Abstract
Background
Medical nutrition therapy is fundamental for managing glycemia and weight in type 1 diabetes, yet dietary guidance specific to this population and relevant subgroups is lacking.
Purpose
We synthesized the interventional literature investigating diet patterns for glycemic and weight management in youth and adults with type 1 diabetes, with attention to interindividual variation that suggests the need for precision approaches. The protocol was prospectively registered (CRD42024519941).
Data Sources
AMED, CINAHL, Cochrane Library, Ovid MEDLINE, Ovid Embase, Google Scholar, and Web of Science Core Collection were searched from January 2011 to June 2024.
Study Selection
Clinical trials ≥4 weeks with ≥10 youth and/or adults diagnosed with type 1 diabetes ≥6 months prior and reporting glycated hemoglobin (HbA1c) or weight were included.
Data Synthesis
Twelve studies with 668 participants were included. Data were pooled by random-effects models for HbA1c and weight. Studies with insufficient data and subgroup differences were narratively synthesized per Synthesis without meta-analysis guidelines. Pooled results of very low to moderate certainty evidence showed no advantage of any particular diet pattern in randomized trials. Very low-quality evidence from single-arm low carbohydrate trials suggested improved HbA1c over time (-0.63% [95% CI, -0.99 to -0.27]; -6.0 mmol/mol [-10.8 to -3.0]). Wide pooled CIs suggested between-person heterogeneity; however, stratification of results by participant characteristics was rarely performed.
Limitations
Limited evidence precluded subgroup analyses to inform precision nutrition approaches.
Conclusion
Randomized trials are needed to confirm the efficacy of specific diets and determine whether precision nutrition therapies optimize glycemia and weight in persons with type 1 diabetes.
Keywords: diet patterns, type 1 diabetes, hemoglobin A1c, weight, meta-analysis, systematic review
The prevalence of macrovascular disease in youth and adults with type 1 diabetes is markedly elevated relative to individuals without diabetes (1). Mitigation of this risk is contingent upon the attainment of glycemic targets in tandem with the prevention of excess weight gain (2) and traditional risk factors such as hypertension. Despite considerable advances in the development of diabetes technologies, a small proportion of youth and adults with type 1 diabetes meet glycemic and weight targets (3-5). Although medical nutrition therapy is a cornerstone of glycemic and weight management, a dearth of literature investigating dietary patterns in persons with type 1 diabetes (6) poses a barrier to achieving clinical targets. Without robust scientific evidence, clinicians may feel ill-equipped to recommend diet approaches to persons with type 1 diabetes (7).
Emerging findings from precision medicine highlight the possibility of augmenting treatment success by tailoring therapies to population subgroups, rather than recommending a one-size-fits-all approach (8, 9). In 2018, the Precision Medicine in Diabetes Initiative was launched by the American Diabetes Association (ADA) and European Association for the Study of Diabetes, with the first consensus report published in 2020 (10). For the type 1 diabetes treatment group, initial efforts focused on the impact of diabetes technologies (11); however, several other areas of interest were identified, with medical nutrition therapy being a top priority. This aligned with a recent focus of the National Institutes of Health (12) on precision nutrition, defined as the stratification of dietary recommendations by population subgroups (8, 13). The most recent systematic review of diet patterns for type 1 and type 2 diabetes management included studies published through the year 2010 and included both observational and experimental evidence (14). Rapid advances in diabetes technology over the past decade have likely augmented the safety and feasibility of diet patterns for type 1 diabetes management, potentially limiting the generalizability of earlier research to contemporary populations. Accordingly, the objective of this systematic review and meta-analysis was to synthesize data from clinical trials published between January 2011 and June 2024 that investigated the effects of diet patterns on glycemia and/or weight in youth and adults with type 1 diabetes, with particular attention to subgroup analyses that indicate the need to tailor treatments according to individual characteristics.
Research Design and Methods
Protocol Development, Data Sources, and Searches
We performed a literature search in accordance with Cochrane recommendations with Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (Table S1, Table S2) (15, 16) and prospectively registered the study protocol with PROSPERO (CRD42024519941, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024519941). Literature searches were conducted in AMED, CINAHL, Cochrane Library, Ovid MEDLINE, Ovid Embase, Google Scholar, and Web of Science Core Collection from January 2011 to June 2024 to identify relevant published literature related to dietary patterns and type 1 diabetes. References of identified studies were manually searched. Table S3 (16) shows full details of the search strategy. Forward and backward citation chasing was performed using CitationChaser.
Study Selection
We included randomized and single-arm clinical trials ≥4 weeks that enrolled participants with type 1 diabetes aged ≥2 years with a diabetes duration of ≥6 months. Trials needed to include ≥10 participants per intervention arm, or a total of 10 participants for crossover trials. One of the coprimary review outcomes of glycated hemoglobin (HbA1c) or weight must have been reported. Full inclusion and exclusion criteria are listed in Table S4 (16).
Abstract and Full-text Screening
Two of 3 reviewers (D.I., L.M.N., and A.A.G.) independently screened articles in a blinded fashion via the Covidence platform. Reviewers met to resolve disagreements.
Data Extraction
E.G.C. and D.I. independently extracted data, which was verified by another reviewer (D.I. or L.M.N.). A standardized template was used for data extraction, which included information about the publication, study design, population, intervention, duration, and outcomes.
Risk of Bias (Quality) Assessment
The Cochrane Risk of Bias 2 (17) tool was used to evaluate the risk of bias for randomized trials in 5 domains. The Risk of Bias in Nonrandomized Studies of Interventions (18) tool was applied to single-arm studies in 7 domains. Results were visualized using the robvis tool (https://www.riskofbias.info/welcome/robvis-visualization-tool).
GRADE Evidence Rating
In accordance with Cochrane guidelines (19), the GRADE approach was used to rate the overall quality of evidence for all studies, including those that could not be included in meta-analyses. Studies were grouped by study design (randomized or single-arm) and diet pattern (general healthful eating, low carbohydrate, Mediterranean, and low fat). Studies were assessed based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. Randomized studies started as high certainty, whereas single-arm studies started as low certainty because of inherent confounding bias (20). Each body of evidence received a final grade of high, moderate, low, or very low certainty. D.I. and L.M.N. independently conducted the GRADE assessments for all included studies and met to reach consensus. L.M.J. and C.G.G. served as adjudicators.
Data Synthesis and Analysis
For the outcomes of HbA1c and weight, we performed meta-analyses using the restricted maximum likelihood (21) to calculate pooled estimates and 95% CIs, stratified by diet pattern and study design. For randomized controlled trials, we compared mean changes in outcomes between the active treatment and control group(s). Results are reported separately for each active treatment-control comparison, including for 1 study that compared a Mediterranean diet and a low-fat diet to a low carbohydrate diet comparator (22). For single-arm nonrandomized studies, postintervention values were compared to baseline. When means or SDs were not reported, we estimated the mean from the median and SD from standard errors or CIs using guidance from the Cochrane Handbook (23). Statistical heterogeneity across studies was assessed using the I2 statistic and Cochrane's Q-statistic (24). All statistical analyses, including the generation of forest plots displaying pooled results, were performed using Stata/BE, version 18.5 (StataCorp, College Station, TX).
