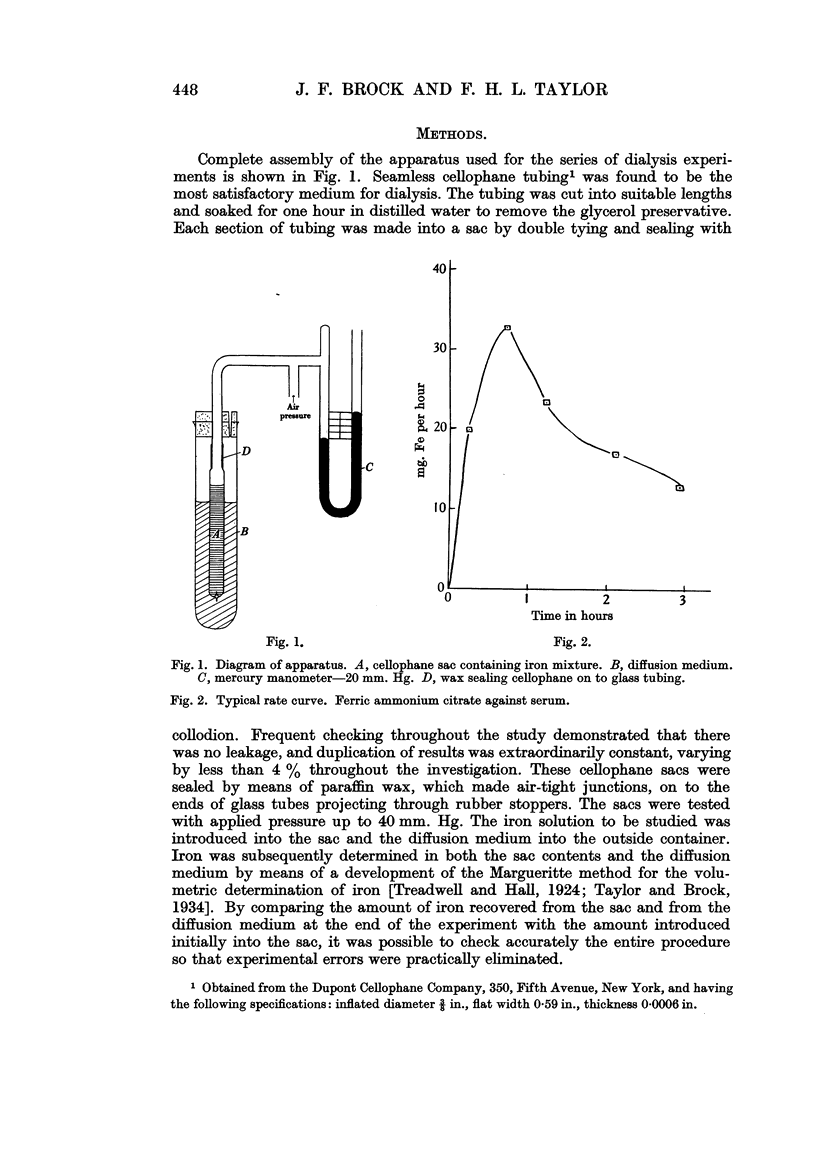
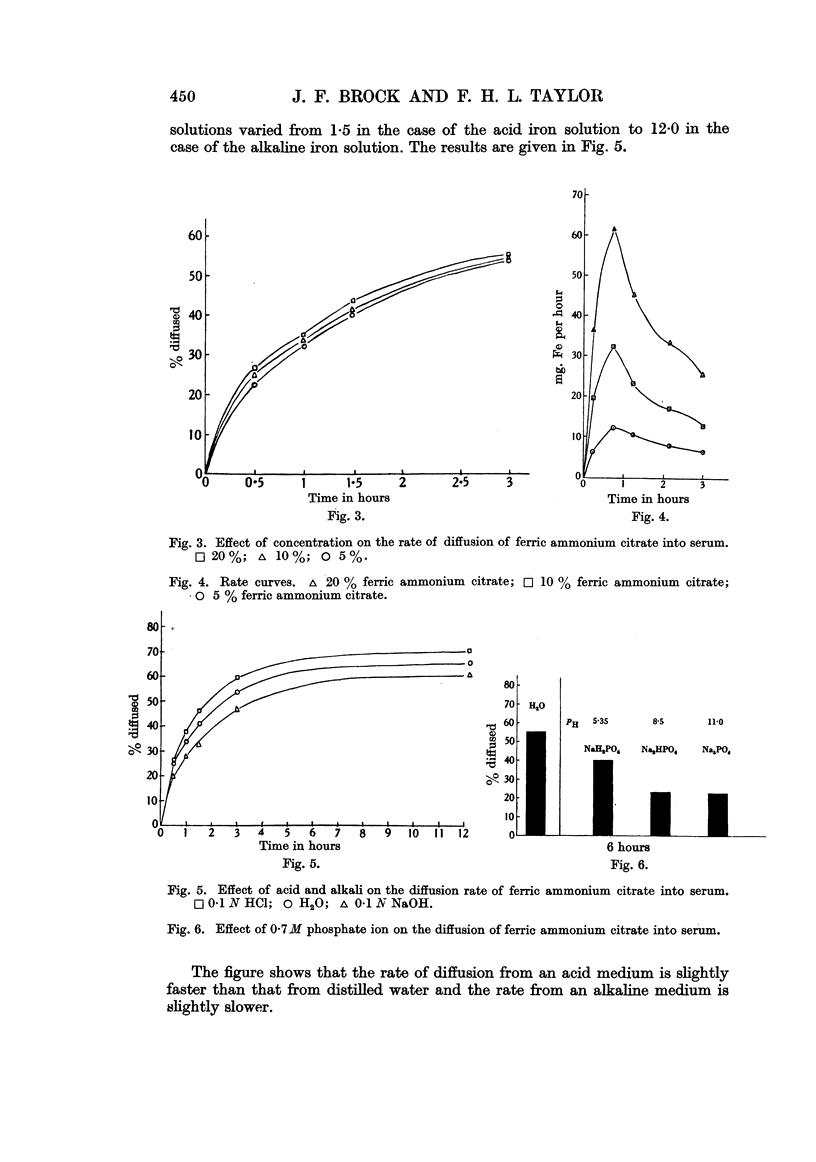
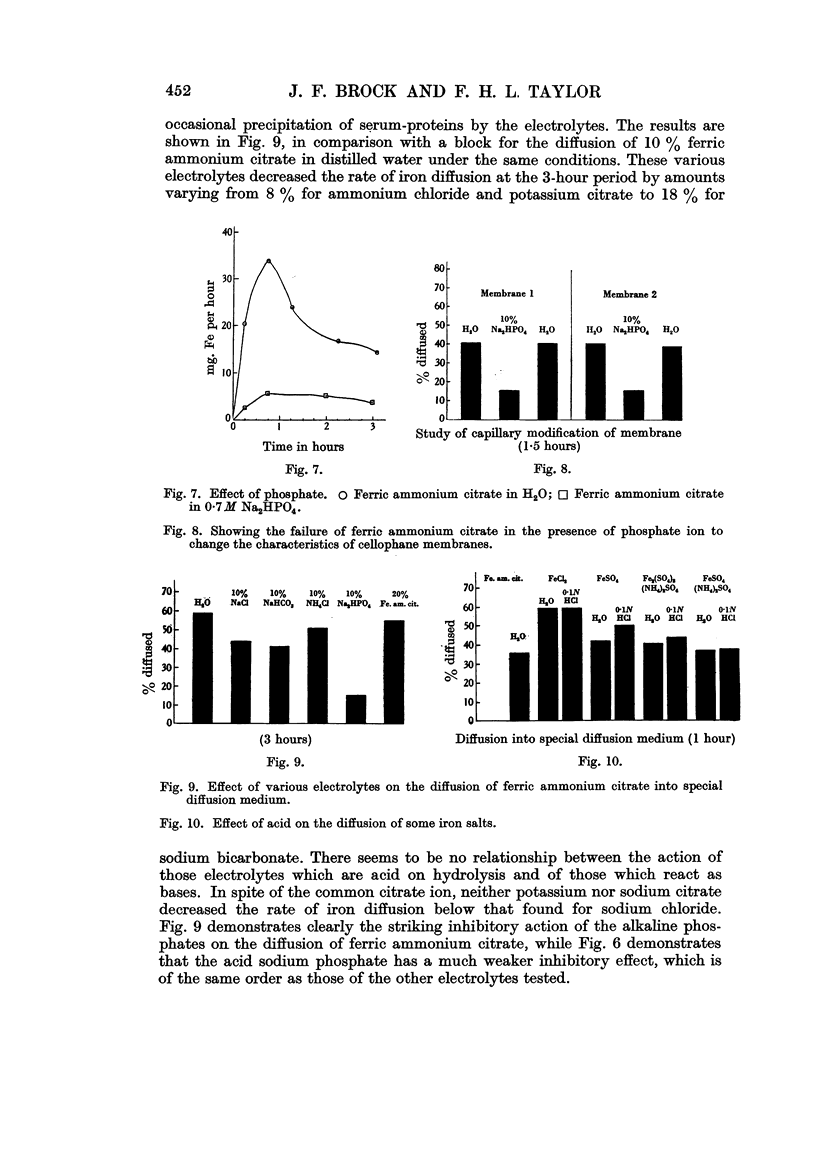
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Heath C. W., Strauss M. B., Castle W. B. QUANTITATIVE ASPECTS OF IRON DEFICIENCY IN HYPOCHROMIC ANEMIA: (The Parenteral Administration of Iron). J Clin Invest. 1932 Nov;11(6):1293–1312. doi: 10.1172/JCI100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macallum A. B. On the Absorption of Iron in the Animal Body. J Physiol. 1894 Apr 17;16(3-4):268–318.10. doi: 10.1113/jphysiol.1894.sp000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettier S. R. THE REACTION OF HUMAN DUODENAL CONTENTS TO ACID AND ALKALINE MEAT MIXTURES. J Clin Invest. 1930 Jun;8(4):561–567. doi: 10.1172/JCI100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. H., Brock J. F. A macro-method for the determination of iron in biological material by a modified permanganate titration. Biochem J. 1934;28(2):442–446. doi: 10.1042/bj0280442. [DOI] [PMC free article] [PubMed] [Google Scholar]


