Abstract
Recently, we identified the 37-kDa laminin receptor precursor (LRP) as an interactor for the prion protein (PrP). Here, we show the presence of the 37-kDa LRP and its mature 67-kDa form termed high-affinity laminin receptor (LR) in plasma membrane fractions of N2a cells, whereas only the 37-kDa LRP was detected in baby hamster kidney (BHK) cells. PrP co-localizes with LRP/LR on the surface of N2a cells and Semliki Forest virus (SFV) RNA transfected BHK cells. Cell-binding assays reveal the LRP/LR-dependent binding of cellular PrP by neuronal and non-neuronal cells. Hyperexpression of LRP on the surface of BHK cells results in the binding of exogenous PrP. Cell binding is similar in PrP+/+ and PrP0/0 primary neurons, demonstrating that PrP does not act as a co-receptor of LRP/LR. LRP/LR-dependent internalization of PrP is blocked at 4°C. Secretion of an LRP mutant lacking the transmembrane domain (aa 86–101) from BHK cells abolishes PrP binding and internalization. Our results show that LRP/LR acts as the receptor for cellular PrP on the surface of mammalian cells.
Keywords: 37-kDa laminin receptor precursor/67-kDa high-affinity laminin receptor/prion receptor/PrP internalization/Semliki Forest virus
Introduction
The prion protein (PrP) is an ubiquitous host protein expressed by all known mammals (Oesch et al., 1985, 1991; Schätzl et al., 1995) predominantly in the brain (Chesebro et al., 1985). While its exact function is still unknown, a role has been proposed in synaptic transmission by neuronal cells (Kitamoto et al., 1992; Collinge et al., 1994; Fournier et al., 1995), in sleep behaviour (Tobler et al., 1996) and in cell survival (Kuwahara et al., 1999; for review see Weissmann, 1996). The Purkinje cell degeneration (Sakaguchi et al., 1996), however, was not due to the lack of PrP, but to overexpression of doppel (Moore et al., 1999). PrP binds copper in vivo (Brown et al., 1997) and reveals signal transduction activity by activating tyrosine kinase Fyn (Mouillet-Richard, 2000). PrP is essential for the development of transmissible spongiform encephalopathies (TSEs) (Bueler et al., 1993) also known as prion diseases, which represent fatal neurodegenerative diseases such as scrapie in sheep, BSE in cattle and Creutzfeldt–Jakob disease (CJD), Gerstmann–Sträussler–Scheinker syndrome (GSS) and fatal familial insomnia (FFI) in humans (for reviews see Weissmann and Aguzzi, 1997; Prusiner et al., 1998; Lasmézas and Weiss, 2000).
It is thought that an abnormal form of PrP, termed PrPres for its partial resistance to proteolytic digestion, which accumulates in the brain of infected individuals, is a major component of the infectious agent of TSEs (Prusiner, 1982). The process leading to the harmful form of the protein results in a conformational change of α-helices or unstructured regions of PrPc to β-sheet structures in PrPres (Caughey et al., 1991). It is still unknown if the neuronal death observed in TSEs is due to a loss of function of PrPc or to the toxicity of PrPres. In this context, the identification of the cellular receptor for PrP would be a key step towards both the understanding of disease pathogenesis and the development of therapeutics.
Within the life cycle of the PrP, PrPc is transported to the cell surface where it remains GPI anchored. PrPc is internalized via clathrin-coated pits (Shyng et al., 1994) or caveolae-like domains (Vey et al., 1996). The conversion of PrPc into PrPres may take place at the cell surface, in endosomes, lysosomes or endolysosomes. This process is thought to be influenced by an unknown protein termed protein X (Telling et al., 1995), which could represent a molecular chaperone such as Hsp60 identified as an interactor for PrPc (Edenhofer et al., 1996). The presence of a specific so far unidentified cell-surface receptor for PrP has been deduced from complementary hydropathy (Martins et al., 1997). Simultaneously, we identified the 37-kDa LRP—which represents the precursor of 67-kDa LR—as an interactor for the PrP in a yeast two-hybrid screen (Rieger et al., 1997) and hypothesized that LRP could act as a receptor or co-receptor for the PrP (for reviews see Rieger et al., 1999; Gauczynski et al., 2001). In the present study, we confirm the presence of the 37-kDa LRP and its mature 67-kDa isoform at the plasma membrane of N2a cells. We found that PrP co-localizes with LRP/LR at the surface of N2a and with LRP on BHK cells, the latter hyperexpressing LRP and PrP by recombinant (rec.) Semliki Forest virus (SFV) vectors (for reviews on the SFV system see Liljestrom and Garoff, 1991; Tubulekas et al., 1997). The relationship between 37-kDa LRP and 67-kDa LR is unknown so far (for review see Gauczynski et al., 2001). As we observed in this study both forms of the receptor in plasma membrane fractions of N2a cells, we suppose that both forms may act as the receptor for cellular PrP. We investigated the role of LRP/LR as a receptor for cellular PrP by the development of various cell-binding/internalization assays for PrP. We further studied by PrP hyperexpression on baby hamster kidney (BHK) and HeLa cells the possible role of endogeneous PrP acting as a co-receptor for LRP/LR on the cell surface. Employing an LRP deletion mutant lacking the transmembrane domain of LRP, termed LRPdelTMD, we also investigated the necessity of LRP for PrP binding and internalization. We conclude from these data that 37-kDa LRP/67-kDa LR acts as the main cell-surface receptor for PrP.
