Abstract
We show for the first time that Cah3, a carbonic anhydrase associated with the photosystem II (PSII) donor side in Chlamydomonas reinhardtii, regulates the water oxidation reaction. The mutant cia3, lacking Cah3 activity, has an impaired water splitting capacity, as shown for intact cells, thylakoids and PSII particles. To compensate this impairment, the mutant overproduces PSII reaction centres (1.6 times more than wild type). We present compelling evidence that the mutant has an average of two manganese atoms per PSII reaction centre. When bicarbonate is added to mutant thylakoids or PSII particles, the O2 evolution rates exceed those of the wild type by up to 50%. The donor side of PSII in the mutant also exhibits a much higher sensitivity to overexcitation than that of the wild type. We therefore conclude that Cah3 activity is necessary to stabilize the manganese cluster and maintain the water-oxidizing complex in a functionally active state. The possibility that two manganese atoms are enough for water oxidation if bicarbonate ions are available is discussed.
Keywords: carbonic anhydrase/Chlamydomonas/manganese cluster/photosystem II/water oxidation complex
Introduction
Oxygen-evolving photosynthesis is the global process where light is absorbed by photosynthetic pigments and converted into chemical energy in plants, algae and cyanobacteria. Photosynthetic water oxidation takes place in photosystem II (PSII), a large multisubunit protein complex, located in the thylakoid membranes of chloroplasts and cyanobacteria, which functions as a light-driven water–plastoquinone oxidoreductase. Despite significant progress in the biochemical, biophysical and structural characterization of PSII (Nield et al., 2000; Zouni et al., 2001), details of the mechanism of water oxidation remain largely unknown (Renger, 2001). Oxygen evolution is catalysed by the water-oxidizing complex (WOC), a manganese cluster and PSII extrinsic proteins on the lumenal side of thylakoid membranes. The electrons and protons released from the water oxidation are transported in a vectorial manner and used to reduce NADP and to generate ATP that reduces CO2 to simple sugars.
However, CO2 (or its ionic species bicarbonate) is not only involved in photosynthetic reactions as the ultimate electron acceptor but also participates in the regulation of the photochemical reactions. More than 40 years ago, Warburg and Krippahl (1958) discovered that bicarbonate could stimulate electron flow in the Hill reaction, but ever since then the effect of bicarbonate on PSII activity has been a matter of debate among the scientific community (Van Rensen et al., 1999). The requirement for bicarbonate at the acceptor side of PSII is now well established (Van Rensen et al., 1999). However, the bicarbonate stimulation of PSII activity on the donor side still remains controversial and has yet to be fully elucidated (Klimov and Baranov, 2001).
One of the early hypotheses to explain the apparent requirement for bicarbonate in PSII was that bicarbonate acts as a direct electron donor to PSII (Metzner, 1978; Stemler, 1980). One prediction arising from the hypothesis was that PSII would need to produce bicarbonate catalytically from CO2 otherwise this reaction would be seriously rate limiting. Carbonic anhydrase (CA) is a zinc metalloenzyme, accelerating the interconversion of CO2 and HCO3– by a factor of >106. It was proposed earlier that a thylakoid CA might be involved in some way in the bicarbonate effect in PSII (Moubarak-Milad and Stemler, 1994). A lot of circumstantial evidence for this possibility has been accumulated over a period of many years (Stemler, 1997), but the putative enzyme involved proved to be elusive. Recently, a novel carbonic anhydrase (Cah3) was identified in the thylakoid lumen of the unicellular green alga Chlamydomonas reinhardtii (Karlsson et al., 1998) and, more importantly, it was shown to bind to PSII (Park et al., 1999).
To elucidate the role of the thylakoid CA in PSII, we have characterized features of the PSII activities in the cia3 mutant of C.reinhardtii, which has been shown to lack an active thylakoid CA (Moroney et al., 1986). Complementation experiments showed that cah3 is the only gene defective in this mutant (Karlsson et al., 1998). Our results provide, for the first time, strong evidence that Cah3 is required for the optimal function of the WOC. In the absence of this CA activity, the manganese cluster becomes unstable and the electron donation side of PSII is impaired. This is the first report on the relationship between a CA activity and the bicarbonate effect on the PSII donor side. We conclude that the catalysed interconversion between the inorganic carbon species at the donor side of PSII is essential for the WOC to function optimally.
Results
Lack of CA makes PSII more sensitive to high light
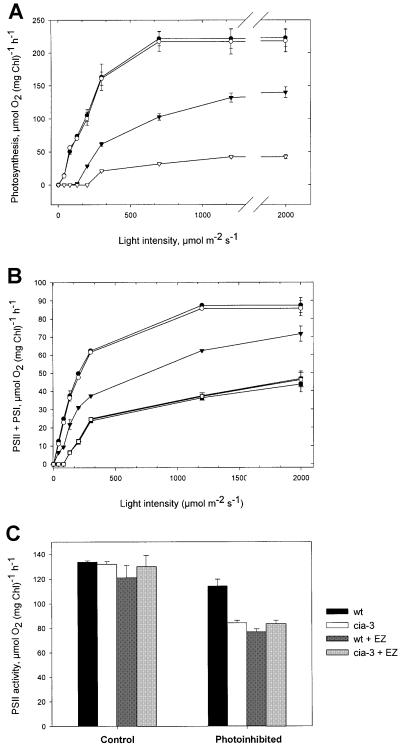
To compare the efficiency of light energy conversion in wild-type and cia3 mutant cells, we measured O2 evolution as a function of light intensity. Figure 1A shows the light response curves of O2 evolution of wild-type and mutant cells, grown continuously at 150 µmol/m2/s before and after exposure to 2200 µmol/m2/s for 1 h. Under control conditions, not only was the light-saturated value of photosynthesis complementary, but also the rate of increase in O2 evolution as a function of light was equal in mutant and wild-type cells (Figure 1A). Therefore, mutant and wild-type cells have an apparent similar efficiency of light utilization. Determination of the linear electron transport flow in isolated thylakoids provided similar results (Figure 1B). There was no difference between the two types of cells at any of the light intensities applied.
