Abstract
To test for a role for the cellular prion protein (PrPc) in cell death, we used a PrPc-binding peptide. Retinal explants from neonatal rats or mice were kept in vitro for 24 h, and anisomycin (ANI) was used to induce apoptosis. The peptide activated both cAMP/protein kinase A (PKA) and Erk pathways, and partially prevented cell death induced by ANI in explants from wild-type rodents, but not from PrPc-null mice. Neuroprotection was abolished by treatment with phosphatidylinositol-specific phospholipase C, with human peptide 106–126, with certain antibodies to PrPc or with a PKA inhibitor, but not with a MEK/Erk inhibitor. In contrast, antibodies to PrPc that increased cAMP also induced neuroprotection. Thus, engagement of PrPc transduces neuroprotective signals through a cAMP/PKA-dependent pathway. PrPc may function as a trophic receptor, the activation of which leads to a neuroprotective state.
Keywords: apoptosis/cell death/neuroprotection/prion/signal transduction
Introduction
Prion diseases are spongiform encephalopathies related to the cellular prion protein (PrPc) abundantly expressed in the central nervous system (CNS) (Prusiner, 1998). Infectious forms are attributed to entrance into normal brain of a proteinaceous particle that leads to accumulation of an abnormally folded form of the protein (scrapie form, or PrPsc) as a consequence of conformational conversion of the endogenous PrPc (for reviews, see Prusiner, 1998; Will et al., 1999). Genetic forms were traced to several mutations in PrPc (for reviews, see Gambetti et al., 1999; Weissmann et al., 1999). In contrast, most cases of prion disease, known as sporadic forms, can neither be attributed to a previous infection nor present identified germline mutations (for reviews, see Prusiner, 1998; Will et al., 1999), and are supposed to occur by stochastic conversion of PrPc into the abnormal form.
These transmissible encephalopathies are viewed as gain-of-function diseases due to accumulation of PrPsc because little evidence is available that the loss of PrPc following conformational conversion has a role in pathogenesis. Notwithstanding this, the possibility that prion diseases have loss-of-function components remains open (Aguzzi and Weissmann, 1997; Samaia and Brentani, 1998), and a critical examination of this hypothesis depends on determining the elusive functions of PrPc in the CNS.
Attempts to unravel these functions by gene knockouts produced controversial results. An early study reported no developmental nor behavioral abnormalities in mice with deletion of the Prnp gene open reading frame (ORF) (Bueler et al., 1992). However, aberrant sleep patterns were found in Prnp knockout mice (Tobler et al., 1996). In addition, while inhibitory avoidance learning and anxiety were similar in wild-type and PrP0/0 mice, the latter showed increased locomotor activity (Roesler et al., 1999). Electrophysiological abnormalities were also described in the brains of PrP0/0 mice (Collinge et al., 1994; Colling et al., 1996), but were not confirmed in other laboratories (Herms et al., 1995; Lledo et al., 1996). Nonetheless, mice lacking PrPc were more sensitive than wild-type animals to seizures induced by various convulsant agents (Walz et al., 1999).
In contrast to the early Prnp knockouts (Bueler et al., 1992), aging mice in which the ORF was deleted together with large portions of both a 5′ intron and the 3′-untranslated region suffered extensive cerebellar degeneration with progressive ataxia (Sakaguchi et al., 1996). The pattern of expression of the PrPc gene homolog Doppel may explain the controversial results of various Prnp knockouts. The explanation is based on possible toxicity of the Doppel protein, which is overexpressed selectively through abnormal splicing following intronic deletions, but not following ORF deletions of the Prnp gene, while toxicity is abolished by the presence of a single wild-type Prnp allele (Moore et al., 1999; Wong et al., 2001).
In addition, copper binding (Brown et al., 1997a) endows PrPc with antioxidant activity, and it has been proposed that loss of PrPc may cause neurodegeneration through increased oxidative stress (for a review, see Wong et al., 2000). Antioxidant properties of PrPc may also be linked to abnormal synaptic activity (for reviews, see Wong et al., 2000; Brown, 2001), which may in turn explain electrophysiological abnormalities detected in some studies of Prnp knockouts.