Results
Co-localization of 37-kDa LRP/67-kDa LR with PrP on the surface of neuroblastoma cells
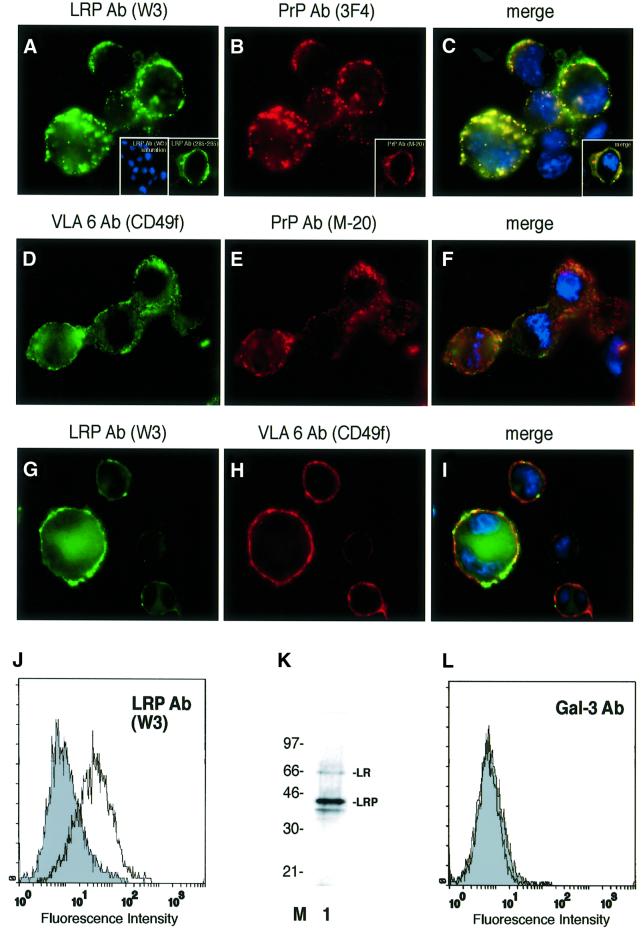
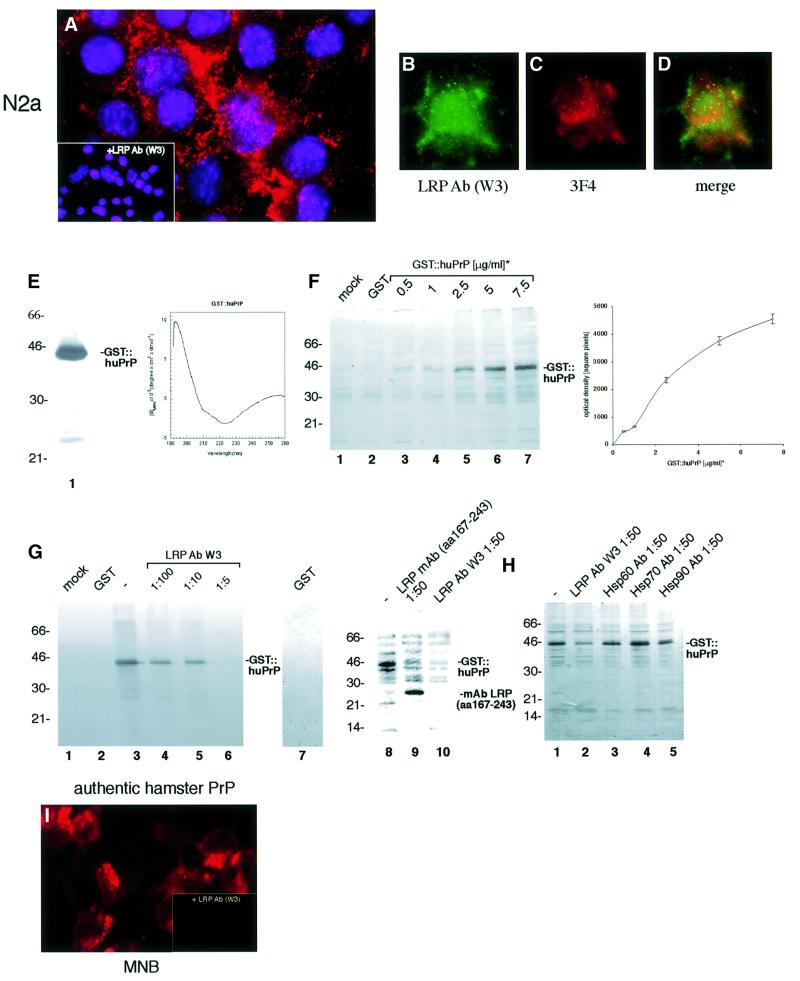
Immunofluorescence (IF) analysis of non-permeabilized murine neuroblastoma cells (N2a[MHM2]) employing LRP- (Figure 1A) and PrP-specific (Figure 1B) antibodies demonstrated that PrP and LRP/LR co-localize on the surface of these cells (Figure 1C). The integrin LR VLA6 failed to co-localize with PrP (Figure 1D–F) and LRP/LR on the cell surface (Figure 1G–I). Fluorescence-activated cell (FAC) scans of non-permeabilized N2a cells employing an LRP-specific antibody confirmed the cell-surface location of LRP/LR (Figure 1J). The β-galactoside lectin galectin-3 (gal-3) (Yang et al., 1996), which was used as a control throughout the experiments because of a previously reported cross-reactivity with LRP (Buto et al., 1998), is not expressed on the surface of N2a cells (Figure 1L). Western blot analysis of cytoplasm free plasma membrane fractions of N2a cells using a monoclonal antibody against LRP/LR revealed that the 37-kDa form (LRP) and to a lesser extent its mature 67-kDa form (LR), are located on the plasma membrane of N2a cells (Figure 1K). IF and FACS scans of non-permeabilized primary cultures of mouse cortical neurons (data not shown) and HeLa cells (Figure 4B) also demonstrated the cell-surface location of LRP/LR on these cells, used for PrP-binding experiments.
Fig. 1. Plasma membrane-associated LRP/LR and PrP co-localize on the surface of neuroblastoma cells. Non-permeabilized N2a [MHM2] cells were incubated with the pAb LRP W3 [sec. Ab fluorescein isothiocyanate (FITC)] (A), pAb LRP W3 saturated with rec. GST::LRP [sec. Ab carbocyanine (Cy2), 4′-6-diamidine-2-phenylindole (DAPI)] (A, left inset) or the mAb LRP (aa 285–295 of LRP, sec. Ab Cy2) (A, right inset) and the mAb PrP 3F4 [sec. Ab indocarbocyanine (Cy3)] (B), or the pAb PrP M-20 (B, inset). Merge of (A) and (B) DAPI staining (C) (magnification ×630). N2a [MHM2] cells were incubated with the mAb VLA6 CD49f (sec. Ab Cy2) (D) and the pAb M-20 (sec. Ab Cy3) CD49f (E). Merge of (D) and (E) DAPI staining (F). N2a [MHM2] cells were incubated with the pAb LRP W3 (sec. Ab Cy2) (G) and the mAb VLA6 (sec. Ab Cy3) (H). Merge of (G) and (H) DAPI staining (I). (J) Non-permeabilized N2a cells were analysed by FACscans. Filled profile, isotype control. non-filled profile, pAb LRP W3. Fluorescence intensity (abscissa) is plotted against relative cell numbers (ordinate). (K) Purified plasma membranes from N2a cells were analysed by western blotting employing a mAb LRP (directed against aa 167–243) (lane 1). Molecular weight markers are indicated. (L) Non-permeabilized N2a cells were analysed by FACscans. Filled profile, isotype control, non-filled profile, anti-gal-3 antibody. Fluorescence intensity (abscissa) is plotted against relative cell numbers (ordinate).
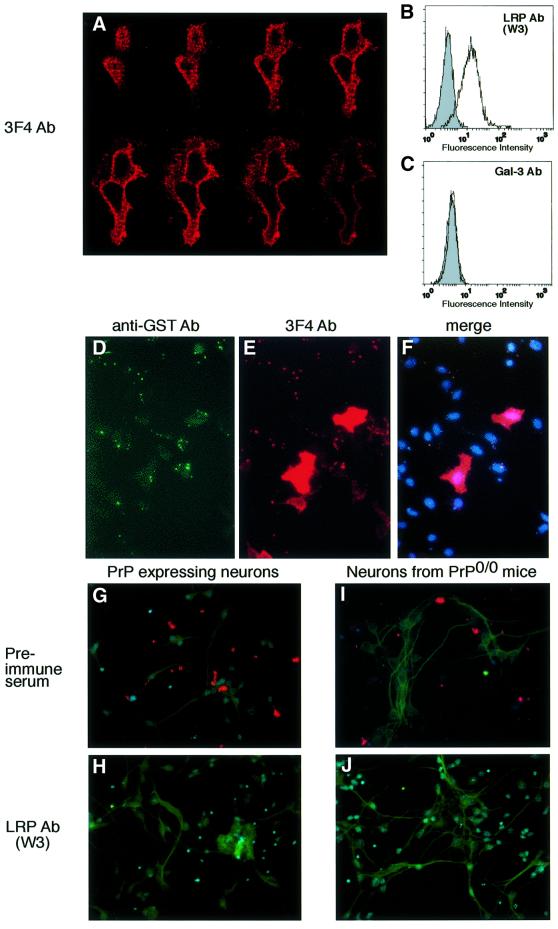
Fig. 4. Endogenous PrP does not act as a co-receptor of LRP/LR for the binding of exogeneous PrP. (A–F) Unaltered GST::huPrP23–230-binding by HeLa cells overexpressing human PrP at the cell surface. (A) Confocal z series of HeLa cells transiently transfected with cDNA encoding for human PrP1–253. Transfected HeLa cells were analysed employing the mAb 3F4 (sec. Ab Texas Red). Confocal scanning was performed from the cell surface (top panel left) towards the interior of the cell (bottom panel, right) (magnification ×630). Non-permeabilized HeLa cells were analysed by FACscans. Filled profile, isotype control (B and C), non-filled profile, pAb LRP W3 (B), pAb-gal-3 (C). Fluorescence intensity (abscissa) is plotted against relative cell numbers (ordinate). (D–F) Binding of GST::huPrP23–230 by HeLa cells transfected with pCR3-uni™-huPrP1–253. (D) Cells were analysed by IF with pAb GST (sec. Ab FITC), (E) Immunostaining mAb 3F4 (sec. Ab Texas Red). (F) Triple labelling with PrP and GST antibodies, DAPI staining. pCR3-uni™-huPrP1-253 transfected cells are red-coloured (magnification ×400). (G–J) LRP-dependent binding of GST::huPrP23–230 by primary culture of neurons isolated from PrP wild-type and PrP0/0 mice. Primary cultures of neurons from wild-type mice (G and H) or PrP0/0 mice (I and J) were incubated with GST::huPrP23–230 (4 µg/ml) after pre-incubation with either pre-immune serum (G and I) or pAb LRP W3 (dilution 1:50) (H and J). Immunostaining was performed with mAb 3F4, DAPI staining and neuron staining with MAP-2 antibody (sec. Ab FITC) (magnification ×400).
