.
References
- Abdel-Malek Z., Suzuki,I., Tada,A., Im,S. and Akcali,C. (1999) The melanocortin-1 receptor and human pigmentation. Ann. NY Acad. Sci., 885, 117–133. [DOI] [PubMed] [Google Scholar]
- Aksan I. and Goding,C.R. (1998) Targeting the microphthalmia basic helix-loop-helix-leucine zipper transcription factor to a subset of E-Box elements in vitro and in vivo. Mol. Cell. Biol., 18, 6930–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert D., Schwenger,P., Han,J. and Vilcek,J. (1999) Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IκB phosphorylation and NF-κB activation. J. Biol. Chem., 274, 22176–22183. [DOI] [PubMed] [Google Scholar]
- Aquilla E., Whelchel,A., Knot,H.J., Nelson,M. and Posada,J. (1996) Activation of multiple mitogen-activated protein kinase signal transduction pathways by the endothelin B receptor requires the cytoplasmic tail. J. Biol. Chem., 271, 31572–31579. [DOI] [PubMed] [Google Scholar]
- Armstrong B.K., Kricker,A. and English,D.R. (1997) Sun exposure and skin cancer. Australas. J. Dermatol., 38, S1–S6. [DOI] [PubMed] [Google Scholar]
- Bentley N.J., Eisen,T. and Goding,C.R. (1994) Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol., 14, 7996–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotto C., Busca,R., Abbe,P., Bille,K., Aberdam,E., Ortonne,J.-P. and Ballotti,R. (1998) Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of Microphthalmia. Mol. Cell. Biol., 18, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein M., Rose,A.B., Holmes,S.G., Allis,C.D. and Broach,J.R. (1993) Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev., 7, 592–604. [DOI] [PubMed] [Google Scholar]
- Cano E. and Mahadevan,L.C. (1995) Parallel signal processing among mammalian MAPKs. Trends Biochem. Sci., 20, 117–122. [DOI] [PubMed] [Google Scholar]
- Clerk A., Michael,A. and Sugden,P.H. (1998) Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: a role in cardiac myocyte hypertrophy? J. Cell Biol., 142, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. (1997) The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol., 7, 353–361. [DOI] [PubMed] [Google Scholar]
- Crews C.M., Alessandrini,A. and Erikson,R.L. (1992) The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science, 258, 478–480. [DOI] [PubMed] [Google Scholar]
- Elwood J.M. (1996) Melanoma and sun exposure. Semin. Oncol., 23, 650–666. [PubMed] [Google Scholar]
- English J.M., Vanderbilt,C.A., Xu,S., Marcus,S. and Cobb,M.H. (1995) Isolation of MEK5 and differential expression of alternatively spliced forms. J. Biol. Chem., 270, 28897–28902. [DOI] [PubMed] [Google Scholar]
- Freshney N.W., Rawlinson,L., Guesdon,F., Jones,E., Cowley,S., Hsuan,J. and Saklatvala,J. (1994) Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell, 78, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Galibert M.-D., Boucontet,L., Goding,C.R. and Meo,T. (1997) Recognition of the E-C4 element from the C4 complement gene promoter by the upstream stimulatory factor-1 transcription factor. J. Immunol., 159, 6167–6183. [PubMed] [Google Scholar]
- Galibert M.-D., Yavuzer,U., Dexter,T.J. and Goding,C.R. (1999) Pax3 and regulation of the melanocyte-specific TRP-1 promoter. J. Biol. Chem., 274, 26894–26900. [DOI] [PubMed] [Google Scholar]
- Ganss R., Schutz,G. and Beermann,F. (1994) The mouse tyrosinase gene. Promoter modulation by positive and negative regulatory elements. J. Biol. Chem., 269, 29808–29816. [PubMed] [Google Scholar]