When meta-analysis could not be performed because of insufficient data, narrative synthesis was employed following Synthesis without meta-analysis guidelines (25) (Table S5) (16). To assess the certainty of narratively synthesized findings, measures of dispersion reported by individual study authors are described in text alongside mean treatment effects, as available. Given the explicit aim to inform future precision nutrition approaches for optimizing health outcomes in persons with type 1 diabetes, we narratively synthesized heterogeneity in intervention effects within sociodemographic and clinical strata or in association with participant characteristics, when reported. Because of the interdependency of changes in weight on insulin dose, we narratively synthesized results for insulin dose.
Results
Searches
Our search yielded 5131 records (Fig. S1) (16). After duplicates were removed and titles and abstracts screened (Table S6) (16), 53 studies were assessed for eligibility during full-text evaluation. Twelve studies that enrolled 668 participants as part of 11 unique clinical trials—7 randomized and 4 single-arm—met prespecified criteria for inclusion (Table 1). One trial reported the results of 2 study arms separately (26, 27). All studies reported HbA1c. Eight studies, including 5 randomized trials (22, 28-34), reported weight. Studies enrolled participants from ages 2 to 70 and spanned 7 countries.
Table 1.
Clinical trials of dietary patterns included in the systematic review
| Randomized clinical trials | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year published (trial name) | Study design | Trial sites | Length of diet | Randomized (N) | Inclusion criteria | Diabetes management | Dietary pattern(s) studied | Comparator | Primary Outcome | Subgroup Analysis | |
| Age (years) | HbA1c | ||||||||||
| Nansel, 2015 (CHEF) | Single-center randomized controlled trial | USA | 18 months | 136 | 8-16.9 | 6.5%-10% (48-86 mmol/mol) | MDI, pump | Whole plant food with family-based approach | Family-based sessions without dietary advice | HbA1c | N/A |
| Fortin, 2018 (MEDIT) | Single-center randomized trial | Canada | 6 months | 28 | 18-65 | N/A | MDI, pump | Mediterranean diet | Low-fat diet | Waist circumference | Sex |
| Schmidt 2019 | Single-center randomized crossover trial | Denmark | 12-week diet with 12-week washout | 14 | ≥18 | >7% (>53 mmol/mol) | Pump (SAP, PLGS, LGS) | Low-carbohydrate diet (<100 g/day) | High-carbohydrate diet (>250 g/day) | % Time in Range 70-180 mg/dL | N/A |
| Isaksson 2021 | Multicenter randomized controlled trial | Sweden | 12 months | 159 | 20-70 | 7.4%-9.3% (57-78 mmol/mol) | MDI, pump | Food-based approach with low glycemic index, carbohydrate counting | Routine care without diet intervention | HbA1c | N/A |
| Duffus 2022 | Single-center randomized trial | USA | 12 weeks | 39 | 13-21 | 7%-10% (53-86 mmol/mol) | Pump (SAP, HCL), CGM | Low-carbohydrate diet (25% calories from carbohydrates), Standard carbohydrate diet (50% calories from carbs) | Diabetes education without diet intervention | HbA1c | N/A |
| Igudesman 2023 (ACTION) | Multicenter randomized trial | USA | 12 weeks | 51 | 19-30 | <13% (<118 mmol/mol) | MDI, pump | Mediterranean, low-fat diet (<30% calories from fat, <10% calories saturated fat) | Low-carbohydrate diet (<14% calories from carbohydrates) | Change in weight, HbA1c, percent time below range | Sex, race and ethnicity |
| Isaksson 2023 | Multicenter randomized crossover trial | Sweden | 4-week diet with 4-week washout | 54 | ≥18 | ≥7.5% (≥58 mmol/mol) | MDI, pump, CGM | Moderate carbohydrate diet (30% calories from carbohydrates) | Traditional diet (50% calories from carbohydrates) | Change in mean CGM glucose levels (final 14 days on diet) | N/A |
| Single-arm clinical trials | |||||||||||
| Cadario 2012 | Single-arm clinical trial | Italy | 6 months | 104 | <19 | N/A | MDI, pump | Mediterranean diet and structured dietician training | Baseline | Nutritional intake and lipid profiles | Sex, puberty status |
| Paul 2022 | Single-arm clinical trial | Australia | 12 weeks | 23 | ≥18 | N/A | MDI, CGM | Low-carbohydrate diet (<100 g/day) | Baseline | HbA1c | Responders, nonresponders |
| Turton 2023 | Single arm clinical trial | Australia | 12 weeks | 20 | 18-70 years | >7% (53 mmol/mol) | MDI, pump, CGM | Low-carbohydrate diet (25-75 g digestible carbs g/day) | Habitual diet (>150 g/day) after a 4-week control period | HbA1c | N/A |
| Levran 2023 A | Single arm clinical trial | Israel | 6 months | 20 | 12-22 | N/A | MDI, pump, CGM | Low-carbohydrate diet (50-80 g/day) | Baseline | Micro-nutrient intake | N/A |
| Levran 2023 B | Single-arm clinical Trial | Israel | 6 months | 20 | 12-21 | N/A | MDI, pump, CGM | Israeli Mediterranean diet | Baseline | Micronutrient intake | N/A |
Abbreviations: CGM, continuous glucose monitor; HbA1c, glycated hemoglobin; HCL, hybrid closed loop; LGS, low glucose suspend; MDI, multiple daily injections; SAP, sensor-augmented pump; PLGS, predictive low glucose suspend.
Diet Patterns Identified
Among the 12 selected studies, there were 6 low or moderate carbohydrate interventions, 4 Mediterranean (including 1 Israeli Mediterranean), 1 low-fat diet, and 2 healthful eating approaches (whole food plant-based, and food-based approach with low glycemic index). The moderate and low carbohydrate diet interventions involved limiting total carbohydrates to 25% (31) or 30% (35) of total caloric intake, less than 100 g per day (29, 32) or a specific range of daily carbohydrate intake (50-80 g per day (33) or 25 to 75 g of digestible carbohydrates per day (26)). Mediterranean diets encouraged use of olive oil, plant-based foods, whole grains, and fish, and limited red meat and processed foods. One study tested a Mediterranean diet and a hypocaloric low fat diet (<30% calories from fat and <10% calories from saturated fat) against a hypocaloric low carbohydrate diet (22). This study was the only one to explicitly recommend caloric reduction given the aim to promote weight loss in participants with overweight or obesity (22). The healthful diet patterns included a family-based behavioral intervention to increase intake of whole plant foods, including fruit, vegetables, whole grains, legumes, nuts, and seeds (30), and a food-based approach encouraging low glycemic index foods (36). A schematic summarizing the identified studies and certainty of evidence (described below in “GRADE Evidence Rating”) by diet pattern, study design, and study outcome is presented in Fig. 1.
Figure 1.
Schematic of identified studies and summary of the certainty of evidence by diet pattern, study design, and study outcome. Figure was created using bioRender.