Fig. 1. Photosynthetic O2 evolution and linear electron transfer versus irradiance, and light-saturated PSII electron transport rates in wild-type and mutant cells and thylakoids. (A) Light response curves of O2 evolution from wild-type (filled symbols) and cia3 mutant (open symbols) cells of C.reinhardtii growing continuously at 150 µmol/m2/s before (circles) and after (inverted triangles) exposure to 2200 µmol/m2/s for 60 min at 26°C. The photoinhibitory treatment was carried out in test tubes of 1 cm diameter, using cell suspensions at 10 µg Chl/ml that were bubbled continuously with air enriched with 5% CO2. (B) Linear electron transport rates (measured in the presence of 1 mM methyl viologen) versus irradiance in thylakoid membranes isolated from wild-type (filled symbols) and cia3 mutant (open symbols) cells before (circles) and after photoinhibition of thylakoid membranes at 600 µmol/m2/s for 10 min in the absence (inverted triangles) or presence (squares) of 0.5 mM EZ. The photoinhibitory treatment was carried out in test tubes of 1 cm diameter. The chlorophyll concentration of the thylakoid preparations used for these experiments was 25 µg Chl/ml. (C) Light-saturated PSII electron transport rates (measured in the presence of 1 mM DCBQ, 1 mM ferricyanide and 10 µM gramicidin D) in thylakoid membranes (25 µg Chl/ml) from wild-type and mutant cells before and after photoinhibition at 600 µmol/m2/s for 10 min. Values are means ± SE (n = 4).
When the sensitivity to high light treatment was compared between wild-type and cia3 cells, an interesting difference started to emerge. A 1 h high light treatment reduced the light-saturated rate of photosynthesis in wild-type cells to 60–70% of the control rates, while in the mutant it decreased to just 20% of control values (Figure 1A).
Figure 1B and C compares rates of linear photosynthetic electron transport and light-saturated PSII-specific electron transport, before and after high light treatment of thylakoid membranes from both types of cells. The results show that the high light-induced decline in oxygen evolution observed in intact cells (Figure 1A) is caused by a concomitant inhibition of the linear electron transport rate in thylakoids (Figure 1B). After high light treatment, PSII activity, measured as electron flow from H2O to the artificial acceptor 2,5-dichloro-p-benzoquinone (DCBQ), was decreased to ∼50% in mutant thylakoids, while in wild type it was only reduced to 80% (Figure 1C). Taken together, these observations suggest that PSII of the mutant exhibited a much higher sensitivity to overexcitation than wild-type PSII. When wild-type thylakoid membranes were photoinhibited in the presence of ethoxyzolamide (EZ), a specific inhibitor of CA activity (Figure 1B and C), identical rates of linear and PSII-specific electron transport were obtained, compared with the mutant. However, EZ did not cause a significant effect on light-saturated PSII electron transport in wild-type or mutant thylakoids that had not been exposed to high light conditions. There was also no effect of EZ on control and photoinhibited thylakoids from the mutant cia3 (Figure 1B and C). These results indicate that the activity of the thylakoid CA is important for stabilizing PSII during high light conditions.
The lumenal CA is associated with PSII
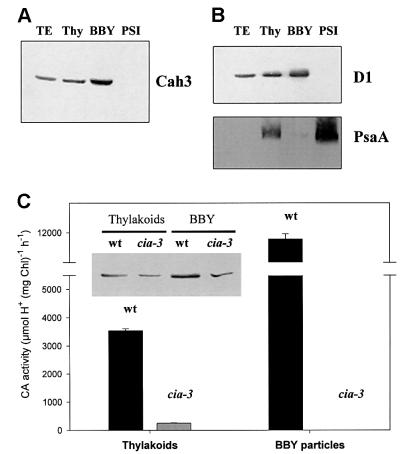
For the above-mentioned hypothesis to be correct, it requires that the thylakoid CA is closely associated with PSII. In fact, an enrichment of Cah3 in PSII preparations was obtained (Figure 2A). BBY particles are known to be enriched in PSII (Andersson, 1986) compared with isolated thylakoid membranes and total cell extracts. Moreover, Cah3 was not detectable in the PSI fraction. Since our BBY preparations are enriched in the D1 protein and contain a barely detectable amount of markers for PSI (Figure 2B), we conclude that CA is associated with PSII. Furthermore, the above results, together with information on the leader sequence of Cah3, typical for lumen-targeted proteins (Karlsson et al., 1998), strongly suggest that Cah3 is located at or close to the donor side of PSII. Both sequence analysis and the data on removal of Cah3 by salt wash treatment predict that Cah3 is not a membrane-spanning polypeptide but rather is attached extrinsically to or at a site close to PSII by ionic and/or hydrophobic interactions. Atomic absorption spectroscopy measurements of the zinc content in BBY preparations support these results. In preparations from wild type, there were close to two atoms of zinc per PSII reaction centre (2.2 ± 0.2) while in the mutant there was less than one (0.9 ± 0.1).
Fig. 2. Subcellular distribution of the Cah3 polypeptide and CA activity. (A) Immunoblot analysis of total cell extracts (TE), total thylakoid membranes (Thy), PSII (BBY) and PSI fractions from wild-type cells of C.reinhardtii with antibodies raised against the overexpressed Cah3 polypeptide. (B) Occurrence of cross-contamination with proteins from PSI and PSII. D1 protein was used as a marker for PSII, and PsaA as a marker for PSI. (C) CA activity measurements in thylakoid membranes and BBY preparations from both wild-type and mutant cells. Values in (C) are means ± SE (n = 4). The inset shows immunoblot analysis of thylakoid membranes and BBY preparations from both wild-type and mutant cells probed with antibodies raised against the overexpressed CA from C.reinhardtii.
Not only the Cah3 polypeptide but also the CA activity was enriched in the BBY preparations from wild-type cells (Figure 2C). In the mutant cia3, the CA activity associated with thylakoid membranes was less than one-tenth of that measured in wild-type samples [245 ± 17 and 3540 ± 80 µmol H+/mg chlorophyll (Chl)/h, respectively] (Figure 2C). The CA activity measured in thylakoids from the mutant was similar to that obtained when thylakoid membranes from the wild type were assayed in the presence of 0.5 mM EZ (245 ± 17 and 315 ± 15 µmol H+/mg Chl/h, respectively). Moreover, the BBY preparations from the mutant cells did not exhibit any measurable CA activity (Figure 2C), although the Cah3 polypeptide was still present in this fraction, albeit in a trace amount compared with the wild type (Figure 2C, inset). The inactivation of the Cah3 in the mutant is caused by a mutation in the region encoding the thylakoid transit peptide (Karlsson et al., 1998) which was suggested to cause a mistargeting of the Cah3 protein. Our results indicate that only a small proportion of the CA is associated with PSII in cia3 and that CA activity at this site is almost undetectable.