Location and orientation of LRP and human PrP on BHK cells transfected by recombinant SFV RNA
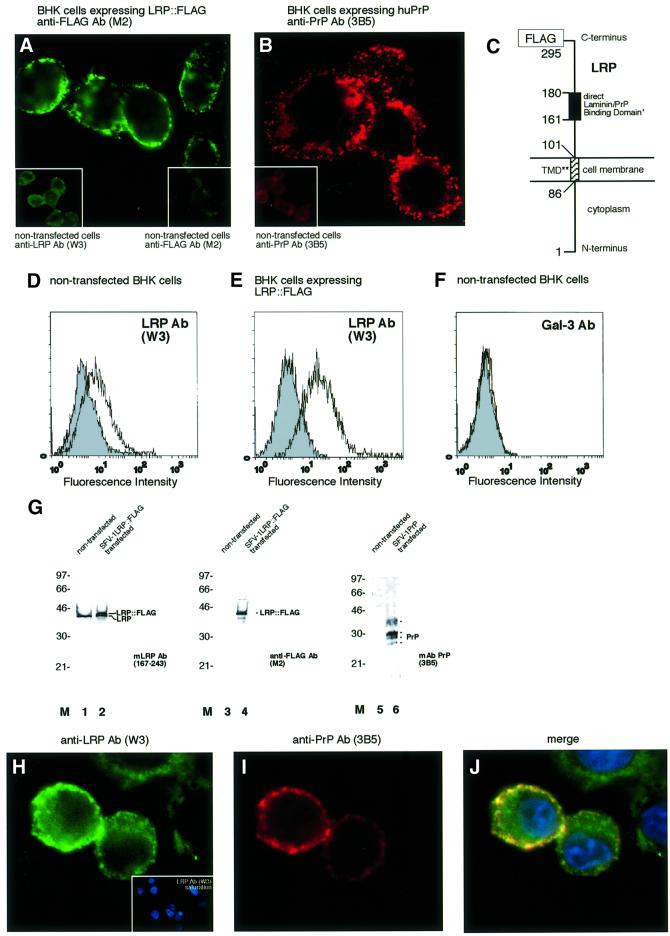
In order to investigate more precisely the localization and orientation of LRP on the surface of mammalian cells, the SFV system was used to express rec. LRP::FLAG in BHK cells. IF analysis (Figure 2A, left inset) and FACS scans (Figure 2D) reveal a low level of endogenous LRP expression. Gal-3 was not expressed on the surface of BHK cells (Figure 2F). Detection of LRP::FLAG at the surface of SFV LRP-FLAG RNA transfected BHK cells with a FLAG antibody (Figure 2A) demonstrates that LRP acts as a type 2 receptor with its C-terminus oriented to the extracellular space. Flow cytometry confirmed the cell-surface location of LRP::FLAG (Figure 2E). Endogeneous LRP (Figure 2G, lane 1), hyperexpressed LRP::FLAG (Figure 2G, lanes 2 and 4) and human PrP (Figure 2G, lane 6) are located at the plasma membrane of BHK cells. Expression of LRP::FLAG in this cell system did not result in the 67-kDa form of the LR. These data lead to the model for LRP depicted in Figure 2C showing the laminin-binding domain (Castronovo et al., 1991) coinciding with the direct PrP-binding site located between amino acids (aa) 161 and 179 (Hundt et al., 2001). An LRP mutant (LRPdelTMD) lacking the proposed transmembrane domain (Castronovo et al., 1991) secreted to the extracellular space of BHK cells (Figure 5D) demonstrating that this region indeed represents the transmembrane domain of LRP. Transfection of SFV human PrP RNA into BHK cells led to the translocation of non-tagged human PrP to the surface of BHK cells (Figure 2B). We then aimed to verify whether the cellular location of LRP and PrP would allow them to interact with each other. Co-expression of LRP::FLAG and human PrP in BHK cells, proved that LRP (Figure 2H) and PrP (Figure 2I) co-localize to a large extent on the cell surface (Figure 2J).
Fig. 2. Orientation, localization of LRP::FLAG and PrP and co-localization of both proteins in BHK cells transfected with rec. SFV RNAs. (A) Immunolocalization of LRP::FLAG to the cell membrane of non-permeabilized BHK cells transfected with rec. SFV LRP-FLAG RNA. Subcellular location was determined by IF using the mAb FLAG M2 (sec. Ab FITC). (Insets) Untransfected BHK cells incubated with the pAb LRP W3 (left), mAb FLAG M2 (right). (B) Immunolocalization of human PrPc to the cell membrane of non-permeabilized BHK cells transfected with rec. SFV-huPrP1–253 RNA. Subcellular location was determined by IF using the mAb PrP 3B5 (sec. Ab Texas Red). Inset: untransfected BHK cells (Ab 3B5). (C) Orientation of LRP on the cell surface. Orientation and localization of LRP on the cell surface is confirmed in (A). *The direct PrP-binding domain suggested by (Rieger et al., 1997) and mapped in detail by (Hundt et al., 2001) is identical with the laminin-binding domain (Castronovo et al., 1991). **The transmembrane domain (TMD) was first suggested by (Castronovo et al., 1991). Secretion of an LRP mutant lacking the transmembrane domain (LRPdelTMD) to the extracellular space of BHK cells (Figure 5D) confirmed that the TMD indeed stretches from aa 86 to 101 of LRP. FACscans of non-permeabilized non-transfected (D) and SFV LRP-FLAG RNA transfected BHK cells (E). Filled profile, isotype control; non-filled profile, pAb LRP W3. (F) FACscans of non-permeabilized non-transfected BHK cells. Filled profile, isotype control; non-filled profile, pAb gal-3. Fluorescence intensity (abscissa) plotted against relative cell numbers (ordinate). (G) Western blot analysis of plasma membrane fractions from non-transfected and rec. SFV transfected BHK cells. Purified plasma membranes from non-transfected cells (lanes 1, 3 and 5) and cells transfected with SFV LRP-FLAG RNA (lanes 2 and 4) or SFV-huPrP1–253 RNA (lane 6) were analysed by western blotting using mAb LRP (aa 167–243) (lanes 1 and 2), mAb FLAG M2 (lanes 3 and 4) or mAb 3B5 (lanes 5 and 6). (H–J) IF analysis of non-permeabilized BHK cells co-transfected with rec. SFV RNAs encoding for LRP::FLAG and human PrP. Immunostaining was performed using (H) the pAb LRP W3 non-sarurated and (H, inset) saturated with rec. GST::LRP (sec. Ab Cy2, DAPI staining) and (I) mAb 3B5 (sec. Ab Cy3). (J) Merge of (H) and (I) DAPI staining (magnification A, B, H–J, ×630).
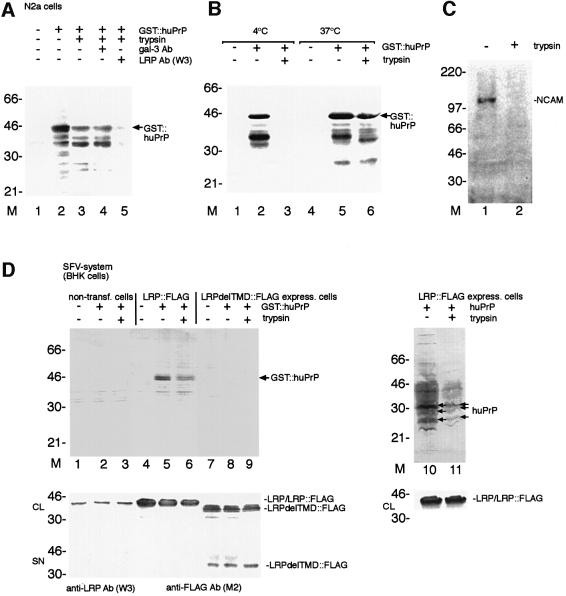
Fig. 5. LRP/LR-dependent binding and internalization of GST::huPrP by N2a cells. An LRP mutant lacking the transmembrane domain (LRPdelTMD) totally abolishes PrP binding and internalization on BHK cells. (A) Internalization of GST::huPrP by N2a cells. N2a cells not pre-incubated with antibodies (lane 1–3), pre-incubated with pAb gal-3 (dilution 1:5; lane 4), pAb LRP W3 (dilution 1:5; lane 5) were incubated with 8 µg/ml of GST::huPrP (lanes 2–5). Non-treated cells (lanes 1 and 2) and trypsin-treated cells (lanes 3–5) were analysed by western blotting employing mAb 3F4. (B) Temperature-dependent internalization of PrP by N2a cells. Cells were incubated with 8 µg/ml of GST::huPrP (lanes 2, 3, 5 and 6) at 4°C (lanes 1–3) and 37°C (lanes 4–6). Non-treated cells (lanes 1, 2, 4 and 5) and trypsin-treated cells (lanes 3 and 6) were analysed by western blotting employing mAb 3F4. (C) Total cell extracts from trypsin-treated N2a cells (lane 2) or non-treated cells (lane 1) were analysed by western blotting employing pAb N-CAM directed against the neuron-specific cell adhesion molecule (N-CAM). (D) Binding and internalization of GST::huPrP by BHK cells hyperexpressing full-length LRP::FLAG or an LRP mutant lacking the transmembrane domain (aa 86–101) termed LRPdelTMD::FLAG. BHK cells either non-transfected (lanes 1–3), hyperexpressing LRP::FLAG (lanes 4–6, 10 and 11) or LRPdelTMD::FLAG (lanes 7–9) by the SFV system were incubated with either 5 µg/ml of GST::huPrP (lanes 1–9) or 5 µg/ml huPrP (generated in the SFV system, lanes 10 and 11). Total cell extracts from non trypsin-treated (lanes 1, 2, 4, 5, 7, 8 and 10) and trypsin-treated cells (lanes 3, 6, 9 and 11) were analysed by western blotting employing the mAb 3F4 (upper panels), pAb LRP W3 (middle/lower panels, lanes 1–3, 10 and 11) or the mAb FLAG M2 (middle/lower panel, lanes 4–9). CL, crude lysate; SN, supernatant.