Risk of Bias
Among the 7 randomized trials reporting HbA1c, 1 was deemed to be at a low risk of bias (30), 4 had some concerns (22, 28, 31, 36), and 2 were judged to have a high risk of bias—1 due to missing data (29) and the second from potential carryover effects in a randomized crossover design (35) (Fig. S2) (16). Of the randomized trials reporting weight, 3 had some concerns (22, 28, 36) and 2 had a high risk of bias because of missing data (29) and bias in the measurement of the outcome (29, 35). All single-arm trials were found to be at an overall high risk of bias for both HbA1c (Fig. S3) and weight (Fig. S4) (16), as none of the included trials adjusted for potential time-varying confounders in their pre-post analyses. With respect to reporting bias, selective nonpublication was deemed to be unlikely given the large proportion of studies with null results. Accordingly, none of the studies was deemed to have been likely to selectively report results.
GRADE Evidence Rating
Rationale for downgrading in each of the GRADE domains is comprehensively outlined in Table S7 and the associated supplemental text (16). All bodies of evidence were downgraded by 1 point in the indirectness domain because of a lack of generalizability. Study participants tended to have lower mean HbA1c values and did not reflect the sociodemographic characteristics, such as race and ethnicity, of the broader target population of individuals living with type 1 diabetes (3) (Table S8) (16). In contrast, no studies were downgraded for publication bias. All single-arm trials were deemed to have very serious limitations and thus downgraded by 2 levels of certainty because of a serious risk of bias in the confounding domain. Bodies of evidence graded as having moderate certainty included randomized trials of healthful diet patterns for the outcomes of HbA1c and weight, and the randomized trial of a low fat diet for the outcome of weight. Low certainty bodies of evidence included randomized trials of Mediterranean diet patterns for both HbA1c and weight, and randomized trials of low-fat diet patterns for HbA1c. All other bodies of evidence, including all evidence from single-arm trials, were graded as very low certainty.
Meta-analyses and Narrative Syntheses
Impact of dietary patterns on hemoglobin A1c
Healthful diet pattern
Two randomized trials investigated healthful diet patterns in youth (30) and adults (36) with type 1 diabetes, with the latter focusing on low glycemic index (36). Control arms included standard of care with carbohydrate counting (36) and usual care without a dietary intervention (30, 36). The pooled result from the meta-analysis of 136 youth and 133 adults was null, indicating no differential effect of healthful diets on HbA1c relative to control (-0.15% [95% CI, -0.40 to 0.11]; -1.6 mmol/mol [95% CI, -4.4 to 1.2]; Table S9) (16).
Low carbohydrate and moderate carbohydrate diet patterns
Six trials—3 randomized (29, 31, 35) and 3 single-arm (26, 32, 33)—studied the effects of low carbohydrate (15%-25% calories from carbohydrates or 25 to <100 g/day) (26, 29, 31-33) or moderate carbohydrate (30% calories from carbohydrates) (35) diets on HbA1c in youth and adults with type 1 diabetes. Control groups included a standard carbohydrate diet (50% calories from carbohydrate) (31, 35), general diabetes education (31), and a high/traditional carbohydrate diet (>250 g/day or 50% calories from carbohydrate) (29, 35). Of the 3 randomized trials investigating low carbohydrate or moderate carbohydrate diets, 2 studies representing 56 participants with type 1 diabetes had available HbA1c data for meta-analysis (29, 31). Pooled results indicated no differential effects of a low carbohydrate diet on HbA1c relative to a high carbohydrate diet (29), a standard carbohydrate diet (31), or general diabetes education (31) over 4.4 months (0.32% [95% CI, -0.07 to 0.72]; 3.5 mmol/mol [95% CI, -0.8 to 7.9]; Fig. 2A). Because of insufficient data, the randomized crossover trial comparing a moderate carbohydrate diet to a traditional diet in 50 adults with type 1 diabetes was not included in pooled analysis but similarly found no difference in HbA1c change between study arms over 4.4 months (-0.10% [95% CI, -0.25 to 0.05]; -1.1 mmol/mol [95% CI, -2.7 to 0.5]) (35). However, time in range (70-180 mg/dL) measured using continuous glucose monitoring (CGM) was nearly 5% points higher during the final 2 weeks of the moderate carbohydrate diet relative to the traditional diet (35). A separate meta-analysis of single-arm trials estimated the change in HbA1c following the 3 single-arm low carbohydrate diet interventions (26, 32, 33) among 19 youth and 38 adults with type 1 diabetes. Pooled results demonstrated an HbA1c reduction of -0.63% (95% CI, -0.99 to -0.27%) (ie, -6.9 mmol/mol [95% CI, -10.8 to -3.0]) over 4.9 ± 0.96 months of a low carbohydrate diet (Fig. 2B; Table S10) (16).
Figure 2.
Forest plots showing pooled estimates and 95% CIs from random effects regression models for randomized (A) and single-arm clinical trials (B) reporting HbA1c. The suffixes “_A” and “_B” were used to report separate effect estimates when more than 1 control arm was included. τ2 (ie, Tau), I2, H2, and θi = θj (ie, the Cochrane Q test based on a chi-square distribution) are tests of heterogeneity; statistical significance (P < .05) would indicate that effects varied across studies, whereas P ≥ .05 indicates failure to reject the null that there is a lack of heterogeneity across studies. The test of θ indicates whether the pooled result for each diet pattern and study type is different from the null. Numbers in parentheses indicate the degrees of freedom representing n-1 studies. The test of group differences (Qb) indicates whether the pooled results for randomized trials vary by diet pattern. Values for Nansel et al estimated from visual presentation of results. HbA1c values for individual studies included in meta-analyses are reported in mmol/mol in Tables S9 and 10 (16). Values were converted from % to mmol/mol using ngsp.org/convert1.asp.