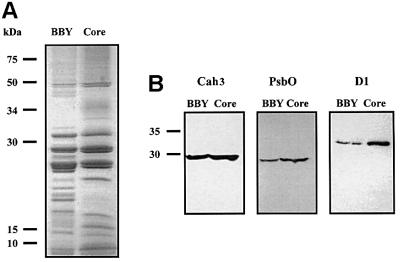
In order to determine further the location of the Cah3 polypeptide, we isolated PSII core complexes from C.reinhardtii wild-type cells. These complexes were highly active. In the presence of CaCl2, the complexes supported oxygen evolution rates of 866 ± 13 µmol O2/mg Chl/h. In addition, the Chl a/b ratio in these samples was >6, indicating the removal of most of the light-harvesting complexes (LHCs). SDS–PAGE profiles of the core complexes and BBY preparations are shown in Figure 3A. The protein composition of the core complex fraction from C.reinhardtii was very similar to that of the spinach LHCII–PSII supercomplexes (Hankamer et al., 1997). Several polypeptides, mainly D1, CP43, CP47, PsbO and some other small polypeptides, are enriched in the core complex fractions when compared with BBY preparations (Figure 3A). These data were confirmed further by immunoblot analysis (Figure 3B). Both D1 and PsbO polypeptides were highly enriched in core complexes compared with BBY preparations. Moreover, the Cah3 polypeptide was also enriched in core fractions compared with BBY preparations (Figure 3B). All this evidence strongly indicates that the Cah3 polypeptide is associated with the PSII core complex.
Fig. 3. Association of the Cah3 polypeptide with PSII core complexes from wild-type C.reinhardtii cells. (A) SDS–PAGE of BBY preparations (BBY) and purified PSII core complexes (Core) stained with Coomassie Blue. The molecular masses of the markers are given in kDa. PSII core complexes and BBY preparations equivalent to 30 µg of protein were loaded. (B) Immunoblot analysis of BBY preparations (BBY) and PSII core complexes (Core) from wild-type cells of C.reinhardtii with antibodies raised against the overexpressed Cah3 polypeptide (Cah3), D1 protein (D1) and PsbO polypeptide (PsbO). The lanes were loaded with 10 µg of protein.
CA is required to stabilize the WOC during overexcitation of PSII
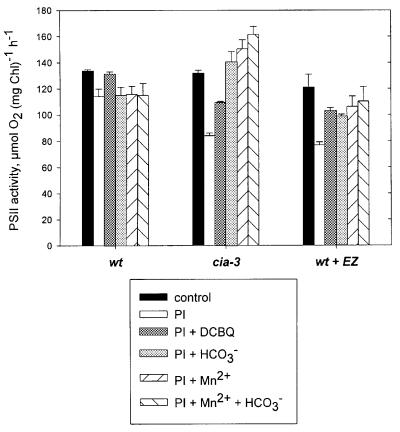
As was shown above, Cah3 is required for stabilizing PSII during overexcitation. Photoinduced damage of PSII occurs mainly through two mechanisms, one acting at the electron acceptor side, and the other at the electron donor side. Acceptor-side photoinhibition takes place at the level of the primary quinone electron acceptor (QA) when it becomes over-reduced, leading to degradation of the D1 polypeptide (Aro et al., 1993). The donor-side mechanism occurs under conditions causing the electron flow from water to be lower than the rate of electron withdrawal on the acceptor side. This also leads to degradation of the D1 polypeptide (Telfer and Barber, 1994). In order to understand the role of CA in these processes, it was of crucial importance to find out whether the photoinduced damage in the mutant cia3 occurs on the donor or acceptor side of PSII. Therefore, wild-type and mutant thylakoids were subjected to damaging light intensities, in both the presence and absence of DCBQ, a compound that accepts electrons from the reduced QA and therefore decreases its photoreduction. The presence of the artificial electron acceptor during the high light treatment completely abolished the photoinduced damage to PSII in wild-type thylakoids, indicating that the damage occurred predominantly at the acceptor side of PSII (Figure 4). On the other hand, the artificial electron acceptor was unable fully to prevent photoinhibition in mutant thylakoids: addition of DCBQ during the high light treatment reduced damage to PSII in the mutant, but only by 20% (Figure 4).
Fig. 4. Effect of the artificial electron acceptor DCBQ, bicarbonate and manganese on the rate of photoinhibition (PI) of light-saturated PSII electron transport rates in thylakoid membranes from wild-type (in the absence or presence of 0.5 mM EZ) and mutant cells. Thylakoid membranes were photoinhibited in the absence (PI) or presence of 1 mM DCBQ (PI + DCBQ), 1 mM HCO3– (PI + HCO3–), 50 µM MnCl2 (PI + Mn2+) or both NaHCO3 and MnCl2 (PI + Mn2+ + HCO3–). Photoinhibition was induced as described in the legend to Figure 1B. Values are means ± SE (n = 4).
These results indicate that the damage caused to PSII by excess light was mediated by two different mechanisms in the wild-type and mutant thylakoids. It is clear that mutant thylakoids, in addition to the acceptor-side effect, also suffered from another type of light-induced damage that was superimposed on the former effect, probably donor-side photoinhibition. It was shown earlier that bicarbonate, in addition to having a binding site at the non-heme iron on the acceptor side of PSII, also plays a role on the donor side (Allakhverdiev et al., 1997; Klimov et al., 1997). These authors postulated that bicarbonate is an essential component of the WOC and is required for both the function and stability of PSII. We therefore subjected thylakoid membranes to a high light treatment in the presence or absence of bicarbonate and/or Mn2+ (an efficient donor to PSII), reasoning that this would allow us to discriminate between the donor and acceptor side effects. Indeed, the addition of 1 mM bicarbonate to mutant thylakoids during the high light treatment protected its oxygen-evolving capacity from damage. Not only were the mutant thylakoids fully protected from photoinhibition, but their oxygen evolution was also stimulated to levels exceeding those of the control samples (Figure 4). When a mixture of 1 mM bicarbonate and 50 µM MnCl2 was added during the photoinhibitory treatment, an even stronger additive stimulation was discovered. In contrast, addition of bicarbonate and/or Mn2+ had no effect whatsoever on wild-type thylakoids. The simple fact that Mn2+ stimulated electron transport in a similar way to bicarbonate is taken as evidence that the main damage in the mutant occurred at the donor side, most probably within the manganese-containing WOC. To the best of our knowledge, the only difference between the two algal strains is that the mutant cia3 lacks a PSII-associated CA activity.
Importantly, similar results to those observed in the mutant were obtained when thylakoids from the wild type were exposed to high light in the presence of 0.5 mM EZ (Figure 4). The high light treatment in the presence of EZ induced a reduction in light-saturated PSII electron transport of 47%. However, the addition of 1 mM bicarbonate during the high light incubation of these samples restored nearly half of the inhibited O2 evolution, although it never exceeded the control values as it did in the mutant thylakoids. A similar restoration was observed when either 50 µM MnCl2 or a combination of bicarbonate and MnCl2 was added.