LRP/LR-dependent binding of human PrP to mammalian cells
To investigate a possible role of LRP/LR for the PrP binding/internalization, we established cell-binding assays with PrP. We confirmed that the PrPc moiety of rec. GST::huPrP, employed in most of our assays, displays a conformation similar to native PrPc by CD spectroscopy (Figure 3E) as shown previously for glutathione S-trans ferase (GST)-fused hamster PrP23–231 (Volkel et al., 1998). The binding of GST::huPrP23–230 to N2a cells (Figure 3A) can be totally abolished by pre-incubating the cells with the LRP antibody W3 (Figure 3A, inset). Exogeneous PrP bound to the cell surface (Figure 3C) and co-localized partly with LRP/LR (Figure 3B and D). The binding curve deduced from western blot quantification of the GST::huPrP binding to N2a cells (Figure 3F) reveals that at a GST::PrPc concentration of 4 µg/ml (used in the co-localization assay) the receptor molecules were not saturated (visible as green dots on the cell surface in Figure 3D). This is a possible explanation for the incomplete co-localization, which is compatible with the kD of 1 × 10–7 mol/l deduced from this binding curve. At this GST::PrPc amount there might also be more receptor molecules on the cell surface than could bind 4 µg of PrP. The association of rec. PrP with N2a cells was competed in a dose-dependent manner with the LRP antibody W3 (Figure 3G; Table I). Recently, a homology of the N-terminus of LRP with members of the Hsp70 family was observed (Ardini et al., 1998) suggesting that LRP/p40 might be involved in protein folding. Antibodies directed against the molecular chaperones Hsp60, 70 or 90 (Figure 3H; Table I), however, did not influence the PrP-binding reaction. GST::huPrP23–230, saturated with a GST antibody prior to exposure, bound also to human NT2 cells (data not shown). Authentic PrPc from hamster brain membrane preparations bound LRP/LR-dependent to MNB cells (Figure 3I). All experiments performed to verify the strict LRP/LR and PrP specificity of the binding reaction are summarized in Table I. Pre-immune serum, antibodies directed against GST, GFAP, laminin or gal-3 revealed no effect. Antibodies against other LRs such as the lutheran protein (El Nemer et al., 1998) and the integrin LR VLA6 (Magnifico et al., 1996) did not inhibit the binding of PrP. In addition, we observed that the lutheran protein failed to interact with LRP in the yeast two-hybrid system (data not shown). Saturation of the rec. protein with the PrP antibody JB007 led to a complete inhibition of the binding. A monoclonal antibody directed against aa 285–295 of LRP/LR failed to compete for the binding of GST::huPrP, whereas the monoclonal LRP/LR antibody directed against aa 167–243 reduced the binding of the PrP to neuronal cells (Figure 3G; Table I).
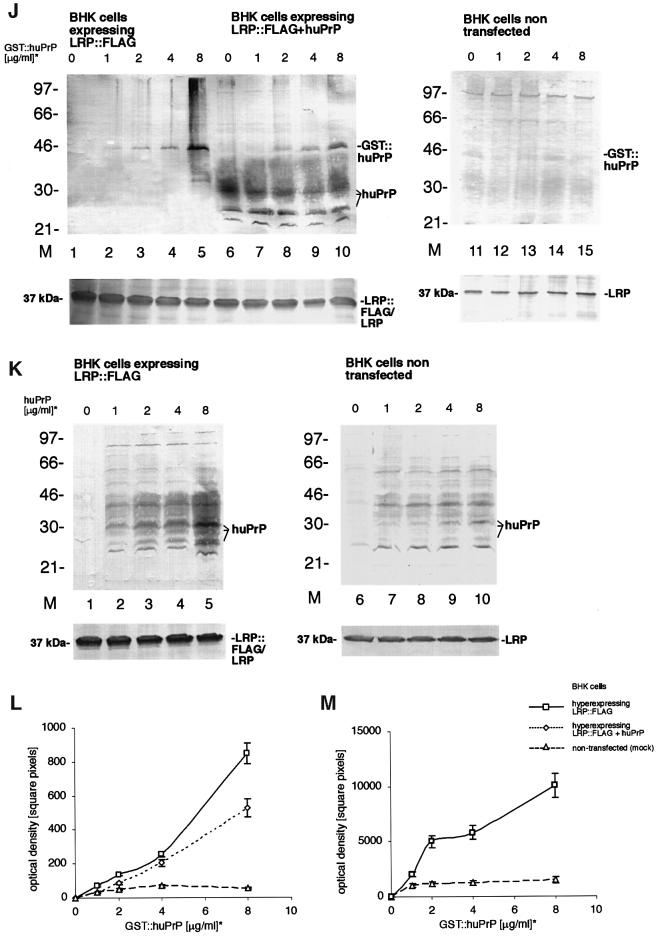
Fig. 3. LRP/LR-dependent binding of PrP by neuronal and BHK cells transfected with rec. SFV RNAs. (A) N2a cells (pre-incubated with pre-immune serum) were incubated with GST::huPrP23–230 (6 µg/ml). Binding of the rec. protein was assessed by IF using mAb GST (sec. Ab Cy3). (Inset) Pre-incubation of cells with pAb LRP W3 (dilution 1:50). (B–D) Co-localization of exogeneous GST::PrP23–230 with endogeneous LRP/LR on non-permeabilized N2a cells. Cells were incubated with GST::huPrP23–230 (4 µg/ml). Endogeneous LRP was detected by IF using the pAb LRP W3 (sec. Ab Cy2, B), exogeneous GST::huPrP was detected by mAb 3F4 (sec. Ab Cy3, C). Merge (D) of (B) and (C) (magnification A–D ×630). (E) Analysis of GST::huPrP23–230 by SDS–PAGE and FAR-UV CD spectroscopy. One microgram of GST::huPrP23–230 (lane 1) was analysed on a 12% SDS–PA-gel stained with silver. FAR-UV CD spectrum (right panel) of GST::huPrP23–230 in 10 mM sodium phosphate buffer, pH 7.4. (F) Western blot analysis of the binding assay illustrated in (A–D). Binding of GST::huPrP to N2a cells: 500 ng/ml (lane 3), 1 µg/ml (lane 4), 2.5 µg/ml (lane 5), 5 µg/ml (lane 6) and 7.5 µg/ml (lane 7) of GST::huPrP23–230, 7.5 µg/ml GST (lane 2) and no protein (lane 1) were incubated with N2a cells. Total cell extracts were loaded. Protein detection by mAb 3F4. The binding curve (right panel) of GST::huPrP23–230 to N2a cells was obtained by densitometric quantification (square pixels) of the western blot signals for GST::huPrP23–230 plotted against the dose of rec. PrP (µg/ml). kD = 1 × 10–7 mol/l (calculation described in Supplementary data). (G) pAb LRP W3 and mAb LRP (aa 167–243) displacement of the GST::huPrP-binding to N2a cells. Cells were incubated in the absence of protein (lane 1), with 7.5 µg/ml GST (lanes 2 and 7), 3 µg/ml GST::huPrP23–230 (lanes 3 and 8), 3 µg/ml GST::huPrP23–230 after pre-incubation with pAb LRP W3 at 1:100 (lane 4), 1:10 (lane 5), 1:5 (lane 6), anti-LRP mAb (aa 167–243) at 1:50 (lane 9) and pAb LRP W3 at 1:50 (lane 10). Proteins were detected by mAb 3 F4 (lanes 1–6 and lanes 8–10) or the pAb GST (lane 7). (H) GST::huPrP displacement on N2a cells with antibodies directed against molecular chaperones. Cells were incubated with 3 µg/ml of GST::huPrP23–230 without antibodies (lane 1) and with pAb LRP W3 (lane 2), antibodies directed against Hsp60 (lane 3), Hsp70 (lane 4) and Hsp90 (lane 5). Antibody dilution: 1:50. Blots were developed with the mAb 3F4. (I) LRP-dependent binding of authentic PrP isolated from hamster brains on MNB cells. MNB cells were incubated with 2 µg/ml of purified PrPc from hamster brain. Immunostaining was performed with the mAb 3F4 (sec. Ab Texas Red). Inset: MNB cells saturated with the pAb LRP W3 (dilution 1:50) prior to PrP treatment (magnification ×630). (J–M) Increased PrP-binding by rec. SFV RNA transfected BHK cells overexpressing LRP at the cell surface. BHK cells (J) were either transfected with SFV LRP-FLAG RNA (lanes 1–5), SFV LRP-FLAG RNA plus SFV-huPrP1–253 RNA (lanes 6–10) or non-transfected (lanes 11–15). Amounts of 0 µg/ml (lanes 1, 6 and 11), 1 µg/ml (lanes 2, 7 and 12), 2 µg/ml (lanes 3, 8 and 13), 4 µg/ml (lanes 4, 9 and 14) and 8 µg/ml (lanes 5, 10 and 15) of GST::huPrP23–230 were added to the cells. Total cell extracts were analysed by western blotting employing the mAb 3F4 (J, upper panels) or the pAb LRP W3 (J, lower panels). Please note that endogenously expressed huPrP appeared as non-, mono- and di-glycosylated isoforms (J). BHK cells (K) were transfected with SFV LRP-FLAG RNA (lanes 1–5) or non-transfected (lanes 6–10). Amounts of 0 µg/ml (lanes 1 and 6), 1 µg/ml (lanes 2 and 7), 2 µg/ml (lanes 3 and 8), 4 µg/ml (lanes 4 and 9) and 8 µg/ml of huPrP23–230 (SFV system) (lanes 5 and 10) were added to the cells. Total cell extracts were analysed by western blotting with the mAb 3F4 (K, upper panels) and the pAb LRP W3 (K, lower panels). Please note that externally added rec. non-tagged human PrP used for binding studies appeared as non-, mono- and di-glycosylated isoforms (K). Binding curves were obtained by quantitating the western blot signals for GST::huPrP in (J) (L) and for huPrP in (K) (M) by densitometry (square pixels). For binding studies the cells were incubated for 18 h with GST::huPrP before staining with the indicated individual antibody was performed. *GST::huPrP and huPrP concentrations represent the concentration of added recombinant protein in the cell media. Values (F, right panel, L and M) were calculated by optical scanning methods (see Supplementary data).