Mediterranean diet pattern
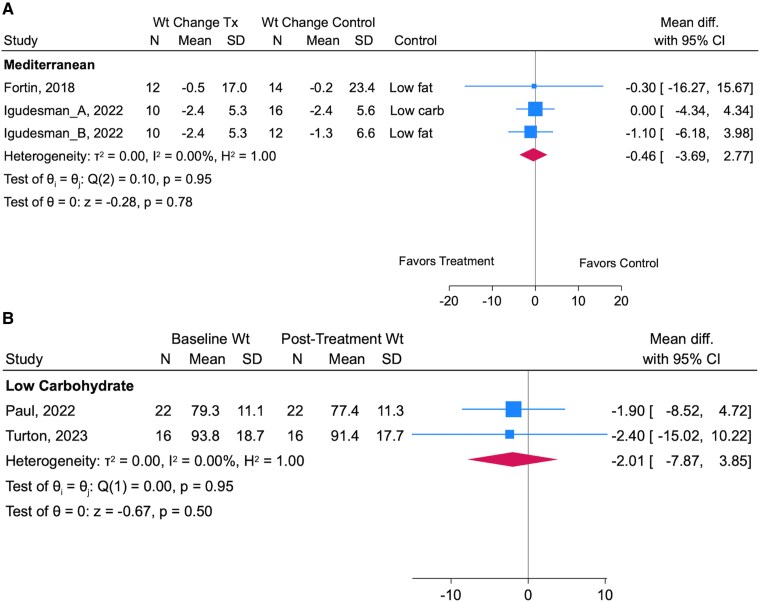
Two randomized (22, 28) and 2 single-arm trials (27, 34) investigated the effects of a eucaloric Mediterranean diet pattern on HbA1c. A meta-analysis of the randomized trials involving 64 adults with type 1 diabetes (duration 4.5 ± 2.1 months) found that a Mediterranean diet without caloric restriction had no differential effects on HbA1c relative to a hypocaloric low-carbohydrate diet (15%-20% calories from carbohydrates) (22), a hypocaloric moderate low-fat diet, and a eucaloric low-fat diet (both low fat diets contained <30% calories from fat) (22, 28) (0.26% [95% CI, -0.40 to 0.92]; 2.8 mmol/mol [95% CI, -4.4 to 10.1]). Supporting the pooled results of the randomized trials in adults, a meta-analysis of the 2 single-arm trials implementing a eucaloric Mediterranean diet (duration 7.4 ± 1.9 months) in 123 youth with type 1 diabetes indicated no change in HbA1c pre- and posttreatment (0.12% [95% CI, -0.39 to 0.15]; 1.3 mmol/mol [95% CI, -4.3 to 1.6]).
Comparison of dietary patterns
The test for group differences across randomized trials indicated no differences in the effects of healthful, low carbohydrate, or Mediterranean diet patterns on HbA1c (P = 0.11). However, there was a statistically significant difference across diet patterns among single-arm interventions (P = .03), reflecting a pooled HbA1c reduction following low carbohydrate but not Mediterranean diets (no healthful diet patterns were included).
Impact of dietary patterns on weight
Healthful diet pattern
Insufficient weight data from 2 randomized trials of healthful diet patterns (30, 36) rendered a meta-analysis of this diet pattern infeasible. Neither study found a differential effect of a healthful diet intervention relative to control (carbohydrate counting (36), routine care without a diet intervention (36), or attentional control matched for frequency of contacts (30)) on weight change over 12 (36) or 18 months (30) among 136 youth (30) and 133 adults (36) with type 1 diabetes.
Low carbohydrate diet pattern
Two randomized crossover trials of low or moderate carbohydrate diets reported weight data (29, 35); however, a meta-analysis was not feasible as the moderate carbohydrate intervention did not report sufficient preintervention data (35). The moderate carbohydrate diet had no differential effect on weight relative to a traditional diet (50% energy from carbohydrates) in 50 adults with type 1 diabetes (0.0 kg difference in weight change [95% CI, -0.4 to 0.4 kg]). The other randomized trial lasting 3 months showed a ∼4 kg greater weight loss in 14 adults following a low carbohydrate compared to a high carbohydrate diet (29). A randomized low carbohydrate diet intervention found no difference in body mass index (BMI) change among 33 adolescents and young adults randomized to 4 months of a low carbohydrate diet, a standard carbohydrate diet, or general diabetes education, although the reported change in carbohydrate consumption was small, with only 1 participant adhering to recommended carbohydrate intake (31). A meta-analysis of 2 low carbohydrate single-arm trials involving 38 adults with type 1 diabetes corroborated the findings from the narratively synthesized randomized trials, showing no statistically significant change in weight over 4.3 ± 0.0 months (-2.01 kg [95% CI, -7.87 to 3.85]; Fig. 3A). However, Levran et al reported a -0.13 (interquartile range -0.29 to -0.02) reduction in BMI z-score in 19 youth and young adults with type 1 diabetes following a 6-month, single-arm, low carbohydrate diet intervention (26).
Figure 3.
Forest plots showing pooled point estimates and 95% CIs from random effects regression models for randomized (A) and single-arm clinical trials (B) reporting weight (kg). The suffixes “_A” and “_B” were used to report separate effect estimates when more than 1 control arm was included. τ2 (ie, Tau), I2, H2, and θi = θj (ie, the Cochrane Q test based on a chi-square distribution) are tests of heterogeneity; statistical significance (P < .05) would indicate that effects varied across studies, whereas P ≥ .05 indicates failure to reject the null that there is a lack of heterogeneity across studies. The test of θ indicates whether the pooled result for each diet pattern and study type is different from the null. Numbers in parentheses indicate the degrees of freedom representing n-1 studies.
Mediterranean diet pattern
The pooled results of a meta-analysis describing the effects of 2 randomized interventions of Mediterranean diets on weight change in 64 adults showed no difference relative to a eucaloric low fat diet (28), a hypocaloric low carbohydrate diet (22), or a hypocaloric low-fat diet (22) (-0.46 kg [95% CI, -3.69 to 2.77 kg]; Fig. 3B). Supporting these results, two single-arm Mediterranean diet trials found no change in BMI z-score among youth and/or young adults with type 1 diabetes over 6 months (26, 34).
Impact of low fat dietary pattern on HbA1c and weight
One study involving 12 participants randomized to 3 months of a hypocaloric low fat diet found no differential change in HbA1c or weight relative to a hypocaloric low carbohydrate diet (n = 16) (22).
Insulin dose
Most studies measured changes in insulin dose via self-report (33, 36) or did not disclose the method of measurement (26, 27, 32, 34, 35). One healthful diet study including 148 adults over 12 months found no difference in total daily insulin relative to carbohydrate counting or usual care (36). One (29) of 3 randomized trials (29, 31, 35) and all 3 single-arm trials (26, 32, 33) of low carbohydrate or moderate carbohydrate diets found 12-week reductions in bolus insulin (29, 32) or in total daily dose over 3 to 6 months in 20 adolescents (26) and 20 adults (Table S11, Table S12) (16). Two of these studies found no change in basal insulin relative to a high carbohydrate diet control (29) or pre-post diet (32). Two single-arm Mediterranean diet studies reported changes in total daily insulin dose in adolescents with type 1 diabetes over 6 months, with 1 finding a 5-unit increase in total daily dose (34), and the other a trend toward a decrease in the insulin to weight ratio (27).
Heterogeneity
I 2 values were 0 for all pooled analyses, indicating a lack of heterogeneity in results across trials included in meta-analyses. A select number of studies reported within-study variation in outcomes, which can only be summarized using narrative synthesis given insufficient data on outcomes of interest. One single-arm trial investigating a low-carbohydrate diet in adults with type 1 diabetes compared the characteristics of responders (n = 10) and nonresponders (n = 12), defined as a ≥10% or <10% relative reduction in HbA1c, respectively (32). Preintervention HbA1c was ~2 percentage points higher in responders, whereas diet adherence and weight loss were not related to treatment response (32).
Two Mediterranean diet interventions reported subgroup analyses by sex (28, 34). Fortin et al found no diet by sex interactions for HbA1c or weight when comparing the effects of a Mediterranean diet with a low fat diet in adults with type 1 diabetes (28). The single-arm Mediterranean diet intervention in youth with type 1 diabetes found that HbA1c decreased by 0.2% (2.2 mmol/mol) over 6 months among pubertal boys, with no change appreciated in prepubertal boys or girls regardless of puberty status (34). In addition, BMI-SD score decreased by ~0.2 units among prepubertal boys and in youth with overweight or obesity but increased among prepubertal girls (34).