In the absence of an active CA (as in either the mutant or wild-type thylakoids plus EZ), it is clear that the donor side of PSII was more sensitive to photoinduced damage caused by excess light. A lack of CA activity can be, partly or fully, compensated by the addition of bicarbonate and/or manganese.
CA is required for the optimal function of PSII
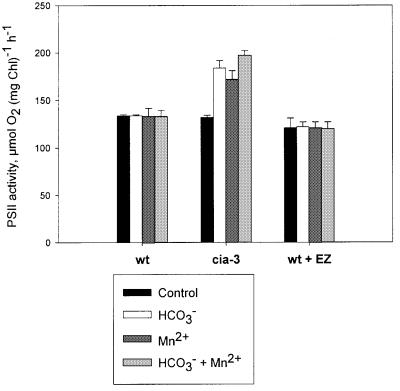
Surprisingly, the addition of bicarbonate to non-photoinhibited cia3 thylakoid membranes also stimulated light-saturated O2 evolution, to rates ∼40% higher than in controls (Figures 4 and 5). When 50 µM MnCl2 or a mixture of bicarbonate and MnCl2 was added, the PSII electron transport rates were also higher than in the control samples (Figure 5). The bicarbonate dependence was increased upon lowering the pH values from 7.6 to 6.0 (data not shown), thus indicating that bicarbonate rather than CO2 was responsible for the stimulation of PSII activity. In addition, it is important to note that we observed a similar stimulation by bicarbonate when BBY preparations were used (Table I). Adding bicarbonate to mutant BBY preparations led to an increase in O2 evolution up to 30% at pH 6.5, and even higher increases (up to 50%) were obtained at pH 5.5. In BBY preparations from the wild type, no stimulation of O2 evolution was found at any pH tested (Table I). We can therefore argue that the dependence on bicarbonate that we obtained in isolated thylakoid membranes was due to a direct effect on PSII.
Fig. 5. Effect of bicarbonate (1 mM) and MnCl2 (50 µM) on light- saturated PSII activity in thylakoid membranes from wild-type cells in the absence (WT) or presence of EZ (WT + EZ), and cia3 mutant cells (cia-3) before photoinhibition. Values are means ± SE (n = 3).
Table I. Light-saturated PSII electron transport rates (µmol O2/ mg Chl/h) in BBY preparations isolated from wild-type and cia3 mutant C.reinhardtii cells.
| Treatment | Wild type | cia3 |
|---|---|---|
| pH 6.5, control | 240 ± 20 | 250 ± 15 |
| pH 6.5 + 1 mM HCO3– | 245 ± 7 | 321 ± 25 |
| pH 5.5, control | 220 ± 6 | 230 ± 10 |
| pH 5.5 + 1 mM HCO3– | 215 ± 14 | 342 ± 8 |
The light-saturated electron flow through PSII in isolated BBY preparations from wild-type and mutant C.reinhardtii cells was measured as O2 evolution in a buffer containing 20 mM MES-KOH, 400 mM sucrose and 35 mM NaCl, at two different pH values, 6.5 and 5.5, in the presence of 1 mM DCBQ and 1 mM K3Fe(CN)6.
Measurements of light-induced electron transfer to the electron acceptor 2,6-dichlorophenolindophenol (DCPIP) in isolated thylakoid membranes confirmed the conclusions drawn from the O2 evolution data. Our results show that the DCPIP reduction rates in the mutant, using water as the electron donor, were similar to those in the wild type (Table II). The addition of bicarbonate and/or manganese to mutant thylakoids also stimulated the rate of DCPIP photoreduction, to levels higher than those observed in the wild type. Notably, in the presence of the exogenous electron donor 2,2′-diphenylcarbonic dihydrazide (DPC), which feeds electrons to PSII at Tyrz, the electron transfer rates that are not dependent on a functional manganese cluster were 90% higher in the mutant than in the wild type (Table II). In the presence of DPC, the addition of bicarbonate and/or MnCl2 did not cause further stimulation (data not shown). These findings allow us to prove a stimulatory effect of bicarbonate on the donor side of PSII before Tyrz. From these results, we can conclude further that PSII in the mutant was functional in all respects except for the water-oxidizing reaction. The addition of bicarbonate and/or manganese to these samples partially overcame their diminished ability to split water.
Table II. PSII electron transfer ratesa (µmol DCPIP reduced/mg Chl/h) in thylakoid membranes isolated from wild-type and cia3 mutant C.reinhardtii cells.
| Treatment | Wild type | cia3 |
|---|---|---|
| Control | 100 ± 10 | 100 ± 10 |
| + 1 mM HCO3– | 102 ± 7 | 144 ± 20 |
| + 50 µM MnCl2 | 98 ± 6 | 121 ± 10 |
| + 1 mM HCO3– + 50 µM MnCl2 | 102 ± 4 | 160 ± 8 |
| + 1 mM DPC | 113 ± 9 | 190 ± 3 |
Electron transfer from water or DPC to DCPIP in isolated thylakoid membranes was measured spectroscopically. Thylakoid membranes were assayed in the absence (control) or presence of 1 mM HCO3–, 50 µM MnCl2, or both HCO3– and MnCl2. Results are the means ± SE of three independent experiments.
aPercentage ± SE of the absolute values obtained from wild-type control thylakoid membranes.
We therefore postulate that CA in vivo is required for the WOC to function optimally. We suggest that one possible role of this CA is to provide the WOC with bicarbonate, which in turn stabilizes the manganese cluster. Atomic absorption spectroscopy measurements of the manganese content in BBY preparations support this contention, since wild-type preparations had 4.5 ± 0.2 atoms of manganese per PSII reaction centre, compared with 1.9 ± 0.1 in preparations from the mutant (Table III). Our results indicate that the manganese cluster in the mutant PSII is less stable than in the wild type. Stabilization of the WOC by bicarbonate has been shown previously during thermo- and photoinactivation of PSII preparations from pea (Klimov et al., 1997). Here we show that the bicarbonate stabilization is required even in undamaged thylakoids and PSII preparations, and is maintained through the activity of a PSII-associated CA.