On the other hand, the introduction into PrP0/0 of N-terminally truncated Prnp alleles lacking either residues 32–121 or 32–134 led to extensive cerebellar degeneration and astrocytosis accompanied by marked ataxia, which were absent with shorter truncations up to residue 106, and were abrogated by the presence of a wild-type Prnp allele (Shmerling et al., 1998). The authors proposed that truncated PrPc molecules compete with other PrP-like molecules for a common ligand. Their results, however, also suggest that deleted domains of PrPc that lead to neurological defects may be essential for the normal functions of PrPc. All deletions examined by Shmerling et al. (1998), and not only those that result in neurodegeneration, removed the N-terminal copper-binding octarepeats (Brown et al., 1997a; Aronoff-Spencer et al., 2000; Whittal et al., 2000), suggesting that other factors besides copper, and related to residues 106–134 of the cellular prion protein, are associated with resistance to neurodegeneration.
Several molecules are capable of interacting with PrPc (Kurschner and Morgan, 1995; Yehiely et al., 1997; Rieger et al., 1997; Graner et al., 2000). In a previous study, a predicted PrPc-binding peptide was designed on the basis of the complementary hydropathy theory (see, for example, Bost et al., 1985; Brentani, 1988; Boquet et al, 1995), and an antibody raised against this peptide recognized a 66 kDa cell surface molecule that binds PrPc (Martins et al., 1997). The immunogenic peptide used in the previous study corresponds to the DNA strand complementary to that of the human Prnp gene that codes for amino acids 114–129 (Martins et al., 1997). The specified amino acid sequence is contained within the domain critical for degenerative effects of PrPc N-terminal deletions (Shmerling et al., 1998). We therefore reasoned that the immunogenic peptide might be useful as a probe to test the hypothesis that PrPc is involved in mechanisms of cell death.
We examined whether the PrPc-binding peptide affects programmed cell death in an in vitro preparation of organotypical retinal explants. This preparation has been used as a model for studies of cell death in the CNS because it maintains the structure of the retinal tissue (for reviews, see Linden et al., 1999; Linden, 2000). The experiments show that, upon engagement with the PrPc-binding peptide as well as with certain antibodies, PrPc transduces neuroprotective signals that affect the sensitivity to induced cell death.
Results
Cellular prion protein is expressed abundantly in the developing retina
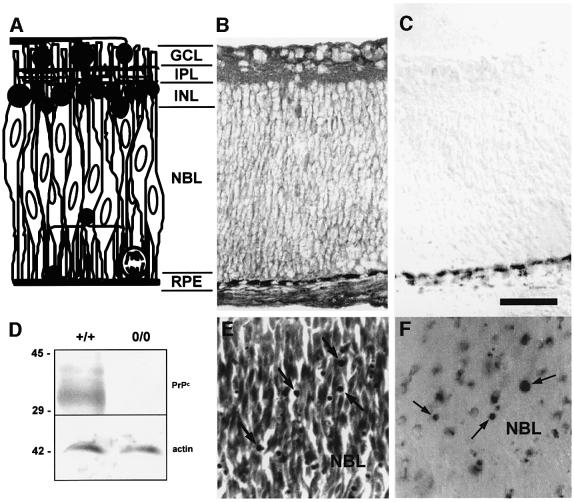
In newborn rats, the retina contains three strata (Figure 1A). The innermost ganglion cell layer (GCL) contains the ganglion cells that project to subcortical visual centers along the optic nerve. The outer cellular stratum is separated from the GCL by the inner plexiform layer (IPL), and contains a few rows of early developing amacrine cells (inner nuclear layer; INL), plus a proliferative zone called the neuroblastic layer (NBL). Besides proliferating cells, the NBL contains post-mitotic cells that recently have left the cell cycle and are migrating towards their definitive positions (for a review, see Linden et al., 1999).