- Giacca M., Gutierrez,M.I., Menzo,S., Di Fagagna,F.D. and Falaschi,A. (1992) A human binding site for transcription factor USF/MLTF mimics the negative regulatory element of human immunodeficiency virus type 1. Virology, 186, 133–147. [DOI] [PubMed] [Google Scholar]
- Goedert M., Cuenda,A., Craxton,M., Jakes,R. and Cohen,P. (1997) Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J., 16, 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves R.F. and O’Hare,P. (1991) Sequence, function and regulation of the Vmw65 gene of herpes simplex virus type 2. J. Virol., 65, 6705–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P.D., Sawadogo,M. and Roeder,R.G. (1990) The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev., 4, 1730–1740. [DOI] [PubMed] [Google Scholar]
- Han J., Lee,J.D., Bibbs,L. and Ulevitch,R.J. (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science, 265, 808–811. [DOI] [PubMed] [Google Scholar]
- Han J., Lee,J.D., Jiang,Y., Li,Z., Feng,L. and Ulevitch,R.J. (1996) Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem., 271, 2886–2891. [DOI] [PubMed] [Google Scholar]
- Hanke T., Szawlowski,P. and Randall,R.E. (1992) Construction of a solid matrix-antibody-antigen complexes containing simian immunodeficiency virus p27 using tag-specific monoclonal antibody and tag-linked antigen. J. Gen. Virol., 73, 653–660. [DOI] [PubMed] [Google Scholar]
- Hara H., Lee,M.H., Chen,H., Luo,D. and Jimbow,K. (1994) Role of gene expression and protein synthesis of tyrosinase, TRP-1, lamp-1 and CD63 in UVB-induced melanogenesis in human melanomas. J. Invest. Dermatol., 102, 495–500. [DOI] [PubMed] [Google Scholar]
- Hemesath T.J. et al. (1994) Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev., 8, 2770–2780. [DOI] [PubMed] [Google Scholar]
- Hodgkinson C.A., Moore,K.J., Nakayama,A., Steingrímsson,E., Copeland,N.G., Jenkins,N.A. and Arnheiter,H. (1993) Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell, 74, 395–404. [DOI] [PubMed] [Google Scholar]
- Howcroft T.K., Murphy,C., Weissman,J.D., Huber,S.J., Sawadogo,M. and Singer,D.S. (1999) Upstream stimulatory factor regulates major histocompatibility complex class I gene expression: the U2ΔE4 splice variant abrogates E-box activity. Mol. Cell. Biol., 19, 4788–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.J., Lingrel,J.B., Krakowsky,J.M. and Anderson,K.P. (1993) A helix-loop-helix transcription factor-like gene is located at the mi locus. J. Biol. Chem., 268, 20687–20690. [PubMed] [Google Scholar]
- Igarashi M. et al. (1999) Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J. Clin. Invest., 103, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G., Miyagishi,M. and Yada,Y. (1995) Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J. Invest. Dermatol, 105, 32–37. [DOI] [PubMed] [Google Scholar]
- Imokawa G., Kobayashi,T., Miyagishi,M., Higashi,K. and Yada,Y. (1997) The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res., 10, 218–228. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Chen,C., Li,Z., Guo,W., Gegner,J.A., Lin,S. and Han,J. (1996) Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem., 271, 17920–17926. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Fitzpatrick,T.B. and Wick,M. (eds) (1991) Biochemistry and Physiology of Melanin Pigmentation. Oxford University Press, New York, NY.