Table III. PSII and PSI characteristics of wild-type and cia3 mutant C.reinhardtii cells.
| Parameter | Wild type | cia3 |
|---|---|---|
| PSII/PSI | 1.58 ± 0.2 | 2.4 ± 0.1 |
| Chl a/b | 2.4 ± 0.05 | 2.9 ± 0.08 |
| Chl/PSII | 272 ± 13.6 | 165 ± 6.6 |
| Manganese content (Mn/PSII)a | 4.5 ± 0.2 | 1.9 ± 0.1 |
Concentrations of PSII and PSI reaction centres in thylakoids were determined from measurements of photoinduced absorbance changes related to the photoreduction of pheophytin and photo-oxidation of P700, respectively. The pigment composition of wild type and mutant was determined spectroscopically. Results are the means ± SE of three independent experiments.
aManganese content was measured on the basis of chlorophyll molecules per PSII reaction centre (4.5 Mn atoms per 272 Chl molecules in wild type, 1.9 atoms per 165 Chl molecules in mutant).
The cia3 mutant overproduces PSII
The higher PSII electron transport rates measured in mutant thylakoids in the presence of bicarbonate and/or MnCl2 can be explained if the mutant contains a higher number of PSII reaction centres compared with wild type. In order to investigate the photosynthetic apparatus organization of the mutant, we estimated the concentrations of PSII and PSI reaction centres by measuring the photoinduced absorbance changes related to the photoreduction of pheophytin and photo-oxidation of P700, respectively (Table III). Our data indicate that there was a shift in the ratio of PSII/PSI from 1.58 ± 0.2 in wild-type cells to 2.44 ± 0.1 in the mutant cells (Table III). In addition, when wild-type and mutant cells were grown under the same light intensity, the Chl a/b ratio in the mutant was higher than in the wild type (Table III). This is consistent with the increased PSII/PSI ratio in the mutant cells. All these data indicated that the mutant thylakoids have a higher PSII content than those from wild-type cells. To verify this hypothesis, thylakoid membranes of the mutant were analysed by immunoblotting (Figure 6) using a collection of antisera raised against individual PSII polypeptides and representative polypeptides of PSI. The level of both plastid- (D1 and CP43 polypeptides) and nuclear-encoded (PsbO and PsbP) subunits of PSII was higher in mutant thylakoids than in wild type (Figure 6). In contrast, the amount of the PsaA and PsaD polypeptides of PSI was similar to that of the wild type. The immunoblot analyses further confirm the results drawn from the spectroscopic measurements. In addition, some of the PSII reaction centres of the mutant are impaired in their ability to split water and require bicarbonate to be fully active (Tables I and II). We propose that the mutant compensates for its less efficient PSII by overproducing PSII reaction centres in order to maintain an optimal linear electron transport rate. The lower PSII electron flow, calculated per reaction centre, in the mutant suggests that Cah3 activity is required for optimal function of the WOC.
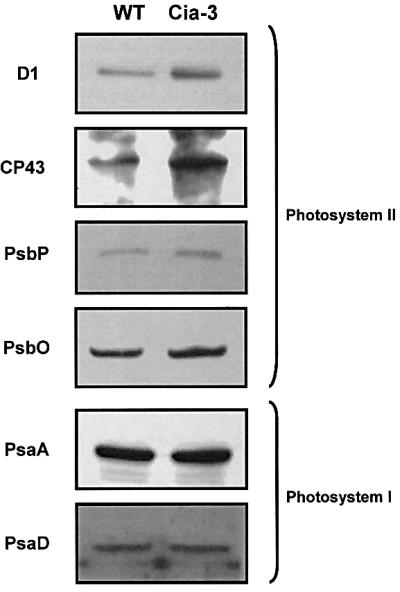
Fig. 6. Immunoblot analysis of thylakoid membrane proteins from mutant and wild type. Size-fractionated membrane proteins were transferred to nitrocellulose, and specific polypeptides were immunodetected with the indicated antisera. The lanes were loaded with 10 µg of protein.
To understand why PSII polypeptides overaccumulate in cia3 mutant cells, the levels of the PSII transcripts were investigated by semiquantitative RT–PCR analysis (Figure 7). The experiments revealed that the amount of all analysed transcripts was not altered in the mutant. The transcript of the PsbP protein was the only one that was higher in the mutant compared with the wild type (Figure 7). In the same set of experiments, no changes in the levels of the PSI transcripts were observed in mutant cells compared with wild type (data not shown).
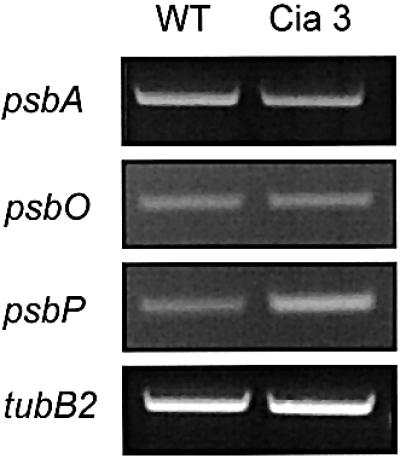
Fig. 7. Expression analysis of PSII genes in wild-type and cia3 mutant C.reinhardtii cells. Semiquantitative RT–PCR analysis of PSII gene expression. Total RNA to be used for reverse transcription was isolated by using Trizol™ reagent according to the manufacturer’s protocol (Life Technologies, USA). Aliquots of the reaction mix were loaded and ethidium bromide stained in 1% agarose gels.
Discussion
In this study, we provide conclusive evidence that a novel CA, Cah3, is associated with PSII in C.reinhardtii and is an essential component of the multiprotein complex that is responsible for the water oxidation reaction. In the absence of CA activity, the manganese cluster becomes unstable and a limitation of linear electron transport occurs that is caused by a lower efficiency of electron donation. We further demonstrate that this restraint in the photosynthetic electron donation can be overcome if exogenous bicarbonate and/or Mn2+ are added to the thylakoids and BBY preparations.
Under normal growth conditions, the light response curves of photosynthetic O2 evolution, calculated on a chlorophyll basis, as well as linear- and PSII-specific electron transport, were identical between wild-type and mutant cells, thylakoids and BBY preparations (Figure 1A and B, and Table I; see also Park et al., 1999). However, the mutant cells showed 1.6 times more PSII reaction centres and an overaccumulation of PSII proteins (Figure 6) compared with the wild type. Taking into account these results, the maximal PSII electron transport rate in the mutant, calculated per reaction centre, was only 60% of that of the wild type unless bicarbonate and/or manganese were added (Figure 4). We therefore draw the conclusion that there is a loss of functional WOC in mutant cells. The direct measurements of the rate of DCPIP photoreduction, in the presence and absence of DPC, clearly indicated that up to half of the WOCs in the mutant were non-functional (Table II). Addition of bicarbonate and/or manganese restored the activity of these complexes.