Igudesman et al reported participant characteristics associated with weight and glycemic outcomes irrespective of whether participants were randomized to a eucaloric Mediterranean diet, a hypocaloric low-carbohydrate diet, or a hypocaloric low-fat diet (22). The authors noted that non-Hispanic White participants had a 0.28 greater percentage point (95% CI, 0.10-1.23) HbA1c improvement than their counterparts from any other racial and ethnic group who comprised ~34% of the sample, and that men had a 0.62 greater HbA1c percentage point reduction (95% CI, 0.02-1.2; ie, 6.8 mmol/mol [95% CI, 0.2-13.1]) than women, after correction for baseline HbA1c (22). Women also lost 2.6 (95% CI, 0.41-4.9) fewer kilograms of weight than men, even after adjusting for baseline weight.
Conclusions
Although medical nutrition therapy is a crucial aspect of diabetes management, the present study highlights the dearth of evidence supporting specific nutrition guidelines for persons with type 1 diabetes in whom unique physiological and behavioral considerations (37) warrant dedicated nutrition research. In this systematic review and meta-analysis, we evaluated 12 studies that addressed the impact of dietary interventions on glycemia and weight in individuals with type 1 diabetes. Our pooled meta-analyses of randomized trials suggested no added benefit of any particular diet pattern on glycemia or weight relative to control interventions. Imprecision was graded as serious, raising the possibility that certain population subgroups may benefit more from specific diets than others. Changes in insulin dose were documented in some but not all studies and often measured via self-report; these data are critical for interpretation and should be rigorously measured in future studies. Given the low certainty of most of the bodies of evidence, these findings are not sufficient to guide changes in clinical decision-making. Rather, existing guidelines (38) that emphasize individualization of diet therapies should continue to be followed until stronger evidence supporting specific diet approaches for use in type 1 diabetes becomes available.
Conversely, pooled results from single-arm interventions suggest reduced HbA1c with a low-carbohydrate diet, albeit from a very low certainty body of evidence. The major caveat noted is that, in addition to lack of a control group, there was no adjustment made for time-varying confounders in any of the included trials, making the probability of confounding bias by changes in physical activity, caloric intake, and other variables likely. At least 1 single-arm low-carbohydrate trial reported reductions in caloric intake (5) over time, which could explain changes in weight, insulin doses, and HbA1c independent of diet composition. Rigorously conducted randomized trials are needed to confirm the safety and efficacy of low carbohydrate diets for managing glycemia and weight in persons with type 1 diabetes. Recognizing these limitations and without assessment of a low carbohydrate intervention in children younger than age 12 years, cautionary guidance has been issued by the International Society for Pediatric and Adolescent Diabetes (ISPAD) and the American Academy of Pediatrics (39, 40) against adopting this diet in children with type 1 diabetes given a lack of long-term safety and efficacy data.
Meta-analyses of low certainty randomized trials testing the effects of generally healthful diet patterns or Mediterranean diets failed to show differential effects on HbA1c or weight. A meta-analysis of very low certainty single-arm Mediterranean diets showed analogous results for HbA1c. Nonetheless, wide 95% CIs indicated the possibility of clinically meaningful heterogeneity that should be explored in future dietary trials of sufficient size and diversity to permit subgroup analysis. Despite lacking sufficient data to perform meta-analyses in sample subgroups to inform future precision nutrition guidelines, here, we present the first narrative synthesis of evidence in support of specific diets according to sex, weight status, pubertal status, and HbA1c, but the low quality of evidence and small number of studies precludes making tailored recommendations. Much of this preliminary evidence is derived from single-arm trials with a high risk of bias or from randomized trials with modest sample sizes, so studies with the explicit aim to study heterogeneity must be conducted.
Precision Nutrition: Future Integration With Published Society Guidelines
The ADA and ISPAD recommend individualized medical nutrition therapy as an essential aspect of treatment for individuals living with type 1 diabetes. The ADA's clinical practice guidelines (ie, Standards of Care in Diabetes) emphasize whole diet patterns that represent dietary intake more globally, as opposed to a reductionist view of macro- and micronutrients (38). The ADA's Standards of Care also underscore the importance of individualizing eating patterns to incorporate a variety of foods that reflect personal and cultural preferences (38), given that there is no one-size-fits-all eating pattern that works for all persons with diabetes. Thus, the existing guidelines emphasize nutrient quality independent of macronutrient intake, as well as the adequacy (eg, nonstarchy vegetables, whole grains, whole fruits, legumes, lean proteins) and moderation (eg, red meat, sugar-sweetened beverages, refined grains, alcohol, foods high in sodium) of specific food groups. Nonetheless, evidence for stratifying recommendations to optimize health within population subgroups is lacking (41, 42). The scant evidence base and lack of clinical practice guidelines addressing diet approaches for managing overweight and obesity in type 1 diabetes (37) warrants particular attention. The ADA Standards of Medical Care focus exclusively on weight in the context of type 2 diabetes in the chapter titled Obesity and Weight Management (43). Our findings highlight that future research is urgently needed to rigorously examine the impact of dietary interventions on weight in those with type 1 diabetes, with particular attention to potential subgroups of interest. In the future, evaluating the effects of dietary interventions on clinical outcomes within specific subgroups, including by age (eg, in youth and older adults), type of insulin regimen, and weight status, will provide insight into the unique behavioral and metabolic factors that influence how diet patterns impact health outcomes in subgroups of persons with type 1 diabetes. Artificial intelligence (AI) algorithms can help to determine whether other characteristics such as gut microbiome composition, genotype, and baseline diet impact behavioral or physiological responses to different diets (8).
Precision nutrition approaches must be vigilant not to widen disparities. This will require the purposive sampling of groups that have been historically underrepresented in research to prevent algorithm bias, which is a consideration for AI algorithms that are becoming commonplace in precision nutrition research. Algorithm bias may lead to suboptimal performance of AI-enabled clinical practice guidelines in underrepresented subgroups (44). Furthermore, it is critical that researchers are cognizant of enrolling older adults with type 1 diabetes. A recent systematic review and summary of clinical practice guidelines addressing nutritional status, dietary intake, and eating behaviors in older adults with type 1 diabetes revealed substantial knowledge gaps in a group already at high risk for suboptimal health outcomes (45). Therefore, several factors need to be considered in trial design, including recruitment across the age spectrum of people with type 1 diabetes from diverse demographic backgrounds, regardless of baseline glycemia as those with an HbA1c substantially above target stand to receive the most benefit. Trial diversity—which was limited in the included studies—will ensure recommended dietary strategies take into account cultural food preferences and affordability and are (45) equitable and sustainable. One previous report addressed the opportunity for healthful eating with food costs similar to that of less healthful diets (46).