Table I. Summary of the displacement capacity of antibodies for the binding of human PrP to neuronal cells.
| Inoculum saturationa | Pre-incubation of cellsa | Dilution | Binding inhibition |
|---|---|---|---|
| pAb anti-PrP (JB007) | +++ | ||
| Pre-immune serum (PrP immunization) | – | ||
| pAb/mAb anti-GST | – | ||
| pAb anti-LRP | 1/50 | +++ | |
| 1/100 | ++ | ||
| 1/500 | + | ||
| 1/1000 | – | ||
| 1/10 000 | – | ||
| pre-immune serum (LRP immunization) | 1/50 | – | |
| mAb anti-LRP 167–243 | 1/5 | ++ | |
| 1/50 | + | ||
| mAb anti-LRP 285–295 | 1/5 | – | |
| 1/50 | – | ||
| pAb anti-GFAP | 1/50 | – | |
| pAb anti-laminin | 1/50 | – | |
| mAb anti-VLA6 | 1/50 | – | |
| mAb anti-lutheran protein | 1/50 | – | |
| pAb anti-gal-3 Ab | 1/50 | – | |
| mAb anti-Hsp60 | 1/50 | – | |
| mAb anti-Hsp70 | 1/50 | – | |
| mAb anti-Hsp90 | 1/50 | – |
aN2a and NT2 cells have been incubated with GST::huPrP23–231 after pre-incubation of the protein with the indicated antibodies (inoculum saturation) or after pre-incubation of the cells with the indicated antibodies. Antibody displacement capacities were analysed by IF. Concentrations of all undiluted antibodies used range between 1.5 and 1.7 mg/ml.
+++, ++, + Inhibition of binding.
– No inhibition of binding.
Hyperexpression of LRP on the cell surface of BHK cells by the SFV system enhanced binding of recombinant PrP
Next we aimed to verify whether a quantitative relationship exists between PrP binding and the amount of LRP available on the cell surface. Untransfected BHK cells with a low level of endogenous LRP (Figure 3J, lower panel and Figure 2A, left inset) in the absence of any detectable LR bind only barely detectable amounts of rec. PrP (Figure 3J, upper panel and Figure 3L, triangles). In contrast, hyperexpression of LRP::FLAG at the surface of BHK cells (Figure 3J, lanes 1–5, lower panel and Figure 2A) led to an enhanced dose-dependent binding of GST::huPrP (Figure 3J, lanes 1–5, upper panel and Figure 3L, squares). Binding of non-, mono- and di-glycosylated human PrP (without any tag) produced in the SFV system was significantly increased when LRP::FLAG was hyperexpressed at the cell surface (Figure 3K, lanes 1–5 versus 6–10 and Figure 3M, squares versus triangles). Next, we wanted to verify whether additional PrP on the cell surface influences the binding of externally added rec. PrP. The co-expression of LRP::FLAG (Figure 3J, lanes 6–10, lower panel) and human PrP (Figure 3J, lanes 6–10, upper panel) on the cell surface reduced the dose-dependent GST::huPrP binding (Figure 3J, lanes 6–10, upper panel and Figure 3L, diamonds) when compared with cells transfected with LRP::FLAG only (Figure 3L, squares). This finding suggests that PrP does not act as a co-receptor for LRP for the binding of externally added PrP on the surface of mammalian cells.
Binding behaviour of PrP to HeLa cells hyperexpressing PrP at the cell surface
The function of PrPc is unknown. However, due to its topography, it has been hypothesized that it could function as a receptor (Weissmann, 1996). We wanted to know whether PrP acts as a co-receptor for LRP/LR. To this purpose, we determined whether transiently transfected HeLa cells (∼10–20% of total cells) with a low level of endogeneous PrP (Figure 4E, non-transfected cells) and a high level of LRP/LR on the cell surface (Figure 4B) hyperexpressing human PrP on their surface (fine red frame, Figure 4A) showed an enhanced binding of externally added rec. PrP compared with non-transfected HeLa cells. The binding of rec. PrP to cells hyperexpressing PrP was not increased compared with normal cells (Figure 4D–F, compare cells stained in red with the others). Both the binding of PrP to transfected and to non-transfected cells could be efficiently inhibited with the LRP-specific antibody (data not shown).
Similar binding of PrP to neurons isolated from PrP0/0 mice and PrP wild-type mice
In order to confirm that PrP at the cell surface does not participate in the binding of rec. PrP, we performed binding assays on primary cultures of neurons from PrP0/0 mice versus wild-type mice. The binding of GST::huPrP was similar for both types of neurons (Figure 4G and I) and was completely abolished by pre-incubating PrP+/+ or PrP0/0 cells with the LRP antibody W3 (Figure 4H and J).
LRP/LR-dependent binding and internalization of recombinant PrP by N2a cells
The internalization of the PrP was shown on N2a cells incubated with GST::huPrP and trypsinized (Figure 5A, lane 3); it was blocked by the LRP antibody (Figure 5A, lane 5), whereas the gal-3 antibody had no effect (Figure 5A, lane 4). Lowering the incubation temperature to 4°C resulted in a complete inhibition of the PrP internalization process (Figure 5B, lane 3) confirming that the process is active and receptor-mediated. These results demonstrate the LRP/LR-dependent internalization of the human PrP.
Secretion of an LRP mutant lacking the transmembrane domain totally abolished PrP binding and internalization
In order to prove the necessity of LRP for the binding and internalization process, we compared the GST::huPrP binding/internalization by BHK cells expressing full-length LRP with cells expressing an LRP mutant lacking the proposed transmembrane domain (aa 86–101) (Castronovo et al., 1991) termed LRPdelTMD. This mutant was detected in the supernatant of the cells and in the crude lysates revealing its presence at high amounts in the secretory pathway and its secretion to the extracellular space (Figure 5D, lanes 7–9, middle and lower panel, respectively), whereas full-length LRP::FLAG was detected in the crude lysate only (Figure 5D, lanes 4–6, middle panel). Binding and internalization of GST:: huPrP was observed in cells expressing wild-type LRP (Figure 5D, upper panel, lanes 5 and 6) but not in those expressing LRPdelTMD (Figure 5D, upper panel, lanes 8 and 9). Untransfected BHK cells having an extremely low level of endogenous LRP bound no or only minimal amounts of externally added GST::huPrP (Figure 5D, lane 2). Binding and internalization of non-tagged highly glycosylated human PrP by LRP::FLAG hyperexpressing cells (Figure 5D, lanes 10 and 11) confirmed the observations made with GST-tagged human PrP. Levels of endogeneous LRP, as well as LRP::FLAG and LRPdel TMD::FLAG were only marginally reduced after trypsin treatment due to the fact that significant amounts of these proteins are located in the secretory pathway. Densito metric measurements revealed that N2a (Figure 5A and B) and BHK cells (Figure 5D) internalize between 25 and 50% of the bound PrP.