Fig. 1. (A) Diagram of the retina of neonatal rodents. The cellular and plexiform strata are indicated, together with a rough schematic representation of the morphology of existing cell types at that stage of development. Dark profiles represent relatively differentiated cells. A mitotic figure is depicted at the lower right corner of the diagram. Elongated profiles with vertically oriented oval nuclei represent both proliferating and early post-mitotic cells, which comprise most of the population within the neuroblastic layer. (B and C) Immunohistochemistry of transverse sections of the retina in a newborn rat, stained either with (B) or without (C) a monoclonal antibody to PrPc. The beaded dark deposits that appear at the bottom of both figures correspond to the melanin-containing retinal pigment epithelium. (D) Example of a western blot of protein extracts from the retinas of wild-type or PrP0/0 mice, probed with an antiserum to PrPc raised in knockout mouse (upper panel), then stripped and re-probed with an antibody to actin as a loading control (lower panel). The monoclonal antibody used for immunohistochemistry in (B) and (C) produced a result similar to (D) in western blots of mouse retinal tissue (data not shown). (E and F) Photomicrographs from the neuroblastic layer of explants from the retina of neonatal rats, treated with anisomycin (1 µg/ml) for 24 h, stained with either neutral red (E) or with the TUNEL technique (F). The arrows indicate degenerating profiles. GCL = ganglion cell layer; IPL = inner plexiform layer; INL = inner nuclear layer; NBL = neuroblastic layer; RPE = retinal pigment epithelium. Bar = 50 µm.
A monoclonal antibody to PrPc produced immunolabeling across the retina (Figure 1B). Cell somas were stained in the NBL, in a pattern consistent with the membrane-associated location of the endogenous PrPc. A few labeled profiles close to the INL resembled migrating cells. Detergent permeabilization of the cryosections resulted in more diffuse labeling, consistent with either increased cytoplasmic labeling or partial release of the glycosylphosphatidylinositol (GPI)-anchored PrPc. Cell somas in both the INL and GCL were also labeled, slightly more intensely in peripheral than in central areas of the retina (data not shown). A similar labeling pattern was obtained with a non-commercial polyclonal antibody (N10, provided by Dr Stanley Prusiner, data not shown), while control sections processed without the primary antibody were unlabeled (Figure 1C). In addition, antibodies raised against the cellular prion protein specifically recognized PrPc in western blots of protein extracts from retinal tissue of wild-type, but not of PrP0/0 mice (Figure 1D). Thus, PrPc is expressed abundantly in the neonatal rodent retina.
A PrPc-binding peptide prevents apoptosis in retinal tissue
We have shown previously that death of ganglion cells, whose axons are damaged when retinal explants are prepared, is blocked by the inhibitor of protein synthesis anisomycin (ANI). However, ANI induces cell death among recent post-mitotic cells within the NBL (Rehen et al., 1996, 1999). To test for effects upon retinal cell death, we used a PrPc-binding peptide (Martins et al., 1997), herein called PrR, and a scrambled peptide, containing the same 16 amino acids as PrR in a distinct order, with a hydropathic profile that is non-complementary to PrPc. The properties of these peptides in a binding assay conformed to their theoretically predicted behavior, and deletion of the putative binding site in PrPc abrogated binding of PrR (Figure 4A and B)
Fig. 4. Peptide binding and further evidence that neuroprotection depends on PrPc. (A) PrPc–PrR binding curves. Biotinylated BSA–PrR or biotinylated BSA alone were incubated with either wild-type His6-moPrPc or His6-moPrPc Δ105–128. Non-specific binding to His6-moPrPc (open squares) was subtracted from total binding (diamonds) to yield PrPc-specific binding to PrR (circles, continuous line). Lack of specific binding to moPrPΔ105–128 is also shown (crosses). All error bars were <5% and were omitted for clarity. (B) Competition assay. Results were expressed as a percentage of the absorbance values corresponding to specific PrPc–PrR binding. Note that both cold PrR (filled bars) and neurotoxic (hatched bars) peptides, but not the scrambled peptide (open bars), displace PrPc–PrR binding. (C) Neurotoxic peptide has no effect in either the presence (filled triangles) or absence (open triangles) of anisomycin 1 µg/ml, while the PrR peptide, that has no effect by itself (open circle), had a neuroprotective effect in the presence of anisomycin (filled circle). Data are means ± SEM of triplicate explants in one representative experiment out of three with similar results made with retinal explants from neonatal rats. (D) Co-incubation with equimolar (80 µM) neurotoxic peptide blocks neuroprotection by the PrR peptide. Data are means ± SEM of triplicate experiments with retinal explants from neonatal rats. *P < 0.01 versus anisomycin alone.
