Abstract
Computational methods have revolutionized NMR spectroscopy, driving significant advancements in structural biology and related fields. This review focuses on recent developments in quantum chemical and machine learning approaches for computational NMR, emphasizing their role in enhancing accuracy, efficiency, and scalability. QM methods provide precise predictions of NMR parameters, enabling detailed structural characterization of diverse systems. ML techniques, leveraging extensive data sets and advanced algorithms, complement QM by efficiently automating spectral assignments, predicting chemical shifts, and analyzing complex data. Together, these approaches have transformed NMR workflows, addressing challenges in metabolomics, protein structure determination, and drug discovery. This review highlights recent progress, emerging tools, and future directions in computational NMR, underscoring its critical role in modern structural science.


1. Introduction
Nuclear magnetic resonance (NMR) is a cornerstone analytical technique with broad applications across physics, chemistry, biology, and medicine, renowned for its ability to provide molecular-level insights into structure, dynamics, and interactions. Developed independently by Felix Bloch and Edward Purcell in the 1940s-a discovery that earned them the 1952 Nobel Prize in Physics-NMR has since become an indispensable tool in structural biology. − Unlike X-ray crystallography − and cryo-electron microscopy, − which often require crystalline or frozen samples, NMR uniquely enables the study of biomolecules in solution under near-native conditions, capturing their conformational flexibility and dynamic behavior. − This capability is critical for understanding the functional roles of proteins and nucleic acids, particularly in dynamic regions essential for binding, catalysis, and regulation. − Moreover, NMR’s versatility extends beyond structural elucidation being also able to probe molecular interactions, identification of ligand-binding sites, and characterizing transient states, which makes NMR invaluable for studying complex biological processes. −
NMR is an invaluable tool in biological research, enabling insights into molecular structure, function, and dynamics across diverse systems. − Its application in metabolomics, highlighted by Wilson and Burlingame’s 1974 study on 13C isotope tracers, has advanced the understanding of metabolic pathways, disease biomarkers, and cellular responses. By the 1980s, technological advancements in NMR spectrometers and magnetic field strengths solidified its role in noninvasive, high-throughput analysis of biofluids, widely applied in clinical diagnostics, pharmacology, and systems biology. − Beyond metabolomics, NMR has contributed to protein structure determination, − enzymatic mechanisms, − and real-time metabolic flux monitoring, − underpinning critical discoveries in disease progression, drug efficacy, and environmental adaptation. − Quantitative data from NMR studies remain essential for modeling complex biochemical networks and understanding organismal physiology. , Beyond biology, NMR plays a pivotal role in chemistry and materials science, − supporting structural elucidation in synthetic chemistry, , natural product isolation, − and reaction mechanism studies. , Solid-state NMR, as used in analyzing amorphous polymers and ceramics, − provides detailed information on molecular arrangements, phase compositions, and interatomic distances, enabling the design of advanced materials with tailored mechanical and thermal properties. −
In the pharmaceutical industry, NMR is widely used in drug discovery and development, supporting every stage from initial screening to quality control. , Once a tool most amenable for applicaton to small, NMR-accessible targets, its role has expanded with advancements in technology and methodology. NMR enables iterative cycles of structure determination, modeling, and chemical refinement, as demonstrated in fragment-based drug design and binding interaction studies. − It characterizes complex drug molecules, monitors kinetics, and ensures batch-to-batch product consistency, with recent innovations like ultrafast data acquisition and automated spectral analysis accelerating lead identification. However, challenges remain, particularly in interpreting complex and overlapping spectra. − Traditional spectral assignment is labor-intensive and expertise-dependent, limiting scalability. To address these limitations, computational methods are increasingly essential, driving advancements in algorithms and models for analyzing larger, more intricate systems. This computational shift enhances NMR’s utility in structural biology, metabolomics, and pharmaceutical research, supporting faster, more precise structural insights.
Computational approaches play a significant role in NMR spectroscopy, − addressing challenges in structure elucidation, , spectral interpretation, , and molecular dynamics studies. , Although NMR provides detailed insights into atomic environments, the interpretation of complex spectral data, particularly for large biomolecules and mixtures, often remains difficult. Computational methods enhance traditional workflows by improving accuracy and efficiency. Quantum chemical methods, including Density Functional Theory (DFT) and Coupled-Cluster (CC) calculations, enable precise prediction of NMR parameters, allowing direct comparison between experimental and simulated spectra for structure determination and verification. − Hybrid QM/MM frameworks extend these predictive capabilities to large biomolecular systems, supporting investigations of protein–ligand interactions and material structures. − Machine learning (ML) algorithms further advance the analysis of extensive data sets, identifying patterns in spectral data and expediting assignments. These advancements reduce dependence on experimental methods, broadening the application of computational NMR to diverse chemical and biological systems.
Quantum chemical methods, while precise, impose substantial computational costs, particularly for large or conformationally diverse molecules. Machine learning models trained on extensive compound databases mitigate this by automating peak assignments in small-molecule characterization and predicting quantum-level chemical shifts with reduced computational effort. − Deep learning further enhances nonlinear modeling between molecular structures and spectra, improving speed and accuracy. − Molecular dynamics (MD) simulations, integrated with NMR data, provide insights into biomolecular motions, aiding studies on protein folding, ligand binding, and enzyme catalysis. − By interpreting parameters like nuclear Overhauser effects (NOEs) and relaxation times, MD enables flexible or disordered protein modeling. , Computational methods integrate diverse NMR experiments with biophysical techniques such as cryo-electron microscopy and X-ray crystallography, while Bayesian inference and probabilistic models fuse heterogeneous data sets for atomic-level resolution. − These approaches expand NMR’s applicability to biomolecules, small molecules, and materials. The growing role of computational NMR continues to reshape structural and functional analysis, enhancing efficiency, accuracy, and applicability across structural biology, metabolomics, and materials science.
Beyond these core approaches, Quantitative Structure–Activity Relationship (QSAR) modeling has proven valuable in chemoinformatics and pharmacology, predicting molecular properties from structural features and linking NMR data to functional outcomes. The integration of QSAR methods with NMR has been instrumental in studying ligand binding, drug discovery, and structural biology workflows. ,
This review explores how computational methods are driving advancements in NMR, integrating quantum chemical models and ML-driven approaches to expand the field’s applicability across structural biology, chemistry, pharmacology, and materials science. By highlighting their unique contributions and synergies, we demonstrate how computational NMR is transforming molecular structure and dynamics studies. Current limitations and future opportunities are discussed to guide researchers in adopting and advancing these tools. Whether applied to metabolite characterization, protein structure elucidation, or materials research, computational NMR continues to bridge experimental data with molecular insights, offering an increasingly powerful framework for diverse scientific applications.
2. Quantum Chemical Methods in Computational NMR
2.1. Overview of DFT’s Role in Predicting Chemical Shifts and Coupling Constants
DFT has established itself as an important tool in computational NMR, offering a balance between computational efficiency and accuracy. , By accurately modeling electronic structures, DFT excels in predicting essential NMR parameters such as chemical shifts and coupling constants, which are critical for spectral interpretation and molecular structure elucidation. Its reliability in handling a diverse range of chemical systems, from small organic molecules to complex biomolecules and materials, has made it a cornerstone method in computational spectroscopy. DFT predictions not only enhance the understanding of experimental spectra but also facilitate the characterization of novel compounds, the elucidation of complex reaction mechanisms, and the optimization of molecular properties for applications in chemistry, biology, and materials science. Furthermore, DFT methods have shown remarkable stability and precision even in challenging cases, such as transition-metal complexes with strong correlation effects, thereby broadening the scope of NMR applications. As advancements in computational power and methodologies continue to refine DFT-based approaches, its integration with experimental NMR is poised to uncover new molecular insights and accelerate progress across multiple scientific disciplines.
In contrast to other spectroscopic and analytical modalities such as mass spectrometry (MS) or chromatography, NMR spectroscopy stands out for its intrinsic computability from first principles. − The chemical shifts and J-couplings observed in NMR are directly linked to a molecule’s electronic structure, making them highly amenable to accurate predictions using quantum chemical methods. Once these parameters are computed-typically using DFT or high-level wave function-based approaches, the entire spectrum can be constructed using density matrix formalism, capturing spin dynamics for any type of 1D or multidimensional NMR experiment. This theoretical completeness is not paralleled in MS or chromatography, where predictive modeling of fragmentation patterns or retention behavior remains largely empirical. , The rigorous quantum mechanical foundations of NMR thus make it uniquely suitable for full spectral simulation and structural verification workflows, enabling direct comparison between computed and experimental data. Kupce and Freeman introduced an integrated NMR acquisition sequence that enables the simultaneous collection of 13C–13C INADEQUATE, multiplicity edited 13C–1H HSQC, HMBC, and standard 13C spectra by utilizing parallel receiver channels and cryogenically cooled probes. This approach streamlines the acquisition of multidimensional NMR data for structural characterization of small molecules. Their methodology, termed PANACEA, , established a workflow in which structural features can be determined directly and reproducibly in a single experiment, under consistent sample conditions, without the need for fragmented data acquisition or retrospective measurements.
MS/MS fragmentation patterns, by comparison, depend on kinetic pathways that are context-dependent, often involving rearrangements, neutral losses, or charge migration phenomena that are challenging to simulate from first principles. Similarly, chromatographic retention is governed by limited characterization of molecular-surface and solvent–solute interactions, which are difficult to generalize or model mechanistically. Even modern ML-based models for LC-MS fragmentation or chromatographic retention often rely on large, system-specific training data sets and lack true first-principles interpretability. By comparison, the ability to simulate NMR spectra solely from computed chemical shifts and scalar couplings has enabled the development of comprehensive simulation platforms. For example, Bak et al. introduced SIMPSON, a general simulation package for solid-state NMR capable of modeling pulse sequences and anisotropic interactions in powdered samples. Smith et al. developed GAMMA, an object-oriented simulation library designed to represent complex spin systems in both solution and solid-state regimes. Hogben et al. advanced this further through the Spinach library, , which incorporates large-scale Liouville space reductions and advanced relaxation modeling to simulate realistic NMR observables efficiently. These simulation frameworks demonstrate the extent to which NMR data can be reconstructed directly from quantum-derived observables, in contrast to the largely heuristic approaches required for MS and chromatography. Recent ML-enhanced protocols such as CASCADE and J-DP4 , further leverage computed NMR parameters to resolve stereochemistry, predict spectra, and benchmark structural candidates with minimal experimental input. The deep theoretical integration, predictive accuracy, and simulation completeness of NMR spectroscopy place it at a distinct computational advantage over other widely used analytical technologies.
2.2. Theoretical Background of NMR Chemical Shifts
To facilitate the analysis of NMR data independent of experimental conditions, the concept of chemical shifts, expressed on the δ-scale, was introduced. ,− This dimensionless parameter represents the relative resonance frequency of nuclei in a sample compared to a reference standard. Mathematically, chemical shifts are defined as the ratio of the frequency difference between the sample and the reference (measured in Hz) to the operating frequency of the NMR spectrometer. Consequently, δ values are expressed in parts per million (ppm), providing a consistent and universally comparable metric for NMR data interpretation.
The chemical shift, δ, is computed using the formula established by the International Union of Pure and Applied Chemistry (IUPAC), which relies on the isotropic absolute NMR shielding constants of the sample (σ sample ) and the reference (σ ref ) The general equation is expressed as
However, in units of parts per million, a simplified equation is used for calculating chemical shifts:
The IUPAC equation ensures precision by correlating the intrinsic electronic environment of nuclei with their resonance frequencies, independent of the spectrometer’s field strength. Chemical shifts offer critical insights into the local electronic and chemical environments of nuclei. Variations in δ values reflect differences in electron density, molecular geometry, and intermolecular interactions, making this parameter relevant for structural elucidation, functional group identification, and dynamic studies in NMR spectroscopy. −
2.3. Chemical Shifts with DFT
DFT provides a robust framework for calculating these shifts by approximating molecular electronic structures through exchange-correlation functionals, as developed by Becke and Lee et al., − and later improved with range-separated hybrids and meta-GGAs. , By capturing electronic shielding effects, DFT enables accurate predictions of shifts for nuclei like 13C, 1H, 15N, and 17O, essential for organic and biomolecular systems. Solvent effects, modeled using approaches like the Polarizable Continuum Model (PCM), enhance alignment with experimental data, particularly for polar molecules. Early work by Bieger et al. introduced the uncoupled perturbation treatment (UCPT) for magnetic shielding, advancing DFT’s application despite limitations in current density functional theory. These developments solidify DFT’s role in interpreting chemical shifts and supporting structural elucidation across diverse chemical systems.
2.4. Coupling Constants and DFT
Coupling constants (J-couplings), critical parameters in NMR spectroscopy, reveal bonding interactions mediated by electrons and molecular geometry. Ramsey’s framework decomposed J-couplings into Fermi-contact (FC), paramagnetic spin–orbit (PSO), diamagnetic spin–orbit (DSO), and spin-dipole (SD) terms, with the FC most often the dominant term for light nuclei. DFT, through coupled-perturbed Kohn–Sham (CP-KS) and finite perturbation theory (FPT), enables accurate predictions by modeling electron density and orbital effects. , Studies by Ziegler et al. and Barone et al. , have demonstrated DFT’s success in challenging cases, including lone-pair nuclei and dispersion-bound complexes. Solvent and relativistic effects, as noted by Mennucci et al., , further enhance accuracy. The interaction between nuclear magnetic moments and electrons is described by Ramsey’s nonrelativistic approach as a sum of four perturbative operators. Mathematically, the Hamiltonian (H) is expressed as
| 1 |
In this framework, the factors γ N and γ M denote the gyromagnetic ratios of nuclei N and M, describing their magnetic moment per unit angular momentum. The spin operators I N and I M account for the nuclear spin interactions in the system. The total spin–spin coupling is decomposed into four key contributions: the Fermi-contact (FC) interaction h N , the diamagnetic spin–orbit (DSO) interaction h NM , the paramagnetic spin–orbit (PSO) contribution h N , and the spin-dipole (SD) term h N . The spin–spin coupling tensor (JNM) is expressed as
| 2 |
E represents the electronic energy of the system. Evaluating NMR spin–spin couplings requires determining the first-order correction to the electronic ground state, which can be derived using quantum chemical approaches. Autschbach et al. explored tensorial J-coupling properties in anisotropic environments like liquid crystals, underscoring DFT’s versatility and accuracy in diverse systems. −
2.5. Gauge-Including Atomic Orbitals (GIAO)
The shielding tensor (σ), a second-order property fundamental to NMR spectroscopy, has been extensively studied through quantum chemical calculations. Among the most impactful methodologies for predicting chemical shifts is the Gauge-Including Atomic Orbitals (GIAO) approach, introduced by Ditchfield and refined by Friedrich et al. within the framework of DFT. By addressing gauge invariance, GIAO enables precise modeling of molecular systems under external magnetic fields, ensuring accurate predictions of chemical shifts. Early implementations, limited by minimal basis sets and the X a approximation, have evolved with modern exchange-correlation functionals and computational techniques, significantly enhancing accuracy. GIAO’s ability to incorporate the magnetic vector potential into atomic orbitals minimizes errors due to gauge origin selection, outperforming earlier methods like individual gauges for localized orbitals (IGLO) − Its accuracy for nuclei such as 1H, 13C, 15N, and 31P has been widely demonstrated, particularly in resolving stereochemistry and structural ambiguities in organic and natural product chemistry. −
Applications of GIAO extend beyond organic systems to enzyme active sites, protein–ligand interactions, and metalloproteins where paramagnetic effects pose challenges. , The method’s reliability is further enhanced by relativistic corrections, enabling accurate chemical shift predictions for heavy nuclei and transition metal complexes. , Advances in linear-scaling algorithms and computational power have broadened GIAO’s scope to include biomolecules and complex materials. While challenges persist in strongly correlated systems, hybrid approaches integrating DFT with post-Hartree–Fock methods are addressing these limitations. Notably, as the importance of relativistic effects becomes increasingly recognized particularly for elements beyond the third period, the integration of relativistic corrections into GIAO frameworks represents a crucial step forward. The continuous evolution of the GIAO methodology reinforces its important role in chemical shift analysis, supporting structural elucidation across diverse chemical and biological systems. −
2.6. Relativistic Corrections in GIAO-Based NMR Calculations
While the GIAO methodology has been successful in predicting chemical shifts across molecular systems, its accuracy becomes limited when relativistic effects are significant. This holds for molecules containing heavy atoms, where relativistic scalar and spin–orbit contributions alter magnetic response properties. As a result, the inclusion of relativistic corrections has shifted from a refinement to a standard component of quantum chemical NMR simulations. Pioneering work by Pyykko and co-workers , introduced a fully relativistic framework for magnetic properties, notably reinterpreting Ramsey’s theory , of spin–spin coupling through Dirac-Hartree–Fock formalism. This early effort highlighted both the isotropic and anisotropic enhancements in the J-coupling tensor for organomercury compounds, underscoring the breakdown of nonrelativistic partitioning into contact, dipolar, and orbital terms. In subsequent reviews, Pyykko emphasized that relativistic effects arise from orbital contraction and expansion, with consequences not only for heavy nuclei but also their lighter bonded neighbors. Such perturbations of the electron density directly influence shielding and spin–spin interactions in both direct and indirect ways.
The total magnetic shielding tensor, σ, in the relativistic regime, is commonly decomposed as
| 3 |
where σ non –rel is the nonrelativistic shielding tensor typically obtained using GIAO within Hartree–Fock or DFT, Δσ scalar accounts for scalar-relativistic corrections such as mass-velocity and Darwin terms, and Δσ SO represents contributions from spin–orbit coupling. These corrections reflect modifications to the electronic structure induced by the relativistic increase in nuclear potential, becoming significant especially for elements beyond the third period. To treat relativistic effects within an efficient and chemically accurate framework, several approximate Hamiltonians have been developed. Among them, the Zeroth-Order Regular Approximation (ZORA) is widely used and expresses the relativistic Hamiltonian as
| 4 |
where V is the scalar potential, p⃗ is the momentum operator, and c is the speed of light. This formulation results from a first-order expansion of the relativistic kinetic energy operator, capturing dominant scalar relativistic corrections without explicitly including spin–orbit terms. It has been successfully applied in systems such as halosilanes, where the presence of heavy halogens like iodine induces large shifts in the NMR parameters of the central silicon atom.
The Douglas–Kroll–Hess (DKH) transformation offers a hierarchy of scalar relativistic approximations by block-diagonalizing the Dirac Hamiltonian. Commonly used levels such as DKH2 or DKH3 provide a balance between accuracy and computational cost and are frequently implemented in conjunction with GIAO methods. , The integration of the DKH transformation with GIAO has been established as a robust approach for the calculation of NMR shielding tensors, particularly for systems in which relativistic effects are pronounced. In this methodology, the DKH transformation is employed to systematically decouple the Dirac Hamiltonian, yielding a quasi-relativistic Hamiltonian that retains the essential scalar-relativistic corrections required for accurate electronic structure descriptions of heavy-element compounds. Subsequently, the GIAO technique is applied to ensure gauge-origin invariance in the evaluation of magnetic properties, thereby eliminating artifacts associated with the choice of origin in the presence of an external magnetic field. Through this combined framework, relativistic corrections are incorporated variationally, and the shielding tensor is obtained by analytical differentiation of the electronic energy with respect to the external magnetic field and the nuclear magnetic moment. The resulting approach has enabled the reliable prediction of NMR shielding constants for transition-metal and lanthanide complexes, where both electron correlation and relativistic effects are significant contributors to the observed chemical shifts.
The nuclear magnetic shielding tensor can be expressed as the second-order mixed derivative of the total electronic energy E with respect to the external magnetic field B⃗ and the nuclear magnetic moment μ⃗ A :
| 5 |
Through this response-theoretic formulation, relativistic corrections can be incorporated directly via the energy functional or through response terms, depending on the method employed. Importantly, the exact two-component (X2C) approach enables simultaneous inclusion of both scalar and spin–orbit effects, yielding results comparable to four-component Dirac methods but with substantially reduced computational demand. Studies involving nuclei such as 195Pt, 199Hg, and 103Rh consistently show that relativistic effects can alter computed chemical shifts by hundreds of ppm, further validating the necessity of these corrections.
The integration of relativistic treatments into GIAO-based NMR calculations has expanded the applicability of computational spectroscopy to systems where relativistic effects influence magnetic response properties. These methods support consistent predictions across the periodic table, including organometallics, heavy-element compounds, and bioinorganic systems, where nonrelativistic approaches lead to systematic errors. The incorporation of these corrections into GIAO frameworks has become a foundational element of predictive NMR protocols, allowing for accurate evaluation of shielding constants and coupling interactions in molecules containing both light and heavy elements.
2.7. Coupled-Cluster and Other High-Level Methods
Coupled-Cluster (CC) theory, introduced by Cizek , and advanced by Bartlett and collaborators, − is renowned for its exceptional accuracy in electronic structure calculations and its pivotal role in computational NMR. − By expanding the wave function as a linear combination of Slater determinants, CC methods efficiently capture correlation energy through connected excitations, as demonstrated by Shavitt and Stanton. Widely used models such as CCSD and CCSD(T) accurately capture electron correlation effects, with CCSDT and CCSDTQ extending this framework to include higher-order excitations, as highlighted by Crawford and Piecuch. , The equation-of-motion CCSD (EOM-CCSD) approach, introduced by Perera and Bartlett, facilitates accurate spin–spin coupling constant predictions, particularly for systems with delocalized electron densities and shielding effects. Stanton and Gauss developed the analytic second-derivative implementation of CCSD, enabling precise computation of indirect spin–spin coupling constants. − These computationally intensive methods, though limited to small molecules, are invaluable for benchmarking other approaches, validating experimental data, and resolving complex spectra, as shown by Kállay, Gauss, and Auer. ,
Coupled-Cluster methods have proven instrumental in studying unusual bonding situations, particularly in heavy-element systems where relativistic effects influence NMR observables. Gauss and co-workers extended the CCSDTQ analytical derivative approach to explore bonding intricacies in organometallic and transition metal complexes, while Neese and colleagues developed parallel algorithms and the DLPNO–CCSD(T) method, , significantly reducing computational costs and enabling CC-level accuracy for larger systems. The integration of CC methods with molecular dynamics (MD) simulations, as demonstrated by Hättig and Piecuch, has allowed dynamic modeling of conformational flexibility, solvent effects, and thermal motion, providing NMR parameters that align closely with experimental data. , Beyond CC theory, the second-order polarization propagator approximation (SOPPA), introduced by Geertsen and Oddershede, offers efficient spin–spin coupling constant calculations, particularly for larger molecules. Variants such as SOPPA(CCSD), employed by Wigglesworth , and Åstrand, address vibrational effects and provide a complementary alternative to CC methods when computational efficiency is critical. These advancements highlight the evolving versatility and accessibility of high-level quantum chemical methods in computational NMR.
Multiconfigurational self-consistent field (MCSCF) methods, such as the complete active space SCF (CASSCF) approach, introduced by Vahtras et al., are relevant for systems with substantial static correlation, achieving accurate results for small molecules like acetylene (C2H2). The restricted active space SCF (RASSCF) extends MCSCF capabilities , by incorporating partial dynamical correlation, as demonstrated by Jordan et al., who combined it with vibrational averaging to study spin–spin coupling in cyanamide derivatives. Full configuration interaction (FCI) theory, the benchmark for QM calculations, inherently accounts for all electronic excitations and has been invaluable for small systems, as highlighted in studies by Crawford et al. However, its steep computational scaling limits practical applications, leading to approximations such as the DLPNO–CCSD(T) framework, which extends FCI-level precision to larger molecules. Together, these high-level methods, supported by vibrational corrections, continue to advance computational NMR, balancing accuracy and scalability in complex systems.
2.8. Applications and Challenges of QM Methods in Computational NMR
Quantum chemical methods are useful in interpreting and predicting NMR spectra, facilitating structural determination and molecular dynamics studies. A key application is structure determination, where quantum methods correlate experimental NMR parameters with molecular geometry. Predictions of chemical shifts and coupling constants are vital for verifying or refining molecular structures, particularly in natural product chemistry, where DFT-based 1H and 13C shift predictions help resolve ambiguities in complex spectral data.
The application of QM methods has been used for spectral assignments where experimental NMR data are insufficient for unambiguous interpretation. Facelli employed DFT calculations to revise the 13C and 15N chemical shifts in methyl bacteriopheophorbide-a (MBPheo-a) and methyl bacteriochlorophyll-a (BChl-a). Using optimized molecular geometries, the calculated 13C chemical shifts showed significantly better alignment with experimental data, enabling the reassignment of pyrrolic ring carbons separated by over 5 ppm. This highlighted the importance of geometry optimization, including proton positions, in improving agreement between computed and experimental values. However, 15N shifts exhibited poorer agreement, emphasizing the need to incorporate environmental effects, such as solvent interactions. Facelli’s study demonstrated the sensitivity of computed shifts to geometry and provided critical evidence for spectral reassignment, advancing the use of quantum chemical methods in NMR analysis.
Since the application of perturbation theory to NMR properties by Ramsey over 70 years ago, QM methods for the computation of NMR parameters have seen significant advances. Numerous reviews highlighting these advances have covered the theoretical and practical aspects of these improvements. Kaupp, Buhl, and Malkin edited a comprehensive book on the calculation of NMR and EPR parameters, providing a foundation for future work. Casabianca and de Dios published a detailed review in 2008, focusing on ab initio NMR chemical shift calculations. Bryce and Wasylishen contributed an extensive review of NMR parameter computations using ab initio methods, while Oldfield reviewed chemical shielding calculations on proteins, peptides, and amino acids, highlighting their relevance in structural biology. Facelli’s review provided insights into the applications of chemical shift calculations across various systems. Key contributions to the development of quantum chemical methods for NMR parameter calculations were reviewed by Gauss et al., Fukui et al., Helgaker et al., Aucar et al., Autschbach et al., Rusakov et al., Facelli, and others. ,, These works have collectively established the computational framework for NMR studies. The early implementations of coupled-cluster theory for NMR parameter calculations, including CCSDT, CCSD(T), and CC3, were pioneered by Auer and Gauss, with later improvements extending computations to larger systems.
Significant advancements have also been made in addressing solvent and electrostatic effects on chemical shifts. Benzi et al. and Bagno et al. , incorporated solvent effects, while Oldfield , applied electrostatic effects to protein structure determination. Tossell used cluster models to compute shielding values for crystals, and Sebastiani reviewed chemical shift calculations in condensed phases. Relativistic effects, crucial for heavy-element compounds, have been explored by Pyykkö and Nakatsuji et al., with applications including spin–orbit coupling and finite-perturbation theory. , Applications of QM methods extend to the determination of relative stereochemistry, regioisomer assignments, and structural elucidation of peptides, proteins, and nucleic acids. Notably, Oldfield pioneered the use of computed chemical shift data for structural information on peptides and proteins. Bryce and Sward summarized quadrupolar halogen studies using solid-state NMR. − Tantillo et al. reviewed the use of ab initio and DFT methods for chemical shift prediction, emphasizing their application to complex molecular systems. Renslow et al. highlighted the role of QM computations in metabolomics, discussing their impact on the omics community for structural predictions. Benchmarking studies on the accuracy of chemical shift predictions, conducted by various researchers, have underscored the need for reliable computational methods. Extensive summaries of advancements in evaluating chemical shielding values have been provided by Jameson and de Dios, , further emphasizing the field’s depth and breadth. This wealth of literature reflects the importance of computational NMR in addressing structural challenges and advancing our understanding of molecular systems.
Sychrovský and co-workers extensively analyzed scalar spin–spin coupling constants (SSCCs) in guanosine and deoxyguanosine using DFT, focusing on their geometrical and solvent-dependent behaviors. The study addressed limitations of empirical Karplus equations, proposing theoretical parametrization to incorporate detailed geometry and solvent effects for improved accuracy. Using CP-DFT with the B3LYP functional, relationships between sugar pucker, glycosidic torsion angles, and SSCCs were established. 1J(C1̀-H1̀) couplings ranged from 154 to 170 Hz, with maxima near anti conformations at X ≈ 180°, consistent with experimental values reported by Kline and Varani and Tinoco. − Variations under solvation and solute–solvent interactions were modeled, enhancing structural interpretations and refining empirical models for nucleosides. Sychrovský’s work highlights quantum chemical calculations as a key tool for refining NMR interpretation and predicting structural details in nucleic acids. ,
The relationships between 31P chemical-shift tensors and backbone conformation in nucleic acids were examined by Přecechtělová et al., focusing on dimethylphosphate (dmp) and dinucleoside-3′,5′-monophosphates. , DFT calculations, both in vacuo and with explicit solvation, revealed significant reductions in chemical shift anisotropy (CSA) due to solvation, with variations up to 30 ppm in principal components δ22 and δ33 linked to torsion angles α and ζ. The isotropic shift (δ iso ) for the gg conformation in solvent aligned with experimental data, affirming dmp as a reliable model. Similarly, Van Mourik and Dingley utilized DFT to study hydrogen-bonding regions in guanine quartets (G-quartets), revealing dependencies of trans-hydrogen-bond couplings and isotropic shifts on bond length and geometry. , Li+, Na+, and K+ ions showed distinct preferences for stabilization positions, with K+ exhibiting higher energy barriers for channel transport, linked to DNA quadruplex dynamics. These studies demonstrate the power of DFT in unraveling the structural and dynamic properties of nucleic acids, offering insights into conformational effects and mechanisms of stabilization.