Discussion
The interaction of PrPc with 37-kDa LRP suggested that LRP and its mature 67-kDa LR might act as a receptor or co-receptor for cellular PrP (Rieger et al., 1997). In order to investigate this hypothesis we initiated a series of cell-binding/internalization assays employing neuronal and non-neuronal cells, recombinant as well as authentic PrPs and a series of recombinant wild-type and mutated LRP molecules.
Localization of LRP/LR
A prerequiste for LRP/LR-dependent binding/internalization of PrP is the cell-surface location of LRP/LR. LRP has been found on 40S ribosomes and was dubbed p40 (Auth and Brawerman, 1992), in the nucleus (Sato et al., 1996) and on the cell surface. The 37-kDa LRP is located in plasma membrane fractions of mosquito cells acting as a receptor for the Venezuelan equine encephalitis virus (Ludwig et al., 1996), in cell-wall fractions of Candida albicans (Lopez-Ribot et al., 1994) and on the cell surface of mammalian cells such as Madin–Darby canine kidney cells (Salas et al., 1992). We showed by IF, flow cytometry and analysis of plasma membrane fractions that the 37-kDa LRP is located on the surface of neuroblastoma cells and non-transfected or LRP::FLAG hyperexpressing BHK cells. The 67-kDa form of the LR locates also to the cell surface (for review see Gauczynski et al., 2001) where it acts as a receptor for the Sindbis virus (Wang et al., 1992). We showed the presence of 67-kDa LR in plasma membrane fractions of N2a cells and concluded that the 37-kDa LRP/67-kDa LR might act as a receptor for PrP at the plasma membrane. The 37-kDa LRP/67-kDa LR polymorphism is unsolved so far. The association of cell-surface molecules such as HSPGs with 37-kDa LRP might explain the appearance of the 67-kDa form of the receptor (Hundt et al., 2001). LRP::FLAG hyperexpressing BHK cells revealed the cell-surface localization of LRP with its C-terminus oriented to the extracellular space enabling PrP to interact with PrP-binding domains on LRP. In summary, we showed (i) the membrane location of LRP/LR and (ii) the co-localization of PrP with LRP/LR on the surface of neuroblastoma cells and LRP/PrP hyperexpressing BHK cells.
LRP/LR-dependent binding of PrP to cells
For PrP-binding and internalization experiments, we used externally added recombinant human PrP or authentic hamster PrP, and a series of mammalian cells including murine neuroblastoma cells (N2a, MNB), primary cultures of neurons, human teratocarcinoma (NT2) and BHK cells. We proved LRP/LR-dependent binding of GST::huPrP and authentic hamster PrP to these cells. The kD for the binding of rec. PrP to N2a cells was of 1 × 10–7 mol/l, which is in good agreement with the kDs of other cell-surface receptors such as the N-formyl peptide receptor (Christophe et al., 2001) or the proteinaceous receptor on the surface of antigen presenting cells (Sondermann et al., 2000).
The strict LRP/LR specificity of the PrP binding to NT2 and N2a cells was demonstrated in competition assays with a series of different antibodies (Table I). Whereas the LRP antibody W3 raised against the entire protein (Rieger et al., 1997) competed totally for the binding of GST::huPrP to neuronal and non-neuronal cells, mAb LRP285–295 did not compete for the binding as aa 285–295 stretches out side the PrP binding domain (Hundt et al., 2001). mAb LRP167–243 encompassing parts of the direct binding domain (Hundt et al., 2001) was able to reduce the binding of PrP. Antibodies against the lutheran protein representing an erythroid receptor for laminin (El Nemer et al., 1998), failed to compete for PrP cell binding. This receptor did not interact with PrP in the yeast two-hybrid system (data not shown). Anti-integrin receptor VLA6 antibodies (Magnifico et al., 1996) and anti-β-galactoside lectin gal-3 antibodies also failed to compete for the PrP-binding reaction. VLA6 does not co-localize with PrP and LRP/LR on the cell surface. The use of LRP hyperexpressing BHK cells demonstrated the quantitative relationship between the number of LRP receptor molecules and the PrP-binding process.
LRP/LR-dependent internalization of PrP
N2a cells internalized 25–50% of the human PrP bound to the cell surface in a LRP/LR-dependent manner. The PrP internalization process represents an active receptor-mediated event, confirmed by lowering the incubation temperature of N2a cells to 4°C resulting in a total blockage of PrP internalization without affecting PrP binding.
Expression of an LRP mutant lacking the putative transmembrane domain (LRPdelTMD) (Castronovo et al., 1991) in BHK cells resulted in secretion of LRPdelTMD to the extracellular space confirming the hitherto indirect evidence that the transmembrane region stretches from aa 86 to 101. In contrast to full-length LRP hyperexpressing BHK cells, LRPdelTMD hyperexpressing cells did not bind or internalize PrP due to the secretion of the mutant to the extracellular space. Untransfected BHK cells similarly failed to bind and internalize PrP due to insufficient amounts of LRP on the cell surface, confirming that LRP is essential for PrP binding and internalization.
Endogeneous PrP does not act as a co-receptor for LRP/LR
The co-localization of LRP/LR and PrP at the surface of mammalian cells raises the possibility that PrP could act as a co-receptor for LRP/LR. Binding of rec. PrP to HeLa or BHK cells expressing additional PrP on the cell surface was not increased. On PrP plus LRP hyperexpressing BHK cells, PrP had even the adverse effect of hampering the increased binding due to LRP hyperexpression, probably by recruiting a proportion of the latter receptor for its own metabolism. Unaltered binding of rec. PrP to primary cortical neurons isolated from PrP knock-out mice confirmed that the absence of PrP on the cell surface had no influence on the LRP/LR-dependent PrP binding, demonstrating that endogeneous PrP does not act as a co-receptor for LRP/LR.
Role of LRP/LR in the metabolism of PrP and implications for the pathogenesis of TSEs
Our study has several implications in terms of both the metabolism of PrPc and the pathogenesis of TSEs. Our co-localization and internalization data suggest that LRP/LR is essential for the normal cell cycle of the PrP by mediating the internalization of PrPc after its exposure at the cell surface. Internalization of PrP might occur via caveolae-like domains (Vey et al., 1996) or via clathrin-coated pits (Shyng et al., 1994). The receptor-mediated endocytosis of the protein (by LRP/LR), would direct the complex into clathrin-coated pits (for reviews see Pley and Parham, 1993; Schmid, 1997) rather than caveolae-like domains (for review Maxfield and Mayor, 1997). The role of LRP/LR as a receptor for the extracellular-matrix proteins laminin and elastin also suggests that its interaction with PrP may induce a signal involved in cell survival. In this respect, it has been shown that primary neurons devoid of PrP are more prone to neuronal death than their PrP expressing counterparts (Kuwahara et al., 1999). One possibility is that the interaction of an LRP/LR receptor on one cell with a PrP molecule on another cell would contribute to cell-to-cell communication essential for cell survival. Recently, a signal transduction activity of PrP by activating tyrosine kinase Fyn was described (Mouillet-Richard, 2000). The plasma membrane- associated LRP/LR (Figures 1 and 2) might mediate the signal transduction of the extracellular GPI-anchored PrP with the intracellular plasma membrane-associated Fyn kinase involving cell-surface HSPGs (Hundt et al., 2001).
The fact that PrPc binds to and is internalized by LRP/LR raises the possibility that PrPres is also bound/internalized by LRP/LR. The expression of LRP/LR in human small intestinal mucosa (Shmakov et al., 2000) suggests that it may represent the portal of entry for PrPres after oral contamination. Our recent finding that LRP levels are increased in only those organs of rodents that accumulate PrPres, indicates that PrPres intervenes in the metabolism of LRP (Rieger et al., 1997). Whether the internalization of PrPres relies on the presence of LRP/LR, PrPc or both may be answered by cell biological studies. The generation of transgenic mice devoid of LRP/LR might also help to determine whether LRP/LR acts as the receptor for the infectious agent.
Also of relevance for pathogenesis, a saturation of the binding sites of LRP/LR may occur as a consequence of PrP accumulation in TSEs rendering the receptor unavailable to its ligand laminin and contributing to neurodegenerative processes. The absence of laminin-binding to its receptor sensitizes neurons to death, as demonstrated in mice affected with the weaver syndrome (Murtomaki et al., 1995). Keeping in mind that laminin plays a central role in cell growth, differentiation and migration and that any interference with these functions may be deleterious for the organism, our findings demonstrating that PrP associates with and is internalized by LRP/LR into the cell open new avenues of research for anti-TSE therapeutics, either to block the entry of the infectious particle, to modify the metabolism of PrP or to interfere with the neurodegenerative process.