- Katoh M., Hirai,M., Sugimura,T. and Terada,M. (1995) Cloning and characterisation of MST, a novel (putative) serine/threonine kinase with SH3 domain. Oncogene, 10, 1445–1447. [PubMed] [Google Scholar]
- Kenny J.J., Millhouse,S., Wotring,M. and Wigdahl,B. (1997) Upstream stimulatory factor family binds to the herpes simplex virus type 1 latency-associated transcript promoter. Virology, 230, 381–391. [DOI] [PubMed] [Google Scholar]
- Krylov D., Kasai,K., Echlin,D.R., Taparowsky,E.J., Arnheiter,H. and Vinson,C. (1997) A general method to design dominant negatives to B-HLHZip proteins that abolish DNA binding. Proc. Natl Acad. Sci. USA, 94, 12274–12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Orsini,M.J., Lee,J.C., McDonnell,P.C., Debouck,C. and Young,P.R. (1996) Activation of the HIV-1 long terminal repeat by cytokines and environmental stress requires an active CSBP/p38 MAP kinase. J. Biol. Chem., 271, 30864–30869. [DOI] [PubMed] [Google Scholar]
- Lee J.C. et al. (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature, 372, 739–746. [DOI] [PubMed] [Google Scholar]
- Li Q., Hu,N., Daggett,M.A., Chu,W.A., Bittel,D., Johnson,J.A. and Andrews,G.K. (1998) Participation of upstream stimulator factor (USF) in cadmium-induction of the mouse metallothionein-I gene. Nucleic Acids Res., 26, 5182–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowings P., Yavuzer,U. and Goding,C.R. (1992) Positive and negative elements regulate a melanocyte-specific promoter. Mol. Cell. Biol., 12, 3653–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.C., Touzjian,N., Stenzel,M., Dorfman,T., Sodroski,J.G. and Haseltine,W.A. (1990) Identification of cis-acting repressive sequences within the negative regulatory element of human immunodeficiency virus type 1. J. Virol., 64, 5226–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E. (2000) p38 MAPK signalling cascades: ancient roles and new functions. BioEssays, 22, 637–645. [DOI] [PubMed] [Google Scholar]
- Nakayama A., Nguyen,M.T., Chen,C.C., Opdecamp,K., Hodgkinson,C.A. and Arnheiter,H. (1998) Mutations in microphthalmia, the mouse homolog of the human deafness gene MITF, affect neuroepithelial and neural crest-derived melanocytes differently. Mech. Dev., 70, 155–166. [DOI] [PubMed] [Google Scholar]
- Nebreda A.R. and Porras,A. (2000) p38 MAP kinases: beyond the stress response. Trends Biochem. Sci., 25, 257–260. [DOI] [PubMed] [Google Scholar]
- Opdecamp K., Nakayama,A., Nguyen,M.T., Hodgkinson,C.A., Pavan,W.J. and Arnheiter,H. (1997) Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development, 124, 2377–2386. [DOI] [PubMed] [Google Scholar]
- Ota A., Park,J.S. and Jimbow,K. (1998) Functional regulation of tyrosinase and LAMP gene family of melanogenesis and cell death in immortal murine melanocytes after repeated exposure to ultraviolet B. Br. J. Dermatol., 139, 207–215. [DOI] [PubMed] [Google Scholar]
- Park H.Y. and Gilchrest,B.A. (1999) Signaling pathways mediating melanogenesis. Cell. Mol. Biol. (Noisy-le-grand), 45, 919–930. [PubMed] [Google Scholar]
- Qyang Y., Luo,X., Lu,T., Ismail,P.M., Krylov,D., Vinson,C. and Sawadogo,M. (1999) Cell-type-dependent activity of the ubiquitous transcription factor USF in cellular proliferation and transcription activation. Mol. Cell. Biol., 19, 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D. and Rotter,V. (1993) The helix-loop-helix containing transcription factor USF binds to and transactivates the promoter of the p53 tumor suppressor gene. Nucleic Acids Res., 21, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J., Cohen,P., Trigon,S., Morange,M., Alonso-Llamazares,A., Zamanillo,D., Hunt,T. and Nebreda,A.R. (1994) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell, 78, 1027–1037. [DOI] [PubMed] [Google Scholar]