The structural reassignment of serlyticin-A, initially isolated from Serratia ureilytica and previously identified for its antioxidative and antiproliferative properties, demonstrates the key role of advanced methods in helping to resolve inaccuracies in natural product characterization. In a recent study, Ka Yi Tsui et al. utilized QM calculations to predict NMR chemical shifts, revealing significant discrepancies between the reported experimental data and the originally proposed structure (structure 1). Structural optimizations and frequency calculations using Gaussian at B3LYP/6-31+G(d,p) , and PCM(MeOH)-B3LYP/6-31+G(d,p) − indicated the thermodynamic instability of the initial assignment. This led to the proposal of alternative structures, such as 3-indolylacetohydroxamic acid (structure 4), which were subsequently validated experimentally. As summarized in Figure , deviations between calculated and experimental 13C and 1H chemical shifts were substantially reduced for structure 4, falling within acceptable limits (≤6 ppm for 13C and ≤ 0.3 ppm for 1H), thus supporting the reassignment. For unsymmetrical dimers, averaged shifts from the two monomers were reported. Another recent study by Armando Navarro-Vázquez (2024) focused on the structural revision of marinoaziridines A and B, originally proposed as aziridine alkaloids. Using a combination of computer-assisted structural elucidation (CASE) and DFT-based chemical shift predictions, including GIAO/PBE0/def2-TZVP with ORCA and CPCM solvation models, , the structures were reassigned as pyrroloquinoline alkaloids. This study emphasized the utility of tailored NMR data analysis in structural elucidation. Both works highlight the impact of advanced techniques in addressing structural misassignments, enhancing the accuracy of natural product characterization, and reinforcing their important role in modern structural studies.
1.
Comparison of calculated and experimental NMR chemical shifts. Left: Deviations between calculated shifts for compound 1 and its experimental values. Center: Deviations between calculated shifts for compound 4 and previously reported experimental data for serlyticin-A. Right: Deviations between calculated and newly determined experimental shifts for compound 4. Deviations are considered acceptable if within 6 ppm for 13C and 0.3 ppm for 1H, values exceeding these thresholds are highlighted in bold. For unsymmetrical dimers, chemical shifts were averaged across monomer units. Reproduced with permission from reference . Copyright (2019) American Chemical Society.
The accurate treatment of solvation has been established as a crucial factor in the quantum chemical prediction of NMR chemical shifts. , Solvent molecules alter the electronic environment of nuclei through dielectric polarization, hydrogen bonding, and short-range specific interactions, which can strongly perturb nuclear shielding, particularly for labile nuclei such as 1H, 15N, and 31P. Implicit solvent models including the Polarizable Continuum Model (PCM), , Integral Equation Formalism PCM (IEF-PCM), and the Conductor-like Screening Model (COSMO) are widely employed for their computational efficiency and ability to approximate bulk electrostatic effects. However, such continuum approaches are often insufficient for capturing directional interactions and dynamic solvent fluctuations near polar functional groups. To address these limitations, explicit solvent models and hybrid QM/MM schemes, have been increasingly adopted to account for local solvent structure and thermal averaging. These strategies enable a more realistic description of solute–solvent interactions and are especially beneficial in systems where hydrogen bonding and conformational flexibility significantly influence the observed chemical shifts.
A recent computational study by Da Silva et al. (2025) has further underscored the critical role of solvent modeling in the accurate prediction of NMR chemical shifts for complex molecular systems. Using azithromycin (AZM), an antibiotic characterized by significant conformational flexibility and multiple polar functional groups as a test system, QM calculations were performed by combining DFT with PCM solvent model to evaluate the influence of solvation on both relative conformer energies and NMR parameters. In addition to continuum solvation, explicit solvent molecules (chloroform, water, and DMSO) were incrementally introduced around key hydrogen bonding sites. It was shown that this hybrid approach significantly improved the agreement between theoretical and experimental NMR chemical shifts, particularly for 1H nuclei involved in hydrogen bonding, such as hydroxyl protons. The explicit solvent models were found to better capture local electrostatic interactions and directional hydrogen-bonding patterns that are otherwise neglected in continuum-only approaches. The use of just five explicit solvent molecules placed at strategic positions, in combination with PCM, was shown to be sufficient for achieving chemically accurate predictions at a manageable computational cost. These results provide a practical guideline for balancing efficiency and accuracy in solution-phase NMR calculations. To illustrate this point, the effect of explicit solvation on predicted NMR accuracy and conformer energetics is summarized in Figure . This study reinforces that the inclusion of explicit solvent molecules in conjunction with implicit models is often necessary for the reliable computational elucidation of molecular structure, particularly in natural products and biomolecules with labile protons and polar functional groups.
2.
Relative electronic energies (ΔE rel ) and RMSD for four conformers of azithromycin (AZM-I to AZM-IV), optimized using PCM with chloroform and various numbers of explicit CHCl3 molecules (n = 0–75). (a) Relative energies calculated at the ωB97x-D/6-31G(d,p)-PCM level. (b) RMSD values for calculated vs experimental 1H NMR shifts (all protons included). (c) RMSD for 13C NMR shifts. (d) RMSD for 1H NMR considering only CHn protons. Explicit solvation effects increasingly stabilize AZM-III and AZM-IV, with 1H NMR profiles showing enhanced agreement with experiment upon inclusion of up to 75 solvent molecules. While relative energies are strongly impacted by solvation, NMR RMSD values remain robust across solvation models, underscoring their reliability for conformer assignment in solution. Reproduced with permission from reference . Copyright (2025) American Chemical Society.
Numerous recent studies have emphasized the role of solvent modeling in improving the accuracy of computed NMR chemical shifts across a wide range of molecular systems. In one study, Cseri and co-workers conducted an experimental and computational investigation of 1H and 13C shifts in green solvents, acids, and organocatalysts, using the IEFPCM model in DMSO. While 13C shifts were reasonably captured, large errors were observed for 1H nuclei in protic and polar species, where continuum models failed to describe localized solute–solvent hydrogen bonding. These results highlighted the limitations of implicit solvation and pointed to the need for hybrid schemes. Live and colleagues examined 15N shifts in gramicidin S and found both local and long-range solvent influences, with protic solvents inducing downfield shifts via H-bonding and disruption of intramolecular H-bond networks. Temperature dependent experiments further revealed solvent modulated couplings across H-bonded amide chains, illustrating solvent’s role as both a direct interaction partner and a remote electrostatic modulator. In another systematic effort, Cohen et al. developed the DELTA50 benchmark data set and compared PCM, CPCM, and SMD models for 1H and 13C shift predictions in drug-like molecules. The SMD model showed improved performance, especially for functional groups involved in hydrogen bonding, emphasizing the importance of solvent model selection. Gao et al. evaluated 11B shift predictions in boron-containing species solvated in THF and showed that including solvent via SMD or CPCM significantly reduced RMSD, even in geometrically constrained systems. De Andrade’s group focused on 99Tc shielding in metal complexes and demonstrated that solvation using the COSMO model was necessary to reproduce deshielding trends in polar media, revealing the sensitivity of metal centered shifts to dielectric screening. Ab initio MD with explicit DMSO solvation was applied to Pt(III) dinuclear complexes by Batista et al., revealing that variations in Pt–N/O bond distances influenced 195Pt shifts effects that were not reproduced by implicit solvation models. Local orbital analysis further revealed that solvent polarization alters virtual orbital energies, driving deshielding. A fully explicit QM/MM protocol using COBRAMM was employed by Calcagno and co-workers to compute 31P shifts in aqueous environments. Their approach sampling hundreds of MD snapshots captured polarization and solvent fluctuation effects more accurately than cluster based or implicit treatments, advocating for explicit embedding in polar phosphorus systems. These studies show that accurate solvent modeling, whether using improved implicit models or fully explicit QM/MM frameworks, is essential for reliable and transferable NMR shift predictions.
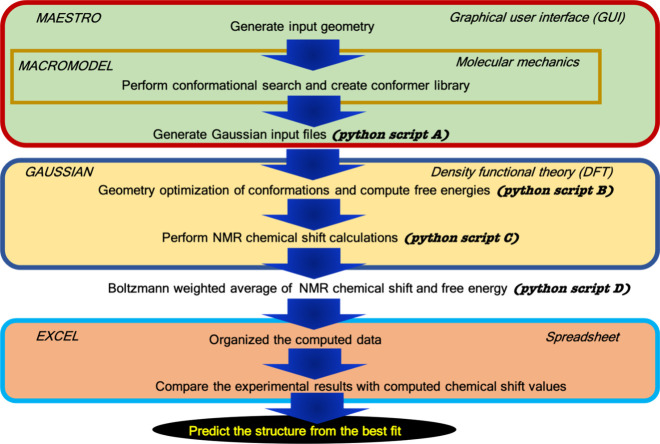
The development of robust computational workflows for NMR chemical shift prediction has significantly advanced structural determination in chemistry, particularly for complex organic molecules. Willoughby et al. , introduced a protocol designed to compute 1H and 13C NMR chemical shifts for structural validation, emphasizing accessibility for bench chemists with limited computational expertise. The workflow involves generating conformers using molecular mechanics software like MacroModel, , followed by geometry optimization and frequency calculations via DFT with the Gaussian software. NMR shielding tensors are computed for each conformer, and Boltzmann-weighted chemical shifts are derived for comparison with experimental data, as outlined in Figure . This approach addresses constitutional and configurational ambiguities, enabling accurate structural assignments for natural products and synthetic compounds. The modular design allows adaptation to complex systems, despite challenges like identifying numerous conformers, extended computational times, and handling multiple candidate structures. By integrating computational rigor with practical usability, the protocol bridges experimental and computational methods, enriching molecular characterization with accurate and reproducible insights.
3.
Schematic protocol for NMR chemical shift prediction as developed by Willoughby et al. The approach integrates molecular conformer generation, DFT-level NMR shift calculations, and statistical analyses to assign shifts to candidate structures. Reproduced with permission from reference . Copyright (2021) American Chemical Society.
Senanayake et al. introduced a workflow combining DFT computations with empirically derived error correction terms, providing a robust method for 13C NMR chemical shift prediction. The procedure (Figure ) begins with candidate structures proposed based on experimental data, followed by conformational searches using MOE , and the LowModeMD algorithm to identify potential energy minima. Conformers undergo geometry optimization in Gaussian. Solvation effects are accounted for using CPCM in solvents such as DMSO, chloroform, and methanol. , 13C shielding constants are computed via the GIAO method, converted to chemical shifts relative to TMS or scaled with linear regression. Employing B3LYP/cc-pVDZ, the method balances accuracy and computational cost, achieving high reliability with systematic error corrections for functional groups. The workflow accelerates structure elucidation, particularly for pharmaceutically relevant compounds, by integrating computational predictions with experimental data to refine molecular characterizations. Its accessibility, combined with user-friendly tools, helps to bridge the gap between experimental and computational approaches.
4.
Workflow for computing NMR chemical shifts as described by Senanayake et al. The protocol involves conformer generation, geometry optimization, vibrational analysis, and DFT-based shielding tensor calculations, followed by Boltzmann weighting and comparison to experimental data. Reproduced with permission from reference . Copyright (2017) American Chemical Society.
Several automated approaches and workflows for predicting NMR-active nuclei chemical shifts have been developed to assist nonexperts in computational NMR, relying on high-level QM methods to produce accurate results. However, their computational cost limits applicability to large data sets, and complex molecules, such as metabolites with numerous rotatable bonds, which challenges existing workflows due to extensive conformational spaces. Current methods often lack validation for such cases, highlighting the need for a high-throughput, automated workflow capable of accurate predictions across diverse structures. Developing such methodologies would enable comprehensive NMR data libraries for complex molecules, advancing structural elucidation and large-scale metabolomics studies.
Merz et al. introduced an efficient computational workflow for predicting NMR chemical shifts of medium-sized organic molecules, including metabolites, with reduced computational costs and high accuracy. The workflow, depicted in Figure , combines force field, machine learning, and quantum mechanical methods, employing steps such as conformation generation, ML-based filtering using the ASE-ANI model, , clustering, DFT-based optimization, and chemical shift calculation. ,, This modular protocol, executed through a series of Python scripts, supports user-defined combinations of DFT theory, solvent models, and NMR-active nuclei. For high-throughput applications, the workflow can be fully automated using managers like Snakemake , or the Galaxy web server, enabling seamless data processing and large-scale QM data set generation. By addressing computational efficiency and accuracy, the workflow provides a robust foundation for advancements in structural assignment and computational NMR applications.
5.

Schematic workflow for Boltzmann-weighted NMR chemical shift prediction as outlined by Merz et al. The process includes conformer generation, filtering, clustering, quantum mechanical optimization, and NMR shift calculations, followed by Boltzmann-weighted structure assignment. Reproduced with permission from reference . Copyright (2020) American Chemical Society.
While DFT-based calculations provide valuable insights into the dependence of chemical shifts on local geometry, empirical and semiempirical approaches have been more successful in modeling local backbone structures. Hunter et al. highlighted semiempirical methods for chemical shift evaluation, while Merz et al. advanced computational NMR through semiempirical and QM/MM methods to address challenges in large biological systems, including proteins and protein–ligand complexes. , Semiempirical methods, using AM1 parameters, offer computationally efficient alternatives to ab initio and DFT calculations, enabling the modeling of macromolecules with acceptable accuracy and reduced costs. Merz et al. further enhanced NMR predictions with QM/MM approaches, partitioning systems into quantum and molecular mechanical regions, as demonstrated in the FKBP-GPI protein–ligand complex, where computed shifts aligned closely with experimental data. Their Automated Fragmentation QM/MM (AF-QM/MM) method refined shielding constant calculations, emphasizing conformational flexibility’s role in accuracy. Additionally, the HECSP model and NMRScore_P scoring function, developed to predict ligand-induced protein chemical shift perturbations and refine protein–ligand structures, demonstrated strong correlations with experimental data. These methodologies, depicted in Figure , underscore their usefulness in computational NMR, providing a robust framework for structural insights and therapeutic discovery advancements.
6.
Merz et al.’s workflow for NMR chemical shift computation using NMRScore_P. (A) Workflow for ranking structures within the NMR ensemble. (B) Workflow for ligand structure refinement. (C) Workflow for structure determination. Reproduced with permission from reference . Copyright (2017) American Chemical Society.
While DFT remains the most widely used approach in NMR computation due to its favorable balance between accuracy and efficiency, its limitations become evident in systems with strong correlation, delocalized electrons, heavy atoms, or subtle vibrational and solvent effects. In such cases, DFT often fails to capture the necessary electron correlation and magnetic response properties with sufficient precision. To overcome these challenges, a range of post-DFT methods including MP2, , CCSD/CCSD(T), DLPNO–CCSD, ,, SOPPA, and CASSCF/RASSCF have been employed to achieve higher accuracy and deeper physical insight. The following discussion highlights benchmark studies that demonstrate the advantages, limitations, and computational trade-offs of these high-level methods in NMR applications.
High-level electron correlation methods have played a critical role in advancing the predictive accuracy of NMR computation, particularly for nuclear spin–spin coupling constants (SSCCs) and shielding tensors. In a comprehensive benchmark by Gleeson et al., vibrational corrections to SSCCs were evaluated across a chemically representative set of 21 small molecules including H2O, CH4, NH3, HCN, C2H2, CO, OHF, and OF2 using CCSD, CCSD(T), and a range of cost-effective alternatives such as SOPPA, and SOPPA(CCSD). Vibrational averaging was incorporated using second-order vibrational perturbation theory, and CCSD-based SSCCs combined with CCSD(T) geometries and cubic force fields (denoted CCSD//CCSD(T)) yielded the closest agreement with experiment (MAD ≈ 1.5 Hz). However, this high level of accuracy came with steep computational costs, restricting full CCSD(T) treatment to molecules containing fewer than five heavy atoms due to the extensive demands of force and property derivative calculations. To mitigate this, mixed protocols were tested wherein high-level equilibrium values from CCSD were paired with vibrational corrections from B3LYP, yielding comparably accurate results (MAD = 1.9 Hz) at a fraction of the cost. Yet, SOPPA-based protocols such as SOPPA//MP2 and SOPPA(CCSD)//MP2, while computationally more feasible for larger systems, introduced triplet instabilities and inaccuracies in geometrical configurations that diminished the benefit of including vibrational corrections. Similarly, in the studies by Faber and Sauer, as well as Jessen and Sauer, CCSD and SOPPA(CCSD) were applied to predict 19F SSCCs across a diverse set of fluorinated systems, including CF4, CH3F, CHF3, C2F6, CH2CHF, CH2CF2, and fluorinated heterocycles and fluoroalkenes. These benchmark studies highlighted the importance of high-level correlation methods and vibrational corrections for achieving quantitative agreement with experimental 19F–19F coupling constants. The full CCSD approach, executed with large aug-cc-pCVQZ basis sets, achieved near-experimental accuracy (MAD ≈ 1.5–2 Hz), but remained computationally prohibitive for molecules with more than 10 heavy atoms. To balance fidelity and feasibility, SOPPA-level spin–spin coupling constants were combined with CCSD optimized geometries and CCSD-derived vibrational corrections in composite SOPPA//CCSD + CCSD protocols. , This hybrid strategy preserved predictive accuracy while mitigating the computational expense of full CCSD treatments. However, the known basis set sensitivity of SOPPA and its inconsistent performance for π-conjugated and unsaturated systems underscore its limitations for routine high-accuracy applications. These studies collectively underscored that while coupled-cluster methods are ideal for benchmark level predictions, their practical scope is best extended through judicious method combinations tailored to system size and chemical complexity.
Efforts to improve computational efficiency without sacrificing accuracy have also been demonstrated through local correlation approaches. Among these, the domain-based local pair natural orbital (DLPNO) methods developed by Neese and co-workers have shown considerable promise for spin–spin coupling constant (SSCC) calculations in small to medium-sized molecules. Preliminary applications to halogenated and polar systems suggest encouraging agreement with canonical CCSD results at a fraction of the computational cost. − The key advantage of DLPNO–CCSD lies in its reduced scaling, enabling systems of ∼ 30 atoms to be treated on standard computational infrastructure. Nonetheless, limitations were noted in delocalized systems such as benzene, where the localization of electron correlation induced marginal overlocalization errors. Complementing these coupled-cluster strategies, Da Silva et al. and Dittmer et al. demonstrated the utility of MP2 in modeling NMR parameters across both solution-phase and solid-state systems. Da Silva et al. studied 1H shifts for nitrogen-containing molecules, valerolactam, formyl anilide, N-methylbenzylamine, phenanthridone in CDCl3 using MP2/6-31G(d,p) with GIAO and PCM, revealing substantial errors for N–H shifts (up to −1.8 ppm) when explicit solvent molecules were not included. Upon inclusion of three to four solvent molecules, N–H agreement improved, albeit at the cost of degraded C–H accuracy. Dittmer et al. applied MP2 within an embedded cluster model to predict 13C and 15N shieldings in urea and formamide crystals, showing improved accuracy over periodic DFT but limited by system size and model complexity. For multireference systems, methods such as CASSCF and RASSCF combined with GIAOs have been applied to compute 15N shieldings with improved accuracy. Nottoli et al. implemented a Cholesky-decomposed CASSCF-GIAO scheme capable of treating systems with ∼ 1,300 basis functions. RASSCF offers a scalable alternative by partitioning active spaces (RAS1/2/3) and is suitable for larger systems. Gauge-origin independence remains critical, typically addressed via GIAOs. These approaches help correct SCF-level errors in 15N shifts, which can exceed tens of ppm in strongly correlated systems. Meanwhile, Gendron et al. , used CASSCF with spin–orbit CI to interpret 1H and 13C shifts in 5f actinide cyclopentadienyl complexes, resolving spin–orbit and magnetic anisotropy effects inaccessible to DFT. Despite offering physically grounded insight, both RASSCF and CASSCF approaches remained computationally expensive and highly sensitive to active space definition, limiting their routine use to systems with ≤ 30 correlated orbitals. These studies suggest that while high-level correlated methods are required for reliable NMR modeling, their broader application must be guided by a balance between electronic accuracy and computational cost.
The application of relativistic corrections has become essential in the accurate calculation of NMR parameters, including chemical shifts and J-couplings, particularly for molecules containing heavy elements or those influenced by heavy-atom neighbors. Pyykko eloquently framed the broader significance of these corrections, emphasizing that relativistic effects are “more common than you thought,” and highlighting their role in a wide range of chemical phenomena, from the color of gold to the covalency trends in f-block complexes, as well as systematic errors in computed NMR parameters if neglected. Comprehensive reviews and studies by Autschbach and co-workers have demonstrated that relativistic quantum chemistry methods, including two- and four-component approaches, are now routinely applied to evaluate nuclear magnetic shielding and indirect spin–spin coupling tensors, with DFT providing a practical framework for large and heavy-element systems. Repisky et al. have detailed the implementation and application of these methods, showing that both scalar and spin–orbit effects can be variationally included to achieve accurate NMR parameters for molecules with heavy atoms. Practical improvements in calculated chemical shifts, often by hundreds of ppm, have been documented for transition metal and main-group compounds when quasirelativistic corrections are included, as shown in studies on molybdenum and tungsten complexes. Reviews by Autschbach and others have also cataloged heavy-neighbor effects, where even lighter atoms in proximity to heavy elements exhibit significant relativistic influences on their NMR shifts. ,,− The HALA (heavy-atom effect on light-atom) and HAHA (heavy-atom effect on heavy-atom) mechanisms, as discussed by M. Kaupp, have been used to interpret changes in shielding tensors due to heavy-atom environments. Applications have spanned main-group, transition-metal, and actinide compounds, where relativistic contributions are consistently found to be significant. Early theoretical work, such as that of Ramsey and subsequent developments, established the foundation for incorporating relativistic corrections into magnetic resonance parameter theory. , These applications underscore the necessity of relativistic treatments for reliable NMR predictions in modern computational chemistry.
In summary, QM methods have significantly advanced computational NMR, offering precise insights into structural biology, metabolomics, and materials science. High-accuracy approaches, such as CCSD(T) and DFT, have enabled reliable predictions of chemical shifts and J-couplings, aiding stereochemical resolution, structural prediction, and protein–ligand interaction analysis. However, challenges persist, including high computational costs, limited scalability for large biomolecules, and dependencies on functionals, basis sets, and approximations. Limitations in modeling solvent effects and conformational flexibility further complicate applications. Machine learning models trained on extensive NMR data sets are emerging as a scalable solution, enabling rapid predictions of NMR parameters. The integration of ML techniques represents a transformative step, bridging the precision of quantum chemistry with the scalability required for real-world systems, thus expanding the scope of computational NMR.
3. Machine Learning Approaches in Computational NMR
3.1. Role of Machine Learning
Empirical approaches often neglect stereochemical and conformational effects, limiting their predictive capacity, while quantum chemical methods are computationally prohibitive for large or complex systems due to their resource-intensive nature. To overcome these limitations, machine learning has emerged, offering scalable and efficient solutions for NMR parameter prediction and spectral interpretation. , By leveraging large data sets, ML algorithms identify patterns and correlations, enabling rapid predictions without relying on first-principles calculations. This data-driven approach has demonstrated remarkable success across diverse applications, including small-molecule characterization, , metabolomics, − and structural biology, − while also addressing the computational challenges posed by traditional methods. Furthermore, ML methods have been applied to correlate experimental and calculated NMR data, facilitating structural elucidation and expanding the accessibility of spectroscopic analysis. − As the field continues to evolve, advances in areas such as spin–spin coupling constant prediction, paramagnetic system analysis, and the integration of ML with molecular dynamics simulations are driving new innovations in NMR. We highlight key contributions in this area, focusing on essential studies while acknowledging the breadth of important work in the field. The selected articles provide an overview of the field and highlight future prospects for this rapidly evolving research area.
3.2. Data Resources for ML-Driven NMR Applications
Databases like NMRShiftDB, , the Natural Products Magnetic Resonance Database (NP-MRD), the Biological Magnetic Resonance Data Bank (BMRB), , the Cambridge Structural Database (CSD), , and the CHESHIRE database ,, are essential resources for advancing machine learning applications in NMR spectroscopy. NMRShiftDB, an open-access database, hosts around 400,000 experimental 13C chemical shifts, along with 1H data, covering natural products, synthetic compounds, and biomolecules. Its curated entries include chemical structures, assigned shifts, and metadata such as solvent conditions and reference standards, providing high-quality training data sets that enable ML models to achieve accurate chemical shift predictions, often rivaling quantum chemical methods. NP-MRD offers a vast repository of NMR data on over 281,859 natural products, including 5.5 million spectra, supporting metabolomics and other omics-based research fields, with enhanced spectral search tools facilitating prediction and analysis. BMRB specializes in biomolecular NMR data, providing detailed 1H, 13C, and 15N assignments for proteins, nucleic acids, and metabolites, aiding ML in tackling complex biological systems. The CHESHIRE database, a specialized resource for computed NMR scaling factors, provides 13C and 1H data alongside coupling constants, supporting the integration of chemical shifts into predictive workflows. CSD complements these resources by linking crystallographic and NMR data to refine structural predictions. The newly developed DFT8K data set comprises 8,000 DFT-optimized structures with 200,000 computed chemical shifts. , Other specialized tools, such as GISSMO, , optimize spin system matrices through experimental data alignment. These curated databases with comprehensive metadata provide diverse, contextualized data sets critical for training ML algorithms, fostering collaborative and open-science initiatives.
A distinctive advantage of NMR over other analytical platforms, such as LC-MS or chromatography, lies in its capacity to generate high-fidelity computational data grounded in quantum mechanics. Whereas most ML models in LC-MS must rely on large volumes of experimental data often constrained by noise, limited chemical diversity, or ambiguous annotations, NMR allows for the creation of large-scale, chemically diverse data sets from first-principles calculations. , This stems from the fact that NMR observables, such as chemical shifts and scalar couplings, are directly linked to the underlying electronic structure of molecules and can be accurately computed using DFT, MP2, or CCSD methods. As a result, ML models can be trained on computed data in regimes where experimental data is sparse or unavailable. This strategy has been used effectively in models such as CASCADE and ML-J-DP4, which leverage computed chemical shift data sets to improve spectral prediction and structural assignment. However, computed data may omit important experimental nuances such as conformational averaging, solvent effects, or systematic instrument-related biases. Therefore, hybrid approaches-which combine the breadth and reproducibility of computed data with the accuracy and realism of experimental data sets are emerging as the most effective strategy for training robust, generalizable models. This hybrid paradigm positions NMR as a uniquely computable and scalable foundation for developing next-generation predictive tools.
3.3. Application and Challenges of ML models for NMR Computation
Applications of machine learning in computational NMR span a wide range of methodologies, including supervised learning, − unsupervised learning, deep learning, − and generative models, , each addressing distinct challenges. Supervised methods, such as support vector machines (SVMs), random forests, and deep neural networks (DNNs), predict chemical shifts with high accuracy by leveraging structural descriptors like atomic charges and interatomic distances. − Unsupervised learning techniques, including k-means clustering, hierarchical clustering, and Gaussian mixture models, assist in peak assignment, spectral clustering, and metabolite identification by uncovering hidden patterns in unlabeled data sets. − Dimensionality reduction methods, such as principal component analysis (PCA) and t-SNE, reduce data complexity, facilitating the analysis of high-dimensional NMR spectra. Deep learning models, particularly convolutional neural networks (CNNs) , and recurrent neural networks (RNNs), , excel at processing spectral data and molecular representations (e.g., graphs or SMILES) to predict chemical shifts or classify peaks in noisy, overlapping regions. Generative models, such as variational autoencoders (VAEs) and generative adversarial networks (GANs), enable the generation of novel molecular conformations and synthetic NMR spectra, enhancing training data sets and bridging the gap between 2D and 3D structural data. Hybrid approaches combining QM with ML methods for chemical shift predictions help improve scalability and precision. Deep reinforcement learning (RL) has also been explored for automating structural elucidation tasks. , Despite their advantages - speed, scalability, and robustness to noise - these methods face challenges such as the need for large, high-quality data sets and the interpretability of complex models. Explainable AI methods are increasingly addressing these issues, fostering confidence in ML-driven NMR applications. ,
By utilizing the aforementioned data sets, Paton et al. recently developed a ML model, CASCADE (depicted in Figure ), for NMR chemical shift predictions. The model builds upon three GNN architectures: DFTNN, ExpNN-dft, and ExpNN-ff. The DFTNN model was trained on the DFT8K data set, which includes chemical shifts computed at the mPW1PW91/6-311+G(d,p) level and geometries optimized at the M06–2X/def2-TZVP level. To enhance accuracy, the authors employed transfer learning to retrain DFTNN against 5,000 experimental chemical shifts from the Exp5K data set, resulting in the ExpNN-dft model. However, the need for structure optimization in ExpNN-dft posed a computational bottleneck, which was addressed by ExpNN-ff, where force field-derived geometries (MMFF94) replaced QM-optimized structures. This optimization reduced the CPU time dramatically. Differences between GNN-predicted chemical shifts and DFT-calculated values yielded mean absolute errors (MAE) of 1.26 ppm for 13C and 0.16 ppm for 1H, with ExpNN-ff achieving comparable accuracy to DFT while being 5,000–10,000 times faster. Inspired by Roitberg et al., the authors incorporated high-quality CCSD(T) values and demonstrated that molecular geometries from inexpensive calculations could maintain predictive accuracy. ,,, CASCADE effectively predicts chemical shifts for large, flexible molecules and addresses challenges in structure elucidation, data reassignment, and regioselectivity predictions.
7.
(a) Schematic representation of the GNN architecture used in CASCADE, illustrating the workflow from molecular input to model training and chemical shift prediction. Molecules are encoded based on atom types and interatomic distances, with iterative message-passing used to generate atomic representations for final predictions. (b) Workflow of data set generation and model development: NMR8K contains unchecked experimental chemical shifts and 2D structures; DFT8K augments this with DFT-optimized 3D structures and calculated shifts; Exp5K includes cleaned experimental shifts. Models trained on these data sets enable rapid and accurate 1H and 13C shift predictions. Schematic representation of the machine learning model used in CASCADE, illustrating the workflow from data preprocessing to model training and prediction. Reproduced with permission . Copyright (2021) Royal Society of Chemistry.