Strengths and Limitations
Strengths
Strengths of this work include a clear, focused research question, quantitative syntheses from stratified meta-analyses, a comprehensive literature search with a transparent, rigorous methodology, and detailed risk of bias and quality assessments. Studies enrolled participants across the lifespan and numerous geographic regions, which facilitates generalizability to a variety of target populations. All studies but one 4-week intervention (35) were 3 months in duration or greater, so nearly all interventions were sufficiently long to observe changes in HbA1c and weight.
Limitations
Our review focused on assignment to an intervention rather than diet adherence. Most studies enrolled relatively few (<50) participants and lasted 6 months or less, making it difficult to evaluate generalizability and longer-term diet sustainability. Some studies were not included in the meta-analysis because of missing data and were instead narratively synthesized, which is less objective than quantitative synthesis even when done systematically (25). Although meta-analyses indicated a lack of heterogeneity between studies, variation in the control groups studied, the populations sampled, and intervention duration made pooled results challenging to interpret. Furthermore, although there was low statistical heterogeneity in our meta-analysis, we noted high imprecision as indicated by the wide CIs. Thus, while there may not be variation in effect sizes between studies (most did not show effects of diet interventions on HbA1c or weight), there is a considerable degree of variability across participants, indicating potential heterogeneity in response. Exclusion of manuscripts published in languages other than English during the screening process may have introduced publication bias and may limit generalizability. Due to only 1 study being available for evaluation, the Inconsistency criterion for GRADE could not be assessed for randomized studies of low carbohydrate diets studying weight, nor for randomized trials of low fat interventions studying HbA1c and weight. Only 1 study explicitly focused on weight loss (22), which may in part explain minimal changes in weight in most other trials.
Racial and Ethnic Diversity
Five of the included studies were conducted outside of the United States (ie, in Israel, Sweden, and Australia) and did not report racial or ethnic data; however, practices for standardizing and reporting racial and ethnic data vary widely across countries, often because of differing legal, cultural, and historical contexts that influence data collection policies and norms. Of the studies that reported race and ethnicity, all studies but 1 (22) enrolled 88% to 100% of participants reporting a White race. These findings highlight the need to include more diverse study participants in future research to ensure equitable representation. Further, Mediterranean dietary patterns were studied in geographic regions in which these diets are common or culturally preferred (eg, Italy, Israel), facilitating access and adherence to the diet. Implementation of any diet pattern requires individualized, culturally tailored guidance to optimize adherence and acceptability. For example, the Mediterranean diet has been adapted to populations residing in the Southeastern United States who are racially and ethnically diverse to promote adherence to this diet pattern (46).
Incorporation of Diabetes Technology
Few of the studies reported CGM-derived outcomes, limiting our ability to assess the impact of diet on important clinical outcomes, including time in range, the frequency and duration of hypoglycemia, and overall glycemic variability, which are vital complements to HbA1c. This underscores the need for future dietary intervention studies to incorporate CGM metrics using standardized reporting frameworks (47). Among the studies included in our review, 6 reported CGM-derived outcomes (22, 27, 31-33, 35). The limited availability of CGM data may reflect the timing of the studies, some of which enrolled participants as early as 2007 when CGM technology was not yet widely accessible, or the geographic settings, in which routine CGM use may not have been integrated into standard diabetes care. This highlights the importance of incorporating diabetes technology into future nutritional research to better reflect real-world diabetes management and to evaluate how dietary interventions interact with evolving treatment modalities. While most studies included participants using an insulin pump, CGM, or both, only 2 studies included those using low-glucose suspend, predictive low-glucose suspend, or hybrid closed-loop technology (29, 31). This may limit generalizability given the potential for automated insulin delivery systems to liberalize dietary intake (eg, by reducing fear of hypoglycemia) and modify the efficacy of medical nutrition therapy—gaps in knowledge that future studies should address. Studies that reported changes in insulin dose often did not disclose measurement methods or collected this information via self-report. Insulin dose data were not meta-analyzed. Changes in background and adjunctive therapies over time may have confounded the results of single-arm trials. Larger, long-term studies with deep participant phenotyping are needed to evaluate the feasibility and sustainability of AI-enabled diet recommendations and address longer-term impacts of nutritional intake on diabetes-related complications.
Future Directions and Implications for Clinical Practice
While optimizing glycemia and weight is critical for cardiovascular disease mitigation, future reviews should evaluate the effects of diet patterns on comprehensive cardiovascular risk factors including hypertension and dyslipidemia as advocated by the ADA (38). CGM data should be collected in all future diet studies enrolling participants with type 1 diabetes to evaluate more granular effects of diet patterns on glycemia as well as the safety of diets—particularly those targeting weight loss through caloric restriction. Future studies should also investigate the effects of diet patterns on enteroendocrine hormone responses that impact postprandial glucose, gastric emptying, insulin needs, appetite, and body adiposity. Other deep phenotypic measures of human physiology and the gut microbiota should be investigated for their usefulness in training AI algorithms to optimize dietary recommendations (48). In addition to physiological measures, psychosocial outcomes are critical for preserving quality of life in type 1 diabetes, and experimental diets have been shown to have discordant effects on psychosocial outcomes and clinical endpoints in persons with type 1 diabetes (49). Future research is needed to guide individualized dietary approaches for subgroups with specific comorbidities (eg, chronic kidney disease, celiac disease), and those taking adjunctive therapies (eg, GLP-1 agonists, SGLT2-inhibitors). Weight loss studies should strive to measure body composition as minimizing lean body mass loss is important, particularly in older adult populations.
Controlled feeding studies such as those employed by the Nutrition for Precision Health Initiative can elucidate physiological responses to diet interventions independent of diet adherence and determine subpopulations that benefit more from specific diet approaches (50). A recent randomized crossover controlled feeding study published after we concluded the present review found that 5 weeks of outpatient low carbohydrate feeding (94.5 ± 4.7 g/day) increased time in target glucose range by ~3 percentage points relative to a recommended carbohydrate diet (191 ± 19.2 g/day) in 34 youth with type 1 diabetes (50). Even with this level of dietary control, the 95% CI for change in time in range spanned 1 to 5 points, indicating the potential for clinically significant heterogeneity between participants. Rigorous assessments of diet adherence with food photography (51), doubly labeled water (52), and novel dietary biomarkers should be employed in future behavioral trials studying the feasibility of diet interventions for type 1 diabetes management (NCT05621863).
Our results support the emphasis of ADA and ISPAD guidelines on personalizing diet patterns to individual goals, preferences, cultures, and physiology. Further research is needed to determine whether nutrition guidelines should be tailored to specific population subgroups to reduce cardiovascular disease burden in persons with type 1 diabetes. Larger studies of longer duration and higher quality are needed to develop the evidence base that will guide AI-enabled precision health approaches, which hold the promise to optimize the clinical management of all individuals living with type 1 diabetes.
Acknowledgments
The authors thank Thomas Mead for his peer review of the search strategy.
Abbreviations
- ADA
American Diabetes Association
- AI
artificial intelligence
- BMI
body mass index
- CGM
continuous glucose monitoring
- HbA1c
glycated hemoglobin
- ISPAD
International Society for Pediatric and Adolescent Diabetes
Contributor Information
Daria Igudesman, Translational Research Institute, AdventHealth, Orlando, FL 32804, USA.