We propose that the mutant compensates for its less efficient PSII by overproducing PSII reaction centres in order to maintain an optimal linear electron transport rate. The ability of mutant cells to overaccumulate PSII subunits is not caused by a general increase in the expression of the genes encoding the PSII subunits, but must be due to post-transcriptional or post-translational alterations or, alternatively, to the higher stability of the PSII subunits once they have been synthesized. It has been shown (Ettinger and Theg, 1991) that an excess of the extrinsic proteins of the PSII exists in the form of a free pool in the thylakoid lumen. Eisenberg-Domovich et al. (1995) showed that the PsbO protein, at least, reassociates with the lumenal side of the thylakoid membranes proportional to the amount of the new D1 protein synthesized during the recovery process after photoinactiva tion of C.reinhardtii cells, without further alterations in the level of expression of that gene. The role of the up-regulation of the expression of the psbP gene remains to be elucidated.
Our results indicate that the CA activity is also crucial under circumstances where the WOC may be damaged, such as during photoinhibition (Figure 4). Stabilization of the WOC by bicarbonate under thermo- and photoinhibitory conditions has been demonstrated with bicarbonate- and Mn-depleted PSII preparations from pea (Klimov et al., 1997; Klimov and Baranov, 2001). Here we show that the stabilization of the WOC under high light treatment is maintained through the activity of the PSII-associated CA. In the absence of CA activity (in either mutant or EZ-inhibited wild-type samples), bicarbonate is not supplied, making the WOC vulnerable to donor-side damage (Figure 4).
The addition of bicarbonate alone was enough to promote stimulation of water splitting in mutant thylakoids, containing an average of two manganese atoms per reaction centre (Figure 5 and Table II). These observations indicate that the manganese cluster is assembled more efficiently and is more active functionally in the presence of bicarbonate. With an average of two atoms of manganese per reaction centre, PSII is more susceptible to photoinduced damage (Figure 4). The mutant cells are not only more sensitive to high light treatment, but also cannot grow under low CO2 concentrations in the medium (Karlsson et al., 1998) or even under low nitrogen (data not shown). We therefore suggest that four manganese atoms per reaction centre are required for the WOC to function optimally under variable environmental conditions.
Our results can, in principle, be interpreted in at least two different ways. The first explanation is that there are two types of PSII reaction centres in the mutant: one is lacking the manganese cluster completely and is not functional in water oxidation and the other has four atoms of manganese and is fully active. It then remains to be explained how bicarbonate, even without manganese, can stimulate O2 evolution in non-functional centres. One possibility could be that the two types of PSII reaction centres in the mutant are sharing one WOC. The other obvious explanation for our results is that all PSII reaction centres in the mutant only have two manganese atoms, which without enough bicarbonate available can only work with 50% efficiency. The role of bicarbonate in this model would then be to stabilize the manganese cluster. From our results, we cannot discriminate between these possibilities.
Our main hypothesis is that the PSII-associated CA is involved in providing the reaction centres with bicarbonate, which in turn stabilizes the manganese cluster at the donor side of the PSII. The low manganese content (an average of two atoms per reaction centre) and the impaired electron donation from water (Table II) in the mutant support this contention. Dimeric Mn–bicarbonate complexes evidently have been important for the evolutionary origin of the WOC (Dismukes, 2001; Dismukes et al., 2001). The donor-side bicarbonate effect is especially pronounced in reconstitution experiments with Mn-depleted PSII preparations from pea (Allakhverdiev et al., 1997; Klimov and Baranov, 2001), and it was postulated that bicarbonate can be involved in direct ligation of the manganese atoms. Together with these observations, our results suggest a model in which the PSII-associated CA is the agent responsible for supplying bicarbonate to the WOC, a step that, in the absence of the enzyme, would be rate limiting especially at the acid lumenal pH during illumination. The proposed role of bicarbonate as an important cofactor in the manganese cluster is supported by our results showing its stimulatory effect on PSII electron transport to be even higher when it is added together with manganese. Allakhverdiev et al. (1997) showed that, in the presence of bicarbonate, only two manganese atoms per reaction centre are enough to restore O2 evolution in Mn-depleted PSII preparations. Taken together, our results and those of Allakhverdiev et al. (1997) imply that, under some conditions, two atoms of manganese may be enough to support PSII-driven electron transport as long as bicarbonate is available.
A complementary hypothesis is that bicarbonate ions might be used to accept protons released as a result of water oxidation. Under illumination, protons are constantly being released in the thylakoid lumen. When the light intensity increases, the rate of proton release will be even higher, resulting in a substantial local acidification (Kramer et al., 1999) that could lead to damage of the WOC and subsequent release of manganese (Virgin et al., 1988). According to this hypothesis, CA would be required to catalyse bicarbonate dehydration at the donor side of the PSII, allowing an immediate removal of protons from the WOC, preventing its damage. CO2, generated as a by-product in this reaction, may also be utilized by the Calvin cycle. This would agree with a recent model postulating that the thylakoid CA is involved in CO2 supply to Rubisco (Raven, 1997). We have to consider that all the experiments presented in this study were performed using cells growing under high CO2 conditions, where there is no inorganic carbon limitation for Rubisco. Our data provide, for the first time, clear evidence for a CA-dependent efficiency of the photosynthetic water oxidation reaction in intact cells, thylakoid membranes and BBY preparations.
The similarity between the algal model system C.reinhardtii and higher plants with respect to both PSII organization and bicarbonate stimulation may be a reason to predict that higher plant thylakoids are also relying on a CA for optimal water oxidation. Until now, no CA has been isolated from thylakoid membranes of higher plants, although they have been shown to possess CA activity (Moubarak-Milad and Stemler, 1994).
Materials and methods
Strains and growth conditions
Chlamydomonas reinhardtii cell wall-deficient mutant 92 (cw92), which is regarded as the standard wild type in photosynthesis studies, was obtained from the Chlamydomonas Culture Collection at Duke University, Durham, NC. The cell wall-deficient, high CO2-requiring cia3 double mutant was kindly provided by J.V.Moroney (Louisiana State University, Baton Rouge, LA) (Moroney et al., 1986). All strains were grown in batch cultures at 25°C under a continuous irradiance of 150 µmol/m2/s supplied from cool, white fluorescent lamps. Cells were cultured in minimal medium (Sueoka, 1960) under aeration with air enriched with 5% CO2. Subsamples from the cell cultures (10 µg Chl/ml) were subjected to a high light treatment in test tubes of 1 cm diameter by exposing them to a light intensity of 2200 µmol/m2/s for 1 h at 26°C (Falk and Samuelsson, 1992). During the treatment, the cultures were bubbled with air enriched with 5% CO2.