Building on the design strategy established in CASCADE, Yang and co-workers proposed a graph neural network architecture that predicts NMR chemical shifts directly from molecular graphs using a message-passing framework, eliminating the need for expert-designed features such as dihedral angles or electronegativity-based descriptors. The model processes molecular fragments where each atom is connected to both covalent and spatially adjacent neighbors through feature-rich edges containing information on bond type and interatomic distance. The architecture comprises edge embeddings, message passing layers with residual connections, and a final readout network that outputs chemical shifts for carbon, nitrogen, and hydrogen atoms. The overall network structure is illustrated in Figure . Unlike earlier models that rely on precomputed torsional data or sequence-derived features, this model is trained end-to-end using only atom types and structural graphs, making it applicable to both macromolecules and small organic species. The authors trained the model using three data sets that include 2405 protein structures from the RefDB database, curated examples from a previously established data set, and 369 metabolites from the Human Metabolome Database. The combined training regime produced over 393,000 protein fragments and several thousand labeled metabolite shifts, allowing the model to generalize across chemically diverse systems.
8.
Graph neural network architecture for NMR chemical shift prediction. The model takes molecular graphs as input, where E0 encodes edge features such as inverse interatomic distances and bond types, and V0 represents the atomic feature matrix based on element types. These inputs are processed through K -MP layers with residual connections, using neighbor list tensors E to propagate local structural information. The resulting atomic representations are passed through L -FC layers, culminating in chemical shift predictions adjusted for element-specific effects. Red and blue blocks represent MP and FC layers, respectively. Reproduced with permission from reference . Copyright (2021) Royal Society of Chemistry.
Performance benchmarks show that the model achieves root mean squared deviations of 0.29 ppm for H α atoms and correlation coefficients approaching 0.88, comparable to or better than existing models under identical input constraints. Furthermore, the model is capable of evaluating one million shifts in approximately five seconds using a single V100 GPU, demonstrating its suitability for high-throughput applications. Importantly, the model captures physically meaningful effects without being explicitly trained on them. It reproduces the downfield shift of H α protons in β sheet structures and predicts chemical shift changes upon breaking a salt bridge between charged residues, aligning well with experimental values reported by White et al. These capabilities make the model well suited for integration into simulation-based structure inference workflows such as metadynamics and ensemble refinement. However, several challenges remain. The authors observed that accuracy decreases slightly when extending the model to multitype atom prediction due to increased data heterogeneity. The model also depends heavily on data quality, as more carefully curated subsets produce superior training curves compared to larger but noisier sets. Additionally, while distance features improved performance marginally, the impact of different graph featurization strategies remains underexplored. Future research will benefit from the development of larger and cleaner data sets, optimization of graph construction protocols, and the inclusion of richer edge features to better encode nonbonded interactions.
A new ML model, ML-J-DP4, developed by Sarotti et al., significantly accelerates structural elucidation with impressive accuracy and efficiency. ML-J-DP4 builds on the J-DP4 framework, a modification of DP4 that incorporates scalar 3JHH couplings to constrain conformational sampling (iJ-DP4) and provides relevant stereochemical insights via a Bayesian probability term (dJ-DP4). Striking an optimal balance between accuracy and computational cost, J-DP4 has been recognized as one of the most effective methods for resolving highly complex molecular structures. ML-J-DP4 further enhances this approach by integrating Karplus-type J-coupling calculations with isotropic shielding predictions at the HF/STO-3G level, refined through Δ-ML techniques. As illustrated in Figure , the workflow involves selecting 17,000 molecules (T17k set) for training and 10,000 molecules (V10k set) for validation from an initial data set of 170,000 structures, achieving mean absolute errors (MAE) of 1.21 ppm for 13C and 0.14 ppm for 1H, with RMS values of 1.63 and 0.19 ppm, respectively. This fully automated workflow, combining Bayesian inference, adaptive learning, and molecular environment refinement, solves complex structures in minutes using standard computational resources. The method’s speed, accuracy, and efficiency make it an important development in applying machine learning to NMR.
9.
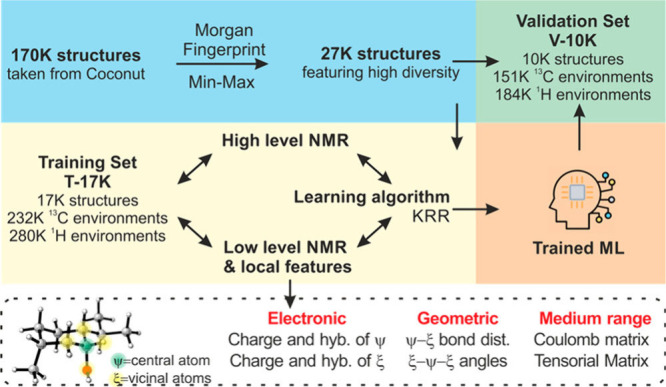
Schematic overview of the ML-J-DP4 method, illustrating a hybrid workflow that combines fast Karplus-type scalar coupling (3J) approximations with ML-refined NMR chemical shift predictions at the HF/STO-3G level. The process accelerates structural elucidation within the J-DP4 formalism, achieving near-DFT accuracy at significantly reduced computational cost. Reproduced with permission from reference . Copyright (2022) American Chemical Society.
Building upon these developments, an improved statistical framework, DP4+, was introduced by Grimblat, Zanardi, and Sarotti to address key deficiencies of the original DP4 method. In response to the well recognized limitations of DP4, particularly its reliance on scaled shift errors and limited accuracy for closely related isomers, DP4+ was formulated with refined error modeling, enhanced prediction accuracy, and chemically informed treatments of NMR data. This enhanced probability model incorporates two foundational improvements. First, it introduces unscaled chemical shift errors alongside traditional scaled ones to better capture environment-specific deviations that are otherwise masked by linear regression. Second, it employs more accurate levels of quantum chemical theory, specifically using B3LYP/6-31G(d) geometries and computing shielding tensors at PCM/mPW1PW91/6-31+G(d,p) level. Benchmarking across a challenging data set of 48 isomeric compounds, DP4+ demonstrated superior performance over traditional DP4, correctly identifying the right structure in cases where the original method failed or provided overconfident yet incorrect predictions. For example, the aldol stereoisomer later confirmed as the correct structure and the natural product cryptomoscatone D2 were both confidently misidentified by the original DP4 method, with confidence scores exceeding 90%. In contrast, DP4+ correctly reassigned their stereochemistry with greater than 99% certainty, underscoring its ability to minimize false positives and enhance structural reliability. This improvement is traced not only to better shielding tensor calculations but also to the treatment of sp2 and sp3 hybridized atoms with separate t-distribution models, resulting in sharper statistical resolution. The performance gain was further quantified using a scoring system that rewards confident assignments: DP4+ achieved scores up to 2.4 times higher than DP4, particularly when both proton and carbon data were integrated. These results collectively suggest that DP4+ provides a more trustworthy and chemically nuanced approach to structure validation using NMR.
One of the key unresolved challenges is the dependency of performance on the accuracy and consistency of the statistical parameters, which must be recalibrated for each new level of theory used in shielding calculations. Although the authors validated the robustness of their parameter set across both training and validation data sets, the complexity of deriving and updating these parameters remains a barrier to broader adoption. Another limitation involves conformational flexibility, which is only partially addressed via Boltzmann-weighted averaging, and may still compromise accuracy in dynamic or solvent-sensitive systems. Future research is expected to refine the parametrization process by leveraging machine learning for adaptive error modeling and to further dissect the respective roles of 1H and 13C data. Interestingly, DP4+ was shown to correct misassignments when either 1H and 13C data alone failed, reinforcing the value of dual-nucleus approaches. The authors also emphasize that while 1H data often exerts stronger discriminative power, this effect varies with the choice of functional and basis set, warranting further studies on nucleus-specific prediction strategies. Overall, DP4+ stands as a critical advancement in NMR-driven structure validation, offering both improved accuracy and a framework for future developments in probabilistic and chemically informed structure elucidation strategies.
The development of ML models for predicting NMR chemical shifts faces several challenges, including the scarcity of high-quality experimental NMR data, solvent effects, and the computational complexity of traditional methods. The limited availability of accurate experimental 1H NMR spectra, particularly for biologically relevant compounds, hampers the ability to create reliable predictive models. Additionally, solvent interactions, which significantly influence chemical shifts, are often neglected in many existing models, leading to inaccuracies when predictions are applied across different solvents. Traditional QM methods, while highly accurate, are time-consuming and not feasible for large-scale applications. In this context, PROSPRE (PROton Shift PREdictor) emerges as a notable advancement in the field, as it addresses these challenges by leveraging a carefully curated, solvent-aware data set and employs a GNN for efficient, accurate predictions. A flowchart illustrating the PROSPRE methodology is depicted in Figure . PROSPRE is capable of accurately predicting 1H chemical shifts (mean absolute error <0.10 ppm), while outperforming previous models, and has been widely applied to over 600,000 biologically relevant compounds. By incorporating solvent-specific corrections, PROSPRE ensures more accurate predictions for compounds in various solvents, particularly water, methanol, chloroform, and dimethyl sulfoxide, making it a useful tool for metabolomics, drug discovery, and natural product research.
10.
A schematic flowchart illustrating (A) the transfer learning methodology employed to train the PROSPRE GNN model, and (B) the workflow for predicting 1H chemical shifts from chemical structures using the PROSPRE model. Reproduced with permission from reference . Copyright (2024) by MDPI, Basel, Switzerland, under the Creative Commons Attribution (CC BY) license.
Recent advancements have significantly improved the accuracy of 1H NMR chemical shift predictions for small molecules by addressing limitations of existing experimental databases and incorporating solvent-aware GNN architectures. − , Extending these approaches to larger, more complex biomolecular systems such as proteins, however, introduces additional challenges, including the accurate representation of protein secondary and tertiary structures, solvent exposure, and conformational flexibility. The recently introduced EFG-CS model by Gu et al. addresses these challenges by integrating sequence-based protein structure prediction and chemical shift estimation into a unified computational pipeline. EFG-CS leverages protein structures predicted from amino acid sequences by the ESMFold algorithm, which uses deep-learning methods trained on extensive sequence data. Chemical shift predictions for backbone atoms are carried out by a transfer-learning-based ML model, utilizing alignment-derived structural and sequence information from reference databases. Additionally, side-chain atom chemical shifts are computed through a GNN-based model, incorporating spatial relationships between atoms within predicted protein structures. The overall computational pipeline employed by EFG-CS is depicted in Figure . In performance evaluations against the UCBShift test set of 200 nonredundant proteins, EFG-CS achieved root-mean-square errors (RMSEs) of 0.43 ppm for H, 0.27 ppm for Hα, 1.13 ppm for Cα, and 2.51 ppm for N atoms, using only ESMFold-predicted structures as input. Despite a modest performance drop relative to models using experimental structures, the results remain robust and comparable in accuracy, underscoring the model’s utility in scenarios where empirical structural data are unavailable. Notably, the prediction errors for H, H α , and C α were tightly clustered around zero, whereas N atoms exhibited greater spread due to inherent challenges in modeling their diverse electronic environments. By eliminating the need for homology modeling or curated experimental input, the EFG-CS pipeline advances the accessibility and scalability of chemical shift prediction in data-limited or high-throughput settings.
11.

Computational pipeline of the EFG-CS model for predicting protein chemical shifts. (a) The user inputs amino acid sequences or protein structures. (b) Protein structure prediction using ESMFold (for sequences) or preprocessing uploaded structures. (c) Hydrogen atom addition to predicted or uploaded structures. (d) Machine learning and graph neural network-based predictions of backbone and side-chain chemical shifts, respectively. (e) Visualization and downloadable chemical shift outputs via the EFG-CS Web server. Reproduced with permission from reference . Copyright (2024) by Wiley Periodicals LLC on behalf of The Protein Society under the Creative Commons Attribution (CC BY) license.
Additional performance evaluations were carried out using the SHIFTX2 test data set for side-chain atom predictions, with EFG-CS achieving RMSEs ranging from 0.58 ppm (Hγ 2) to 1.26 ppm (Hε 2). These values, obtained without data curation or manual preprocessing, reflect the model’s strong generalizability and alignment with realistic application environments. However, the results also highlight remaining challenges particularly in modeling structurally flexible residues, disordered regions, or post-translational modifications, where broader RMSE distributions were observed. To further enhance prediction accuracy, future development may focus on improving long-range electrostatic modeling, refining treatment of solvent accessibility, and incorporating uncertainty quantification within deep learning architectures. The convergence of end-to-end protein structure prediction with chemical shift estimation in EFG-CS exemplifies the accelerating shift toward integrated learning-based models that advance both mechanistic insight and translational discovery in NMR.
The evolution of machine learning in structural biology has been marked by a shift from broad-spectrum predictors to models tailored to the hierarchical and dynamic complexity of proteins. Among these developments, a model called LEGOLAS (neuraL nEtwork enGine fOr caLculating chemicAl Shifts) was introduced by Darrows et al. This model was designed using the TorchANI framework. It has been optimized for the rapid and accurate prediction of protein NMR chemical shifts. A critical challenge in the field, the need for fast, differentiable, and accurate models across large structural ensembles has been addressed by LEGOLAS, particularly for systems encountered during MD simulations. By encoding atomic environments through atomic environment vectors (AEVs) derived from symmetry functions, LEGOLAS circumvents the limitations of discrete structure encodings. The model supports end-to-end GPU acceleration and differentiability, thereby enabling direct integration into refinement protocols and large-scale dynamic sampling pipelines. When benchmarked against SHIFTX2, LEGOLAS achieved similar or better accuracy across key nuclei (e.g., C α ,C β , H α ) with a 10-fold increase in computational speed. Its ensemble architecture of five independently trained neural networks contributed to improved generalization and noise robustness. Importantly, this approach enables time-averaged prediction across MD snapshots-a capability that remains largely unmet in earlier methods. The model architecture and deployment pipeline are schematically depicted in Figure . Thus, LEGOLAS represents a critical advancement by directly targeting both the accuracy and scalability requirements needed for real time, structure-aware NMR modeling in proteins.
12.
(a) Representation of the atomic environment vector (AEV) radial cutoff sphere (5.1 Å) centered on a carbonyl carbon, illustrating the spatial encoding of neighboring atoms within the LEGOLAS framework. (b) Schematic of the LEGOLAS neural network architecture for protein NMR chemical shift prediction. Input protein structures or trajectories are processed into atom-specific AEVs incorporating radial and angular distance metrics, as well as residue-type embeddings. Each AEV, of length 570, is passed through two fully connected hidden layers (256 and 64 neurons, respectively) with exponential linear unit (ELU) activation functions, culminating in the predicted chemical shift output (δPRED). An ensemble of five independently trained networks per backbone atom type is employed for robust prediction. The model is optimized by minimizing the mean squared error between predicted and experimental shifts. Reproduced with permission from reference . Copyright (2025) American Chemical Society.
Compared to other contemporary ML models for NMR chemical shift prediction, LEGOLAS distinguishes itself through its atom-wise architecture and systematic handling of protein structure as radial and angular atomic environment vectors (AEVs). In contrast to CASCADE, which applies graph neural networks to small organic molecules by learning spectral shifts through message passing among molecular graph nodes, LEGOLAS encodes detailed spatial and chemical features via predefined AEVs and performs independent predictions for each backbone atom type, enhancing modularity and interpretability. The use of multiple independently trained networks, one for each atom class, has been shown to improve robustness across varying residue types. While PROSPRE integrates equivariant neural networks with ESM-derived embeddings for sequence-informed protein structure encoding, its reliance on large protein language models introduces additional computational cost and complexity. By comparison, LEGOLAS leverages direct structural inputs from PDB or trajectory files without sequence-based pretraining, achieving competitive accuracy for 1H, 13C, and 15N shifts. Furthermore, unlike the NMR-GNN framework, which incorporates attention mechanisms over chemical bond types and 3D geometry for general-purpose shift prediction across atom types, LEGOLAS maintains a biologically specialized design tailored to protein backbone prediction. This focused scope facilitates more efficient training and accurate reproduction of experimental chemical shifts, particularly in structured protein regions, and demonstrates the value of atom-wise modular design in biomolecular NMR modeling.
To address the fragmentation of prediction strategies across liquid-state and solid-state NMR applications, Xu et al. , proposed a unified ML framework called NMRNet, which leverages a two-stage approach involving large-scale pretraining and task-specific fine-tuning. The motivation behind NMRNet stems from a pressing need for models that can operate across diverse chemical domains, ranging from small organic molecules in solution to periodic solids in materials chemistry, without sacrificing either speed or accuracy. The framework, schematically illustrated in Figure , consists of four main modules including data preparation, large-scale structural pretraining, supervised fine-tuning on NMR data sets, and flexible inference modes for spectral analysis. During pretraining, over 4.8 million 3D structures were utilized to learn robust atomic environment representations, mitigating limitations imposed by sparse experimental NMR data. For the fine-tuning stage, two curated data sets for solution phase spectra and ShiftML for solid state predictions were used to optimize the model for different applications. The result is a system capable of achieving state-of-the-art accuracy across multiple elements and chemical systems. In liquid-state testing, NMRNet reduced the mean absolute error for 1H and 13C shifts to 0.181 and 1.098 ppm, respectively, even when applied to molecules with over 150 atoms. In solid-state cases, the model demonstrated strong correlation with DFT-derived values for elements such as 15N and 17O. These benchmarks highlight the model’s generalization capacity and set a new standard for cross domain chemical shift prediction. Importantly, NMRNet was designed not only for numerical prediction but also for advanced inference tasks such as peak assignment and configuration determination, including stereoisomer resolution in chiral systems. The ability to handle such tasks without requiring extensive domain specific feature engineering or hand-curated rule sets marks a significant methodological advancement in the use of deep learning for NMR interpretation.
13.
Schematic representation of the NMRNet framework for chemical shift prediction. Left: data preparation pipeline integrating structural information from both liquid-state (LSNMR) and solid-state NMR (SSNMR) sources. Right top: self-supervised pretraining using molecular structures to perform masked atom type recovery and 3D coordinate reconstruction. Right middle: supervised fine-tuning phase leveraging annotated NMR chemical shift data sets. Right bottom: inference phase in which pretrained and fine-tuned model weights are applied to downstream prediction tasks. Coord = coordinate and Dist = distance. Adapted from Xu et al., arXiv:2408.15681v1, Copyright (2025), under a Creative Commons CC BY 4.0 license.
In a critical assessment of its performance and limitations, Xu et al. conducted several validation experiments to explore the boundaries of NMRNet’s predictive capacity and its alignment with structural complexities. In fine-tuned applications involving nerve agents and high-molecular-weight compounds, the model maintained R2 values above 0.95 for both 1H and 13C shifts, confirming that predictive accuracy was retained across increasing molecular size. When benchmarked against previous models on the QM9-NMR and nmrshiftdb2 data sets, NMRNet achieved a reduction in mean absolute error from 0.054 to 0.020 ppm for 1H and from 0.520 to 0.262 ppm for 13C, demonstrating clear improvements over established baselines. In the domain of peak assignment, a 94% accuracy rate was obtained for 13C NMR in nerve agent molecules, although performance for 1H lagged at 72%, revealing challenges related to signal crowding and resonance overlap. One of the key unresolved challenges is the model’s limited ability to generalize solvation effects and temperature induced chemical shift variability, which are often critical in experimental settings but poorly captured in available training data. Additionally, stereochemical discrimination in chiral systems remains partly dependent on dual nucleus inputs, highlighting a need for improved single-nucleus resolution strategies. Xu et al. also emphasized that performance gains were largely attributable to large-scale pretraining, particularly in data-sparse regimes, suggesting future directions involving curated multimodal data sets, integration with quantum mechanical descriptors, and incorporation of domain adaptation techniques. Therefore, NMRNet serves as both a functional tool for current NMR modeling and a template for next-generation ML pipelines aimed at comprehensive spectrum-structure correlation across chemical systems. Its introduction represents a critical step toward solving the long-standing problem of generalizability in NMR prediction and contributes essential insights into the design of models that are simultaneously accurate, scalable, and chemically informed.
An especially impactful advancement in ML-augmented NMR modeling is the DU8ML framework developed by Novitskiy and Kutateladze. DU8ML is a hybrid protocol that builds upon the DU8+ method by combining lower-level DFT calculations with machine-learned substructure-specific correction terms to significantly enhance accuracy and throughput in predicting 13C chemical shifts and proton–proton spin–spin coupling constants. Trained on over 11000 experimental 13C shifts and 4000 J-couplings primarily in chloroform, DU8ML achieves RMSDs of approximately 0.95 ppm for 13C and 0.28 Hz for 1H–1H J values, enabling rapid and reliable validation of large natural products and alkaloids. The approach successfully revised 35 misassigned structures from a test set of 170 alkaloids, demonstrating its practical utility in resolving common challenges such as heavy atom misassignments, epimerization errors, and N-oxide stereochemistry. The DU8ML protocol and model architecture are schematically illustrated in Figure . Notably, DU8ML bypasses the need for exhaustive spin–orbit coupling calculations by applying substructure-aware empirical corrections, providing speed and accuracy without resorting to expensive relativistic methods. With typical computation times under 20 min per conformer on standard HPC nodes, DU8ML offers a robust solution for high-throughput structure verification and reassignment in complex natural product discovery pipelines. One of the key remaining challenges is extending the model’s applicability beyond alkaloid and natural product scaffolds, particularly for flexible systems, varied solvents, and heteroatom-rich environments not well represented in the training set.
14.
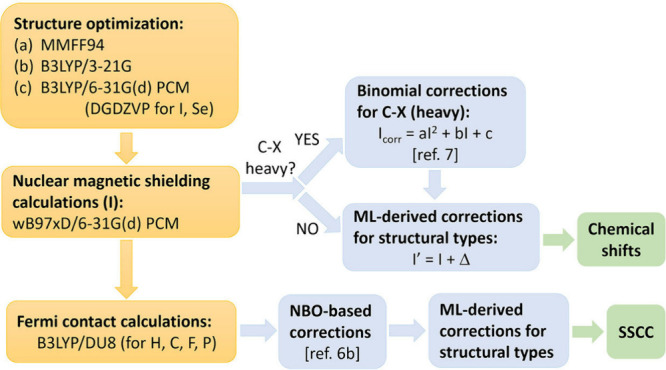
Workflow of the DU8ML protocol for high-throughput NMR chemical shift and scalar spin–spin coupling constant (SSCC) prediction. Orange modules denote quantum chemical calculations performed at various levels of theory, including structure optimization shielding constant evaluation and Fermi contact calculations. Blue modules represent empirical corrections, including binomial corrections for heavy atom-bearing carbons (C-X), NBO-based corrections, and ML-derived corrections based on substructure types. These refinements yield chemically accurate predictions of NMR observables with minimal computational cost. Reproduced with permission from reference . Copyright (2022) American Chemical Society.
While models like DU8ML have demonstrated remarkable accuracy in small-molecule and natural product prediction through hybrid QM-ML strategies, recent efforts in protein NMR have shifted toward integrating high-resolution structural features with learning-based frameworks. One such advancement is UCBShift 2.0, which introduces a modular, physics-aware approach to predicting protein chemical shifts across diverse backbone and side-chain environments. Developed by Ptaszek et al., UCBShift 2.0 fuses two complementary components: UCBShift-X, which uses a comprehensive set of physics-informed structural features derived from protonated X-ray structures, and UCBShift-Y, which applies transfer learning from homologous proteins using TM-align-based structural alignment. These modules are integrated into a multistage random forest regression model that predicts both backbone and side chain chemical shifts, offering flexibility in cases where homologous templates are available. As depicted in Figure , the model was trained on 851 protein structures from the RefDB database paired with BMRB chemical shift data, and evaluated on an independent test set of 200 proteins. UCBShift 2.0 achieved mean absolute errors of 0.80 ppm for 13C, 0.16 ppm for 1H, and 0.99 ppm for 15N atoms in side chains, outperforming SHIFTX2 across all atom types. In particular, the model demonstrated improved accuracy for atom environments that are traditionally difficult to predict, such as isoleucine CD1, where conformational variability due to rotamers and, in other cases, π-stacking interactions significantly influence chemical shifts.
15.

Schematic workflow of the UCBShift model for protein chemical shift prediction. The UCBShift framework integrates two complementary modules: UCBShift-X (left path), a feature-based ML pipeline employing extra tree and random forest regressors trained on structural descriptors extracted from X-ray protein structures and UCBShift-Y (right path), a transfer-learning module that identifies homologous sequences and structures from a reference database to assign chemical shifts via similarity weighted averaging. The final predictions are produced by a combined random forest model that integrates outputs from both modules, enhancing accuracy across both backbone and side chain atom types. Reproduced with permission from reference . Copyright (2024) American Chemical Society.
Critically, the improved performance of UCBShift 2.0 stems from both its refined input representations and its hybrid learning strategy. Features such as backbone and side chain dihedral angles, hydrogen bond counts, B-factors, solvent accessibility, and local electric field vectors contribute to the model’s chemical sensitivity. The TM-align based homology module enables conditional predictions tailored to structural analogs, contributing to a 0.5 and 0.2 ppm improvement in 13C and 1H shift accuracy, respectively, over the standalone X-module. The model was also able to resolve protonation-state dependent chemical shift perturbations in charged residues, such as histidine and glutamine, which are difficult to capture using simpler backbone-focused models. The open-source implementation and modular design of UCBShift make it particularly suited for large-scale protein NMR analysis, ensemble refinement, and real-time assignment validation. One of the key unresolved challenges is the model’s limited nucleus coverage, especially for nonstandard side chains and metal-binding environments, which restricts its applicability in metalloproteins and complex cofactors. Future efforts will benefit from expanding training sets to include a broader diversity of structural motifs, improving nucleus-specific parametrization, and incorporating dynamic ensemble averaging to further enhance prediction accuracy in flexible or partially disordered regions.
The interpretive bottleneck in structure elucidation often lies not in spectral acquisition but in resolving subtle spectroscopic distinctions among stereoisomers and constitutional analogues. To address this longstanding challenge, Marcarino et al. , developed the Artificial Neural Network-Pattern Recognition Algorithm (ANN-PRA), a data-driven method designed to systematically correlate theoretical and experimental NMR chemical shifts for robust structure verification. The protocol combines artificial neural networks with unsupervised clustering and chemometric scoring to rank candidate structures based on shift agreement, while accounting for solvent effects and conformational variability. By mapping input features such as weighted statistical shift errors and chemical environment fingerprints, ANN-PRA learns patterns that distinguish between correct and incorrect molecular connectivities, even when traditional error metrics like mean absolute deviation or DP4 probability produce ambiguous results. In benchmark tests involving a range of organic compounds, including stereoisomers with near-identical 1H and 13C shift patterns, ANN-PRA demonstrated a marked improvement in false-positive reduction, correctly identifying erroneous proposals in cases where DFT-based shift matching alone failed. Furthermore, the method is solvent-aware, enabling improved ranking accuracy across polar and nonpolar media by dynamically adjusting shift interpretation using reference data sets. The overall structure of the ANN-PRA workflow is illustrated in Figure . The model’s explainability was enhanced by saliency maps, which highlight the most chemically influential atoms in driving structure discrimination, offering insight into NMR-descriptor relevance within the network.
16.
Overview of the ANN-PRA framework for structural validation using NMR. (a) Illustration of the structure validation problem where only a single proposed structure is available and must be evaluated against experimental data. (b) Schematic of the ANN-PRA architecture, which applies a two-stage neural network system: the first trained on 1D 13C NMR correlations to assess atom connectivity, and the second using combined 1H, 13C, and HSQC 2D data to evaluate stereochemical configurations. (c) Examples of natural products that were originally misassigned, with stereocenters requiring revision indicated by green dots. Reproduced with permission from reference . Copyright (2020) American Chemical Society.
Beyond its standalone utility, ANN-PRA offers a blueprint for hybridizing pattern recognition with QM data in high-throughput structure validation pipelines. The approach is particularly effective for detecting misassigned connectivities and regioisomeric confusion in systems where stereochemistry plays a minimal role but structural ambiguity remains high. One of the key challenges identified by Sarotti and co-workers is the diminished predictive confidence when trained models are applied to highly strained or nonbenzenoid scaffolds outside the chemical domain of the training set. The authors propose that model robustness may be further improved by integrating fragmentation-based alignment procedures and by retraining networks using curated error prone data sets with known experimental misassignments. Another promising direction involves coupling ANN-PRA outputs with automated spectral assignment tools to enable end-to-end decision-making in spectroscopic workflows. , As machine learning continues to redefine how chemists interpret NMR data, methods like ANN-PRA underscore the growing importance of feature engineering, domain transferability, and model transparency in the deployment of predictive NMR analytics. These innovations pave the way for more intelligent, interpretable, and context-aware structure verification systems capable of operating across broad chemical and spectral domains.
In pursuit of fully autonomous stereochemical elucidation, Howarth et al. introduced DP4-AI, a high-throughput, open-source platform that automates the entire NMR structure validation workflow, encompassing spectral processing, chemical shift prediction, assignment, and configurational probability scoring. The motivation stems from limitations in traditional DP4 methods, which rely on manual spectrum interpretation and are thus prone to user bias, limited scalability, and low throughput. DP4-AI replaces these steps with automated modules for Fourier transformation, phasing, solvent peak identification, baseline correction, and multiplet deconvolution, followed by probabilistic structure ranking using the Bayesian DP4 framework. The full architecture is depicted in Figure . When evaluated on a test set of 47 chemically diverse molecules including 21 natural products, 10 synthetic intermediates, and 16 stereochemically complex scaffolds the system correctly identified the correct isomer in 43 out of 47 cases, yielding an overall assignment accuracy of 91%. Importantly, 16 of these molecules contained four or more stereocenters, and 10 included flexible rings or acyclic segments where conformational averaging complicates shift prediction. For the natural product NP2, a particularly challenging 10-membered macrolide with six stereocenters, DP4-AI assigned the correct structure from a pool of 64 possible diastereomers using only 1H and 13C 1D spectra in under 12 min. These performance metrics mark a significant leap in both accuracy and automation compared to earlier semiautomated methods that required days of human input. Moreover, spectral referencing and assignment steps are dynamically optimized by internal confidence metrics, allowing DP4-AI to adjust to noisy or partially resolved experimental data. Despite these successes, one of the key unresolved challenges is the treatment of conformationally dynamic systems, particularly those with multiple low-energy conformers that contribute nonlinearly to averaged NMR shifts. Current GIAO-based predictions do not sufficiently capture this complexity without extensive conformational sampling, which remains computationally intensive and often inaccurate in solvent-sensitive systems. The current implementation assumes monocomponent mixtures and performs less reliably on overlapping multiplets or mixture spectra. The authors highlight promising future directions including the incorporation of 2D NMR data sets, improved conformational sampling algorithms, and a generalization of the Bayesian model to account for correlated prediction errors across nuclei. DP4-AI not only delivers an end-to-end automated solution for routine NMR-based stereochemical assignment but also establishes a critical foundation for future developments that couple experimental spectra with statistically grounded quantum predictions at scale.