Laura M Nally, Department of Pediatrics, Yale School of Medicine, New Haven, CT 06511, USA.
Alyssa A Grimshaw, Harvey Cushing/John Hay Whitney Medical Library, Yale University, New Haven, CT 06510, USA.
Craig G Gunderson, Department of Pediatrics, Yale School of Medicine, New Haven, CT 06511, USA.
Elizabeth G Considine, Department of Pediatrics, Yale School of Medicine, New Haven, CT 06511, USA.
Laura M Jacobsen, Departments of Pediatrics and Pathology, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Mustafa Tosur, Texas Children's Hospital, Baylor College of Medicine, Houston, TX 77030, USA; USDA/ARS, Children's Nutrition Research Center, Houston, TX 77030, USA.
Peter A Gottlieb, Barbara Davis Center for Diabetes, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Irl B Hirsch, Division of Metabolism, Endocrinology, and Nutrition, Department of Medicine, University of Washington School of Medicine, Seattle, WA 98109, USA.
Lori M Laffel, Joslin Diabetes Center, Harvard Medical School, Boston, MA 02215, USA.
Jennifer L Sherr, Department of Pediatrics, Yale School of Medicine, New Haven, CT 06511, USA.
Chantal Mathieu, Department of Endocrinology, University Leuven, Leuven 3000, Belgium.
Richard E Pratley, Translational Research Institute, AdventHealth, Orlando, FL 32804, USA.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23 DK128560.
Author Contributions
All authors contributed to the study concept. D.I., L.M.N., and A.A.G. designed the search strategy, and A.A.G. performed the search. D.I. and L.M.N. take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. D.I. and L.M.N. reviewed and analyzed the data, drafted the manuscript, prepared tables and figures, and finalized the manuscript. A.A.G. and C.G.G. contributed to the initial manuscript. C.G.G. performed statistical analyses. All authors reviewed and approved the final manuscript.
Disclosures
L.M.N. receives research support from the National Institutes of Health and is a consultant for Medtronic, WebMD, and Calm. J.L.S. serves, or has served, on advisory panels for Bigfoot Biomedical, Cecelia Health, Insulet Corporation, Mannkind, Medtronic Diabetes, StartUp Health Diabetes Moonshot, and Vertex. She has served as a consultant to Abbott Diabetes, Bigfoot Biomedical, Insulet, Medtronic Diabetes, and Zealand. Yale School of Medicine has received research support for J.L.S. from Abbott Diabetes, JAEB Center for Health Research, JDRF, Insulet, Medtronic, NIH, and Prevention Bio. C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, Eli Lilly and Company, Novartis, Dexcom, Boehringer Ingelheim, Bayer, Roche, Abbott, Medtronic, Insulet, Biomea Fusion, SAB Bio, and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for C.M. from Medtronic, Novo Nordisk, and Sanofi; C.M. serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Eli Lilly and Company, Medtronic, Dexcom, Insulet, Abbott, Vertex, and Boehringer Ingelheim. Financial compensation for these activities has been received by KU Leuven. I.B.H. reports research funding from Tandem and Dexcom; and consulting fees from Abbott, Roche, GWave, and Vertex. L.M.L. reports consulting for Dexcom, Boehringer Ingelheim, Medtronic, Provention Bio, Sanofi, Medtronic, Sequel MedTech, Vertex, and Tandem Diabetes. P.A.G. has served as an advisor to Viacyte/Vertex, Imcyse, JDRF T1D Fund, and GentiBio; has received research support from Novo Nordisk, Imcyse, Novartis, Mercia/Nova, Provention Bio, ActoBio Therapeutics, Helmsley Foundation, JDRF, and NIH; and is a cofounder, Chief Medical Officer, and shareholder of ImmunoMolecular Therapeutics, Inc. M.T. served as an advisory board member for Provention Bio in 2020 and 2021. R.E.P. has received the following (through 12/31/2023 directed to his institution; as of 1/1/2024 directed to R.E.P. personally): speaker fees from Lilly, Merck and Novo Nordisk; consulting fees from Bayer AG, Bayer HealthCare Pharmaceuticals, Inc., Corcept Therapeutics Incorporated, Dexcom, Endogenex, Inc., Gasherbrum Bio, Inc., Genprex, Getz Pharma, Hanmi Pharmaceutical Co., Hengrui (USA) Ltd., Intas Pharmaceuticals, Inc., Lilly, Merck, Novo Nordisk, Pfizer, Rivus Pharmaceuticals Inc., Sanofi, and Sun Pharmaceutical Industries; and grants from Biomea Fusion, Carmot Therapeutics, Dompe, Endogenex, Inc., Fractyl, Lilly, Novo Nordisk, and Sanofi. All other authors report no conflict of interest.
Data Availability
Template data collection forms; data extracted from included studies; and analytic code will be made available upon reasonable request.
PROSPERO Registration
PROSPERO registration code: CRD42024519941.