Materials and methods
SFV system
pSFV1-LRP::FLAG, pSFV1-LRPdelTMD::FLAG and pSFV1-huPrP1-253 were constructed as described in the Supplementary data, available at The EMBO Journal Online. pSFV-1 (Liljestrom and Garoff, 1991), pSFV3-lacZ (Life Technologies) and the ORF from human PrP (Krasemann et al., 1996) were used. Transfections of BHK-21 C13 cells with rec. SFV RNAs (transfection efficiencies = 90–100%) are described (see Supplementary data).
HeLa cells expressing huPrP
Human epitheloid carcinoma of cervix HeLa cells (ATCC CCL2) were transfected with pCR3-uni™-huPrP1–253 containing human Prn-p cDNA (Jaegly et al., 1998) for huPrP expression as described (see Supplementary data).
Tissue culture of N2a, N2a [MHM2], MNB, NT2, HeLa, BHK, Sf9 cells, primary mouse cortical neurons, PrP0/0 neuronal cultures
N2a, N2a [MHM2], MNB, NT2, HeLa, BHK, Sf9 cells, primary mouse cortical cultures and PrP0/0 neuronal cultures (C.Weissmann, Zürich) were cultivated and prepared as described (see Supplementary data).
Generation of recombinant and authentic prion proteins
pAcSecG2T-huPrP was generated and rec. baculoviruses produced as described (see Supplementary data). Rec. GST, GST::huPrP23–230, dialysed against 20 mM HEPES pH 7.4, were expressed in the baculovirus system as described for GST::haPrP proteins (Weiss et al., 1995; Weiss et al., 1996). Authentic PrPc was prepared from hamster brain membrane fractions (Meyer et al., 1986). Human PrP was expressed in the SFV system (see Supplementary data).
Far-UV circular dichroism analysis
CD spectra of GST::huPrP23–230 were recorded as described (see Supplementary data).
PrP-binding/internalization assays followed by IF analysis, confocal microscopy or western blotting
For competition studies the cells were either pre-incubated with the individual antibody before the addition of rec. protein or the rec. protein was pre-incubated with the individual antibody before addition to the cells (inoculum saturation). After 18 h of incubation, cells were processed (with or without trypsin treatment) for IF-staining, confocal microscopy or western blotting as described in the Supplementary data.
FACS analysis (flow cytometry)
Single-cell suspensions were prepared, cells treated and data acquisition obtained as described (see Supplementary data).
Isolation of plasma membranes
Plasma membrane preparations were done according to (Vleurick et al., 1999).
Calculation of binding curves for recombinant PrP to cells and determination of the kD for the interaction of PrP with LRP/LR
Calculations (NIH-Image)/kD determination (Prism 3) were performed as described (see Supplementary data).
Antibodies
The antibodies used are described in the Supplementary data. For saturation of pAb LRP W3 with rec. PrP, immobilized GST::LRP was incubated with pAb LRP W3 and the supernatant assayed by IF on N2a/BHK cells.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank J.-P.Cartron (INTS, Paris, France) for antibodies and cDNA directed against and encoding for the Lutheran protein, to J.P.Houchins (Minneapolis, MN) for the monoclonal LRP antibodies, to J.Grassi (CEA-Saclay, France) for SAF70 antibody, to G.Hunsmann for the 3B5 antibody, to H.-J.Gabius (LMU, Munich) for anti-gal-3 antibodies and rec. gal-3, to H.A.Kretzschmar for huPrP encoding cDNA, to S.B.Prusiner (San Francisco, CA) for N2a[MHM2] cells, to C.Weissmann (London, UK) for PrP knock-out mice, to B.Chesebro and B.Caughey (Rocky Mountain Laboratories, Hamilton, MT) for MNB cells. We thank S.Janetzky, S.Hengge, A.Pahlich and K.Krüger (Munich) for excellent technical assistance and M.Ried (Munich) for technical support. This work was supported by grants from DRET (Direction des Recherches et Techniques, Paris, France), from INSERM (Paris, France) and from the European Union (BIOMED PL-97-6054, QLRT-2000-02085 and FAIR-CT-98-7020). S.Weiss acknowledges the support by grants KI-01-9760 and KO-01-1541-01 (BMBF), LMU 3 and 4 (Bavarian Prion Research Foundation) and thanks R.Grosschedl and E.-L.Winnacker for valuable advice and continuous support.
References
- Ardini E., Pesole,G., Tagliabue,E., Magnifico,A., Castronovo,V., Sobel,M.E., Colnaghi,M.I. and Menard,S. (1998) The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol. Biol. Evol., 15, 1017–1025. [DOI] [PubMed] [Google Scholar]
- Auth D. and Brawerman,G. (1992) A 33-kDa polypeptide with homology to the laminin receptor: component of translation machinery. Proc. Natl Acad. Sci. USA, 89, 4368–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R. et al. (1997) The cellular prion protein binds copper in vivo. Nature, 390, 684–687. [DOI] [PubMed] [Google Scholar]
- Bueler H., Aguzzi,A., Sailer,A., Greiner,R.A., Autenried,P., Aguet,M. and Weissmann,C. (1993) Mice devoid of PrP are resistant to scrapie. Cell, 73, 1339–1347. [DOI] [PubMed] [Google Scholar]
- Buto S. et al. (1998) Formation of the 67-kDa laminin receptor by acylation of the precursor. J. Cell. Biochem., 69, 244–251. [DOI] [PubMed] [Google Scholar]
- Castronovo V., Taraboletti,G. and Sobel,M.E. (1991) Functional domains of the 67-kDa laminin receptor precursor. J. Biol. Chem., 266, 20440–20446. [PubMed] [Google Scholar]
- Caughey B.W., Dong,A., Bhat,K.S., Ernst,D., Hayes,S.F. and Winslow,S.C. (1991) Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry, 30, 7672–7680. [DOI] [PubMed] [Google Scholar]
- Chesebro B. et al. (1985) Identification of scrapie prion protein-specific mRNA in scrapie infected and uninfected brain. Nature, 315, 331–333. [DOI] [PubMed] [Google Scholar]
- Christophe T., Karlsson,A., Dugave,C., Rabiet,M.J., Boulay,F. and Dahlgren,C. (2001) The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/LXA4R and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J. Biol. Chem., 276, 21585–21593. [DOI] [PubMed] [Google Scholar]
- Collinge J., Whittington,M.A., Sidle,K.C.L., Smith,C.J., Palmer,M.S., Clarke,A.R. and Jefferys,J.G.R. (1994) Prion protein is necessary for normal synaptic function. Nature, 370, 295–297. [DOI] [PubMed] [Google Scholar]
- Edenhofer F., Rieger,R., Famulok,M., Wendler,W., Weiss,S. and Winnacker,E.L. (1996) Prion protein PrPc interacts with molecular chaperones of the Hsp60 family. J. Virol., 70, 4724–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Nemer W., Gane,P., Colin,Y., Bony,V., Rahuel,C., Galacteros,F., Cartron,J.P. and Le Van Kim,C. (1998) The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem., 273, 16686–16693. [DOI] [PubMed] [Google Scholar]
- Fournier J.-G., Escaign-Haye,F., Villemeur,T.B.D. and Robain,O. (1995) Ultrastructural localisation of cellular prion protein in synaptic boutons of normal hamster hippocampus. C.R. Acad. Sci. (Paris), 318, 339–344. [PubMed] [Google Scholar]
- Gauczynski S., Hundt,C., Leucht,C. and Weiss,S. (2001) Interaction of prion proteins with cell surface receptors, molecular chaperones and other molecules. Adv. Protein Chem., 57, 229–272. [DOI] [PubMed] [Google Scholar]
- Hundt C. et al. (2001) Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J., 20, 5876–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegly A., Mouthon,F., Peyrin,J.-M., Camugli,B., Deslys,J.-P. and Dormont,D. (1998) Search for a nuclear localization signal in the prion protein. Mol. Cell. Neurosci., 11, 127–133. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Shin,R.W., Dohura,K., Tomokane,N., Miyazono,M., Muramoto,T. and Tateishi,J. (1992) Abnormal isoform of prion proteins accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt–Jakob disease. Am. J. Pathol., 140, 1285–1294. [PMC free article] [PubMed] [Google Scholar]
- Krasemann S., Groschup,M.H., Harmeyer,S., Hunsmann,G. and Bodemer,W. (1996) Generation of monoclonal antibodies against human prion proteins in prp0/0 mice. Mol. Med., 2, 725–734. [PMC free article] [PubMed] [Google Scholar]
- Kuwahara C. et al. (1999) Prions prevent neuronal cell-line death. Nature, 400, 225–226. [DOI] [PubMed] [Google Scholar]
- Lasmézas C.I. and Weiss,S. (2000) Molecular biology of prion diseases. In Cary,J.W., Linz,J.E. and Bhatnagar,D. (eds), Microbial Foodborne Diseases. Mechanisms of Pathogenicity and Toxin Synthesis. Technomic Publishing Co., Inc., Lancaster, PA, pp. 495–537.