17.
Schematic workflow of the DP4-AI framework for automated structure validation using raw 1D NMR spectra. The pipeline begins with raw spectral data, which undergoes automated phase correction, baseline adjustment, peak picking, solvent identification, and integration to extract multiplet centers and integrals. These features are matched to DFT-calculated NMR shifts using an assignment algorithm, followed by DP4 statistical analysis to yield stereochemical probabilities. The system enables fully automated, high-throughput diastereomeric discrimination with minimal user intervention. Reproduced with permission from reference . Copyright (2020) The Royal Society of Chemistry.
Building upon the automation framework of DP4 AI, Howarth and Goodman introduced the DP5 probability model to tackle a critical gap in structure verification, namely the absence of statistical rigor when only a single structure candidate is available. While DP4 assumes the correct structure is present in a set of diastereomers, DP5 abandons this constraint by quantifying the absolute probability that a proposed structure is correct, based solely on 13C NMR data and DFT calculated shifts. The DP5 workflow is depicted in Figure . In this framework, atomic-level prediction errors extracted from over 63000 DFT-computed NMR shift deviations across a reference set of 5140 molecules are processed using Gaussian kernel density estimation to construct atom-specific probability distributions. These atomic probabilities are then combined using a Boltzmann weighted geometric mean and calibrated through Bayesian correction to yield a normalized molecular level probability. When benchmarked on 13 real world structure reassignments from the literature, DP5 assigned near zero probabilities to all initially incorrect structures and significantly higher confidence to their correct revisions, demonstrating its practical value in detecting subtle errors in stereochemistry or substitution. In a cross validation test designed to match correct and incorrect spectra with equivalent DFT mean absolute errors of approximately 1.6 ppm, DP5 maintained its discriminatory power, underscoring its ability to distinguish candidates that would otherwise confound an expert chemist. One major challenge is posed by the intrinsic accuracy limits of DFT, which restrict the maximum DP5 probability to 72% even for correct structures. This ceiling is caused by uncertainties in atomic environments and DFT functional errors. Future DP5 versions are expected to include multidimensional NMR data, better conformer handling, and integration with generative design workflows. By offering a mathematically grounded, structure-independent confidence score, DP5 is positioned to enhance NMR analysis for cases where a single structure must be evaluated in isolation.
18.

Schematic overview of the DP5 probability framework for single-structure validation using 13C NMR data. Starting from a candidate molecular structure and raw NMR spectrum, the pipeline integrates automated DFT shift prediction (via DP4-AI) with automated spectral processing (via NMR-AI). Atomic representations are used to quantify DFT prediction error probabilities using atom-environment-specific statistical models. These probabilities are aggregated into a molecular-level probability and refined through a Bayesian correction function to yield the final DP5 probability, which represents the likelihood that the proposed structure is correct. Reproduced with permission from reference . Copyright (2022) The Royal Society of Chemistry.
In addition to the models discussed in detail above, several other ML methods have been developed that extend the applicability of NMR predictions across chemical and biomolecular domains. A diverse range of ML models has emerged in recent years for predicting NMR parameters, each tailored to specific molecular classes, nuclei, or spectral types. Notable among these is IMPRESSION (IMPRESSION-G2), , which leverages kernel ridge regression trained on DFT-level data to predict 1H, 13C chemical shifts, and 1JCH scalar couplings with near-quantum accuracy for organic molecules. Similarly, the MPNN-NMRML approach has deployed ensembles of message-passing neural networks to enhance 13C chemical shift predictions in small molecules, with a strong emphasis on data set diversity and uncertainty quantification. ShiftML has focused on crystalline materials, particularly for solid-state NMR, using Gaussian approximation potentials and symmetry-aware atomic descriptors to predict shielding tensors. Expanding to protein-scale predictions, models such as PLM-CS (protein-language-model-based chemical shift prediction) integrate protein language models with downstream neural networks to predict backbone shifts without requiring explicit structure inputs, while EFG-CS combines ESMFold-generated structures with hybrid models transfer learning for backbone atoms and GNNs for side chains to enable full-protein chemical shift prediction pipelines.
Beyond these, additional models have been introduced to address niche applications and specialized NMR modalities. The GT-NMR framework, built on graph transformers, has reported state-of-the-art performance on small-molecule 1H and 13C shift prediction, whereas TransPeakNet targets HSQC data sets from HMDB 5.0 using multitask deep learning to resolve cross-peaks and correlation patterns. Solid-state-focused models include Solid-state GNN, which predicts shielding tensors such as those of SiO2, and 17O-specific predictors that utilize molecular descriptors for oxide environments. Specialized efforts for zeolites have yielded ML models for 27Al shift prediction, conditioned on framework type and extrinsic factors. Importantly, tools like 2DNMRGym have also emerged as curated data sets that accelerate the training of ML models on 2D NMR spectra. Meanwhile, earlier statistical or empirical approaches, SHIFTX2, SPARTA+, CamShift, and PROSHIFT pioneered the domain of protein chemical shift prediction by combining torsion angle potentials, empirical corrections, and chemical environment descriptors, though they have now largely been supplanted by neural and hybrid architectures that better accommodate structural flexibility and residue interactions. More recently, the MIM-ML model has been developed as a fragment-based random forest approach trained exclusively on DFT-derived data for nucleic acids, enabling accurate prediction of both 1H and 13C chemical shifts without experimental fitting. These developments illustrate the accelerating momentum and increasing granularity with which ML models are addressing the multifaceted challenge of NMR shift prediction.
4. NMR Shifts in QSAR Modeling
NMR chemical shifts have long served as molecular descriptors in QSAR studies, providing insights into electronic and steric influences on biological activity. Foundational work by Pople and Karplus established correlations between chemical shifts and molecular geometry, enabling their application in predicting physicochemical properties and biological activity. Integration of 13C and 1H NMR shifts into QSAR models has enhanced drug design, toxicity assessments, and bioactivity predictions, supported by advancements in quantum chemical calculations for complex systems. − The incorporation of machine learning, including graph neural networks, has further refined shift predictions and improved QSAR model performance. Computational frameworks such as CS-ROSETTA and CS23D have bridged NMR data with predictive modeling, streamlining structural elucidation and cheminformatics applications. ,
The application of 13C chemical shifts in QSAR has demonstrated correlations with electronic parameters such as Hammett substituent constants (σ, σ+ ,σ), atomic charges, and frontier molecular orbitals (HOMO, LUMO). Chemical shifts of substituent-bearing carbons often correlate directly with substituent electronic properties, making them useful predictors of electronic interactions in molecules. Downfield chemical shifts are typically associated with electron-withdrawing substituents, while electron-donating substituents produce upfield shifts. This relationship has facilitated numerous studies involving structure–activity predictions for diverse biological end points, such as antimicrobial, antiviral, and anticancer activities, as well as reactivity and stability assessments. ,−
Quantitative Spectrometric Data–Activity Relationship (QSDAR) methodologies explicitly integrate chemical shift data into QSAR models, enhancing their predictive capabilities. Techniques like Comparative Structural Connectivity Spectra Analysis (CoSCoSA) combine structural and spectral data, providing deeper insight into the relationship between chemical structure and biological activity. The combination of chemical shifts with classical molecular descriptors-including electronegativity, polarizability, bond dissociation energies, and ionization potentials-further strengthens the predictive power of QSAR models. These integrated approaches have been effectively employed to predict enzyme inhibition, receptor–ligand interactions, environmental persistence, and compound toxicity. −
The evolution of QSAR modeling with NMR data continues to be driven by high-throughput spectral databases and AI-driven methodologies, underscoring the critical role of chemical shifts in modern cheminformatics. Rapid advancements in computational chemistry and machine learning have significantly accelerated the ability to interpret complex spectral data, allowing extensive QSAR model training and validation against large chemical data sets. These developments highlight the ongoing importance of NMR chemical shifts as fundamental descriptors in QSAR, paving the way for enhanced molecular design and predictive accuracy in pharmaceutical, environmental, and chemical research.
5. Challenges and Future Prospects
Computational NMR methodologies, encompassing quantum chemical calculations, machine learning predictions, and QSAR techniques have advanced significantly over the years yet persistent challenges remain. Quantum chemical NMR calculations, often utilizing density functional theory, encounter inherent limitations related to electron correlation, relativistic effects, and the static approximation of molecular structures. Although quantum mechanical NMR computations can predict molecular structures with high accuracy, they are computationally expensive and become prohibitively costly as system size increases. Additionally, DFT frequently fails to capture the temporal evolution of solvation structures under varying conditions such as temperature, pressure, pH, and composition. These computational approaches often struggle to accurately reflect solvation effects, rovibrational dynamics, and conformational flexibility, factors essential for precise chemical shift predictions. Consequently, developments in advanced sampling methods, polarizable force fields, and explicit relativistic corrections remain critical to overcome these intrinsic computational limitations.
Machine learning has emerged prominently within computational NMR, offering significant reductions in computational time compared to classical quantum methods. However, ML-based methods require extensive, high-quality data sets for effective training, presenting a substantial bottleneck due to the limited availability and chemical diversity of current data sets. Techniques such as ShiftML, IMPRESSION, CASCADE, and ML-J-DP4 demonstrate that ML models trained on DFT-generated chemical shifts can achieve high predictive accuracy with drastically reduced computational costs, although these methods remain dependent on the quality of input structures. Future directions necessitate expanding training data sets, refining algorithms for accuracy, and enhancing interpretability to clarify underlying physicochemical relationships.
In the QSAR domain, significant challenges arise from the complexity of spectral data analysis and the difficulty in reliably extracting meaningful quantitative descriptors from NMR spectra due to spectral overlap and variations caused by experimental conditions. QSAR models must also robustly integrate spectral data with physicochemical and omics data. Improved statistical methods and data preprocessing techniques capable of accurately parsing complex spectral data sets will substantially enhance QSAR’s applicability and reliability.
Looking ahead, one of the most promising frontiers in computational NMR lies in the development of robust machine learning models trained on quantum-derived data sets. Unlike many analytical modalities that are limited by sparse or inconsistent experimental data, NMR provides a pathway to systematically generate large, chemically diverse, and physically grounded data sets using first-principles methods. This makes it possible to construct predictive models without requiring massive experimental campaigns. However, challenges remain in reconciling computed and experimental data, especially in capturing effects such as solvation, conformational dynamics, and instrument-specific variations. Future research should focus on hybrid modeling strategies that integrate computed chemical shifts and couplings with curated experimental data sets through transfer learning or domain adaptation techniques. Such approaches are likely to improve model generalizability, reduce overfitting to synthetic data artifacts, and enable more reliable spectral prediction across novel compound classes. The unique ability to train on accurate, scalable, and interpretable computational data solidifies NMR’s position at the forefront of data-driven chemical analysis.
6. Quantum Utility in NMR Simulations: Progress, Prospects, and Challenges
Quantum computing has emerged as a compelling paradigm for simulating NMR-relevant phenomena, particularly as classical methods encounter exponential scaling barriers. Algorithms such as the Quantum Phase Estimation (QPE) , and Variational Quantum Eigensolver (VQE) have been deployed to simulate spin Hamiltonians and molecular electronic structures central to NMR interpretation. Notably, Burov et al. (2024) demonstrated the first high-field NMR spectral simulations using gate-based quantum processors. By encoding NMR-relevant Hamiltonians comprising chemical shift and scalar coupling terms into a digitally simulated spin model using first-order Trotterization, and executing the circuit on IBM’s 127-qubit quantum processor (Eagle r3), gate-based quantum simulations of liquid-state, high-field NMR spectra were performed for molecular systems containing up to 11 proton spins (approximately 47 atoms). In this study, spectral features of biologically relevant molecules, including ATP and 4-methylvaleric acid, were reproduced with qualitative agreement to experimental 400 MHz high-field NMR data. Compressed sensing techniques and built-in circuit-level error mitigation were applied to improve spectral resolution and reduce sampling cost. This work, conducted by Burov et al., represents a foundational demonstration of quantum simulation of liquid-state NMR using NISQ processors, establishing a critical benchmark for advancing quantum utility in spectroscopic applications. The integration of dynamic circuit compilation with scalable gate-based execution has provided a practical route for modeling complex spin systems that are increasingly challenging for classical algorithms. Complementary efforts by HQS Quantum Simulations under the EU-funded NextNMR initiative , are building hybrid quantum-classical platforms for structure verification and spectral deconvolution in complex biological matrices. Merz et al. have made significant strides in extending the applicability of Sample-based Quantum Diagonalization (SQD) − to chemically relevant molecular systems, establishing a robust foundation for its future deployment in quantum NMR simulations. The SQD algorithm, originally developed in collaboration with IBM quantum researchers, was designed to deliver Full CI-level chemical accuracy through measurement-based diagonalization strategies. Quantum-accurate SQD simulations were demonstrated for supramolecular interactions, validating the method against classical reference data. Fragment-based Density Matrix Embedding Theory (DMET) was integrated with SQD to enable the simulation of large molecular systems using quantum processors. Implicit solvation effects were incorporated through IEF-PCM, allowing the quantum simulation of solvated polar molecules. These advances demonstrate a clear path toward the utility of quantum processors in the simulation of nontrivial systems relevant to spectroscopy, catalysis, and drug discovery. Collectively, these efforts signal that quantum-enhanced NMR, once aspirational, has entered the proof-of-concept stage with steadily expanding applicability to realistic chemical property computations.
Despite demonstrable progress, several hardware-centric limitations continue to constrain the scalability and accuracy of quantum NMR simulations. Noise and decoherence inherent in NISQ devices significantly degrade circuit fidelity, especially for simulations involving intricate spin–spin couplings and subtle chemical shift perturbations. As circuit depth increases often scaling quadratically with the number of spins accumulated gate errors and limited coherence times obscure fine spectral features such as J-splitting or long-range correlations. Qubit connectivity and gate calibration further influence simulation tractability, necessitating substantial transpilation overhead and introducing architecture-specific biases. Additionally, challenges in quantum state preparation for entangled spin networks, and imperfect readout processes, compound these issues. The reliance on low-order Trotter decompositions, while computationally efficient, restricts the accuracy of simulated Hamiltonian dynamics. The hardware-agnostic implementation of SQD, combined with the LUCJ ansatz, offers an alternative to traditional eigenvalue solvers by relying on quantum-sampled expectation values to reconstruct eigenstates. This makes SQD especially attractive for NMR simulations, where many-body correlations and sparse spectra dominate. Their protocol’s compatibility with shallow circuits aligns well with the capabilities of current quantum devices, mitigating several bottlenecks posed by depth-intensive approaches like QPE. These innovations underscore the necessity of algorithm–hardware codesign in overcoming persistent limitations.
Efforts to overcome these challenges have spurred a diverse portfolio of error mitigation and hybrid algorithmic strategies. IBM’s Qiskit Runtime supports circuit reparameterization, zero-noise extrapolation, and dynamic decoupling sequences that extend coherence and reduce operational infidelity. Virtual purification and probabilistic error cancellation have improved the accuracy of postprocessed observables, while tensor-network-inspired recompilation schemes reduce effective circuit depth. HQS Quantum Simulations has piloted digital-analog hybrid frameworks that exploit native hardware interactions to simulate NMR observables with lower resource demands. Merz et al.’s fragment-based SQD framework leverages DMET to partition large molecules, enabling scalable simulations of complex chemical systems by isolating active subspaces. This hybrid decomposition strategy facilitates accurate treatment of electron correlation and spin interactions relevant to NMR without the exponential resource demands of global treatment. Spin-squeezing protocols and advanced quantum state engineering techniques are also being explored to enhance spectral readout. As IBM’s roadmap , continues with devices like Osprey, Heron, and the modular System Two, the path toward simulating significantly larger systems such as peptides or metalloprotein clusters becomes increasingly realizable.
Future trajectories in quantum NMR hinge on algorithm-hardware codesign, rigorous benchmarking, Hardware efficient Ansatz design and expanding molecular applicability. As illustrated by Burov et al., scaling to 20-spin systems is plausible with near-term devices supporting 1000+ two-qubit gates. This opens the door to simulating spectroscopically rich systems such as small conformationally flexible biomolecules, catalytic centers, and reactive intermediates. IBM’s strategic focus on quantum advantage through accessible APIs, dynamic scheduling, and scalable hardware modularity continues to guide the field’s infrastructure. Coupling quantum simulations with classical machine learning models such as for shift prediction or spin topology inference may accelerate convergence toward utility. Recent development of the SQD formalism to include the IEF-PCM solvent model by Merz and colleagues, implemented via IBM’s Qiskit addon, provides a foundation for NMR-relevant simulations of solvated polar systems, a crucial step toward biologically and pharmaceutically meaningful applications. Meanwhile, NextNMR continues to bridge classical spectral processing with quantum backends, targeting applications in personalized medicine and mixture deconvolution. Ultimately, realizing robust quantum advantage in NMR will depend on sustained progress in error correction, coherence management, and hybrid algorithm design. The convergence of these efforts establishes a credible roadmap toward quantum-accelerated NMR spectroscopy.
7. Concluding Remarks
This review highlights the transformative impact of quantum chemical and machine learning methods in computational NMR, driving advancements in structural biology and related disciplines. Quantum mechanical approaches, such as density functional theory and coupled-cluster calculations, remain central to the accurate prediction of NMR parameters. These methodologies facilitate precise structure determination and validation across diverse chemical and biological systems. Additionally, QSAR models significantly contribute by enabling the quantitative interpretation of complex structure–activity relationships, further enhancing the understanding of molecular interactions and chemical properties.
Machine learning methods complement these quantum chemical approaches by leveraging extensive data sets to automate spectral assignments, enhance predictive accuracy, and efficiently analyze complex, multidimensional data. The synergy between QM precision, and ML-driven automation has substantially advanced computational NMR methods development, providing powerful tools capable of addressing increasingly intricate molecular systems. As computational techniques continue to evolve, integrating QM, and ML approaches promises significant improvements in the accuracy, scalability, and practical applicability of NMR. Such integration will shape future developments in structural and molecular sciences, bridging theoretical predictions and experimental validations to accelerate scientific discovery.
Acknowledgments
The authors gratefully acknowledge financial support from the National Science Foundation (NSF) through CSSI Frameworks Grant OAC-2209717 and from the National Institutes of Health (Grant Number GM130641). The authors also thank Mithony Keng for useful feedback on the manuscript.
Glossary
Abbreviations
- NMR
Nuclear Magnetic Resonance
- DFT
Density Functional Theory
- HF
Hartree–Fock
- CC
Coupled-Cluster
- QM
Quantum Mechanics
- FF
Force Field
- ML
Machine Learning
- DL
Deep Learning
- MD
Molecular Dynamics
- AI
Artificial Intelligence
- SMILES
Simplified Molecular Input Line Entry System
- UCPT
Uncoupled perturbation treatment
- PCM
Polarizable Continuum Models
- GNN
Graph Neural Network
- MPNN
Message Passing Neural Network
- CNN
Convolutional Neural Network
- RNN
Recurrent Neural Network
- RL
Reinforcement Learning
- VAE
Variational Autoencoder
- GAN
Generative Adversarial Network
- QSAR
Quantitative Structure–Activity Relationship
- QSPR
Quantitative Structure–Property Relationship
- QSDAR
Quantitative Spectrometric Data-Activity Relationship
- HOMO
Highest Occupied Molecular Orbital
- LUMO
Lowest Unoccupied Molecular Orbital
- NOE
Nuclear Overhauser Effect
- PCA
Principal Component Analysis
- EPR
Electron Paramagnetic Resonance
- GIAO
Gauge-Including Atomic Orbital
- SOAP
Smooth Overlap of Atomic Positions
- TL
Transfer Learning
- XAI
Explainable Artificial Intelligence
- MMFF
Merck Molecular Force Field
- FC
Fermi Contact
- SD
Spin-Dipole
- PSO
Paramagnetic Spin–Orbit
- DSO
Diamagnetic Spin–Orbit
- SSCC
Spin–Spin Coupling Constant
- CSD
Cambridge Structural Database
- BMRB
Biological Magnetic Resonance Data Bank
- NP-MRD
Natural Products Magnetic Resonance Database
- GISSMO
Guided Ideographic Spin System Model Optimization
- AZM
Azithromycin
- ANN
Artificial Neural Network
- MCSCF
Multiconfigurational self-consistent field methods
- CASSCF
Complete active space self-consistent field
- RASSCF
The restricted active space self-consistent field
- FCI
Full configuration interaction theory
- DLPNO
Domain-Based Local Pair Natural Orbital
- CCSD(T)
Coupled-Cluster with Single, Double, and Perturbative Triple excitations
- CP-DFT
Coupled-Perturbed Density Functional Theory
- CSA
Chemical Shift Anisotropy
- CASE
Computer-Assisted Structural Elucidation
- CPCM
Conductor-like Polarizable Continuum Model
- TMS
Tetramethylsilane
- QM/MM
Quantum Mechanics/Molecular Mechanics
- AF-QM/MM
Automated Fragmentation QM/MM
- CHESHIRE
Chemical Shift Repository for Computed NMR Scaling Factors
- SVM
Support Vector Machines
- VAE
Variational Autoencoders
- MAE
Mean Absolute Errors
- RMS
Root Mean Square
- GIPAW DFT
Gauge-Including Projector Augmented Wave Density Functional Theory
- CoSCoSA
Comparative Structural Connectivity Spectra Analysis
- CCS
Collisional Cross Sections
- DP4-AI
Probability-Based NMR Structure Validation with AI
- DP5
Standalone Probability Framework for NMR Structure Validation
- ML-J-DP4
Machine Learning-enhanced J-DP4 Structural Assignment Method
- CASCADE
ChemicAl Shift CAlculation with DEep learning
- ExpNN
Experimental Neural Network
- DFTNN
DFT-based Neural Network
- LEGOLAS
neuraL nEtwork enGine fOr caLculating chemical Shifts
- IMPRESSION
Intelligent Machine Prediction of Shift and Scalar Information of Nuclei
- DMSO
Dimethyl Sulfoxide
- QC
Quantum computing
- QPE
Quantum Phase Estimation
- VQE
Variational Quantum Eigensolver
- SQD
Sample-based Quantum Diagonalization
- DMET
Density Matrix Embedding Theory
Biographies
Susanta Das is a Research Associate at the Lerner Research Institute, Cleveland Clinic, where he collaborates with Dr. Kenneth M. Merz, Jr. Trained as a quantum chemist, his research lies at the intersection of quantum mechanics, machine learning, and cheminformatics, with a focus on developing automated workflows for molecular structure elucidation. He has designed high-throughput pipelines that predict NMR chemical shifts and ion mobility-derived collisional cross sections (CCS) directly from SMILES strings, integrating advanced conformational sampling, deep learning potentials, unsupervised clustering, and DFT-based calculations. These computational protocols are rigorously benchmarked against experimental data and have demonstrated high accuracy and scalability for metabolite structure annotation. His work contributes significantly to the development of open-source, reproducible tools that bridge experimental and theoretical metabolomics, enabling rapid and reliable identification of unknown small molecules in complex biological systems.
Kenneth M. Merz, Jr., is a Full Staff scientist at the Lerner Research Institute of the Cleveland Clinic as well as the Joseph Zichis Chair in Chemistry at Michigan State University. He is also the Editor-in-Chief of the Journal of Chemical Information and Modeling which is part of the ACS family of journals. His research interests lie in the development of theoretical and computational tools and their application to chemical and biological problems including structure and ligand-based drug design, mechanistic enzymology, transition ion modeling, and methodological verification and validation. He has received numerous honors, including election as an ACS Fellow, the 2010 ACS Award for Computers in Chemical and Pharmaceutical Research, election as a fellow of the American Association for the Advancement of Science, and a John Simon Guggenheim Fellowship. When not doing science, he enjoys reading across a range of genres, but especially (re)reading the novels of Jane Austen.