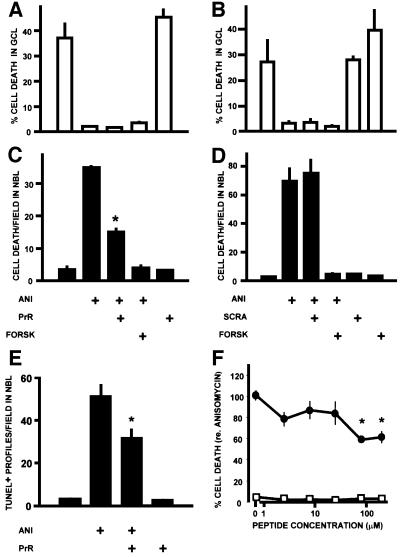
Cell death was quantitated in rat retinal explants, by counting the pyknotic, homogeneously stained profiles (Figure 1E) that correspond to dying cells (Rehen et al., 1996). The PrR peptide had no effect on ganglion cell death, which was completely blocked by ANI (Figure 2A). In contrast, PrR reduced ANI-induced cell death by ∼50% in the NBL (Figure 2C). The effect was less than with forskolin, which completely abolished ANI-induced cell death in the NBL (Figure 2C). The scrambled peptide had no effect (Figure 2B and D).
Fig. 2. Effect of the PrPc-binding peptide (PrR) upon retinal cell death. The data are shown as means ± SEM of either the percentage of degenerating cells in the single-cell thick ganglion cell layer (GCL), or the densitiy of degenerating profiles in the neuroblastic layer (NBL), in retinal explants from neonatal rats. Treatments in (A–E) are: ANI = anisomycin 1 µg/ml; PrR = PrR peptide 80 µM; SCRA = scrambled peptide 80 µM; FORSK = forskolin 10 µM. The rates of cell death evaluated by counts of pyknotic profiles in the GCL (A and B) and NBL (C and D) are shown for two representative experiments made in triplicate with either the PrR (A and C) or the scrambled control (B and D) peptides. *P < 0.01 versus anisomycin alone. In this and the following illustrations, statistical significance is indicated only for the most relevant among the multiple comparisons tabulated in the Duncan’s test. (E) Blockade of anisomycin-induced apoptosis in the NBL detected with the TUNEL technique (triplicate explants), following treatment with the PrR peptide. *P < 0.01 versus anisomycin alone. (F) Dose–response curve for the PrR peptide, either in the presence (filled circles) or in the absence of anisomycin 1 µg/ml. Each data point is the mean ± SEM of 8–16 explants from a total of five experiments, and normalized with respect to the rate of cell death (100%) induced by anisomycin. *P < 0.01 versus anisomycin alone.
Degenerating profiles in the NBL can be stained with both the TUNEL technique (Figure 1F) and with an antibody to the activated form of caspase-3 (Namura et al., 1998), indicating that cell death is at least in part due to apoptosis. The PrR peptide also significantly reduced the incidence of TUNEL-positive profiles in the NBL (Figure 2E).
Concentrations of the PrR peptide between 2.5 and 25 µM yielded little effect upon cell death in the NBL, whereas either 80 or 200 µM significantly reduced anisomycin-induced cell death to 58.7 ± 2.8 and 61.2 ± 5.8% of control values, respectively (Figure 2F; means ± SEM, n = 15 and 9, respectively).