References
- 1. de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843‐2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Purnell JQ, Braffett BH, Zinman B, et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Care. 2017;40(12):1756‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malik FS, Sauder KA, Isom S, et al. Trends in glycemic control among youth and young adults with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2022;45(2):285‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wallace AS, Chang AR, Shin JI, et al. Obesity and chronic kidney disease in US adults with type 1 and type 2 diabetes Mellitus. J Clin Endocrinol Metab. 2022;107(5):1247‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim G, Divers J, Fino NF, et al. Trends in prevalence of cardiovascular risk factors from 2002 to 2012 among youth early in the course of type 1 and type 2 diabetes. The SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2019;20(6):693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campos A, Gutierrez RR, Galindo RJ, McCoy RG, Hurtado Andrade MD. Managing obesity in adults with type 1 diabetes. Diabetes Res Clin Pract. 2025;220:111983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang M, Jeon Y, Echouffo-Tcheugui JB, Selvin E. Prevalence and management of obesity in U.S. adults with type 1 diabetes. Ann Intern Med. 2023;176(3):427‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079‐1094. [DOI] [PubMed] [Google Scholar]
- 9. Tobias DK, Merino J, Ahmad A, et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat Med. 2023;29(10):2438‐2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(7):1617‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobsen LM, Sherr JL, Considine E, et al. Utility and precision evidence of technology in the treatment of type 1 diabetes: a systematic review. Commun Med (Lond). 2023;3(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institutes of Health . Nutrition for Precision Health, Powered by the All of Us Research Program; 2024. Accessed March 4, 2025. https://commonfund.nih.gov/nutritionforprecisionhealth
- 13. Zeisel SH. Precision (personalized) nutrition: understanding metabolic heterogeneity. Annu Rev Food Sci Technol. 2020;11(1):71‐92. [DOI] [PubMed] [Google Scholar]
- 14. Wheeler ML, Dunbar SA, Jaacks LM, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012;35(2):434‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igudesman D. Supplemental Data—Dietary patterns for weight and glycemic management in persons with type 1 diabetes: a precision nutrition-focused systematic review and meta-analysis of clinical trials: figshare; 2025. [DOI] [PMC free article] [PubMed]
- 17. Sterne JAC, Savovic J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 18. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raudenbush SW. Analyzing effect sizes: random-effects models. The handbook of research synthesis and meta-analysis. 2009;2:295‐316. [Google Scholar]
- 22. Igudesman D, Crandell J, Corbin KD, et al. Weight management in young adults with type 1 diabetes: the advancing care for type 1 diabetes and obesity network sequential multiple assignment randomized trial pilot results. Diabetes Obes Metab. 2023;25(3):688‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JPT, Thomas J, Chandler TJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 1st ed. John Wiley & Sons, Ltd; 2019:143‐176. [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levran N, Levek N, Sher B, et al. The impact of a low-carbohydrate diet on micronutrient intake and Status in adolescents with type 1 diabetes. Nutrients. 2023;15(6):1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levran N, Levek N, Sher B, et al. The Mediterranean diet for adolescents with type 1 diabetes: a prospective interventional study. Nutrients. 2023;15(21):4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fortin A, Rabasa-Lhoret R, Lemieux S, Labonte ME, Gingras V. Comparison of a Mediterranean to a low-fat diet intervention in adults with type 1 diabetes and metabolic syndrome: a 6-month randomized trial. Nutr Metab Cardiovasc Dis. 2018;28(12):1275‐1284. [DOI] [PubMed] [Google Scholar]
- 29. Schmidt S, Christensen MB, Serifovski N, et al. Low versus high carbohydrate diet in type 1 diabetes: a 12-week randomized open-label crossover study. Diabetes Obes Metab. 2019;21(7):1680‐1688. [DOI] [PubMed] [Google Scholar]
- 30. Nansel TR, Laffel LM, Haynie DL, et al. Improving dietary quality in youth with type 1 diabetes: randomized clinical trial of a family-based behavioral intervention. Int J Behav Nutr Phys Act. 2015;12(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duffus SH, Slaughter JC, Cooley W, et al. A pragmatic low carbohydrate diet intervention changes neither carbohydrate consumption nor glycemia in adolescents and young adults with type 1 diabetes in a randomized trial. Pediatr Diabetes. 2022;23(7):1088‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paul J, Jani R, Jones M, Davoren P, Knight-Agarwal C. Association between a low-carbohydrate diet, glycemic control, and quality of life in Australian adults living with type 1 diabetes: a pilot study. Endocr Pract. 2022;28(11):1125‐1131. [DOI] [PubMed] [Google Scholar]
- 33. Turton JL, Brinkworth GD, Parker HM, et al. Effects of a low-carbohydrate diet in adults with type 1 diabetes management: a single arm non-randomised clinical trial. PLoS One. 2023;18(7):e0288440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cadario F, Prodam F, Pasqualicchio S, et al. Lipid profile and nutritional intake in children and adolescents with type 1 diabetes improve after a structured dietician training to a Mediterranean-style diet. J Endocrinol Invest. 2012;35(2):160‐168. [DOI] [PubMed] [Google Scholar]
- 35. Isaksson SS, Olafsdottir AF, Ivarsson S, et al. The effect of carbohydrate intake on glycaemic control in individuals with type 1 diabetes: a randomised, open-label, crossover trial. Lancet Reg Health Eur. 2024;37:100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Isaksson SS, Bacos MB, Eliasson B, et al. Effects of nutrition education using a food-based approach, carbohydrate counting or routine care in type 1 diabetes: 12 months prospective randomized trial. BMJ Open Diabetes Res Care. 2021;9(1):e001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corbin KD, Driscoll KA, Pratley RE, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev. 2018;39(5):629‐663. [DOI] [PubMed] [Google Scholar]
- 38. American Diabetes Association Professional Practice Committee . 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2025. Diabetes Care. 2025;48(Suppl_1):S86‐S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Annan SF, Higgins LA, Jelleryd E, et al. ISPAD clinical practice consensus guidelines 2022: nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2022;23(8):1297‐1321. [DOI] [PubMed] [Google Scholar]
- 40. Neyman A, Hannon TS, Committee On N. Low-carbohydrate diets in children and adolescents with or at risk for diabetes. Pediatrics. 2023;152(4):e2023063755. [DOI] [PubMed] [Google Scholar]
- 41. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46(2):578‐588. [DOI] [PubMed] [Google Scholar]
- 42. Franco RZ, Fallaize R, Weech M, Hwang F, Lovegrove JA. Effectiveness of web-based personalized nutrition advice for adults using the eNutri web app: evidence from the EatWellUK randomized controlled trial. J Med Internet Res. 2022;24(4):e29088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Diabetes Association Professional Practice Committee . 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2025. Diabetes Care. 2025;48(Suppl_1):S167‐S180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nazer LH, Zatarah R, Waldrip S, et al. Bias in artificial intelligence algorithms and recommendations for mitigation. PLOS Digit Health. 2023;2(6):e0000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarteau A C, Ercolino G, Muthukkumar R, Fruik A, Mayer-Davis EJ, Kahkoska AR. Nutritional Status, dietary intake, and nutrition-related interventions among older adults with type 1 diabetes: a systematic review and call for more evidence toward clinical guidelines. Diabetes Care. 2024;47(9):1468‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Embree GG, Samuel-Hodge CD, Johnston LF, et al. Successful long-term weight loss among participants with diabetes receiving an intervention promoting an adapted Mediterranean-style dietary pattern: the heart healthy lenoir project. BMJ Open Diabetes Res Care. 2017;5(1):e000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2023;11(1):42‐57. [DOI] [PubMed] [Google Scholar]
- 48. Shilo S, Godneva A, Rachmiel M, et al. Prediction of personal glycemic responses to food for individuals with type 1 diabetes through integration of clinical and microbial data. Diabetes Care. 2022;45(3):502‐511. [DOI] [PubMed] [Google Scholar]
- 49. Paul J, Jani R, Thorning S, Obucina M, Davoren P, Knight-Agarwal C. Low carbohydrate diets, glycaemic control, enablers, and barriers in the management of type 1 diabetes: a mixed methods systematic review. Diabetol Metab Syndr. 2024;16(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neuman V, Plachy L, Drnkova L, et al. Low-carbohydrate diet in children and young people with type 1 diabetes: a randomized controlled trial with cross-over design. Diabetes Res Clin Pract. 2024;217:111844. [DOI] [PubMed] [Google Scholar]
- 51. Martin CK, Correa JB, Han H, et al. Validity of the remote food photography method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity (Silver Spring). 2012;20(4):891‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Speakman JR, Yamada Y, Sagayama H, et al. A standard calculation methodology for human doubly labeled water studies. Cell Rep Med. 2021;2(2):100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Igudesman D. Supplemental Data—Dietary patterns for weight and glycemic management in persons with type 1 diabetes: a precision nutrition-focused systematic review and meta-analysis of clinical trials: figshare; 2025. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Template data collection forms; data extracted from included studies; and analytic code will be made available upon reasonable request.