K.M.M. supervised and conceptualized the review manuscript. SD drafted the initial version, while K.M.M. contributed to writing, reviewing, editing, and finalizing the manuscript. K.M.M. also provided supervision, managed the project, and secured funding. All authors have read and approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Emsley J. W., Feeney J.. Forty years of Progress in Nuclear Magnetic Resonance Spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2007;50(4):179–198. doi: 10.1016/j.pnmrs.2007.01.002. [DOI] [Google Scholar]
- Becker, E. D. Development of NMR: An Update. In eMagRes., 1996. 10.1002/9780470034590.emrhp1088 [DOI] [Google Scholar]
- Slichter C. P.. The Golden Anniversary of Nuclear Magnetic Resonance NMR: Fifty Years of Surprises. Proceedings of the American Philosophical Society. 1998;142(4):533–556. [Google Scholar]
- Woolfson, M. M. An introduction to X-ray crystallography; Cambridge University Press, 1997. [Google Scholar]
- Smyth M., Martin J.. x Ray crystallography. Molecular Pathology. 2000;53(1):8. doi: 10.1136/mp.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg L.. X-ray crystallography. Sci. Am. 1968;219(1):58–70. doi: 10.1038/scientificamerican0768-58. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Authier, A. Early days of X-ray crystallography; OUP Oxford, 2013. [Google Scholar]
- Drenth, J. Principles of protein X-ray crystallography; Springer Science & Business Media, 2007. [Google Scholar]
- Adrian M., Dubochet J., Lepault J., McDowall A. W.. Cryo-electron microscopy of viruses. Nature. 1984;308(5954):32–36. doi: 10.1038/308032a0. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Benjin X., Ling L.. Developments, applications, and prospects of cryo-electron microscopy. Protein Sci. 2020;29(4):872–882. doi: 10.1002/pro.3805. From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danev R., Yanagisawa H., Kikkawa M.. Cryo-Electron Microscopy Methodology: Current Aspects and Future Directions. Trends Biochem. Sci. 2019;44(10):837–848. doi: 10.1016/j.tibs.2019.04.008. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Fernandez-Leiro R., Scheres S. H.. Unravelling biological macromolecules with cryo-electron microscopy. Nature. 2016;537(7620):339–346. doi: 10.1038/nature19948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J. L., Borgnia M. J., Bartesaghi A., Tran E. E., Earl L. A., Schauder D. M., Lengyel J., Pierson J., Patwardhan A., Subramaniam S.. Cryo-electron microscopy-a primer for the non-microscopist. FEBS journal. 2013;280(1):28–45. doi: 10.1111/febs.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishima R., Torchia D. A.. Protein dynamics from NMR. Nature structural biology. 2000;7(9):740–743. doi: 10.1038/78963. [DOI] [PubMed] [Google Scholar]
- Kearns D. R., James T. L.. NMR studies of conformational states and dynamics of DNA. Critical Reviews in Biochemistry. 1984;15(3):237–290. doi: 10.3109/10409238409102803. [DOI] [PubMed] [Google Scholar]
- Kafle N., Makita Y., Zheng Y., Schwarz D., Kurosu H., Pan P., Eagan J. M., Nakama Y., Hayano S., Miyoshi T.. Roles of conformational flexibility in the crystallization of stereoirregular polymers. Macromolecules. 2021;54(12):5705–5718. doi: 10.1021/acs.macromol.1c00888. [DOI] [Google Scholar]
- Vogel A., Reuther G., Roark M. B., Tan K.-T., Waldmann H., Feller S. E., Huster D.. Backbone conformational flexibility of the lipid modified membrane anchor of the human N-Ras protein investigated by solid-state NMR and molecular dynamics simulation. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2010;1798(2):275–285. doi: 10.1016/j.bbamem.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Tsou C.-L.. The role of active site flexibility in enzyme catalysis. Biochemistry-New York-English Translation of Biokhimiya. 1998;63(3):253–258. [PubMed] [Google Scholar]
- Hammes G. G., Benkovic S. J., Hammes-Schiffer S.. Flexibility, diversity, and cooperativity: pillars of enzyme catalysis. Biochemistry. 2011;50(48):10422–10430. doi: 10.1021/bi201486f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A.. Computer simulations of enzyme catalysis: methods, progress, and insights. Annual review of biophysics and biomolecular structure. 2003;32(1):425–443. doi: 10.1146/annurev.biophys.32.110601.141807. [DOI] [PubMed] [Google Scholar]
- Villà J., Warshel A.. Energetics and Dynamics of Enzymatic Reactions. J. Phys. Chem. B. 2001;105(33):7887–7907. doi: 10.1021/jp011048h. [DOI] [Google Scholar]
- Palmer A. G. III. NMR probes of molecular dynamics: overview and comparison with other techniques. Annual review of biophysics and biomolecular structure. 2001;30(1):129–155. doi: 10.1146/annurev.biophys.30.1.129. [DOI] [PubMed] [Google Scholar]
- Pellecchia M.. Solution nuclear magnetic resonance spectroscopy techniques for probing intermolecular interactions. Chemistry & biology. 2005;12(9):961–971. doi: 10.1016/j.chembiol.2005.08.013. [DOI] [PubMed] [Google Scholar]
- El Seoud O. A.. Use of NMR to probe the structure of water at interfaces of organized assemblies. Journal of molecular liquids. 1997;72(1–3):85–103. doi: 10.1016/S0167-7322(97)00032-9. [DOI] [Google Scholar]
- Gjuroski I., Furrer J., Vermathen M.. Probing the interactions of porphyrins with macromolecules using NMR spectroscopy techniques. Molecules. 2021;26(7):1942. doi: 10.3390/molecules26071942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche C., Otting G.. NMR studies of ligand binding. Curr. Opin. Struct. Biol. 2018;48:16–22. doi: 10.1016/j.sbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Kodama Y., Takeuchi K., Shimba N., Ishikawa K., Suzuki E.-i., Shimada I., Takahashi H.. Rapid identification of ligand-binding sites by using an assignment-free NMR approach. J. Med. Chem. 2013;56(22):9342–9350. doi: 10.1021/jm4014357. [DOI] [PubMed] [Google Scholar]
- Meyer B., Peters T.. NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors. Angew. Chem., Int. Ed. 2003;42(8):864–890. doi: 10.1002/anie.200390233. [DOI] [PubMed] [Google Scholar]
- Anthis N. J., Clore G. M.. Visualizing transient dark states by NMR spectroscopy. Q. Rev. Biophys. 2015;48(1):35–116. doi: 10.1017/S0033583514000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ådén J., Wolf-Watz M.. NMR identification of transient complexes critical to adenylate kinase catalysis. J. Am. Chem. Soc. 2007;129(45):14003–14012. doi: 10.1021/ja075055g. [DOI] [PubMed] [Google Scholar]
- Iwahara J., Clore G. M.. Detecting transient intermediates in macromolecular binding by paramagnetic NMR. Nature. 2006;440(7088):1227–1230. doi: 10.1038/nature04673. [DOI] [PubMed] [Google Scholar]
- Dannatt H. R., Felletti M., Jehle S., Wang Y., Emsley L., Dixon N. E., Lesage A., Pintacuda G.. Weak and Transient Protein Interactions Determined by Solid-State NMR. Angew. Chem., Int. Ed. 2016;55(23):6638–6641. doi: 10.1002/anie.201511609. [DOI] [PubMed] [Google Scholar]
- Kupce E., Freeman R.. Molecular structure from a single NMR experiment. J. Am. Chem. Soc. 2008;130(32):10788–10792. doi: 10.1021/ja8036492. [DOI] [PubMed] [Google Scholar]
- Jardetzky, O. ; Roberts, G. C. K. . NMR in molecular biology; Academic Press, 2013. [Google Scholar]
- Liang B., Tamm L. K.. NMR as a tool to investigate the structure, dynamics and function of membrane proteins. Nature structural & molecular biology. 2016;23(6):468–474. doi: 10.1038/nsmb.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittermaier A., Kay L. E.. New tools provide new insights in NMR studies of protein dynamics. science. 2006;312(5771):224–228. doi: 10.1126/science.1124964. [DOI] [PubMed] [Google Scholar]
- Palmer A. G. III. NMR characterization of the dynamics of biomacromolecules. Chem. Rev. 2004;104(8):3623–3640. doi: 10.1021/cr030413t. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Burlingame A. L., Cronholm T., Sjövall J.. Deuterium and carbon-13 tracer studies of ethanol metabolism in the rat by 2H, 1H-decoupled 13C nuclear magnetic resonance. Biochem. Biophys. Res. Commun. 1974;56(3):828–835. doi: 10.1016/0006-291X(74)90680-9. [DOI] [PubMed] [Google Scholar]
- Hawkins D. R., Midgley I.. The use of 13C-nmr spectroscopy for the detection and identification of metabolites of carbon-13 labelled amitriptyline. J. Pharm. Pharmacol. 1978;30(1):547–553. doi: 10.1111/j.2042-7158.1978.tb13321.x. [DOI] [PubMed] [Google Scholar]
- Williams T. H., Sasso G. J., Ryan J. J., Schwartz M. A.. Novel application of proton nuclear magnetic resonance spectroscopy in the identification of 2’-chloronordiazepam metabolites in the dog. J. Med. Chem. 1979;22(4):436–440. doi: 10.1021/jm00190a016. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Shulman R. G.. 13C NMR studies of gluconeogenesis in rat liver cells: utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc. Natl. Acad. Sci. U. S. A. 1979;76(4):1603–1609. doi: 10.1073/pnas.76.4.1603. From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hyder F., Shulman R. G.. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annual review of physiology. 2003;65(1):401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- Weiner M. W., Hetherington H., Hubesch B., Karczmar G., Massie B., Maudsley A., Meyerhoff D. J., Sappey-Marinier D., Schaefer S., Twieg D. B.. et al. Clinical magnetic resonance spectroscopy of brain, heart, liver, kidney, and cancer. A quantitative approach. NMR in Biomedicine. 1989;2(5–6):290–297. doi: 10.1002/nbm.1940020519. [DOI] [PubMed] [Google Scholar]
- Bock J. L.. Analysis of serum by high-field proton nuclear magnetic resonance. Clinical Chemistry. 1982;28(9):1873–1877. doi: 10.1093/clinchem/28.9.1873. [DOI] [PubMed] [Google Scholar]
- Bales J. R., Higham D. P., Howe I., Nicholson J. K., Sadler P. J.. Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clinical Chemistry. 1984;30(3):426–432. doi: 10.1093/clinchem/30.3.426. [DOI] [PubMed] [Google Scholar]
- Fossel E. T., Carr J. M., McDonagh J.. Detection of malignant tumors. New England Journal of Medicine. 1986;315(22):1369–1376. doi: 10.1056/NEJM198611273152201. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Jeyarajah E. J., Bennett D. W.. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clinical Chemistry. 1991;37(3):377–386. doi: 10.1093/clinchem/37.3.377. [DOI] [PubMed] [Google Scholar]
- Cavalli A., Salvatella X., Dobson C. M., Vendruscolo M.. Protein structure determination from NMR chemical shifts. Proc. Natl. Acad. Sci. U. S. A. 2007;104(23):9615–9620. doi: 10.1073/pnas.0610313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K.. The way to NMR structures of proteins. Nature structural biology. 2001;8(11):923–925. doi: 10.1038/nsb1101-923. [DOI] [PubMed] [Google Scholar]
- Wüthrich K.. Protein structure determination in solution by nuclear magnetic resonance spectroscopy. Science. 1989;243(4887):45–50. doi: 10.1126/science.2911719. [DOI] [PubMed] [Google Scholar]
- López-Méndez B., Güntert P.. Automated protein structure determination from NMR spectra. J. Am. Chem. Soc. 2006;128(40):13112–13122. doi: 10.1021/ja061136l. [DOI] [PubMed] [Google Scholar]
- Güntert P.. Automated structure determination from NMR spectra. Eur. Biophys. J. 2009;38:129–143. doi: 10.1007/s00249-008-0367-z. [DOI] [PubMed] [Google Scholar]
- Boehr D. D., Dyson H. J., Wright P. E.. An NMR perspective on enzyme dynamics. Chem. Rev. 2006;106(8):3055–3079. doi: 10.1021/cr050312q. [DOI] [PubMed] [Google Scholar]
- Frey, P. A. ; Hegeman, A. D. . Enzymatic reaction mechanisms; Oxford University Press, 2007. [Google Scholar]
- He X., Agnihotri G., Liu H.-w.. Novel enzymatic mechanisms in carbohydrate metabolism. Chem. Rev. 2000;100(12):4615–4662. doi: 10.1021/cr9902998. [DOI] [PubMed] [Google Scholar]
- Alger J., Shulman R.. NMR studies of enzymatic rates in vitro and in vivo by magnetization transfer. Q. Rev. Biophys. 1984;17(1):83–124. doi: 10.1017/S0033583500005266. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Jardetzky O.. Monitoring of cellular metabolism by NMR. Biochimica et Biophysica Acta (BBA)-Reviews on Bioenergetics. 1981;639(1):53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Szyperski T.. 13C-NMR, MS and metabolic flux balancing in biotechnology research. Q. Rev. Biophys. 1998;31(1):41–106. doi: 10.1017/S0033583598003412. [DOI] [PubMed] [Google Scholar]
- Yu X., White L. T., Doumen C., Damico L. A., LaNoue K. F., Alpert N. M., Lewandowski E. D.. Kinetic analysis of dynamic 13C NMR spectra: metabolic flux, regulation, and compartmentation in hearts. Biophysical journal. 1995;69(5):2090–2102. doi: 10.1016/S0006-3495(95)80080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers R.. NMR metabolomics and drug discovery. Magn. Reson. Chem. 2009;47(S1):S2–S11. doi: 10.1002/mrc.2461. [DOI] [PubMed] [Google Scholar]
- Zhang Z.-Z., Fan M.-L., Hao X., Qin X.-M., Li Z.-Y.. Integrative drug efficacy assessment of Danggui and European Danggui using NMR-based metabolomics. J. Pharm. Biomed. Anal. 2016;120:1–9. doi: 10.1016/j.jpba.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Shivapurkar R., Hingamire T., Kulkarni A. S., Rajamohanan P., Reddy D. S., Shanmugam D.. Evaluating antimalarial efficacy by tracking glycolysis in Plasmodium falciparum using NMR spectroscopy. Sci. Rep. 2018;8(1):18076. doi: 10.1038/s41598-018-36197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard M. E., Stanley E. G., Lindon J. C., Nicholson J. K., Holmes E.. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo. 2005;18(3):143–162. doi: 10.1002/nbm.935. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Rothman D. L.. 13C NMR of intermediary metabolism: implications for systemic physiology. Annu. Rev. Physiol. 2001;63(1):15–48. doi: 10.1146/annurev.physiol.63.1.15. [DOI] [PubMed] [Google Scholar]
- Blumich, B. NMR imaging of materials; OUP Oxford, 2000. [Google Scholar]
- Vankerckhoven H., Lambrechts P., Van Beylen M., Vanherle G.. Materials Science: Characterization of Composite Resins by NMR and TEM. Journal of dental research. 1981;60(12):1957–1965. doi: 10.1177/00220345810600120601. [DOI] [PubMed] [Google Scholar]
- Haouas M., Taulelle F., Martineau C.. Recent advances in application of 27Al NMR spectroscopy to materials science. Progress in nuclear magnetic resonance spectroscopy. 2016;94:11–36. doi: 10.1016/j.pnmrs.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Breitmaier, E. Structure elucidation by NMR in organic chemistry: a practical guide; John Wiley & Sons, 2002. [Google Scholar]
- Sarker S. D., Latif Z., Gray A.. An introduction to natural products isolation. Methods Mol. Biol. 2006;864:1–25. doi: 10.1385/1-59259-955-9:1. [DOI] [PubMed] [Google Scholar]
- Pauli G. F., Jaki B. U., Lankin D. C.. Quantitative 1H NMR: development and potential of a method for natural products analysis. J. Nat. Prod. 2005;68(1):133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- Sprogøe K., Stærk D., Jäger A. K., Adsersen A., Hansen S. H., Witt M., Landbo A.-K. R., Meyer A. S., Jaroszewski J. W.. Targeted natural product isolation guided by HPLC-SPE-NMR: constituents of Hubertia species. J. Nat. Prod. 2007;70(9):1472–1477. doi: 10.1021/np0702741. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Fry D. C.. NMR studies of the mechanism of enzyme action. Adv. Enzymol. Relat. Areas Mol. Biol. 1987;59:241–313. doi: 10.1002/9780470123058.ch6. [DOI] [PubMed] [Google Scholar]
- Ben-Tal Y., Boaler P. J., Dale H. J., Dooley R. E., Fohn N. A., Gao Y., García-Domínguez A., Grant K. M., Hall A. M., Hayes H. L.. et al. Mechanistic analysis by NMR spectroscopy: A users guide. Progress in nuclear magnetic resonance spectroscopy. 2022;129:28–106. doi: 10.1016/j.pnmrs.2022.01.001. [DOI] [PubMed] [Google Scholar]
- Blümich B., Hagemeyer A., Schaefer D., Schmidt-Rohr K., Spiess H. W.. Solid State NMR spectroscopy in polymer science. Adv. Mater. 1990;2(2):72–81. doi: 10.1002/adma.19900020203. [DOI] [Google Scholar]
- Kaźmierski S., Pawlak T., Jeziorna A., Potrzebowski M.. Modern solid state NMR techniques and concepts in structural studies of synthetic polymers. Polym. Adv. Technol. 2016;27(9):1143–1155. doi: 10.1002/pat.3780. [DOI] [Google Scholar]
- Horii F., Kaji H., Ishida H., Kuwabara K., Masuda K., Tai T.. Solid-state NMR analyses of the structure and dynamics of polymers in the different states. Journal of molecular structure. 1998;441(2–3):303–311. doi: 10.1016/S0022-2860(97)00303-7. [DOI] [Google Scholar]
- Hatfield G. R., Carduner K. R.. Solid state NMR: Applications in high performance ceramics. J. Mater. Sci. 1989;24:4209–4219. doi: 10.1007/BF00544489. [DOI] [Google Scholar]
- Gervais C.. Solid-state NMR applied to Si-based polymer derived ceramics: A review. Open Ceramics. 2023;15:100376. doi: 10.1016/j.oceram.2023.100376. [DOI] [Google Scholar]
- Gervais C., Maquet J., Babonneau F., Duriez C., Framery E., Vaultier M., Florian P., Massiot D.. Chemically derived BN ceramics: Extensive 11B and 15N solid-state NMR study of a preceramic polyborazilene. Chemistry of materials. 2001;13(5):1700–1707. doi: 10.1021/cm001244l. [DOI] [Google Scholar]
- Peng J., Xiao Y., Clarkson D. A., Greenbaum S. G., Zawodzinski T. A., Chen X. C.. A Nuclear magnetic resonance study of cation and anion dynamics in polymer-ceramic composite solid electrolytes. ACS Applied Polymer Materials. 2020;2(3):1180–1189. doi: 10.1021/acsapm.9b01068. [DOI] [Google Scholar]
- Schuhmacher J., Berger F., Weinmann M., Bill J., Aldinger F., Müller K.. Solid-state NMR and FT IR studies of the preparation of Si-B-C-N ceramics from boron-modified polysilazanes. Applied organometallic chemistry. 2001;15(10):809–819. doi: 10.1002/aoc.235. [DOI] [Google Scholar]
- Kirchhain H., van Wüllen L.. Solid state NMR at very high temperatures. Prog. Nucl. Magn. Reson. Spectrosc. 2019;114:71–85. doi: 10.1016/j.pnmrs.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Everett, J. R. ; Harris, R. K. ; Lindon, J. C. ; Wilson, I. D. . NMR in Pharmaceutical Science; John Wiley & Sons, 2015. [Google Scholar]
- Kiss R., Fizil Á., Szántay C. Jr. What NMR can do in the biopharmaceutical industry. J. Pharm. Biomed. Anal. 2018;147:367–377. doi: 10.1016/j.jpba.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Holzgrabe U.. Quantitative NMR spectroscopy in pharmaceutical applications. Prog. Nucl. Magn. Reson. Spectrosc. 2010;57(2):229–240. doi: 10.1016/j.pnmrs.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Lepre C. A., Moore J. M., Peng J. W.. Theory and applications of NMR-based screening in pharmaceutical research. Chem. Rev. 2004;104(8):3641–3676. doi: 10.1021/cr030409h. [DOI] [PubMed] [Google Scholar]
- Harner M. J., Frank A. O., Fesik S. W.. Fragment-based drug discovery using NMR spectroscopy. Journal of biomolecular NMR. 2013;56:65–75. doi: 10.1007/s10858-013-9740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mureddu L. G., Vuister G. W.. Fragment-based drug discovery by NMR. Where are the successes and where can it be improved? Frontiers in molecular biosciences. 2022;9:834453. doi: 10.3389/fmolb.2022.834453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages J., Coles M., Kessler H.. NMR-based screening: a powerful tool in fragment-based drug discovery. Analyst. 2007;132(7):692–705. doi: 10.1039/B709658P. [DOI] [PubMed] [Google Scholar]
- Giraudeau P.. Challenges and perspectives in quantitative NMR. Magn. Reson. Chem. 2017;55(1):61–69. doi: 10.1002/mrc.4475. [DOI] [PubMed] [Google Scholar]
- Giraudeau P.. Quantitative NMR spectroscopy of complex mixtures. Chem. Commun. 2023;59(44):6627–6642. doi: 10.1039/D3CC01455J. [DOI] [PubMed] [Google Scholar]
- Gowda G. N., Raftery D.. Can NMR solve some significant challenges in metabolomics? J. Magn. Reson. 2015;260:144–160. doi: 10.1016/j.jmr.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivdin L. B.. Computational 1H NMR: Part 1. Theoretical background. Magn. Reson. Chem. 2019;57(11):897–914. doi: 10.1002/mrc.4873. [DOI] [PubMed] [Google Scholar]
- Krivdin L. B.. Computational 1H NMR: part 2. Chemical applications. Magn. Reson. Chem. 2020;58(1):5–14. doi: 10.1002/mrc.4896. [DOI] [PubMed] [Google Scholar]
- Castro, A. C. ; Swart, M. . Recent Advances in Computational NMR Spectrum Prediction. In Computational Techniques for Analytical Chemistry and Bioanalysis, Wilson, P. B. , Grootveld, M. , Eds.; The Royal Society of Chemistry, 2020; p 0. [Google Scholar]
- Vaara J.. Theory and computation of nuclear magnetic resonance parameters. Phys. Chem. Chem. Phys. 2007;9(40):5399–5418. doi: 10.1039/b706135h. 10.1039/B706135H. [DOI] [PubMed] [Google Scholar]
- Das S., Edison A. S., Merz K. M. Jr.. Metabolite Structure Assignment Using In Silico NMR Techniques. Anal. Chem. 2020;92(15):10412–10419. doi: 10.1021/acs.analchem.0c00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyashberg M., Williams A., Martin G.. Computer-assisted structure verification and elucidation tools in NMR-based structure elucidation. Prog. Nucl. Magn. Reson. Spectrosc. 2008;53(1–2):1–104. doi: 10.1016/j.pnmrs.2007.04.003. [DOI] [Google Scholar]
- Semenov V. A., Krivdin L. B.. Computational NMR of natural products. Russ. Chem. Rev. 2022;91(5):RCR5027. doi: 10.1070/RCR5027. [DOI] [PubMed] [Google Scholar]
- Orekhov V. Y., Ibraghimov I. V., Billeter M.. MUNIN: a new approach to multi-dimensional NMR spectra interpretation. Journal of biomolecular NMR. 2001;20:49–60. doi: 10.1023/A:1011234126930. [DOI] [PubMed] [Google Scholar]
- Mazurek A. H., Szeleszczuk Ł., Pisklak D. M.. A review on combination of ab initio molecular dynamics and nmr parameters calculations. International Journal of Molecular Sciences. 2021;22(9):4378. doi: 10.3390/ijms22094378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühl M., van Mourik T.. NMR spectroscopy: quantum-chemical calculations. Wiley Interdisciplinary Reviews: Computational Molecular Science. 2011;1(4):634–647. doi: 10.1002/wcms.63. [DOI] [Google Scholar]
- Marcarino M. O., Zanardi M. a. M., Cicetti S., Sarotti A. M.. NMR calculations with quantum methods: development of new tools for structural elucidation and beyond. Acc. Chem. Res. 2020;53(9):1922–1932. doi: 10.1021/acs.accounts.0c00365. [DOI] [PubMed] [Google Scholar]
- Helgaker T., Jaszuński M., Pecul M.. The quantum-chemical calculation of NMR indirect spin-spin coupling constants. Prog. Nucl. Magn. Reson. Spectrosc. 2008;53(4):249–268. doi: 10.1016/j.pnmrs.2008.02.002. [DOI] [Google Scholar]
- de Dios A. C.. Ab initio calculations of the NMR chemical shift. Prog. Nucl. Magn. Reson. Spectrosc. 1996;29(3):229–278. doi: 10.1016/S0079-6565(96)01029-1. [DOI] [Google Scholar]
- Rusakova I. L.. Quantum chemical approaches to the calculation of NMR parameters: From fundamentals to recent advances. Magnetochemistry. 2022;8(5):50. doi: 10.3390/magnetochemistry8050050. [DOI] [Google Scholar]
- Warshel A., Levitt M.. Theoretical studies of enzymic reactions: Dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J. Mol. Biol. 1976;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- Morzan U. N., Alonso de Armino D. J., Foglia N. O., Ramirez F., Gonzalez Lebrero M. C., Scherlis D. A., Estrin D. A.. Spectroscopy in complex environments from QM-MM simulations. Chem. Rev. 2018;118(7):4071–4113. doi: 10.1021/acs.chemrev.8b00026. [DOI] [PubMed] [Google Scholar]
- Wang B., Merz K. M.. A Fast QM/MM (Quantum Mechanical/Molecular Mechanical) Approach to Calculate Nuclear Magnetic Resonance Chemical Shifts for Macromolecules. J. Chem. Theory Comput. 2006;2(1):209–215. doi: 10.1021/ct050212s. [DOI] [PubMed] [Google Scholar]
- Yu Z., Li P., Merz K. M. Jr.. Using Ligand-Induced Protein Chemical Shift Perturbations To Determine Protein-Ligand Structures. Biochemistry. 2017;56(18):2349–2362. doi: 10.1021/acs.biochem.7b00170. [DOI] [PubMed] [Google Scholar]
- He X., Wang B., Merz K. M. Jr. Protein NMR chemical shift calculations based on the automated fragmentation QM/MM approach. J. Phys. Chem. B. 2009;113(30):10380–10388. doi: 10.1021/jp901992p. [DOI] [PubMed] [Google Scholar]
- Guan Y., Shree Sowndarya S. V., Gallegos L. C., St John P. C., Paton R. S.. Real-time prediction of 1H and 13C chemical shifts with DFT accuracy using a 3D graph neural network. Chemical Science. 2021;12(36):12012–12026. doi: 10.1039/D1SC03343C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard W., Bratholm L. A., Packer M. J., Mulholland A. J., Glowacki D. R., Butts C. P.. IMPRESSION-prediction of NMR parameters for 3-dimensional chemical structures using machine learning with near quantum chemical accuracy. Chemical science. 2020;11(2):508–515. doi: 10.1039/C9SC03854J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrows M. Y., Kodituwakku D., Xue J., Pickering I., Terrel N. S., Roitberg A. E.. LEGOLAS: A Machine Learning Method for Rapid and Accurate Predictions of Protein NMR Chemical Shifts. J. Chem. Theory Comput. 2025;21(8):4266–4275. doi: 10.1021/acs.jctc.5c00026. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Tsai Y. H., Amichetti M., Zanardi M. M., Grimson R., Daranas A. H., Sarotti A. M.. ML-J-DP4: An Integrated Quantum Mechanics-Machine Learning Approach for Ultrafast NMR Structural Elucidation. Org. Lett. 2022;24(41):7487–7491. doi: 10.1021/acs.orglett.2c01251. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Li C., Cong Y., Deng W.. Identifying molecular functional groups of organic compounds by deep learning of NMR data. Magn. Reson. Chem. 2022;60(11):1061–1069. doi: 10.1002/mrc.5292. [DOI] [PubMed] [Google Scholar]
- Cobas C.. NMR signal processing, prediction, and structure verification with machine learning techniques. Magn. Reson. Chem. 2020;58(6):512–519. doi: 10.1002/mrc.4989. [DOI] [PubMed] [Google Scholar]
- Lu X.-Y., Wu H.-P., Ma H., Li H., Li J., Liu Y.-T., Pan Z.-Y., Xie Y., Wang L., Ren B.. et al. Deep Learning-Assisted Spectrum-Structure Correlation: State-of-the-Art and Perspectives. Anal. Chem. 2024;96(20):7959–7975. doi: 10.1021/acs.analchem.4c01639. [DOI] [PubMed] [Google Scholar]
- Mocci, F. ; Laaksonen, A. . Combining MD simulations and NMR spectroscopy for molecular insight and methodological synergy: the integrated MD-NMR method. In Nuclear Magnetic Resonance: Vol. 44, Kamienska-Trela, K. , Ed.; Vol. 44; The Royal Society of Chemistry, 2015; p 0. [Google Scholar]
- Ghitti, M. ; Musco, G. ; Spitaleri, A. . NMR and Computational Methods in the Structural and Dynamic Characterization of Ligand-Receptor Interactions. In Protein Conformational Dynamics, Han, K.-l. , Zhang, X. , Yang, M.-j. , Eds.; Springer International Publishing, 2014; pp 271–304. [DOI] [PubMed] [Google Scholar]
- Unione L., Ortega G., Mallagaray A., Corzana F., Pérez-Castells J., Canales A., Jiménez-Barbero J., Millet O.. Unraveling the conformational landscape of ligand binding to glucose/galactose-binding protein by paramagnetic NMR and MD simulations. ACS Chem. Biol. 2016;11(8):2149–2157. doi: 10.1021/acschembio.6b00148. [DOI] [PubMed] [Google Scholar]
- Stiller J. B., Jordan Kerns S., Hoemberger M., Cho Y.-J., Otten R., Hagan M. F., Kern D.. Probing the transition state in enzyme catalysis by high-pressure NMR dynamics. Nature Catalysis. 2019;2(8):726–734. doi: 10.1038/s41929-019-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Yuwen T., Zhu F., Skrynnikov N. R.. Role of electrostatic interactions in binding of peptides and intrinsically disordered proteins to their folded targets. 1. NMR and MD characterization of the complex between the c-Crk N-SH3 domain and the peptide Sos. Biochemistry. 2014;53(41):6473–6495. doi: 10.1021/bi500904f. [DOI] [PubMed] [Google Scholar]
- Konrat R.. NMR contributions to structural dynamics studies of intrinsically disordered proteins. J. Magn. Reson. 2014;241:74–85. doi: 10.1016/j.jmr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. S., Lindorff-Larsen K., Bonomi M.. What Will Computational Modeling Approaches Have to Say in the Era of Atomistic Cryo-EM Data? J. Chem. Inf. Model. 2020;60(5):2410–2412. doi: 10.1021/acs.jcim.0c00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyashberg M., Argyropoulos D.. Computer Assisted Structure Elucidation (CASE): Current and future perspectives. Magn. Reson. Chem. 2021;59(7):669–690. doi: 10.1002/mrc.5115. [DOI] [PubMed] [Google Scholar]