Neuroprotection by the PrPc-binding peptide depends on PrPc
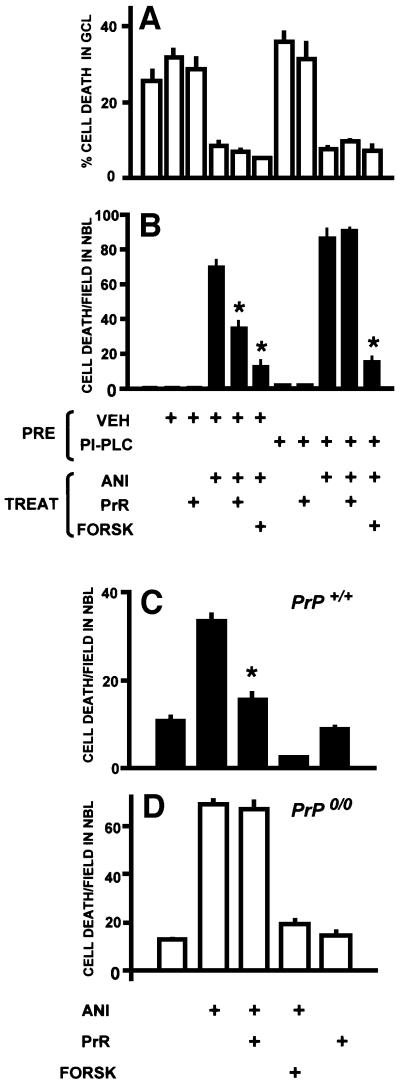
Hydrolysis of GPI anchors releases PrPc from the plasma membrane and is expected to block PrPc-mediated effects. Since the half-life of PrPc has been estimated at ∼6 h (Taraboulos et al., 1992), and ANI at 1 µg/ml blocks protein synthesis almost completely in retinal tissue (Rehen et al., 1996), replenishment of surface PrPc during a 24 h treatment with ANI following hydrolysis of GPI anchors is unlikely. Treatment of rat retinal explants with phosphatidylinositol-specific phospholipase C (PI-PLC) abolished the neuroprotective effect of the PrR peptide. In these conditions, the enzyme had no effect upon either ganglion cell death or ANI-induced cell death in the NBL, nor upon the protective effect of forskolin. The only observable effect was upon the protective action of PrR (Figure 3A and B).
Fig. 3. Neuroprotection depends on the cellular prion protein. (A and B) Hydrolysis of GPI anchors prevents neuroprotection by the PrR peptide. Explants from rat retinas were pre-treated (PRE) with either vehicle or PI-PLC, then washed and treated with various combinations of anisomycin (1 µg/ml), the PrR peptide (80 µM) and forskolin (10 µM). Note that PI-PLC treatment prevents the neuroprotection by the PrR peptide in the NBL. *P < 0.01 versus anisomycin alone. (C and D) Neuroprotection by the PrR peptide occurs in the retina of wild-type (C), but not Prnp-knockout mice (D). Data are means ± SEM counts of pyknotic profiles from triplicate explants. *P < 0.01 versus anisomycin alone.
Likewise, ANI induced apoptosis in the NBL of both wild-type and PrP0/0 mice, while preventing ganglion cell death. Similarly to rat retina, the PrR peptide reduced the rate of cell death by ∼50% in the NBL of wild-type mouse retinal explants (Figure 3C), but had no effect upon explants from PrP0/0 mice (Figure 3D). In contrast, forskolin counteracted ANI-induced cell death in both wild-type and PrP0/0 explants.
The preceding experiments suggested that protection by the PrR peptide depends on the presence of the endogenous PrPc on the surface of the retinal cells. It is, however, conceivable that interaction of PrPc with the 66 kDa PrPc-binding protein (Martins et al., 1997) may sensitize retinal cells to degeneration, and that the peptide might reduce cell death by competing against the 66 kDa protein for the binding site in PrPc.
To test this hypothesis, we used a human neurotoxic peptide (106–126), which is similar to the sequence of amino acids 105–125 in both rat and mouse PrPc, and induces cell death in dissociated brain cell cultures, depending on the presence of the endogenous PrPc and mediated by glia (Forloni et al., 1993; Giese et al., 1998; Brown, 1999). Since this sequence of amino acids overlaps the binding site predicted for PrR, it should disrupt interactions of PrPc with the 66 kDa PrPc-binding protein. Indeed, peptide 106–126 (here called NTX) completely displaced the binding between the PrPc-binding peptide and PrPc (Figure 4B).