- Sadowski I. and Ptashne,M. (1989) A vector for expressing GAL4(1–147) fusions in mammalian cells. Nucleic Acids Res., 17, 7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G.A. and Cassidy,L. (1998) Rac1 mediates dendrite formation in response to melanocyte stimulating hormone and ultraviolet light in a murine melanoma model. J. Invest. Dermatol., 111, 243–250. [DOI] [PubMed] [Google Scholar]
- Sieweke M.H., Tekotte,H., Jarosch,U. and Graf,T. (1998) Cooperative interaction of ets-1 with USF-1 required for HIV-1 enhancer activity in T cells. EMBO J., 17, 1728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirito M., Walker,S., Lin,Q., Kozlowski,M.T., Klein,W.H. and Sawadogo,M. (1992) Members of the USF family of helix-loop-helix proteins bind DNA as homo- as well as heterodimers. Gene Expr., 2, 231–240. [PMC free article] [PubMed] [Google Scholar]
- Sirito M., Lin,Q., Maity,T. and Sawadogo,M. (1994) Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res., 22, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley K. and Eisen,T. (2000) The involvement of p38 mitogen-activated protein kinase in the α-melanocyte stimulating hormone (α-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett., 476, 198–202. [DOI] [PubMed] [Google Scholar]
- Soares K., Hwang,D.Y., Ramakrishnan,R., Schmidt,M.C., Fink,D.J. and Glorioso,J.C. (1996) cis-acting elements involved in transcriptional regulation of the herpes simplex virus type 1 latency-associated promoter 1 (LAP1) in vitro and in vivo. J. Virol., 70, 5384–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsson E. et al. (1994) Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nature Genet., 8, 256–263. [DOI] [PubMed] [Google Scholar]
- Sturm R.A., O’Sullivan,B.J., Thomson,J.A., Jamshidi,N., Pedley,J. and Parsons,P.G. (1994) Expression studies of pigmentation and POU-domain genes in human melanoma cells. Pigment Cell Res., 7, 235–240. [DOI] [PubMed] [Google Scholar]
- Thody A.J. and Graham,A. (1998) Does α-MSH have a role in regulating skin pigmentation in humans? Pigment Cell Res., 11, 265–274. [DOI] [PubMed] [Google Scholar]
- Thomson S., Clayton,A.L., Hazzalin,C.A., Rose,S., Barratt,M.J. and Mahadevan,L.C. (1999) The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J., 18, 4779–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L., Pav,S., White,D.M., Rogers,S., Crane,K.M., Cywin,C.L., Brown,M.L. and Pargellis,C.A. (1997) A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nature Struct. Biol., 4, 311–316. [DOI] [PubMed] [Google Scholar]
- Vallet V.S., Henrion,A.A., Bucchini,D., Casado,M., Raymondjean,M., Kahn,A. and Vaulont,S. (1997) Glucose-dependent liver gene expression in upstream stimulatory factor 2 –/– mice. J. Biol. Chem., 272, 21944–21949. [DOI] [PubMed] [Google Scholar]
- Viollet B., Lefrancois-Martinez,A.M., Henrion,A., Kahn,A., Raymondjean,M. and Martinez,A. (1996) Immunochemical characterization and transacting properties of upstream stimulatory factor isoforms. J. Biol. Chem., 271, 1405–1415. [DOI] [PubMed] [Google Scholar]
- Wu M., Hemesath,T.J., Takemoto,C.M., Horstmann,M.A., Wells,A.G., Price,E.R., Fisher,D.Z. and Fisher,D.E. (2000) c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev., 14, 301–312. [PMC free article] [PubMed] [Google Scholar]
- Xu W., Gong,L., Haddad,M.M., Bischof,O., Campisi,J., Yeh,E.T. and Medrano,E.E. (2000) Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp. Cell Res., 255, 135–143. [DOI] [PubMed] [Google Scholar]
- Yavuzer U., Keenan,E., Lowings,P., Vachtenheim,J., Currie,G. and Goding,C.R. (1995) The Microphthalmia gene product interacts with the retinoblastoma protein in vitro and is a target for deregulation of melanocyte-specific transcription. Oncogene, 10, 123–134. [PubMed] [Google Scholar]
- Yasumoto K., Yokoyama,K., Shibata,K., Tomita,Y. and Shibahara,S. (1994) Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol. Cell. Biol., 14, 8058–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos G., Clements,B. and Conner,J. (1999) Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem., 274, 5097–5103. [DOI] [PubMed] [Google Scholar]