- Sels D., Dashti H., Mora S., Demler O., Demler E.. Quantum approximate Bayesian computation for NMR model inference. Nature machine intelligence. 2020;2(7):396–402. doi: 10.1038/s42256-020-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R. P., Hansch C.. Use of 13C NMR chemical shift as QSAR/QSPR descriptor. Chem. Rev. 2011;111(4):2865–2899. doi: 10.1021/cr100125d. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Hansch C.. The physicochemical approach to drug design and discovery (QSAR) Drug Dev. Res. 1981;1(4):267–309. doi: 10.1002/ddr.430010403. [DOI] [Google Scholar]
- Verma J., Khedkar V. M., Coutinho E. C.. 3D-QSAR in drug design-a review. Current topics in medicinal chemistry. 2010;10(1):95–115. doi: 10.2174/156802610790232260. [DOI] [PubMed] [Google Scholar]
- Bühl M., Kaupp M., Malkina O. L., Malkin V. G.. The DFT route to NMR chemical shifts. J. Comput. Chem. 1999;20(1):91–105. doi: 10.1002/(SICI)1096-987X(19990115)20:1<91::AID-JCC10>3.0.CO;2-C. [DOI] [Google Scholar]
- Emwas, A.-H. M. The Strengths and Weaknesses of NMR Spectroscopy and Mass Spectrometry with Particular Focus on Metabolomics Research. In Metabonomics: Methods and Protocols, Bjerrum, J. T. , Ed.; Springer New York, 2015; pp 161–193. [DOI] [PubMed] [Google Scholar]
- Letertre, M. P. M. ; Giraudeau, P. ; de Tullio, P. . Nuclear Magnetic Resonance Spectroscopy in Clinical Metabolomics and Personalized Medicine: Current Challenges and Perspectives. Frontiers in Molecular Biosciences 2021, Volume 8, Review. 10.3389/fmolb.2021.698337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S.. NMR metabolomics: A look ahead. J. Magn. Reson. 2019;306:155–161. doi: 10.1016/j.jmr.2019.07.013. [DOI] [PubMed] [Google Scholar]
- Scheubert K., Hufsky F., Böcker S.. Computational mass spectrometry for small molecules. Journal of Cheminformatics. 2013;5(1):12. doi: 10.1186/1758-2946-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinitcyn P., Rudolph J. D., Cox J.. Computational Methods for Understanding Mass Spectrometry-Based Shotgun Proteomics Data. Annual Review of Biomedical Data Science. 2018;1:207–234. doi: 10.1146/annurev-biodatasci-080917-013516. [DOI] [Google Scholar]
- Kupce E., Freeman R.. High-resolution NMR correlation experiments in a single measurement (HR-PANACEA) Magn. Reson. Chem. 2010;48(5):333–336. doi: 10.1002/mrc.2595. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Kupce E., Yong J. R. J., Widmalm G., Claridge T. D. W.. Parallel NMR Supersequences: Ten Spectra in a Single Measurement. JACS Au. 2021;1(11):1892–1897. doi: 10.1021/jacsau.1c00423. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupce E., Freeman R.. Molecular structure from a single NMR sequence (fast-PANACEA) J. Magn. Reson. 2010;206(1):147–153. doi: 10.1016/j.jmr.2010.06.018. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Bak M., Rasmussen J. T., Nielsen N. C.. SIMPSON: a general simulation program for solid-state NMR spectroscopy. J. Magn. Reson. 2000;147(2):296–330. doi: 10.1006/jmre.2000.2179. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Levante T. O., Meier B. H., Ernst R. R.. Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. Journal of Magnetic Resonance, Series A. 1994;106(1):75–105. doi: 10.1006/jmra.1994.1008. [DOI] [Google Scholar]
- Hogben H. J., Krzystyniak M., Charnock G. T., Hore P. J., Kuprov I.. Spinach-a software library for simulation of spin dynamics in large spin systems. J. Magn. Reson. 2011;208(2):179–194. doi: 10.1016/j.jmr.2010.11.008. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Krzystyniak M., Edwards L. J., Kuprov I.. Destination state screening of active spaces in spin dynamics simulations. J. Magn. Reson. 2011;210(2):228–232. doi: 10.1016/j.jmr.2011.03.010. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Zanardi M. M., Suarez A. G., Sarotti A. M.. Determination of the Relative Configuration of Terminal and Spiroepoxides by Computational Methods. Advantages of the Inclusion of Unscaled Data. J. Org. Chem. 2017;82(4):1873–1879. doi: 10.1021/acs.joc.6b02129. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Dickinson W. C.. The Time Average Magnetic Field at the Nucleus in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1951;81(5):717–731. doi: 10.1103/PhysRev.81.717. [DOI] [Google Scholar]
- Jameson C. J.. Understanding NMR chemical shifts. Annu. Rev. Phys. Chem. 1996;47(1):135–169. doi: 10.1146/annurev.physchem.47.1.135. [DOI] [Google Scholar]
- Gauss J.. Accurate calculation of NMR chemical shifts. Berichte der Bunsengesellschaft für physikalische Chemie. 1995;99(8):1001–1008. doi: 10.1002/bbpc.199500022. [DOI] [Google Scholar]
- Nerli S., McShan A. C., Sgourakis N. G.. Chemical shift-based methods in NMR structure determination. Prog. Nucl. Magn. Reson. Spectrosc. 2018;106–107:1–25. doi: 10.1016/j.pnmrs.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takis P. G., Aggelidou V. A., Sands C. J., Louka A.. Mapping of 1H NMR chemical shifts relationship with chemical similarities for the acceleration of metabolic profiling: Application on blood products. Magn. Reson. Chem. 2023;61(12):759–769. doi: 10.1002/mrc.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando I.. Some aspects of the NMR chemical shift/structure correlation in the structural characterization of polymers and biopolymers. Polym. J. 2012;44(8):734–747. doi: 10.1038/pj.2012.95. [DOI] [Google Scholar]
- Robustelli P., Cavalli A., Vendruscolo M.. Determination of protein structures in the solid state from NMR chemical shifts. Structure. 2008;16(12):1764–1769. doi: 10.1016/j.str.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Wishart D. S.. Interpreting protein chemical shift data. Progress in nuclear magnetic resonance spectroscopy. 2011;58(1–2):62–87. doi: 10.1016/j.pnmrs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Berjanskii M. V., Wishart D. S.. Unraveling the meaning of chemical shifts in protein NMR. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2017;1865(11):1564–1576. doi: 10.1016/j.bbapap.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Wagner G., Pardi A., Wuethrich K.. Hydrogen bond length and proton NMR chemical shifts in proteins. J. Am. Chem. Soc. 1983;105(18):5948–5949. doi: 10.1021/ja00356a056. [DOI] [Google Scholar]
- Edison A. S., Colonna M., Gouveia G. J., Holderman N. R., Judge M. T., Shen X., Zhang S.. NMR: unique strengths that enhance modern metabolomics research. Analytical chemistry. 2021;93(1):478–499. doi: 10.1021/acs.analchem.0c04414. [DOI] [PubMed] [Google Scholar]
- Becke A. D.. Completely numerical calculations on diatomic molecules in the local-density approximation. Phys. Rev. A. 1986;33(4):2786–2788. doi: 10.1103/PhysRevA.33.2786. [DOI] [PubMed] [Google Scholar]
- Lee C., Yang W., Parr R. G.. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37(2):785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Becke A. D.. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988;38(6):3098–3100. doi: 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Kurth S., Perdew J. P., Blaha P.. Molecular and solid-state tests of density functional approximations: LSD, GGAs, and meta-GGAs. International journal of quantum chemistry. 1999;75(4–5):889–909. doi: 10.1002/(SICI)1097-461X(1999)75:4/5<889::AID-QUA54>3.0.CO;2-8. [DOI] [Google Scholar]
- Reiter K., Mack F., Weigend F.. Calculation of magnetic shielding constants with meta-GGA functionals employing the multipole-accelerated resolution of the identity: implementation and assessment of accuracy and efficiency. J. Chem. Theory Comput. 2018;14(1):191–197. doi: 10.1021/acs.jctc.7b01115. [DOI] [PubMed] [Google Scholar]
- Bieger W., Seifert G., Eschrig H., Grossmann G.. LCAO Xα calculations of nuclear magnetic shielding in molecules. Chem. Phys. Lett. 1985;115(3):275–280. doi: 10.1016/0009-2614(85)80027-0. [DOI] [Google Scholar]
- Aucar G. A.. Understanding NMR J-couplings by the theory of polarization propagators. Concepts in Magnetic Resonance Part A. 2008;32A(2):88–116. doi: 10.1002/cmr.a.20108. [DOI] [Google Scholar]
- Helgaker T., Jaszunski M., Ruud K.. Ab initio methods for the calculation of NMR shielding and indirect spin-spin coupling constants. Chem. Rev. 1999;99:293–352. doi: 10.1021/cr960017t. [DOI] [PubMed] [Google Scholar]
- Fukui H.. Theory and calculation of nuclear spin-spin coupling constants. Prog. Nucl. Magn. Reson. Spectrosc. 1999;35(3):267–294. doi: 10.1016/S0079-6565(99)00012-6. [DOI] [Google Scholar]
- Khandogin J., Ziegler T.. A density functional study of nuclear magnetic resonance spin-spin coupling constants in transition-metal systems. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 1999;55(3):607–624. doi: 10.1016/S1386-1425(98)00265-0. [DOI] [Google Scholar]
- Adamo C., Barone V.. Toward chemical accuracy in the computation of NMR shieldings: the PBE0 model. Chem. Phys. Lett. 1998;298(1–3):113–119. doi: 10.1016/S0009-2614(98)01201-9. [DOI] [Google Scholar]
- Bienati M., Adamo C., Barone V.. Performance of a new hybrid Hartree-Fock/Kohn-Sham model (B98) in predicting vibrational frequencies, polarisabilities and NMR chemical shifts. Chemical physics letters. 1999;311(1–2):69–76. doi: 10.1016/S0009-2614(99)00776-9. [DOI] [Google Scholar]
- Klein R. A., Mennucci B., Tomasi J.. Ab Initio Calculations of 17O NMR-Chemical Shifts for Water. The Limits of PCM Theory and the Role of Hydrogen-Bond Geometry and Cooperativity. J. Phys. Chem. A. 2004;108(27):5851–5863. doi: 10.1021/jp0487408. [DOI] [Google Scholar]
- Mennucci, B. ; Cammi, R. . Continuum solvation models in chemical physics: from theory to applications; John Wiley & Sons, 2008. [Google Scholar]
- Autschbach J., Ziegler T.. Relativistic calculations of spin-spin coupling constants of heavy nuclei. Calculation of NMR and EPR parameters: Theory and applications. 2004:249–264. doi: 10.1002/3527601678.ch15. [DOI] [Google Scholar]
- Autschbach, J. ; Ziegler, T. . Relativistic computation of NMR shieldings and spin-spin coupling constants. eMagRes. 2007. 10.1002/9780470034590.emrstm0451 [DOI] [Google Scholar]
- Kaltsoyannis N., McGrady J., Autschbach J.. The calculation of NMR parameters in transition metal complexes. Principles and Applications of Density Functional Theory in Inorganic Chemistry I. 2004;112:1–48. doi: 10.1007/b97936. [DOI] [Google Scholar]
- Autschbach J.. Density functional theory applied to calculating optical and spectroscopic properties of metal complexes: NMR and optical activity. Coord. Chem. Rev. 2007;251(13):1796–1821. doi: 10.1016/j.ccr.2007.02.012. [DOI] [Google Scholar]
- Ditchfield R.. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974;27(4):789–807. doi: 10.1080/00268977400100711. [DOI] [Google Scholar]
- Friedrich K., Seifert G., Großmann G.. Nuclear magnetic shielding in molecules. The application of GIAO's in LCAO-X α-calculations. Z Phys. D: At.,Mol. Clusters. 1990;17:45. doi: 10.1007/BF01437495. [DOI] [Google Scholar]
- Heine T., Schleyer P. v. R., Corminboeuf C., Seifert G., Reviakine R., Weber J.. Analysis of Aromatic Delocalization: Individual Molecular Orbital Contributions to Nucleus-Independent Chemical Shifts. J. Phys. Chem. A. 2003;107(33):6470–6475. doi: 10.1021/jp035163z. [DOI] [Google Scholar]
- Korzhnev D. M., Billeter M., Arseniev A. S., Orekhov V. Y.. NMR studies of Brownian tumbling and internal motions in proteins. Progress in Nuclear magnetic resonance spectroscopy. 2001;38(3):197–266. doi: 10.1016/S0079-6565(00)00028-5. [DOI] [Google Scholar]
- Walling A. E., Pargas R. E., de Dios A. C.. Chemical shift tensors in peptides: a quantum mechanical study. J. Phys. Chem. A. 1997;101(39):7299–7303. doi: 10.1021/jp971783m. [DOI] [Google Scholar]
- Kutzelnigg W.. Theory of Magnetic Susceptibilities and NMR Chemical Shifts in Terms of Localized Quantities. Isr. J. Chem. 1980;19(1–4):193–200. doi: 10.1002/ijch.198000020. [DOI] [Google Scholar]
- Schindler M., Kutzelnigg W.. Theory of magnetic susceptibilities and NMR chemical shifts in terms of localized quantities. II. Application to some simple molecules. J. Chem. Phys. 1982;76(4):1919–1933. doi: 10.1063/1.443165. [DOI] [Google Scholar]
- Alkorta I., Elguero J.. Ab initio (GIAO) calculations of absolute nuclear shieldings for representative compounds containing 1 (2) H, 6 (7) Li, 11 B, 13 C, 14 (15) N, 17 O, 19 F, 29 Si, 31 P, 33 S, and 35 Cl nuclei. Structural Chemistry. 1998;9:187–202. doi: 10.1023/A:1022419030317. [DOI] [Google Scholar]
- Alkorta I., Elguero J.. GIAO calculations of chemical shifts in heterocyclic compounds. Structural Chemistry. 2003;14:377–389. doi: 10.1023/A:1024402027760. [DOI] [Google Scholar]
- Gholivand K., Maghsoud Y., Hosseini M., Kahnouji M.. A theoretical study on 1H/13C/31P NMR chemical shifts, and the correlation between 2JP-H and the electronic structure of different phosphoryl benzamide derivatives. J. Mol. Struct. 2019;1183:230–240. doi: 10.1016/j.molstruc.2019.01.081. [DOI] [Google Scholar]
- Siudem P., Paradowska K., Bukowicki J.. Conformational analysis of capsaicin using 13C, 15N, MAS NMR, GIAO DFT and GA calculations. J. Mol. Struct. 2017;1146:773–781. doi: 10.1016/j.molstruc.2017.05.142. [DOI] [Google Scholar]
- Shan H., Wilson W. K., Kamaric E.. NOESY and DFT-GIAO calculations reveal pervasive errors in C20 configurations of taraxastane-3, 20-diols: proposals to improve NMR structure determinations. Org. Lett. 2020;22(5):1714–1719. doi: 10.1021/acs.orglett.9b04566. [DOI] [PubMed] [Google Scholar]
- Wolff S., Ziegler T.. Calculation of DFT-GIAO NMR shifts with the inclusion of spin-orbit coupling. J. Chem. Phys. 1998;109(3):895–905. doi: 10.1063/1.476630. [DOI] [Google Scholar]
- Vıcha J., Novotný J., Straka M., Repisky M., Ruud K., Komorovsky S., Marek R.. Structure, solvent, and relativistic effects on the NMR chemical shifts in square-planar transition-metal complexes: Assessment of DFT approaches1. Phys. Chem. Chem. Phys. 2015;17:24944–24955. doi: 10.1039/C5CP04214C. [DOI] [PubMed] [Google Scholar]
- Pyykko P.. Relativistic theory of nuclear spin-spin coupling in molecules. Chem. Phys. 1977;22(2):289–296. doi: 10.1016/0301-0104(77)87013-4. [DOI] [Google Scholar]
- Knowles S. L., Roberts C. D., Augustinovic M., Flores-Bocanegra L., Raja H. A., Heath-Borrero K. N., Burdette J. E., Falkinham J. O. III, Pearce C. J., Oberlies N. H.. Opportunities and limitations for assigning relative configurations of antibacterial bislactones using GIAO NMR shift calculations. J. Nat. Prod. 2021;84(4):1254–1260. doi: 10.1021/acs.jnatprod.0c01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colherinhas G., Ludwig V., da Costa Ludwig Z. M.. GIAO-NMR spectroscopy of the xanthine’s structures in water solution using S-MC/QM methodology: An evaluation of the DFT-functionals’ efficiency. J. Mol. Liq. 2022;347:117955. doi: 10.1016/j.molliq.2021.117955. [DOI] [Google Scholar]
- Zhuang Y., Yang F., Menon A., Song J. M., Espinoza R. V., Schultz P. J., Garner A. L., Tripathi A.. An ECD and NMR/DP4+ computational pipeline for structure revision and elucidation of diphenazine-based natural products. J. Nat. Prod. 2023;86(7):1801–1814. doi: 10.1021/acs.jnatprod.3c00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy S. K., Raghavachari K.. Accurate and cost-effective NMR chemical shift predictions for nucleic acids using a molecules-in-molecules fragmentation-based method. J. Chem. Theory Comput. 2023;19(2):544–561. doi: 10.1021/acs.jctc.2c00967. [DOI] [PubMed] [Google Scholar]
- Pyykko P.. Relativistic effects in structural chemistry. Chem. Rev. 1988;88(3):563–594. doi: 10.1021/cr00085a006. [DOI] [Google Scholar]
- Ramsey N. F., Purcell E. M.. Interactions between Nuclear Spins in Molecules. Phys. Rev. 1952;85(1):143–144. doi: 10.1103/PhysRev.85.143. [DOI] [Google Scholar]
- Ramsey N. F.. Electron Coupled Interactions between Nuclear Spins in Molecules. Phys. Rev. 1953;91(2):303–307. doi: 10.1103/PhysRev.91.303. [DOI] [Google Scholar]
- Pyykko P.. Relativistic effects in chemistry: more common than you thought. Annu. Rev. Phys. Chem. 2012;63:45–64. doi: 10.1146/annurev-physchem-032511-143755. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- van Lenthe E., Snijders J. G., Baerends E. J.. The zero-order regular approximation for relativistic effects: The effect of spin-orbit coupling in closed shell molecules. J. Chem. Phys. 1996;105(15):6505–6516. doi: 10.1063/1.472460. [DOI] [Google Scholar]
- Reiher M.. Douglas-Kroll-Hess Theory: a relativistic electrons-only theory for chemistry. Theor. Chem. Acc. 2006;116:241–252. doi: 10.1007/s00214-005-0003-2. [DOI] [Google Scholar]
- Nakajima T., Hirao K.. The Douglas-Kroll-Hess approach. Chem. Rev. 2012;112(1):385–402. doi: 10.1021/cr200040s. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Hada M.. Gauge-origin dependence of NMR shielding constants in the Douglas-Kroll-Hess method. Chem. Phys. Lett. 2015;618:132–141. doi: 10.1016/j.cplett.2014.10.066. [DOI] [Google Scholar]
- Autschbach J.. Relativistic Effects on Electron-Nucleus Hyperfine Coupling Studied with an Exact 2-Component (X2C) Hamiltonian. J. Chem. Theory Comput. 2017;13(2):710–718. doi: 10.1021/acs.jctc.6b01014. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Kaupp, M. Relativistic Effects on NMR Chemical Shifts. In Relativistic Electronic Structure Theory - Part 2. Applications, Schwerdtfeger, P. , Ed.; Theoretical and Computational Chemistry, Vol. 14; Elsevier, 2004; pp 552–597. [Google Scholar]
- Čížek J.. On the Correlation Problem in Atomic and Molecular Systems. Calculation of Wavefunction Components in Ursell-Type Expansion Using Quantum-Field Theoretical Methods. J. Chem. Phys. 1966;45(11):4256–4266. doi: 10.1063/1.1727484. [DOI] [Google Scholar]
- Paldus J., Čížek J., Takahashi M.. Approximate account of the connected quadruply excited clusters in the coupled-pair many-electron theory. Phys. Rev. A. 1984;30(5):2193. doi: 10.1103/PhysRevA.30.2193. [DOI] [Google Scholar]
- Sekino H., Bartlett R. J.. A linear response, coupled-cluster theory for excitation energy. Int. J. Quantum Chem. 1984;26(S18):255–265. doi: 10.1002/qua.560260826. [DOI] [Google Scholar]
- Bartlett R. J.. Coupled-cluster theory: An overview of recent developments. Modern Electronic Structure Theory: Part II. 1995;2:1047–1131. doi: 10.1142/9789812832115_0005. [DOI] [Google Scholar]
- Bartlett R. J.. Perspective on Coupled-cluster Theory. The evolution toward simplicity in quantum chemistry. Phys. Chem. Chem. Phys. 2024;26(10):8013–8037. doi: 10.1039/D3CP03853J. [DOI] [PubMed] [Google Scholar]
- Gauss J., Stanton J. F.. Coupled-cluster calculations of nuclear magnetic resonance chemical shifts. J. Chem. Phys. 1995;103(9):3561–3577. doi: 10.1063/1.470240. [DOI] [Google Scholar]
- Krivdin L. B.. Computational NMR of charged systems. Magn. Reson. Chem. 2022;60(1):8–79. doi: 10.1002/mrc.5201. [DOI] [PubMed] [Google Scholar]
- Wilson D. J.. Extreme NMR shielding in fluoro-nitrogen cations. Phys. Chem. Chem. Phys. 2023;25(37):25420–25434. doi: 10.1039/D3CP03399F. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Hedin N.. Electron correlation and vibrational effects in predictions of paramagnetic NMR shifts. Phys. Chem. Chem. Phys. 2022;24(25):15230–15244. doi: 10.1039/D2CP01206E. [DOI] [PubMed] [Google Scholar]
- Piecuch P., Kucharski S. A., Kowalski K., Musiał M.. Efficient computer implementation of the renormalized coupled-cluster methods: the r-ccsd [t], r-ccsd (t), cr-ccsd [t], and cr-ccsd (t) approaches. Comput. Phys. Commun. 2002;149(2):71–96. doi: 10.1016/S0010-4655(02)00598-2. [DOI] [Google Scholar]
- Crawford T. D., Schaefer H. F. III. An introduction to coupled cluster theory for computational chemists. Reviews in computational chemistry. 2000;14:33–136. doi: 10.1002/9780470125915.ch2. [DOI] [Google Scholar]
- Byrd, J. N. ; Rishi, V. ; Perera, A. ; Bartlett, R. J. . Approximating electronically excited states with equation-of-motion linear coupled-cluster theory. J. Chem. Phys. 2015, 143 (16). 10.1063/1.4934232. [DOI] [PubMed] [Google Scholar]
- Perera A.. What made possible the accurate calculations of NMR spin-spin coupling constants? Mol. Phys. 2010;108(21–23):3017–3025. doi: 10.1080/00268976.2010.512567. [DOI] [Google Scholar]
- Stanton J. F., Gauss J.. Many-body methods for excited state potential energy surfaces. II. Analytic second derivatives for excited state energies in the equation-of-motion coupled cluster method. J. Chem. Phys. 1995;103(20):8931–8943. doi: 10.1063/1.470083. [DOI] [Google Scholar]
- Stanton J. F., Gauss J.. Analytic second derivatives in high-order many-body perturbation and coupled-cluster theories: Computational considerations and applications. Int. Rev. Phys. Chem. 2000;19(1):61–95. doi: 10.1080/014423500229864. [DOI] [Google Scholar]
- Dračínský, M. ; Kaminský, J. ; Bouř, P. . Relative importance of first and second derivatives of nuclear magnetic resonance chemical shifts and spin-spin coupling constants for vibrational averaging. J. Chem. Phys. 2009, 130 (9). 10.1063/1.3081317. [DOI] [PubMed] [Google Scholar]
- Auer A. A., Gauss J.. Triple excitation effects in coupled-cluster calculations of indirect spin-spin coupling constants. J. Chem. Phys. 2001;115(4):1619–1622. doi: 10.1063/1.1386698. [DOI] [Google Scholar]
- Gauss J., Kállay M., Neese F.. Calculation of Electronic g-Tensors using Coupled Cluster Theory. J. Phys. Chem. A. 2009;113(43):11541–11549. doi: 10.1021/jp9028535. [DOI] [PubMed] [Google Scholar]
- Matthews, D. A. ; Cheng, L. ; Harding, M. E. ; Lipparini, F. ; Stopkowicz, S. ; Jagau, T.-C. ; Szalay, P. G. ; Gauss, J. ; Stanton, J. F. . Coupled-cluster techniques for computational chemistry: The CFOUR program package. J. Chem. Phys. 2020, 152 (21). 10.1063/5.0004837. [DOI] [PubMed] [Google Scholar]
- Neese F.. The ORCA program system. WIREs Computational Molecular Science. 2012;2(1):73–78. doi: 10.1002/wcms.81. [DOI] [Google Scholar]
- Liakos D. G., Guo Y., Neese F.. Comprehensive Benchmark Results for the Domain Based Local Pair Natural Orbital Coupled Cluster Method (DLPNO-CCSD(T)) for Closed- and Open-Shell Systems. J. Phys. Chem. A. 2020;124(1):90–100. doi: 10.1021/acs.jpca.9b05734. [DOI] [PubMed] [Google Scholar]
- Deustua, J. E. ; Yuwono, S. H. ; Shen, J. ; Piecuch, P. . Accurate excited-state energetics by a combination of Monte Carlo sampling and equation-of-motion coupled-cluster computations. J. Chem. Phys. 2019, 150 (11). 10.1063/1.5090346. [DOI] [PubMed] [Google Scholar]
- Hättig C.. Geometry optimizations with the coupled-cluster model CC2 using the resolution-of-the-identity approximation. J. Chem. Phys. 2003;118(17):7751–7761. doi: 10.1063/1.1564061. [DOI] [Google Scholar]
- Geertsen J., Oddershede J.. Second-order polarization propagator calculations of indirect nuclear spin-spin coupling tensors in the water molecule. Chem. Phys. 1984;90(3):301–311. doi: 10.1016/0301-0104(84)85327-6. [DOI] [Google Scholar]
- Wigglesworth R. D., Raynes W. T., Kirpekar S., Oddershede J., Sauer S. P.. A. Erratum: “Nuclear spin-spin coupling in the acetylene isotopomers calculated from ab initio correlated surfaces for J1(C, H), J1(C, C), J2(C, H), and J3(H, H)” [J. Chem. Phys. 112, 3735 (2000)] J. Chem. Phys. 2001;114(20):9192–9192. doi: 10.1063/1.1369154. [DOI] [Google Scholar]
- Wigglesworth R. D., Raynes W. T., Kirpekar S., Oddershede J., Sauer S. P. A.. Nuclear spin-spin coupling in the acetylene isotopomers calculated from ab initio correlated surfaces for 1J(C,H), 1J(C,C), 2J(C,H), and 3J(H,H) J. Chem. Phys. 2000;112(8):3735–3746. doi: 10.1063/1.480525. [DOI] [Google Scholar]
- Åstrand P.-O., Ruud K., Mikkelsen K. V., Helgaker T.. Rovibrationally averaged magnetizability, rotational g factor, and indirect spin-spin coupling of the hydrogen fluoride molecule. J. Chem. Phys. 1999;110(19):9463–9468. doi: 10.1063/1.478912. [DOI] [Google Scholar]
- Vahtras O., Ågren H., Jørgensen P., Jensen H. J. A., Padkjær S. B., Helgaker T.. Indirect nuclear spin-spin coupling constants from multiconfiguration linear response theory. J. Chem. Phys. 1992;96(8):6120–6125. doi: 10.1063/1.462654. [DOI] [Google Scholar]
- Jaszuński M. X., Ruud K.. Spin-spin coupling constants in C 2 H 2. Chem. Phys. Lett. 2001;336(5–6):473–478. doi: 10.1016/S0009-2614(01)00123-3. [DOI] [Google Scholar]
- Pecul M., Helgaker T.. The spin-spin coupling constants in ethane, methanol and methylamine: a comparison of DFT, MCSCF and CCSD results. International Journal of Molecular Sciences. 2003;4(3):143–157. doi: 10.3390/i4030143. [DOI] [Google Scholar]
- Malmqvist P. Å., Rendell A., Roos B. O.. The restricted active space self-consistent-field method, implemented with a split graph unitary group approach. J. Phys. Chem. 1990;94(14):5477–5482. doi: 10.1021/j100377a011. [DOI] [Google Scholar]
- Jordan M. J. T., Toh J. S. S., Del Bene J. E.. Vibrational averaging of NMR properties for an N-H-N hydrogen bond. Chem. Phys. Lett. 2001;346(3–4):288–292. doi: 10.1016/S0009-2614(01)00978-2. [DOI] [Google Scholar]
- Pecul M.. The nuclear spin-spin coupling constant in He2. J. Chem. Phys. 2000;113(24):10835–10836. doi: 10.1063/1.1332994. [DOI] [Google Scholar]
- Feller, D. ; Peterson, K. A. ; Crawford, T. D. . Sources of error in electronic structure calculations on small chemical systems. J. Chem. Phys. 2006, 124 (5). 10.1063/1.2137323. [DOI] [PubMed] [Google Scholar]
- Facelli J. C.. Density Functional Theory Calculations of the Structure and the 15N and 13C Chemical Shifts of Methyl Bacteriopheophorbide a and Bacteriochlorophyll a. J. Phys. Chem. B. 1998;102(11):2111–2116. doi: 10.1021/jp9800970. [DOI] [Google Scholar]
- Ramsey N. F.. Magnetic shielding of nuclei in molecules. Phys. Rev. 1950;78(6):699. doi: 10.1103/PhysRev.78.699. [DOI] [Google Scholar]
- Kaupp M., Bühl M., Malkin V. G.. Introduction: The Quantum Chemical Calculation of NMR and EPR Parameters. Calculation of NMR and EPR Parameters. 2004:1–5. doi: 10.1002/3527601678.ch1. [DOI] [Google Scholar]
- Casabianca L. B., de Dios A. C.. Ab initio calculations of NMR chemical shifts. J. Chem. Phys. 2008;128(5):052201. doi: 10.1063/1.2816784. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Vaara J., Jokisaari J., Wasylishen R. E., Bryce D. L.. Spin-spin coupling tensors as determined by experiment and computational chemistry. Prog. Nucl. Magn. Reson. Spectrosc. 2002;41(3):233–304. doi: 10.1016/S0079-6565(02)00050-X. [DOI] [Google Scholar]
- Oldfield E.. CHEMICAL SHIFTS IN AMINO ACIDS, PEPTIDES, AND PROTEINS: From Quantum Chemistry to Drug Design. Annu. Rev. Phys. Chem. 2002;53:349–378. doi: 10.1146/annurev.physchem.53.082201.124235. [DOI] [PubMed] [Google Scholar]
- Facelli J. C.. Chemical shift tensors: Theory and application to molecular structural problems. Progress in nuclear magnetic resonance spectroscopy. 2011;58(3–4):176. doi: 10.1016/j.pnmrs.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss J., Stanton J. F.. Electron-Correlated Methods for the Calculation of NMR Chemical Shifts. Calculation of NMR and EPR Parameters. 2004:123–139. doi: 10.1002/3527601678.ch8. [DOI] [Google Scholar]
- Fukui, H. ; Aliev, A. E. ; Paduano, L. ; Fukui, H. ; Jameson, C. J. ; Kuroki, S. ; Kurosu, H. ; Ludwig, R. ; Matthews, S. J. ; Prior, M. J. W. ; et al. Theoretical aspects of spin-spin couplings. In Nuclear Magnetic Resonance, Webb, G. A. , Kamienska-Trela, K. , Eds.; Vol. 39; The Royal Society of Chemistry, 2010; p 0. [Google Scholar]
- Aucar G. A., Romero R. H., Maldonado A. F.. Polarization propagators: A powerful theoretical tool for a deeper understanding of NMR spectroscopic parameters. Int. Rev. Phys. Chem. 2010;29(1):1–64. doi: 10.1080/01442350903432865. [DOI] [Google Scholar]
- Ziegler T., Autschbach J.. Theoretical methods of potential use for studies of inorganic reaction mechanisms. Chem. Rev. 2005;105(6):2695–2722. doi: 10.1021/cr0307188. [DOI] [PubMed] [Google Scholar]