Isolation of PSII membrane fragments and PSII core complexes, and spectroscopy
Thylakoid membranes were isolated according to Allen and Staehelin (1994). The PSII membrane fragments (BBY preparations) from both wild-type and mutant cia3 were isolated as previously described for higher plants (Berthold et al., 1981). PSII core complexes were isolated from BBY preparations according to Hankamer et al. (1997). Concentrations of PSII and PSI reaction centres in thylakoids were determined from measurements of photoinduced absorbance changes related to the photoreduction of pheophytin and photo-oxidation of P700, respectively, as described (Klimov et al., 1980). Chlorophyll concentrations in algal subcellular fractions were determined spectroscopically after extraction in absolute methanol (Porra et al., 1989).
Carbonic anhydrase activity measurements
Carbonic anhydrase activity was measured electrometrically by monitoring the rate of pH change immediately following an addition of bicarbonate solution (23 mM, final concentration) at 20°C. The accuracy and rapid response required for this analysis were assured by using a fast-response microelectrode (MI-710 ‘blue glass’; Microelectrodes Inc.) and an 18-bit A/D converter (IOtech Inc.). The reaction kinetics after the addition of bicarbonate solution were recorded for 20 s. Differences in the rate of bicarbonate dehydration after the bicarbonate addition to sample and control (buffer) solutions were used to calculate CA activity, which was expressed as µmol protons consumed/mg Chl/h.
Photosynthetic response curves
Photosynthetic O2 evolution was measured at different incident photon flux densities in a Clark-type oxygen electrode (CB1D, Hansatech, Norfolk, UK). Measurements of whole-cell O2 evolution were conducted at 26°C on vigorously stirred samples containing 10 µg Chl/ml as previously described (Falk and Samuelsson, 1992).
In vitro measurements of electron flow
The linear electron transport rates in thylakoid membranes were measured at different incident photon fluxes by determining O2 uptake in the presence of 1 mM methyl viologen. The assay was performed at a concentration of 25 µg Chl/ml in a buffer containing 50 mM HEPES-KOH pH 7.5, 100 mM sucrose, 20 mM KCl and 5 mM MgCl2. The light-saturated electron flow through PSII in isolated thylakoids was measured as O2 evolution in the same buffer and in the presence of 1 mM K3Fe(CN)6, 1 mM DCBQ and 10 µM gramicidin D. The light-saturated electron flow through PSII in BBY preparations was measured in a buffer containing 20 mM MES-KOH, 400 mM sucrose and 35 mM NaCl, at two different pH values, pH 6.5 and pH 5.5, in the presence of 1 mM K3Fe(CN)6 and 1 mM DCBQ. Electron transfer from water or DPC to DCPIP in isolated thylakoid membranes was measured spectroscopically as previously described (Rova et al., 1994). The light-saturated rates of oxygen evolution in PSII core complexes were measured in a buffer containing 25 mM MES-KOH pH 6.5, 0.3 M sucrose, 10 mM NaCl and 50 mM CaCl2, in the presence of 1 mM DCBQ and 1 mMK3Fe(CN)6 as described by Bumann and Oesterhelt (1994).
Mn and Zn content in PSII membrane fragments
The amount of manganese and zinc bound to PSII membrane fragments was determined by reference to internal standards with an atomic absorption spectrophotometer.
Western blot analysis
Isolated thylakoid membranes, PSII membrane fragments, total cell proteins and PSII core complexes were separated on 12% SDS–polyacrylamide gels (Laemmli, 1970). After electrophoresis, proteins were blotted onto a nitrocellulose membrane. Immunoblotting was performed as described in the protocol supplied by Bio-Rad Laboratories. Horseradish peroxidase-labelled secondary antibodies and enhanced chemiluminescence (ECL, Amersham International) were used to detect the antibody–antigen conjugate.
Estimation of relative transcript levels with RT–PCR
To determine specifically the relative transcript levels of genes encoding proteins of PSII and PSI, RT–PCR assays were performed. Total RNA was isolated from 2 ml of culture by using the Trizol™ reagent according to the manufacturer’s protocol (Life Technologies, USA). A 2.5 ng aliquot of total RNA from wild-type and mutant cia3 was pre-heated to 70°C for 20 min prior to the RT–PCRs. An Advantage One-step RT–PCR Kit (Clontech Laboratories, Inc., CA) was used and ∼10 µl of each reaction mix were run on a 1% agarose gel. The following primers were used for the different transcripts: tubB2, 5′-CTTGTTCTGCACGTTCAGC-3′ and 5′-AAGCAGATGTCGTACAGG-3′; psbA, 5′-ATACAACGGTGGTCCTTACC-3′ and 5′-TCTTGTCCGAAACGGTAACC-3′; psbO, 5′-TCTTCACGTTCTCCTTCAGC-3′ and 5′-ACAAGCTGGAGAACTTCTGC-3′; psbP, 5′-CGCCAATCTGGATCTTGATG-3′ and 5′-GAACAACCTGGTCGTCATTG-3′.
Acknowledgments
Acknowledgements
We thank Professor Sane and Dr A.Ivanov for critically reading the manuscript, and A.Nordin for atomic absorption spectroscopy measurements. J.-D.Rochaix and S.Jansson kindly provided antibodies against PsaA and PsaD protein, respectively. T.Hundal and B.Andersson provided antibodies against D1, CP43, PsbO and PsbP proteins. This work was supported by research grants from the Swedish National Research Council, the Kempe Foundation and the Swedish Academy of Sciences to G.S.