- Liljestrom P. and Garoff,H. (1991) A new generation of animal cell expression vectors based on the Semliki–Forest virus replicon. Biotechnology, 9, 1356–1361. [DOI] [PubMed] [Google Scholar]
- Lopez-Ribot J.L., Casanova,M., Monteagudo,C., Sepulveda,P. and Martinez,J.P. (1994) Evidence for the presence of a high-affinity laminin receptor-like molecule on the surface of Candida albicans yeast cells. Infect. Immun., 62, 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig G.V., Kondig,J.P. and Smith,J.F. (1996) A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol., 70, 5592–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnifico A., Tagliabue,E., Buto,S., Ardini,E., Castronovo,V., Colnaghi,M.I. and Menard,S. (1996) Peptide G, containing the binding site of the 67-kDa laminin receptor, increases and stabilizes laminin binding to cancer cells. J. Biol. Chem., 271, 31179–31184. [DOI] [PubMed] [Google Scholar]
- Martins V.R., Graner,E., Garcia-Abreu,J., de SouzaS.J., Mercadante, A.F., Veiga,S.S., Zanata,S.M., Neto,V.M. and Brentani,R.R. (1997) Complementary hydropathy identifies a cellular prion protein receptor. Nat. Med., 3, 1376–1382. [DOI] [PubMed] [Google Scholar]
- Maxfield F.R. and Mayor,S. (1997) Cell surface dynamics of GPI-anchored proteins. Adv. Exp. Med. Biol., 419, 355–364. [DOI] [PubMed] [Google Scholar]
- Meyer R.K., McKinley,M.P., Bowman,K.A., Braunfeld,M.B., Barry,R.A. and Prusiner,S.B. (1986) Separation and properties of cellular and scrapie prion proteins. Proc. Natl Acad. Sci. USA, 83, 2310–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.C. et al. (1999) Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol., 292, 797–817. [DOI] [PubMed] [Google Scholar]
- Mouillet-Richard S., Ermonval,M., Chebassier,C., Laplanche,J.L., Lehmann,S., Launay,J.M. and Kellermann,O. (2000) Signal transduction through prion protein. Science, 289, 1925–1928. [DOI] [PubMed] [Google Scholar]
- Murtomaki S., Trenkner,E., Wright,J.M., Saksela,O. and Liesi,P. (1995) Increased proteolytic activity of the granule neurons may contribute to neuronal death in the weaver mouse cerebellum. Dev. Biol., 168, 635–648. [DOI] [PubMed] [Google Scholar]
- Oesch B. et al. (1985) A cellular gene encodes scrapie PrP 27–30 protein. Cell, 40, 735–746. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway,D. and Prusiner,S.B. (1991) Prion protein genes: evolutionary and functional aspects. In Chesebro,B.W. (ed.), Current Topics in Microbiology and Immunology. Transmissible Spongiform Encephalopathies: Scrapie, BSE and Related Disorders. Springer-Verlag, Berlin, Germany, pp. 109–124. [DOI] [PubMed]
- Pley U. and Parham,P. (1993) Clathrin: its role in receptor-mediated vesicular transport and specialized functions in neurons. Crit. Rev. Biochem. Mol. Biol., 28, 431–464. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. (1982) Novel proteinaceous infectious particles cause scrapie. Science, 216, 136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B., Scott,M.R., DeArmond,S.J. and Cohen,F.E. (1998) Prion protein biology. Cell, 93, 337–348. [DOI] [PubMed] [Google Scholar]
- Rieger R., Edenhofer,F., Lasmezas,C.I. and Weiss,S. (1997) The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat. Med., 3, 1383–1388. [DOI] [PubMed] [Google Scholar]
- Rieger R., Lasmezas,C.I. and Weiss,S. (1999) Role of the 37 kDa laminin receptor precursor in the life cycle of prions. Transfus. Clin. Biol., 6, 7–16. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. et al. (1996) Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature, 380, 528–531. [DOI] [PubMed] [Google Scholar]
- Salas P.J., Ponce,M.I., Brignoni,M. and Rodriguez,M.L. (1992) Attachment of Madin-Darby canine kidney cells to extracellular matrix: role of a laminin binding protein related to the 37/67 kDa laminin receptor in the development of plasma membrane polarization. Biol. Cell, 75, 197–210. [DOI] [PubMed] [Google Scholar]
- Sato M., Kinoshita,K., Kaneda,Y., Saeki,Y., Iwamatsu,A. and Tanaka,K. (1996) Analysis of nuclear localization of laminin binding protein precursor p40 (LBP/p40). Biochem. Biophys. Res. Commun., 229, 896–901. [DOI] [PubMed] [Google Scholar]
- Schätzl H.M., Da-Costa,M., Taylor,L., Cohen,F.E. and Prusiner,S.B. (1995) Prion protein gene variation among primates. J. Mol. Biol., 245, 362–374. [DOI] [PubMed] [Google Scholar]
- Schmid S.L. (1997) Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem., 66, 511–548. [DOI] [PubMed] [Google Scholar]
- Shmakov A.N., Bode,J., Kilshaw,P.J. and Ghosh,S. (2000) Diverse patterns of expression of the 67-kDa laminin receptor in human small intestinal mucosa: potential binding sites for prion proteins? J. Pathol., 191, 318–322. [DOI] [PubMed] [Google Scholar]
- Shyng S.L., Heuser,J.E. and Harris,D.A. (1994) A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J. Cell Biol., 125, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H., Becker,T., Mayhew,M., Wieland,F. and Hartl,F.U. (2000) Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol. Chem., 381, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Telling G.C., Scott,M., Mastrianni,J., Gabizon,R., Torchia,M., Cohen,F.E., DeArmond,S.J. and Prusiner,S.B. (1995) Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell, 83, 79–90. [DOI] [PubMed] [Google Scholar]
- Tobler I. et al. (1996) Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature, 380, 639–642. [DOI] [PubMed] [Google Scholar]
- Tubulekas I., Berglund,P., Fleeton,M. and Liljestrom,P. (1997) Alphavirus expression vectors and their use as recombinant vaccines: a minireview. Gene, 190, 191–195. [DOI] [PubMed] [Google Scholar]
- Vey M., Pilkuhn,S., Wille,H., Nixon,R., DeArmond,S.J., Smart,E.J., Anderson,R.G., Taraboulos,A. and Prusiner,S.B. (1996) Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl Acad. Sci. USA, 93, 14945–14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleurick L., Kuhn,E.R., Decuypere,E. and Van,V.P. (1999) Isolation of plasma membranes and Golgi apparatus from a single chicken liver homogenate. J. Cell. Biochem., 72, 349–355. [DOI] [PubMed] [Google Scholar]
- Volkel D., Blankenfeldt,W. and Schomburg,D. (1998) Large-scale production, purification and refolding of the full-length cellular prion protein from Syrian golden hamster in Escherichia coli using the glutathione S-transferase fusion system. Eur. J. Biochem., 251, 462–471. [DOI] [PubMed] [Google Scholar]
- Wang K.S., Kuhn,R.J., Strauss,E.G., Ou,S. and Strauss,J.H. (1992) High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol., 66, 4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Famulok,M., Edenhofer,F., Wang,Y.H., Jones,I.M., Groschup,M. and Winnacker,E.L. (1995) Overexpression of active Syrian golden hamster prion protein PrPc as a glutathione S-transferase fusion in heterologous systems. J. Virol., 69, 4776–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Rieger,R., Edenhofer,F., Fisch,E. and Winnacker,E.-L. (1996) Recombinant prion protein rPrP27–30 from Syrian Golden Hamster reveals proteinase K sensitivity. Biochem. Biophys. Res. Commun., 219, 173–179. [DOI] [PubMed] [Google Scholar]
- Weissmann C. (1996) PrP effects clarified. Curr. Biol., 6, 1359. [DOI] [PubMed] [Google Scholar]
- Weissmann C. and Aguzzi,A. (1997) Bovine spongiform encephalopathy and early onset variant Creutzfeldt–Jakob disease. Curr. Opin. Neurobiol., 7, 695–700. [DOI] [PubMed] [Google Scholar]
- Yang R.Y., Hsu,D.K. and Liu,F.T. (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl Acad. Sci. USA, 93, 6737–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]