- Rusakova I. L., Krivdin L. B.. Relativistic effects in the NMR spectra of compounds containing heavy chalcogens. Mendeleev Commun. 2018;28(1):1–13. doi: 10.1016/j.mencom.2018.01.001. [DOI] [Google Scholar]
- Lodewyk M. W., Siebert M. R., Tantillo D. J.. Computational prediction of 1H and 13C chemical shifts: a useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012;112(3):1839–1862. doi: 10.1021/cr200106v. [DOI] [PubMed] [Google Scholar]
- Schastnev P. V., Cheremisin A. A.. Quantum-chemical calculation of NMR chemical shifts. Journal of Structural Chemistry. 1982;23(3):440–472. doi: 10.1007/BF00753484. [DOI] [Google Scholar]
- Benzi C., Crescenzi O., Pavone M., Barone V.. Reliable NMR chemical shifts for molecules in solution by methods rooted in density functional theory. Magn. Reson. Chem. 2004;42(S1):S57–S67. doi: 10.1002/mrc.1447. [DOI] [PubMed] [Google Scholar]
- Bagno A., Rastrelli F., Saielli G.. Prediction of the 1H and 13C NMR spectra of α-D-glucose in water by DFT methods and MD simulations. Journal of Organic Chemistry. 2007;72(19):7373–7381. doi: 10.1021/jo071129v. [DOI] [PubMed] [Google Scholar]
- Bagno A., Scorrano G., Stiz S.. Preferential solvation of neutral species in binary solvent mixtures characterized by 1H NOESY NMR spectroscopy. J. Am. Chem. Soc. 1997;119(9):2299–2300. doi: 10.1021/ja963707n. [DOI] [Google Scholar]
- Oldfield E.. Chemical shifts and three-dimensional protein structures. Journal of biomolecular NMR. 1995;5:217–225. doi: 10.1007/BF00211749. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Oldfield E.. NMR hyperfine shifts in blue copper proteins: A quantum chemical investigation. J. Am. Chem. Soc. 2008;130(12):3814–3823. doi: 10.1021/ja075978b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossell J.. Quantum mechanical calculation of 23 Na NMR shieldings in silicates and aluminosilicates. Physics and Chemistry of Minerals. 1999;27:70–80. doi: 10.1007/s002690050242. [DOI] [Google Scholar]
- SEBASTIANI D.. AB-INITIO CALCULATIONS OF NMR PARAMETERS IN CONDENSED PHASES. Modern Physics Letters B. 2003;17(25):1301–1319. doi: 10.1142/S0217984903006372. [DOI] [Google Scholar]
- Fukuda R., Hada M., Nakatsuji H.. Quasirelativistic theory for magnetic shielding constants. II. Gauge-including atomic orbitals and applications to molecules. J. Chem. Phys. 2003;118(3):1027–1035. doi: 10.1063/1.1528934. [DOI] [Google Scholar]
- Xu Y., Szell P. M., Kumar V., Bryce D. L.. Solid-state NMR spectroscopy for the analysis of element-based non-covalent interactions. Coord. Chem. Rev. 2020;411:213237. doi: 10.1016/j.ccr.2020.213237. [DOI] [Google Scholar]
- Bryce D. L., Viger-Gravel J.. Solid-state NMR study of halogen-bonded adducts. Halogen Bonding I: Impact on Materials Chemistry and Life Sciences. 2014;358:183–203. doi: 10.1007/128_2014_542. [DOI] [PubMed] [Google Scholar]
- Bryce D. L., Sward G. D.. Chlorine-35/37 NMR spectroscopy of solid amino acid hydrochlorides: refinement of hydrogen-bonded proton positions using experiment and theory. J. Phys. Chem. B. 2006;110(51):26461–26470. doi: 10.1021/jp065878c. [DOI] [PubMed] [Google Scholar]
- Borges R. M., Colby S. M., Das S., Edison A. S., Fiehn O., Kind T., Lee J., Merrill A. T., Merz K. M. Jr., Metz T. O.. et al. Quantum Chemistry Calculations for Metabolomics. Chem. Rev. 2021;121(10):5633–5670. doi: 10.1021/acs.chemrev.0c00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson C. J., de Dios A. C.. Theoretical and physical aspects of nuclear shielding. NUCLEAR MAGNETIC RESONANCE-LONDON- 2000;29:41–84. doi: 10.1039/9781847553850-00041. [DOI] [Google Scholar]
- de Dios A. C., Jameson C. J.. Recent advances in nuclear shielding calculations. Annu. Rep. NMR Spectrosc. 2012;77:1–80. doi: 10.1016/B978-0-12-397020-6.00001-5. [DOI] [Google Scholar]
- Sychrovský V., Gräfenstein J., Cremer D.. Nuclear magnetic resonance spin-spin coupling constants from coupled perturbed density functional theory. J. Chem. Phys. 2000;113(9):3530–3547. doi: 10.1063/1.1286806. [DOI] [Google Scholar]
- Kline P. C., Serianni A. S.. Chiral hydroxymethyl groups: 1H NMR assignments of the prochiral C-5′ protons of ribonucleosides. Magnetic resonance in chemistry. 1988;26(2):120–123. doi: 10.1002/mrc.1260260207. [DOI] [Google Scholar]
- Shajani Z., Varani G.. 13C NMR relaxation studies of RNA base and ribose nuclei reveal a complex pattern of motions in the RNA binding site for human U1A protein. Journal of molecular biology. 2005;349(4):699–715. doi: 10.1016/j.jmb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Shajani Z., Varani G.. NMR studies of dynamics in RNA and DNA by 13C relaxation. Biopolymers: Original Research on Biomolecules. 2007;86(5–6):348–359. doi: 10.1002/bip.20650. [DOI] [PubMed] [Google Scholar]
- Tinoco I. Jr. Physical chemistry of nucleic acids. Annu. Rev. Phys. Chem. 2002;53(1):1–15. doi: 10.1146/annurev.physchem.53.082001.144341. [DOI] [PubMed] [Google Scholar]
- Varani G., Tinoco I. Jr. RNA structure and NMR spectroscopy. Q. Rev. Biophys. 1991;24(4):479–532. doi: 10.1017/S0033583500003875. [DOI] [PubMed] [Google Scholar]
- Fukal J., Buděšínský M., Páv O., Jurečka P., Zgarbová M., Šebera J., Sychrovský V.. The Ad-MD method to calculate NMR shift including effects due to conformational dynamics: The 31P NMR shift in DNA. J. Comput. Chem. 2022;43(2):132–143. doi: 10.1002/jcc.26778. [DOI] [PubMed] [Google Scholar]
- Sychrovský V., Buděšínský M., Benda L., Špirko V., Vokacova Z., Šebestík J., Bouř P.. Dependence of the L-alanyl-L-alanine conformation on molecular charge determined from Ab initio computations and NMR spectra. J. Phys. Chem. B. 2008;112(6):1796–1805. doi: 10.1021/jp076557j. [DOI] [PubMed] [Google Scholar]
- Přecechtělová J., Munzarova M. L., Novák P., Sklenář V.. Relationships between 31P chemical shift tensors and conformation of nucleic acid backbone: a DFT study. J. Phys. Chem. B. 2007;111(10):2658–2667. doi: 10.1021/jp0668652. [DOI] [PubMed] [Google Scholar]
- Přecechtělová J., Padrta P., Munzarova M. L., Sklenář V.. 31P chemical shift tensors for canonical and non-canonical conformations of nucleic acids: a DFT study and NMR implications. J. Phys. Chem. B. 2008;112(11):3470–3478. doi: 10.1021/jp076073n. [DOI] [PubMed] [Google Scholar]
- Van Mourik T., Dingley A. J.. Characterization of the monovalent ion position and hydrogen-bond network in guanine quartets by DFT calculations of NMR parameters. Chem. Eur. J. 2005;11(20):6064–6079. doi: 10.1002/chem.200500198. [DOI] [PubMed] [Google Scholar]
- van Mourik, T. Density functional theory reveals an increase in the amino H1 chemical shift in guanine due to hydrogen bonding with water. J. Chem. Phys. 2006, 125 (19). 10.1063/1.2400028. [DOI] [PubMed] [Google Scholar]
- Tsui K. Y., Tombari R. J., Olson D. E., Tantillo D. J.. Reconsidering the Structure of Serlyticin-A. J. Nat. Prod. 2019;82(12):3464–3468. doi: 10.1021/acs.jnatprod.9b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becke A. D.. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98(7):5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- Tomasi J., Mennucci B., Cammi R.. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005;105(8):2999–3094. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- Leszczynski, J. Computational chemistry: reviews of current trends; World Scientific, 2003. [Google Scholar]
- Cheeseman J. R., Trucks G. W., Keith T. A., Frisch M. J.. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996;104(14):5497–5509. doi: 10.1063/1.471789. [DOI] [Google Scholar]
- Navarro-Vazquez A.. Computational NMR Structural Revision of Marinoaziridines A and B. Magn. Reson. Chem. 2025;63(3):216–219. doi: 10.1002/mrc.5505. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Mennucci B., Tomasi J.. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997;106(12):5151–5158. doi: 10.1063/1.473558. [DOI] [Google Scholar]
- Live D. H., Davis D. G., Agosta W. C., Cowburn D.. Long range hydrogen bond mediated effects in peptides: nitrogen-15 NMR study of gramicidin S in water and organic solvents. J. Am. Chem. Soc. 1984;106(7):1939–1941. doi: 10.1021/ja00319a006. [DOI] [Google Scholar]
- Laszlo P.. Chapter 6 Solvent effects and nuclear magnetic resonance. Prog. Nucl. Magn. Reson. Spectrosc. 1967;3:231–402. doi: 10.1016/0079-6565(67)80016-5. [DOI] [Google Scholar]
- Mennucci B.. Polarizable continuum model. WIREs Computational Molecular Science. 2012;2(3):386–404. doi: 10.1002/wcms.1086. [DOI] [Google Scholar]
- Cossi M., Barone V., Cammi R., Tomasi J.. Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996;255(4):327–335. doi: 10.1016/0009-2614(96)00349-1. [DOI] [Google Scholar]
- Cancès E., Mennucci B., Tomasi J.. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997;107(8):3032–3041. doi: 10.1063/1.474659. [DOI] [Google Scholar]
- Klamt A., Schüürmann G.. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc., Perkin Trans. 2. 1993;(5):799–805. doi: 10.1039/P29930000799. [DOI] [Google Scholar]
- Dracinsky M., Bour P.. Computational Analysis of Solvent Effects in NMR Spectroscopy. J. Chem. Theory Comput. 2010;6(1):288–299. doi: 10.1021/ct900498b. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Szanto J. K., Dietschreit J. C. B., Shein M., Schutz A. K., Ochsenfeld C.. Systematic QM/MM Study for Predicting (31)P NMR Chemical Shifts of Adenosine Nucleotides in Solution and Stages of ATP Hydrolysis in a Protein Environment. J. Chem. Theory Comput. 2024;20(6):2433–2444. doi: 10.1021/acs.jctc.3c01280. From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva H. C., Hernandes I. S., De Almeida W. B.. Modeling Solvent Effects in Quantum Chemical Calculation of Relative Energies and NMR Chemical Shifts for Azithromycin. J. Phys. Chem. A. 2025;129(9):2200–2216. doi: 10.1021/acs.jpca.4c08015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseri L., Kumar S., Palchuber P., Szekely G.. NMR Chemical Shifts of Emerging Green Solvents, Acids, and Bases for Facile Trace Impurity Analysis. ACS Sustainable Chem. Eng. 2023;11(14):5696–5725. doi: 10.1021/acssuschemeng.3c00244. [DOI] [Google Scholar]
- Cohen R. D., Wood J. S., Lam Y. H., Buevich A. V., Sherer E. C., Reibarkh M., Williamson R. T., Martin G. E.. DELTA50: A Highly Accurate Database of Experimental (1)H and (13)C NMR Chemical Shifts Applied to DFT Benchmarking. Molecules. 2023;28(6):2449. doi: 10.3390/molecules28062449. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Wang X., Huang Z., Yu H.. (11)B NMR Chemical Shift Predictions via Density Functional Theory and Gauge-Including Atomic Orbital Approach: Applications to Structural Elucidations of Boron-Containing Molecules. ACS Omega. 2019;4(7):12385–12392. doi: 10.1021/acsomega.9b01566. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade T., Dos Santos H. F., Fonseca Guerra C., Paschoal D. F. S.. Computational Prediction of Tc-99 NMR Chemical Shifts in Technetium Complexes with Radiopharmaceutical Applications. J. Phys. Chem. A. 2022;126(32):5434–5448. doi: 10.1021/acs.jpca.2c01617. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Batista P. R., Ducati L. C., Autschbach J.. Solvent effect on the (195)Pt NMR properties in pyridonate-bridged Pt(III) dinuclear complex derivatives investigated by ab initio molecular dynamics and localized orbital analysis. Phys. Chem. Chem. Phys. 2021;23(22):12864–12880. doi: 10.1039/D0CP05849A. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Calcagno F., Maryasin B., Garavelli M., Avagliano D., Rivalta I.. Modeling solvent effects and convergence of (31)P-NMR shielding calculations with COBRAMM. J. Comput. Chem. 2024;45(18):1562–1575. doi: 10.1002/jcc.27338. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Willoughby P. H., Jansma M. J., Hoye T. R.. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nature protocols. 2014;9(3):643–660. doi: 10.1038/nprot.2014.042. [DOI] [PubMed] [Google Scholar]
- Willoughby P. H., Jansma M. J., Hoye T. R.. Addendum: A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nat. Protoc. 2020;15(7):2277–2277. doi: 10.1038/s41596-020-0293-9. [DOI] [PubMed] [Google Scholar]
- Mohamadi F., Richards N. G. J., Guida W. C., Liskamp R., Lipton M., Caufield C., Chang G., Hendrickson T., Still W. C.. Macromodelan integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990;11(4):440–467. doi: 10.1002/jcc.540110405. [DOI] [Google Scholar]
- Watts K. S., Dalal P., Tebben A. J., Cheney D. L., Shelley J. C.. Macrocycle Conformational Sampling with MacroModel. J. Chem. Inf. Model. 2014;54(10):2680–2696. doi: 10.1021/ci5001696. [DOI] [PubMed] [Google Scholar]
- Gaussian 16 Rev. C.01; Wallingford, CT, 2016. [Google Scholar]
- Xin D., Sader C. A., Chaudhary O., Jones P.-J., Wagner K., Tautermann C. S., Yang Z., Busacca C. A., Saraceno R. A., Fandrick K. R.. et al. Development of a 13C NMR Chemical Shift Prediction Procedure Using B3LYP/cc-pVDZ and Empirically Derived Systematic Error Correction Terms: A Computational Small Molecule Structure Elucidation Method. Journal of Organic Chemistry. 2017;82(10):5135–5145. doi: 10.1021/acs.joc.7b00321. [DOI] [PubMed] [Google Scholar]
- Vilar S., Cozza G., Moro S.. Medicinal chemistry and the molecular operating environment (MOE): application of QSAR and molecular docking to drug discovery. Current topics in medicinal chemistry. 2008;8(18):1555–1572. doi: 10.2174/156802608786786624. [DOI] [PubMed] [Google Scholar]
- Molecular Operating Environment (MOE) , Suite #910, Montreal, QC, Canada, H3A 2R7, 2015. [Google Scholar]
- Labute P.. LowModeMDImplicit Low-Mode Velocity Filtering Applied to Conformational Search of Macrocycles and Protein Loops. J. Chem. Inf. Model. 2010;50(5):792–800. doi: 10.1021/ci900508k. [DOI] [PubMed] [Google Scholar]
- Das S., Merz K. M. Jr.. Molecular Gas-Phase Conformational Ensembles. J. Chem. Inf. Model. 2024;64(3):749–760. doi: 10.1021/acs.jcim.3c01309. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Nebgen B. T., Zubatyuk R., Lubbers N., Devereux C., Barros K., Tretiak S., Isayev O., Roitberg A. E.. Approaching coupled cluster accuracy with a general-purpose neural network potential through transfer learning. Nat. Commun. 2019;10(1):2903. doi: 10.1038/s41467-019-10827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Zubatyuk R., Nebgen B., Lubbers N., Barros K., Roitberg A. E., Isayev O., Tretiak S.. The ANI-1ccx and ANI-1x data sets, coupled-cluster and density functional theory properties for molecules. Scientific data. 2020;7(1):134. doi: 10.1038/s41597-020-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemura K. A., Das S., Merz K. M. Jr.. AutoGraph: Autonomous Graph-Based Clustering of Small-Molecule Conformations. J. Chem. Inf. Model. 2021;61(4):1647–1656. doi: 10.1021/acs.jcim.0c01492. [DOI] [PubMed] [Google Scholar]
- Manathunga M., Miao Y., Mu D., Götz A. W., Merz K. M. Jr.. Parallel Implementation of Density Functional Theory Methods in the Quantum Interaction Computational Kernel Program. J. Chem. Theory Comput. 2020;16(7):4315–4326. doi: 10.1021/acs.jctc.0c00290. [DOI] [PubMed] [Google Scholar]
- Manathunga M., Aktulga H. M., Götz A. W., Merz K. M. Jr.. Quantum Mechanics/Molecular Mechanics Simulations on NVIDIA and AMD Graphics Processing Units. J. Chem. Inf. Model. 2023;63(3):711–717. doi: 10.1021/acs.jcim.2c01505. [DOI] [PubMed] [Google Scholar]
- Das S., Dinpazhoh L., Tanemura K. A., Merz K. M. Jr.. Rapid and Automated Ab Initio Metabolite Collisional Cross Section Prediction from SMILES Input. J. Chem. Inf. Model. 2023;63(16):4995–5000. doi: 10.1021/acs.jcim.3c00890. [DOI] [PubMed] [Google Scholar]
- Molder, F. ; Jablonski, K. ; Letcher, B. ; Hall, M. ; Tomkins-Tinch, C. ; Sochat, V. ; Forster, J. ; Lee, S. ; Twardziok, S. ; Kanitz, A. ; et al. Sustainable data analysis with Snakemake [version 1; peer review: 1 approved, 1 approved with reservations]. F1000Research 2021, 10 (33), 33. 10.12688/f1000research.29032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Community T. G.. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024;52(W1):W83–W94. doi: 10.1093/nar/gkae410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Brothers E. N., van der Vaart A., Merz K. M. Jr.. Fast semiempirical calculations for nuclear magnetic resonance chemical shifts: A divide-and-conquer approach. J. Chem. Phys. 2004;120(24):11392–11400. doi: 10.1063/1.1752877. [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Packer M. J., Zonta C.. From structure to chemical shift and vice-versa. Prog. Nucl. Magn. Reson. Spectrosc. 2005;47(1):27–39. doi: 10.1016/j.pnmrs.2005.06.001. [DOI] [Google Scholar]
- Dixon S. L., Merz K. M. Jr. Fast, accurate semiempirical molecular orbital calculations for macromolecules. J. Chem. Phys. 1997;107(3):879–893. doi: 10.1063/1.474386. [DOI] [Google Scholar]
- Merz K. M. Jr. Using quantum mechanical approaches to study biological systems. Acc. Chem. Res. 2014;47(9):2804–2811. doi: 10.1021/ar5001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, S. ; Lipparini, F. ; Gauss, J. ; Stopkowicz, S. . NMR chemical shift computations at second-order Møller-Plesset perturbation theory using gauge-including atomic orbitals and Cholesky-decomposed two-electron integrals. J. Chem. Phys. 2021, 155 (7). 10.1063/5.0059633. [DOI] [PubMed] [Google Scholar]
- Gauss J., Werner H.-J.. NMR chemical shift calculations within local correlation methods: the GIAO-LMP2 approach. Phys. Chem. Chem. Phys. 2000;2(10):2083–2090. doi: 10.1039/b000024h. [DOI] [Google Scholar]
- Gleeson R., Aggelund P. A., Ostergaard F. C., Schaltz K. F., Sauer S. P. A.. Exploring Alternate Methods for the Calculation of High-Level Vibrational Corrections of NMR Spin-Spin Coupling Constants. J. Chem. Theory Comput. 2024;20(3):1228–1243. doi: 10.1021/acs.jctc.3c01223. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Hedin N.. Electron correlation and vibrational effects in predictions of paramagnetic NMR shifts. Phys. Chem. Chem. Phys. 2022;24(25):15230–15244. doi: 10.1039/D2CP01206E. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Dittmer A., Stoychev G. L., Maganas D., Auer A. A., Neese F.. Computation of NMR Shielding Constants for Solids Using an Embedded Cluster Approach with DFT, Double-Hybrid DFT, and MP2. J. Chem. Theory Comput. 2020;16(11):6950–6967. doi: 10.1021/acs.jctc.0c00067. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron F., Autschbach J.. Ligand NMR Chemical Shift Calculations for Paramagnetic Metal Complexes: 5f(1) vs 5f(2) Actinides. J. Chem. Theory Comput. 2016;12(11):5309–5321. doi: 10.1021/acs.jctc.6b00462. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Faber R., Sauer S. P.. On the discrepancy between theory and experiment for the F-F spin-spin coupling constant of difluoroethyne. Phys. Chem. Chem. Phys. 2012;14(47):16440–16447. doi: 10.1039/c2cp42198d. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Jessen, L. M. ; Sauer, S. P. A. . On the performance of HRPA(D) for NMR spin-spin coupling constants: Smaller molecules, aromatic and fluoroaromatic compounds. J. Chem. Phys. 2024, 160 (6). 10.1063/5.0189932 From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Jaszunski M., Sauer S. P. A., Faber R., Wilson D. J. D.. NMR parameters of FNNF as a test for coupled-cluster methods: CCSDT shielding and CC3 spin-spin coupling. Phys. Chem. Chem. Phys. 2020;22(37):21350–21359. doi: 10.1039/D0CP02730H. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Riplinger C., Sandhoefer B., Hansen A., Neese F.. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 2013;139(13):134101. doi: 10.1063/1.4821834. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Riplinger C., Neese F.. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys. 2013;138(3):034106. doi: 10.1063/1.4773581. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Pantazis D. A., Neese F.. All-Electron Scalar Relativistic Basis Sets for the Lanthanides. J. Chem. Theory Comput. 2009;5(9):2229–2238. doi: 10.1021/ct900090f. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Da Silva H. C., De Almeida W. B.. Theoretical calculations of 1H NMR chemical shifts for nitrogenated compounds in chloroform solution. Chem. Phys. 2020;528:110479. doi: 10.1016/j.chemphys.2019.110479. [DOI] [Google Scholar]
- Nottoli T., Burger S., Stopkowicz S., Gauss J., Lipparini F.. Computation of NMR shieldings at the CASSCF level using gauge-including atomic orbitals and Cholesky decomposition. J. Chem. Phys. 2022;157(8):084122. doi: 10.1063/5.0101838. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Lischka H., Nachtigallova D., Aquino A. J. A., Szalay P. G., Plasser F., Machado F. B. C., Barbatti M.. Multireference Approaches for Excited States of Molecules. Chem. Rev. 2018;118(15):7293–7361. doi: 10.1021/acs.chemrev.8b00244. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Gendron F., Sharkas K., Autschbach J.. Calculating NMR Chemical Shifts for Paramagnetic Metal Complexes from First-Principles. J. Phys. Chem. Lett. 2015;6(12):2183–2188. doi: 10.1021/acs.jpclett.5b00932. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Repisky, M. ; Komorovsky, S. ; Bast, R. ; Ruud, K. . Relativistic Calculations of Nuclear Magnetic Resonance Parameters. In Gas Phase NMR, Jackowski, K. , Jaszuński, M. , Eds.; The Royal Society of Chemistry, 2016; pp 267–303. [Google Scholar]
- Autschbach, J. ; Zheng, S. . Chapter 1 Relativistic Computations of NMR Parameters from First Principles: Theory and Applications. In Annu. Rep. NMR Spectrosc., Webb, G. A. Ed.; Annual Reports on NMR Spectroscopy, Vol. 67; Academic Press, 2009; pp 1–95. [Google Scholar]
- Pyykko P., Gorling A., Rosch N.. A transparent interpretation of the relativistic contribution to the N.M.R. ‘heavy atom chemical shift’. Mol. Phys. 1987;61(1):195–205. doi: 10.1080/00268978700101071. [DOI] [Google Scholar]
- Vi Cha J., Novotny J., Komorovsky S., Straka M., Kaupp M., Marek R.. Relativistic Heavy-Neighbor-Atom Effects on NMR Shifts: Concepts and Trends Across the Periodic Table. Chem. Rev. 2020;120(15):7065–7103. doi: 10.1021/acs.chemrev.9b00785. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Pyykkö P.. Theory of NMR Parameters. From Ramsey to Relativity, 1953 to 1983. Calculation of NMR and EPR Parameters. 2004:7–19. doi: 10.1002/3527601678.ch2. [DOI] [Google Scholar]
- Cortés I., Cuadrado C., Hernández Daranas A., Sarotti A. M.. Machine learning in computational NMR-aided structural elucidation. Frontiers in Natural Products. 2023;2:1122426. doi: 10.3389/fntpr.2023.1122426. [DOI] [Google Scholar]
- Colby S. M., Nuñez J. R., Hodas N. O., Corley C. D., Renslow R. R.. Deep learning to generate in silico chemical property libraries and candidate molecules for small molecule identification in complex samples. Analytical chemistry. 2020;92(2):1720–1729. doi: 10.1021/acs.analchem.9b02348. [DOI] [PubMed] [Google Scholar]
- Sajed T., Sayeeda Z., Lee B. L., Berjanskii M., Wang F., Gautam V., Wishart D. S.. Accurate Prediction of (1)H NMR Chemical Shifts of Small Molecules Using Machine Learning. Metabolites. 2024;14(5):290. doi: 10.3390/metabo14050290. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C., Vasi S., Neri F., Mezzasalma A. M., Neri G., Fazio E.. NMR in metabolomics: From conventional statistics to machine learning and neural network approaches. Applied Sciences. 2022;12(6):2824. doi: 10.3390/app12062824. [DOI] [Google Scholar]
- Pomyen Y., Wanichthanarak K., Poungsombat P., Fahrmann J., Grapov D., Khoomrung S.. Deep metabolome: Applications of deep learning in metabolomics. Computational and Structural Biotechnology Journal. 2020;18:2818–2825. doi: 10.1016/j.csbj.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J., Mayonu M., Jiang L., Wang B.. The development of machine learning approaches in two-dimensional NMR data interpretation for metabolomics applications. Anal. Biochem. 2024;695:115654. doi: 10.1016/j.ab.2024.115654. [DOI] [PubMed] [Google Scholar]
- Debik J., Sangermani M., Wang F., Madssen T. S., Giskeødegård G. F.. Multivariate analysis of NMR-based metabolomic data. NMR in Biomedicine. 2022;35(2):e4638. doi: 10.1002/nbm.4638. [DOI] [PubMed] [Google Scholar]
- Cramer P.. AlphaFold2 and the future of structural biology. Nature structural & molecular biology. 2021;28(9):704–705. doi: 10.1038/s41594-021-00650-1. [DOI] [PubMed] [Google Scholar]
- Shukla V. K., Heller G. T., Hansen D. F.. Biomolecular NMR spectroscopy in the era of artificial intelligence. Structure. 2023;31(11):1360–1374. doi: 10.1016/j.str.2023.09.011. [DOI] [PubMed] [Google Scholar]
- Klukowski P., Riek R., Güntert P.. Rapid protein assignments and structures from raw NMR spectra with the deep learning technique ARTINA. Nat. Commun. 2022;13(1):6151. doi: 10.1038/s41467-022-33879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimblat N., Sarotti A. M.. Computational chemistry to the rescue: Modern toolboxes for the assignment of complex molecules by GIAO NMR calculations. Chem. Eur. J. 2016;22(35):12246–12261. doi: 10.1002/chem.201601150. [DOI] [PubMed] [Google Scholar]
- Hu F., Chen M. S., Rotskoff G. M., Kanan M. W., Markland T. E.. Accurate and efficient structure elucidation from routine one-dimensional NMR spectra using multitask machine learning. ACS Central Science. 2024;10(11):2162–2170. doi: 10.1021/acscentsci.4c01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Ye Q., Zhuang X., Chen Q., Zhang J., Wu J., Xu X.. Elucidating structures of complex organic compounds using a machine learning model based on the 13C NMR chemical shifts. Precision Chemistry. 2023;1(1):57–68. doi: 10.1021/prechem.3c00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck C., Krause S., Kuhn S.. NMRShiftDB constructing a free chemical information system with open-source components. Journal of chemical information and computer sciences. 2003;43(6):1733–1739. doi: 10.1021/ci0341363. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Kolshorn H., Steinbeck C., Schlörer N.. Twenty years of nmrshiftdb2: A case study of an open database for analytical chemistry. Magn. Reson. Chem. 2024;62(2):74–83. doi: 10.1002/mrc.5418. [DOI] [PubMed] [Google Scholar]
- Wishart D. S., Sayeeda Z., Budinski Z., Guo A., Lee B. L., Berjanskii M., Rout M., Peters H., Dizon R., Mah R.. NP-MRD: the natural products magnetic resonance database. Nucleic Acids Res. 2022;50(D1):D665–D677. doi: 10.1093/nar/gkab1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich E. L., Akutsu H., Doreleijers J. F., Harano Y., Ioannidis Y. E., Lin J., Livny M., Mading S., Maziuk D., Miller Z.. et al. BioMagResBank. Nucleic acids research. 2007;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. C., Baskaran K., Burr H., Chin J., Eghbalnia H. R., Fujiwara T., Gryk M. R., Iwata T., Kojima C., Kurisu G.. et al. Biological magnetic resonance data bank. Nucleic acids research. 2023;51(D1):D368–D376. doi: 10.1093/nar/gkac1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen F. H., Taylor R.. Research applications of the Cambridge structural database (CSD) Chem. Soc. Rev. 2004;33(8):463–475. doi: 10.1039/b309040j. [DOI] [PubMed] [Google Scholar]
- Groom C. R., Bruno I. J., Lightfoot M. P., Ward S. C.. The Cambridge structural database. Structural Science. 2016;72(2):171–179. doi: 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodewyk M. W., Soldi C., Jones P. B., Olmstead M. M., Rita J., Shaw J. T., Tantillo D. J.. The Correct Structure of Aquatolide Experimental Validation of a Theoretically-Predicted Structural Revision. J. Am. Chem. Soc. 2012;134(45):18550–18553. doi: 10.1021/ja3089394. [DOI] [PubMed] [Google Scholar]
- Lodewyk M. W., Tantillo D. J.. Prediction of the structure of nobilisitine A using computed NMR chemical shifts. J. Nat. Prod. 2011;74(5):1339–1343. doi: 10.1021/np2000446. [DOI] [PubMed] [Google Scholar]