References
- Allakhverdiev S.I., Yruela,I., Picorel,R. and Klimov,V.V. (1997) Bicarbonate is an essential constituent of the water-oxidizing complex of photosystem II. Proc. Natl Acad. Sci. USA, 94, 5050–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K.D. and Staehelin,L.A. (1994) Polypeptide composition, assembly and phosphorylation patterns of the photosystem II antenna system of Chlamydomonas reinhardtii. Planta, 194, 42–54. [Google Scholar]
- Andersson B. (1986) Characterization of the thylakoid membrane by subfractionation analyses. Methods Enzymol., 118, 325–338. [Google Scholar]
- Aro E.-M., Virgin,I. and Andersson,B. (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta, 1143, 113–134. [DOI] [PubMed] [Google Scholar]
- Berthold D.A., Babcock,G.T. and Yocum,C.F. (1981) A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett., 134, 231–234. [Google Scholar]
- Bumann D. and Oesterhelt,D. (1994) Purification and characterization of oxygen-evolving photosystem II core complexes from the green alga Chlamydomonas reinhardtii. Biochemistry, 33, 10906–10910. [DOI] [PubMed] [Google Scholar]
- Dismukes G.C. (2001) Splitting water. Science, 292, 447–448. [DOI] [PubMed] [Google Scholar]
- Dismukes G.C., Klimov,V.V., Baranov,S.V., Kozlov,Y.N., Dasgupta,J. and Tyrishkin,A. (2001) The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis. Proc. Natl Acad. Sci. USA, 98, 2170–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg-Domovich Y., Oelmüller,R., Hermann,G. and Ohad,I. (1995) Role of the RCII-D1 protein in the reversible association of the oxygen-evolving complex proteins with the lumenal side of photosystem II. J. Biol. Chem., 270, 30181–30186. [DOI] [PubMed] [Google Scholar]
- Ettinger W.F. and Theg,S.M. (1991) Physiologically active chloroplasts contain pools of unassembled extrinsic proteins of the photosynthetic oxygen-evolving enzyme complex in the thylakoid lumen. J. Cell Biol., 115, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S. and Samuelsson,G. (1992) Recovery of photosynthesis and photosystem II fluorescence in Chlamydomonas reinhardtii after exposure to three levels of high light. Physiol. Plant., 85, 61–68. [Google Scholar]
- Hankamer B., Nield,J., Zheleva,D., Boekema,E., Jansson,S. and Barber,J. (1997) Isolation and biochemical characterisation of monomeric and dimeric photosystem II complexes from spinach and their relevance to the organisation of photosystem II in vivo. Eur. J. Biochem., 243, 422–429. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Clarke,A.K., Chen,Z.-Y., Hugghins,S.Y., Park,Y.-L., Husic,H.D., Moroney,J.V. and Samuelsson,G. (1998) A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J., 17, 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimov V.V. and Baranov,S.V. (2001) Bicarbonate requirement for the water-oxidizing complex of photosystem II. Biochim. Biophys. Acta, 1503, 187–196. [DOI] [PubMed] [Google Scholar]
- Klimov V.V., Allakhverdiev,S.I., Shutilova,N.I. and Krasnovsky,A.A. (1980) Study of photoreduction of pheophytin and photooxidation of P680 in photosystem II preparations from chloroplasts of pea and Chlamydomonas reinhardtii. Plant Physiol. (Moscow), 27, 315–325. [Google Scholar]
- Klimov V.V., Baranov,S.V. and Allakhverdiev,S.I. (1997) Bicarbonate protects the donor side of photosystem II against photoinhibition and thermoinactivation. FEBS Lett., 418, 243–246. [DOI] [PubMed] [Google Scholar]
- Kramer D.M., Sacksteder,C.A. and Cruz,J,A, (1999) How acidic is the lumen? Photosynth. Res., 60, 151–163. [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Metzner H. (1978) Oxygen evolution as energetic problem. In Metzner,H. (ed.), Photosynthetic Oxygen Evolution. Academic Press, London, UK, pp. 59–76.
- Moroney J.V., Tolbert,N.E. and Sears,B.B. (1986) Complementation analysis of the inorganic carbon concentrating mechanism of Chlamydomonas reinhardtii. Mol. Gen. Genet., 204, 199–203. [Google Scholar]
- Moubarak-Milad A and Stemler,A. (1994) Oxidation-reduction potential dependence of photosystem II carbonic anhydrase in maize thylakoids. Biochemistry, 33, 4432–4438. [DOI] [PubMed] [Google Scholar]
- Nield J., Kruse,O., Ruprecht,J., Fonseca,P., Büchel,C. and Barber,J. (2000) 3D structure of Chlamydomonas reinhardtii and Synechococcus elongatus photosystem II complexes allows for comparison of their OEC organisation. J. Biol. Chem., 275, 27940–27946. [DOI] [PubMed] [Google Scholar]
- Park Y-L., Karlsson,J., Rojdestvenski,I., Pronina,N., Klimov,V.V., Öquist,G. and Samuelsson,G. (1999) Role of a novel photosystem II-associated carbonic anhydrase in photosynthetic carbon assimilation in Chlamydomonas reinhardtii. FEBS Lett., 444, 102–105. [DOI] [PubMed] [Google Scholar]
- Porra R.J., Thompson,W.A. and Kriedemann,P.E. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentrations of chlorophyll standards by absorption spectroscopy. Biochim. Biophys. Acta, 975, 384–394. [Google Scholar]
- Raven J.A. (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ., 20, 147–154. [Google Scholar]
- Renger G. (2001) Photosynthetic water oxidation to molecular oxygen: apparatus and mechanism. Biochim. Biophys. Acta, 1503, 210–228. [DOI] [PubMed] [Google Scholar]
- Rova M., Franzén,L.G., Fredriksson,P.-O. and Styring,S. (1994) Photosystem II in a mutant of Chlamydomonas reinhardtii lacking the 23 kDa psbP protein shows increased sensitivity to photoinhibition in the absence of chloride. Photosynth. Res., 39, 75–83. [DOI] [PubMed] [Google Scholar]
- Stemler A. (1980) Inhibition of photosystem II by formate: possible evidence for a direct role of bicarbonate in photosynthetic oxygen evolution. Biochim. Biophys. Acta, 593, 103–112. [DOI] [PubMed] [Google Scholar]
- Stemler A. (1997) The case for chloroplast thylakoid carbonic anhydrase. Physiol. Plant., 99, 348–353. [Google Scholar]
- Sueoka N. (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA, 46, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A. and Barber,J. (1994) Elucidating the molecular mechanisms of photoinhibition by studying isolated photosystem II reaction centres. In Baker,N.R and Bowler,J.P. (eds), Photoinhibition of Photosynthesis from Molecular Mechanism to the Field. BIOS, Oxford, UK, pp. 25–49.
- Van Rensen J.J.S., Xu,C. and Govindjee (1999) Role of bicarbonate in photosystem II, the water–plastoquinone oxido-reductase of plant photosynthesis. Physiol. Plant., 105, 585–592. [Google Scholar]
- Virgin I., Styring,S. and Andersson,B. (1988) Photosystem II disorganization and manganese release after photoinhibition of isolated spinach thylakoid membranes. FEBS Lett., 233, 408–412. [Google Scholar]
- Warburg O. and Krippahl,G. (1958) Hill-Reaktionen. Z. Naturforsch., 13b, 509–514. [PubMed] [Google Scholar]
- Zouni A., Witt,H.T., Kern,J., Fromme,P., Krauβ,N., Saenger,W. and Orth,P. (2001) Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature, 409, 739–743. [DOI] [PubMed] [Google Scholar]