- Guan Y., Sowndarya S. S., Gallegos L. C., st John P. C., Paton R. S.. Real-time prediction of 1 H and 13 C chemical shifts with DFT accuracy using a 3D graph neural network. Chemical Science. 2021;12(36):12012–12026. doi: 10.1039/D1SC03343C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti H., Wedell J. R., Westler W. M., Tonelli M., Aceti D., Amarasinghe G. K., Markley J. L., Eghbalnia H. R.. Applications of Parametrized NMR Spin Systems of Small Molecules. Anal. Chem. 2018;90(18):10646–10649. doi: 10.1021/acs.analchem.8b02660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti H., Westler W. M., Tonelli M., Wedell J. R., Markley J. L., Eghbalnia H. R.. Spin System Modeling of Nuclear Magnetic Resonance Spectra for Applications in Metabolomics and Small Molecule Screening. Anal. Chem. 2017;89(22):12201–12208. doi: 10.1021/acs.analchem.7b02884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. G., Muhoberac M., Randolph C. E., Beveridge C. H., Wijewardhane P. R., Kenttamaa H. I., Chopra G.. Recent Developments in Machine Learning for Mass Spectrometry. ACS Meas Sci. Au. 2024;4(3):233–246. doi: 10.1021/acsmeasuresciau.3c00060. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdemir D., Rio S., Isidro y Sánchez J.. Trainsel: an r package for selection of training populations. Frontiers in genetics. 2021;12:655287. doi: 10.3389/fgene.2021.655287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdadi L., Lambert J., Telfah A., Hergenröder R., Wöhler C.. Automated metabolic assignment: Semi-supervised learning in metabolic analysis employing two dimensional Nuclear Magnetic Resonance (NMR) Computational and Structural Biotechnology Journal. 2021;19:5047–5058. doi: 10.1016/j.csbj.2021.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Salces R. M., Gallo B., Collado M. I., Sasía-Arriba A., Viacava G. E., García-González D. L., Toschi T. G., Servili M., Berrueta L. Á.. 1H-NMR fingerprinting and supervised pattern recognition to evaluate the stability of virgin olive oil during storage. Food Control. 2021;123:107831. doi: 10.1016/j.foodcont.2020.107831. [DOI] [Google Scholar]
- Tiwari, P. ; Madabhushi, A. ; Rosen, M. . A hierarchical unsupervised spectral clustering scheme for detection of prostate cancer from magnetic resonance spectroscopy (MRS). In International Conference on Medical Image Computing and Computer-Assisted Intervention, 2007; Springer: pp 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Liao Y., Wang Y., Wang S., Du W., Lu H., Kong B., Yang H., Zhang Z.. Deep learning-based method for compound identification in NMR spectra of mixtures. Molecules. 2022;27(12):3653. doi: 10.3390/molecules27123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H., Liu J., Fang Q., Chen X., Hu L.. Accelerated pure shift NMR spectroscopy with deep learning. Anal. Chem. 2024;96(4):1515–1521. doi: 10.1021/acs.analchem.3c04007. [DOI] [PubMed] [Google Scholar]
- Klukowski P., Riek R., Güntert P.. Time-optimized protein NMR assignment with an integrative deep learning approach using AlphaFold and chemical shift prediction. Science Advances. 2023;9(47):eadi9323. doi: 10.1126/sciadv.adi9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Judge M. T., Athersuch T., Xiang Y., Liu Z., Jiménez B., Ebbels T. M.. Resolution enhancement of metabolomic J-Res NMR spectra using deep learning. Anal. Chem. 2024;96(29):11707–11715. doi: 10.1021/acs.analchem.4c00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S., Shah S. L., Marrie T. J., Slupsky C. M.. Analysis of metabolomic data using support vector machines. Analytical chemistry. 2008;80(19):7562–7570. doi: 10.1021/ac800954c. [DOI] [PubMed] [Google Scholar]
- Parfait S., Walker P. M., Créhange G., Tizon X., Miteran J.. Classification of prostate magnetic resonance spectra using support vector machine. Biomedical Signal Processing and Control. 2012;7(5):499–508. doi: 10.1016/j.bspc.2011.09.003. [DOI] [Google Scholar]
- Fathi F., Majari-Kasmaee L., Mani-Varnosfaderani A., Kyani A., Rostami-Nejad M., Sohrabzadeh K., Naderi N., Zali M. R., Rezaei-Tavirani M., Tafazzoli M.. et al. 1H NMR based metabolic profiling in Crohn’s disease by random forest methodology. Magn. Reson. Chem. 2014;52(7):370–376. doi: 10.1002/mrc.4074. [DOI] [PubMed] [Google Scholar]
- Chandy S. K., Raghavachari K.. MIM-ML: A Novel Quantum Chemical Fragment-Based Random Forest Model for Accurate Prediction of NMR Chemical Shifts of Nucleic Acids. J. Chem. Theory Comput. 2023;19(19):6632–6642. doi: 10.1021/acs.jctc.3c00563. [DOI] [PubMed] [Google Scholar]
- Lovatti B. P., Nascimento M. H., Rainha K. P., Oliveira E. C., Neto Á. C., Castro E. V., Filgueiras P. R.. Different strategies for the use of random forest in NMR spectra. Journal of Chemometrics. 2020;34(12):e3231. doi: 10.1002/cem.3231. [DOI] [Google Scholar]
- Kuhn S., Egert B., Neumann S., Steinbeck C.. Building blocks for automated elucidation of metabolites: Machine learning methods for NMR prediction. BMC bioinformatics. 2008;9:1–19. doi: 10.1186/1471-2105-9-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. F.. Using deep neural networks to reconstruct non-uniformly sampled NMR spectra. Journal of biomolecular NMR. 2019;73(10):577–585. doi: 10.1007/s10858-019-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V. K., Karunanithy G., Vallurupalli P., Hansen D. F.. A combined NMR and deep neural network approach for enhancing the spectral resolution of aromatic side chains in proteins. Science Advances. 2024;10(51):eadr2155. doi: 10.1126/sciadv.adr2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.-W., Hansen A. L., Yuan C., Bruschweiler-Li L., Brüschweiler R.. DEEP picker is a deep neural network for accurate deconvolution of complex two-dimensional NMR spectra. Nat. Commun. 2021;12(1):5229. doi: 10.1038/s41467-021-25496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithy G., Hansen D. F.. FID-Net: A versatile deep neural network architecture for NMR spectral reconstruction and virtual decoupling. Journal of biomolecular NMR. 2021;75(4):179–191. doi: 10.1007/s10858-021-00366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding B. D., Hu Z., Hiett A., Delaglio F., Henzler-Wildman K. A., Rienstra C. M.. Enhancing Spectrometer Performance with Unsupervised Machine Learning. J. Phys. Chem. B. 2024;128(42):10397–10407. doi: 10.1021/acs.jpcb.4c05109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos M., Isamura B. K., Popelier P. L., Martín Pendás A. n.. An Unsupervised Machine Learning Approach for the Automatic Construction of Local Chemical Descriptors. J. Chem. Inf. Model. 2024;64(8):3059–3079. doi: 10.1021/acs.jcim.3c01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Zhang D., Xia S., Zhang Y.. Accurate Prediction of NMR Chemical Shifts: Integrating DFT Calculations with Three-Dimensional Graph Neural Networks. J. Chem. Theory Comput. 2024;20(12):5250–5258. doi: 10.1021/acs.jctc.4c00422. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettelkamp, J. ; Romanin, L. ; Piccini, D. ; Priya, S. ; Jacob, M. . Motion Compensated Unsupervised Deep Learning for 5D MRI. In International Conference on Medical Image Computing and Computer-Assisted Intervention, 2023; Springer: pp 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull H., Fischer M., Kuhn S.. NMR shift prediction from small data quantities. Journal of Cheminformatics. 2023;15(1):114. doi: 10.1186/s13321-023-00785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J., Kushibar K., Asfaw D. S., Valverde S., Oliver A., Martí R., Lladó X.. Deep convolutional neural networks for brain image analysis on magnetic resonance imaging: a review. Artificial intelligence in medicine. 2019;95:64–81. doi: 10.1016/j.artmed.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Kern S., Liehr S., Wander L., Bornemann-Pfeiffer M., Müller S., Maiwald M., Kowarik S.. Artificial neural networks for quantitative online NMR spectroscopy. Anal. Bioanal. Chem. 2020;412:4447–4459. doi: 10.1007/s00216-020-02687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong E., Jang J., Kim J.-h., Kim H.. Recurrent neural network-aided processing of incomplete free induction decays in 1H-MRS of the brain. J. Magn. Reson. 2024;368:107762. doi: 10.1016/j.jmr.2024.107762. [DOI] [PubMed] [Google Scholar]
- Xu C., Deng X., Lu Y., Yu P.. Generation of molecular conformations using generative adversarial neural networks. Digital Discovery. 2025;4(1):161–171. doi: 10.1039/D4DD00179F. [DOI] [Google Scholar]
- Devata S., Sridharan B., Mehta S., Pathak Y., Laghuvarapu S., Varma G., Priyakumar U. D.. DeepSPInN - deep reinforcement learning for molecular structure prediction from infrared and 13C NMR spectra. Digital Discovery. 2024;3(4):818–829. doi: 10.1039/D4DD00008K. [DOI] [Google Scholar]
- Aggarwal R., Koes D. R.. PharmRL: pharmacophore elucidation with deep geometric reinforcement learning. BMC Biology. 2024;22(1):301. doi: 10.1186/s12915-024-02096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H., Tipirneni-Sajja A.. Explainable AI to Facilitate Understanding of Neural Network-Based Metabolite Profiling Using NMR Spectroscopy. Metabolites. 2024;14(6):332. doi: 10.3390/metabo14060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J., Bocklitz T.. Explainable artificial intelligence for spectroscopy data: a review. Pflügers Archiv - European Journal of Physiology. 2025;477:603. doi: 10.1007/s00424-024-02997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo C., Barone V.. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998;108(2):664–675. doi: 10.1063/1.475428. [DOI] [Google Scholar]
- Zhao Y., Truhlar D. G.. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120(1):215–241. doi: 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Gao X., Ramezanghorbani F., Isayev O., Smith J. S., Roitberg A. E.. TorchANI: a free and open source PyTorch-based deep learning implementation of the ANI neural network potentials. J. Chem. Inf. Model. 2020;60(7):3408–3415. doi: 10.1021/acs.jcim.0c00451. [DOI] [PubMed] [Google Scholar]
- Devereux C., Smith J. S., Huddleston K. K., Barros K., Zubatyuk R., Isayev O., Roitberg A. E.. Extending the applicability of the ANI deep learning molecular potential to sulfur and halogens. J. Chem. Theory Comput. 2020;16(7):4192–4202. doi: 10.1021/acs.jctc.0c00121. [DOI] [PubMed] [Google Scholar]
- Yang Z., Chakraborty M., White A. D.. Predicting chemical shifts with graph neural networks. Chem. Sci. 2021;12(32):10802–10809. doi: 10.1039/D1SC01895G. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Neal S., Wishart D. S.. RefDB: a database of uniformly referenced protein chemical shifts. J. Biomol NMR. 2003;25(3):173–195. doi: 10.1023/A:1022836027055. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Wishart D. S., Tzur D., Knox C., Eisner R., Guo A. C., Young N., Cheng D., Jewell K., Arndt D., Sawhney S.. et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35(Database issue):D521–526. doi: 10.1093/nar/gkl923. From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. D., Voth G. A.. Efficient and Minimal Method to Bias Molecular Simulations with Experimental Data. J. Chem. Theory Comput. 2014;10(8):3023–3030. doi: 10.1021/ct500320c. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Grimblat N., Zanardi M. M., Sarotti A. M.. Beyond DP4: an Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015;80(24):12526–12534. doi: 10.1021/acs.joc.5b02396. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Smith S. G., Goodman J. M.. Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability. J. Am. Chem. Soc. 2010;132(37):12946–12959. doi: 10.1021/ja105035r. [DOI] [PubMed] [Google Scholar]
- Gu X., Myung Y., Rodrigues C. H. M., Ascher D. B.. EFG-CS: Predicting chemical shifts from amino acid sequences with protein structure prediction using machine learning and deep learning models. Protein Sci. 2024;33(8):e5096. doi: 10.1002/pro.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Bennett K. C., Liu Y., Martin M. V., Head-Gordon T.. Accurate prediction of chemical shifts for aqueous protein structure on “Real World” data. Chemical Science. 2020;11(12):3180–3191. doi: 10.1039/C9SC06561J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Liu Y., Ginzinger S. W., Wishart D. S.. SHIFTX2: significantly improved protein chemical shift prediction. Journal of biomolecular NMR. 2011;50:43–57. doi: 10.1007/s10858-011-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Guo W., Wang F., Yao L., Wang H., Tang F., Gao Z., Zhang L., E W., Tian Z. Q.. et al. Toward a unified benchmark and framework for deep learning-based prediction of nuclear magnetic resonance chemical shifts. Nat. Comput. Sci. 2025;5(4):292–300. doi: 10.1038/s43588-025-00783-z. From NLM PubMed-not-MEDLINE. [DOI] [PubMed] [Google Scholar]
- Xu F., Guo W., Wang F., Yao L., Wang H., Tang F., Gao Z., Zhang L., Tian Z.-Q., Cheng J.. Towards a Unified Benchmark and Framework for Deep Learning-Based Prediction of Nuclear Magnetic Resonance Chemical Shifts. arXiv e-prints. 2024:arXiv:2408.15681. doi: 10.48550/arXiv.2408.15681. [DOI] [PubMed] [Google Scholar]
- Cordova M., Engel E. A., Stefaniuk A., Paruzzo F., Hofstetter A., Ceriotti M., Emsley L.. A Machine Learning Model of Chemical Shifts for Chemically and Structurally Diverse Molecular Solids. J. Phys. Chem. C. 2022;126(39):16710–16720. doi: 10.1021/acs.jpcc.2c03854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Chakraborty S., Ramakrishnan R.. Revving up 13C NMR shielding predictions across chemical space: benchmarks for atoms-in-molecules kernel machine learning with new data for 134 kilo molecules. Machine Learning: Science and Technology. 2021;2(3):035010. doi: 10.1088/2632-2153/abe347. [DOI] [Google Scholar]
- Kuhn S., Schlörer N. E.. Facilitating quality control for spectra assignments of small organic molecules: nmrshiftdb2 - a free in-house NMR database with integrated LIMS for academic service laboratories. Magn. Reson. Chem. 2015;53(8):582–589. doi: 10.1002/mrc.4263. [DOI] [PubMed] [Google Scholar]
- Garcia-Viloca M., Gelabert R., González-Lafont À., Moreno M., Lluch J. M.. Temperature Dependence of Proton NMR Chemical Shift As a Criterion To Identify Low-Barrier Hydrogen Bonds. J. Am. Chem. Soc. 1998;120(39):10203–10209. doi: 10.1021/ja9742141. [DOI] [Google Scholar]
- Novitskiy I. M., Kutateladze A. G.. DU8ML: Machine Learning-Augmented Density Functional Theory Nuclear Magnetic Resonance Computations for High-Throughput In Silico Solution Structure Validation and Revision of Complex Alkaloids. J. Org. Chem. 2022;87(7):4818–4828. doi: 10.1021/acs.joc.2c00169. From NLM Medline. [DOI] [PubMed] [Google Scholar]
- Ptaszek A. L., Li J., Konrat R., Platzer G., Head-Gordon T.. UCBShift 2.0: Bridging the Gap from Backbone to Side Chain Protein Chemical Shift Prediction for Protein Structures. J. Am. Chem. Soc. 2024;146(46):31733–31745. doi: 10.1021/jacs.4c10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarotti A. M.. Successful combination of computationally inexpensive GIAO 13C NMR calculations and artificial neural network pattern recognition: a new strategy for simple and rapid detection of structural misassignments. Organic & Biomolecular Chemistry. 2013;11(29):4847–4859. doi: 10.1039/c3ob40843d. [DOI] [PubMed] [Google Scholar]
- Batista A. N. L., Martorano L. H., Valverde A. L., dos Santos F. M.. Combining the power of DP4+ and ANN-PRA analysis on stereochemical assignments: structural validation of bioactive cyclic peroxide from the marine sponge Plakortis angulospiculatus. New J. Chem. 2023;47(22):10834–10841. doi: 10.1039/D3NJ01762A. [DOI] [Google Scholar]
- Howarth A., Ermanis K., Goodman J. M.. DP4-AI automated NMR data analysis: straight from spectrometer to structure. Chemical Science. 2020;11(17):4351–4359. doi: 10.1039/D0SC00442A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés, I. ; Cuadrado, C. ; Hernández Daranas, A. ; Sarotti, A. M. . Machine learning in computational NMR-aided structural elucidation. Frontiers in Natural Products 2023, Volume 2. 10.3389/fntpr.2023.1122426. [DOI] [Google Scholar]
- Howarth A., Goodman J. M.. The DP5 probability, quantification and visualisation of structural uncertainty in single molecules. Chem. Sci. 2022;13(12):3507–3518. doi: 10.1039/D1SC04406K. From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu C., Honoré B., Gerrard W., Napolitano-Farina J., Russell D., Trist I. M. L., Dooley R., Butts C. P.. IMPRESSION generation 2 - accurate, fast and generalised neural network model for predicting NMR parameters in place of DFT. Chemical Science. 2025;16(19):8377–8382. doi: 10.1039/D4SC07858F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D., Ponte S., Iglesias I., Tonge N., Cobas C., Kemsley E. K.. Chemical shift prediction in 13C NMR spectroscopy using ensembles of message passing neural networks (MPNNs) J. Magn. Reson. 2024;368:107795. doi: 10.1016/j.jmr.2024.107795. [DOI] [PubMed] [Google Scholar]
- Zhu H., Hu L., Yang Y., Chen Z.. A novel approach to protein chemical shift prediction from sequences using a protein language model. Digital Discovery. 2025;4(2):331–337. doi: 10.1039/D4DD00367E. [DOI] [Google Scholar]
- Lin Z., Akin H., Rao R., Hie B., Zhu Z., Lu W., Smetanin N., Verkuil R., Kabeli O., Shmueli Y.. et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science. 2023;379(6637):1123–1130. doi: 10.1126/science.ade2574. [DOI] [PubMed] [Google Scholar]
- Chen H., Liang T., Tan K., Wu A., Lu X.. GT-NMR: a novel graph transformer-based approach for accurate prediction of NMR chemical shifts. Journal of Cheminformatics. 2024;16(1):132. doi: 10.1186/s13321-024-00927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu H., Kumar A., Wang D.-S., Heiss C., Azadi P., Hong P.. TransPeakNet for solvent-aware 2D NMR prediction via multi-task pre-training and unsupervised learning. Communications Chemistry. 2025;8(1):51. doi: 10.1038/s42004-025-01455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S., Guo A., Oler E., Wang F., Anjum A., Peters H., Dizon R., Sayeeda Z., Tian S., Lee Brian L.. et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 2022;50(D1):D622–D631. doi: 10.1093/nar/gkab1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S., Wang Y., Cai W.. Generalizability of Graph Neural Network Force Fields for Predicting Solid-State Properties. Advanced Theory and Simulations. 2025;8(4):2401058. doi: 10.1002/adts.202401058. [DOI] [Google Scholar]
- Gaumard R., Dragún D., Pedroza-Montero J. N., Alonso B., Guesmi H., Malkin Ondík I., Mineva T.. Regression Machine Learning Models Used to Predict DFT-Computed NMR Parameters of Zeolites. Computation. 2022;10(5):74. doi: 10.3390/computation10050074. [DOI] [Google Scholar]
- Li, Y. ; Xu, H. ; Hong, P. . 2DNMRGym: An Annotated Experimental Dataset for Atom-Level Molecular Representation Learning in 2D NMR via Surrogate Supervision. arXiv, 2025. 10.48550/arXiv.2505.18181. [DOI] [Google Scholar]
- Shen Y., Bax A.. SPARTA+: a modest improvement in empirical NMR chemical shift prediction by means of an artificial neural network. Journal of Biomolecular NMR. 2010;48(1):13–22. doi: 10.1007/s10858-010-9433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhoff K. J., Robustelli P., Cavalli A., Salvatella X., Vendruscolo M.. Fast and Accurate Predictions of Protein NMR Chemical Shifts from Interatomic Distances. J. Am. Chem. Soc. 2009;131(39):13894–13895. doi: 10.1021/ja903772t. [DOI] [PubMed] [Google Scholar]
- Meiler J., Baker D.. Coupled prediction of protein secondary and tertiary structure. Proc. Natl. Acad. Sci. U. S. A. 2003;100(21):12105–12110. doi: 10.1073/pnas.1831973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pople, J. ; Schneider, W. ; Bernstein, H. . High-Resolution Nuclear Magnetic Resonance; M. Hill Book Company: New York, 1959. [Google Scholar]
- Pople J. A.. The theory of chemical shifts in nuclear magnetic resonance II. Interpretation of proton shifts. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 1957;239(1219):550–556. doi: 10.1098/rspa.1957.0061. [DOI] [Google Scholar]
- Karplus M.. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963;85(18):2870–2871. doi: 10.1021/ja00901a059. [DOI] [Google Scholar]
- Karplus M.. THE ANALYSIS OF MOLECULAR WAVE FUNCTIONS BY NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY1a. J. Phys. Chem. 1960;64(12):1793–1798. doi: 10.1021/j100841a001. [DOI] [Google Scholar]
- Shen Y., Vernon R., Baker D., Bax A.. De novo protein structure generation from incomplete chemical shift assignments. Journal of Biomolecular NMR. 2009;43(2):63–78. doi: 10.1007/s10858-008-9288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S., Arndt D., Berjanskii M., Tang P., Zhou J., Lin G.. CS23D: a web server for rapid protein structure generation using NMR chemical shifts and sequence data. Nucleic acids research. 2008;36(suppl_2):W496–W502. doi: 10.1093/nar/gkn305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuvonen H., Neuvonen K., Koch A., Kleinpeter E., Pasanen P.. Electron-withdrawing substituents decrease the electrophilicity of the carbonyl carbon. An investigation with the aid of 13C NMR chemical shifts, ν (CO) frequency values, charge densities, and isodesmic reactions to interprete substituent effects on reactivity. Journal of Organic Chemistry. 2002;67(20):6995–7003. doi: 10.1021/jo020121c. [DOI] [PubMed] [Google Scholar]
- Vanderhoeven S. J., Troke J., Tranter G. E., Wilson I. D., Nicholson J. K., Lindon J. C.. Nuclear magnetic resonance (NMR) and quantitative structure-activity relationship (QSAR) studies on the transacylation reactivity of model 1β-O-acyl glucuronides. II: QSAR modelling of the reaction using both computational and experimental NMR parameters. Xenobiotica. 2004;34(10):889–900. doi: 10.1080/00498250400005674. [DOI] [PubMed] [Google Scholar]
- Mercader, A. G. ; Duchowicz, P. R. ; Sivakumar, P. . Chemometrics applications and research: QSAR in medicinal chemistry; CRC Press, 2016. [Google Scholar]
- Beger R. D.. Computational modeling of biologically active molecules using NMR spectra. Drug Discovery Today. 2006;11(9):429–435. doi: 10.1016/j.drudis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Beger R. D., Buzatu D. A., Wilkes J. G., Jackson. Comparative Structural Connectivity Spectra Analysis (CoSCoSA) Models of Steroid Binding to the Corticosteroid Binding Globulin. J. Chem. Inf. Comput. Sci. 2002;42(5):1123–1131. doi: 10.1021/ci025511e. [DOI] [PubMed] [Google Scholar]
- Todeschini, R. ; Consonni, V. . Handbook of molecular descriptors; John Wiley & Sons, 2008. [Google Scholar]
- Steinmetz W. E., Rodarte C. B., Lin A.. 3D QSAR study of the toxicity of trichothecene mycotoxins. European journal of medicinal chemistry. 2009;44(11):4485–4489. doi: 10.1016/j.ejmech.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Mor M., Rivara S., Lodola A., Lorenzi S., Bordi F., Plazzi P. V., Spadoni G., Bedini A., Duranti A., Tontini A.. et al. Application of 3D-QSAR in the Rational Design of Receptor Ligands and Enzyme Inhibitors. Chemistry & biodiversity. 2005;2(11):1438–1451. doi: 10.1002/cbdv.200590117. [DOI] [PubMed] [Google Scholar]
- Leniak A., Pietruś W., Kurczab R.. From NMR to AI: Designing a Novel Chemical Representation to Enhance Machine Learning Predictions of Physicochemical Properties. J. Chem. Inf. Model. 2024;64(8):3302–3321. doi: 10.1021/acs.jcim.3c02039. [DOI] [PubMed] [Google Scholar]
- Paruzzo F. M., Hofstetter A., Musil F., De S., Ceriotti M., Emsley L.. Chemical shifts in molecular solids by machine learning. Nat. Commun. 2018;9(1):4501. doi: 10.1038/s41467-018-06972-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burov, A. ; Nagl, O. ; Javerzac-Galy, C. . Towards quantum utility for NMR quantum simulation on a NISQ computer. arXiv, 2024. 10.48550/arXiv.2404.17548. [DOI] [Google Scholar]
- Shor P. W.. Algorithms for quantum computation: discrete logarithms and factoring. Proceedings 35th Annual Symposium on Foundations of Computer Science. 1994:124–134. doi: 10.1109/SFCS.1994.365700. 20–22 Nov. 1994. [DOI] [Google Scholar]
- Wocjan P., Chiang C.-F., Nagaj D., Abeyesinghe A.. Quantum algorithm for approximating partition functions. Phys. Rev. A. 2009;80(2):022340. doi: 10.1103/PhysRevA.80.022340. [DOI] [Google Scholar]
- Peruzzo, A. A variational eigenvalue solver on a quantum processor. eprint. arXiv, 2013. 10.48550/arXiv.1304.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam K., Biswas D., Noel C., Risinger A., Zhu D., Katz O., Chattopadhyay S., Cetina M., Monroe C., Demler E.. et al. Digital quantum simulation of NMR experiments. Science Advances. 2023;9(46):eadh2594. doi: 10.1126/sciadv.adh2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedri, A. ; Stadler, P. ; Bark, K. ; Lodi, M. ; Reiner, R. ; Vogt, N. ; Marthaler, M. ; Leppäkangas, J. . The impact of noise on the simulation of NMR spectroscopy on NISQ devices. arXiv, 2024. 10.48550/arXiv.2404.18903. [DOI] [Google Scholar]
- HQS Quantum Simulations HQS Quantum Simulations. 2025. (acccessed 2025/06/17).
- HQS Quantum Simulations Next-Gen NMR Prediction and Analysis: A Cutting-Edge Tool for High-Precision Structure Identification (HQS-NextNMR). 2025. (acccessed 2025/06/17).
- Robledo-Moreno, J. ; Motta, M. ; Haas, H. ; Javadi-Abhari, A. ; Jurcevic, P. ; Kirby, W. ; Martiel, S. ; Sharma, K. ; Sharma, S. ; Shirakawa, T. . Chemistry beyond exact solutions on a quantum-centric supercomputer. arXiv, 2024, 10.48550/arXiv.2405.05068. [DOI] [PubMed] [Google Scholar]
- Barison S., Robledo Moreno J., Motta M.. Quantum-centric computation of molecular excited states with extended sample-based quantum diagonalization. Quantum Science and Technology. 2025;10(2):025034. doi: 10.1088/2058-9565/adb781. [DOI] [Google Scholar]
- Kanno, K. ; Kohda, M. ; Imai, R. ; Koh, S. ; Mitarai, K. ; Mizukami, W. ; Nakagawa, Y. O. . Quantum-selected configuration interaction: Classical diagonalization of Hamiltonians in subspaces selected by quantum computers. arXiv, 2023. 10.48550/arXiv.2302.11320. [DOI] [Google Scholar]
- Nakagawa Y. O., Kamoshita M., Mizukami W., Sudo S., Ohnishi Y.-y.. ADAPT-QSCI: Adaptive Construction of an Input State for Quantum-Selected Configuration Interaction. J. Chem. Theory Comput. 2024;20(24):10817–10825. doi: 10.1021/acs.jctc.4c00846. [DOI] [PubMed] [Google Scholar]
- Kaliakin, D. ; Shajan, A. ; Moreno, J. R. ; Li, Z. ; Mitra, A. ; Motta, M. ; Johnson, C. ; Saki, A. A. ; Das, S. ; Sitdikov, I. . Accurate quantum-centric simulations of supramolecular interactions. arXiv, 2024. 10.48550/arXiv.2410.09209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajan, A. ; Kaliakin, D. ; Mitra, A. ; Moreno, J. R. ; Li, Z. ; Motta, M. ; Johnson, C. ; Saki, A. A. ; Das, S. ; Sitdikov, I. . Towards quantum-centric simulations of extended molecules: sample-based quantum diagonalization enhanced with density matrix embedding theory. arXiv, 2024. 10.48550/arXiv.2411.09861. [DOI] [PubMed] [Google Scholar]
- Kaliakin D., Shajan A., Liang F., Merz K. M. Jr.. Implicit Solvent Sample-Based Quantum Diagonalization. J. Phys. Chem. B. 2025;129(23):5788–5796. doi: 10.1021/acs.jpcb.5c01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M., Sung K. J., Whaley K. B., Head-Gordon M., Shee J.. Bridging physical intuition and hardware efficiency for correlated electronic states: the local unitary cluster Jastrow ansatz for electronic structure. Chemical Science. 2023;14(40):11213–11227. doi: 10.1039/D3SC02516K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsroom, I. B. M. IBM Sets the Course to Build World’s First Large-Scale, Fault-Tolerant Quantum Computer at New IBM Quantum Data Center. (Yorktown Heights, NY), 2025/06/10, 2025. https://newsroom.ibm.com/2025-06-10-IBM-Sets-the-Course-to-Build-Worlds-First-Large-Scale,-Fault-Tolerant-Quantum-Computer-at-New-IBM-Quantum-Data-Center.
- Newsroom, I. B. M. IBM Unveils 400-Qubit-Plus Quantum Processor and Next-Generation IBM Quantum System Two. (Yorktown Heights, NY), 2022/11/09, 2022. https://newsroom.ibm.com/2022-11-09-IBM-Unveils-400-Qubit-Plus-Quantum-Processor-and-Next-Generation-IBM-Quantum-System-Two.
- Saki, A. A. ; Barison, S. ; Fuller, B. ; Garrison, J. R. ; Glick, J. R. ; Johnson, C. ; Mezzacapo, A. ; Robledo-Moreno, J. ; Rossmannek, M. ; Schweigert, P. . Qiskit addon: sample-based quantum diagonalization. 2024.