Incubation of retinal explants with the NTX peptide did not lead to cell death above control levels. This is probably due to the use of fresh solutions prepared immediately before the experiments, while it has been shown that only aged solutions of NTX may cause retinal cell degeneration (Ettaiche et al., 2000). In addition, in contrast to the PrR peptide, NTX did not block ANI-induced cell death (Figure 4C). However, pre-incubation of NTX with PrR abolished the neuroprotective effect of the latter (Figure 4D). The results are consistent with the hypothesis that rather than disruption of PrPc–p66 binding, it is the interaction of PrPc with PrR that induces neuroprotection.
PrPc-binding peptide activates both the cAMP-dependent protein kinase and the Erk pathways in the retina
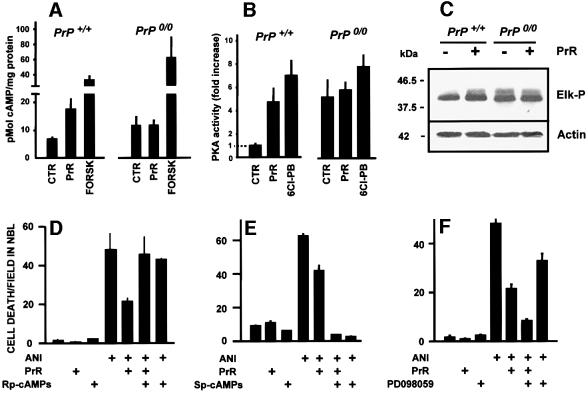
Incubation of retinal explants from wild-type mice with the PrR peptide was followed by an increase in the intracellular concentration of cAMP, which was not observed in similarly treated PrP0/0 explants (Figure 5A).
Fig. 5. PrPc-mediated neuroprotective signaling. (A and B) Responses of the cAMP/PKA signaling pathway to the PrR peptide (PrR) in the retina of either wild-type or PrP0/0 mice. Positive controls were either forskolin (FORSK) or a D1-like dopaminergic receptor agonist (6-Chloro-PB). Note the cAMP (A) and PKA (B) responses restricted to wild-type retinal tissue, and the higher basal values in knockout retinas. (C) Western blots for phospho-Elk (top) and loading control with actin (bottom), following treatment of either wild-type or PrP0/0 mouse retinal tissue with the PrR peptide. Note the activation of the Erk pathway restricted to wild-type tissue, as well as the higher basal activity in knockout tissue. (D–F) PrPc-mediated neuroprotective signaling through the cAMP-PKA pathway. Retinal explants from neonatal rats were treated with anisomycin (1 µg/ml), the PrR peptide (PrR 80 µM) in the presence of either 100 µM Rp-cAMP-s (D) or 100 µM Sp-cAMP-s (E), respectively an inhibitor and an activator of cAMP-dependent protein kinase, or in the presence of 30 µM PD98059, an inhibitor of the Erk-activating MEK enzyme (F). Note the reversion of the neuroprotective effect with the PKA inhibitor, and the potentiation of the neuroprotective effect with the Erk pathway inhibitor.
The activity of protein kinase A (PKA) was also increased in wild-type retinas incubated with the peptide, to a level close to that with the D1-dopaminergic agonist 6-Cl-PB. The latter result is consistent with the presence of functional D1-like receptors in the retina of developing rodents (e.g. Shearman et al., 1997). In contrast, PKA activity was not increased above resting levels in PrP0/0 explants treated with the peptide (Figure 5B). However, the levels by which forskolin stimulated PKA relative to unstimulated controls were similar in both wild-type and PrP0/0 explants (data not shown). Interestingly, both the basal, unstimulated levels of cAMP and the resting activity of PKA were higher in PrP0/0 retina than in wild type.
The PrR peptide also activated the Erk pathway. Both the level of Erk phosphorylation (data not shown) and the ability of the activated enzyme to phosphorylate its substrate Elk-1 (Figure 5C) were increased in wild-type retinas treated with the PrR peptide. Densitometric measurements (means ± SEM from three independent experiments) indicated a fold increase of 2.4 ± 0.3 in phospho-Elk detection following incubation with immunoprecipitated phospho-Erk from wild-type mouse retinas treated with the PrR peptide, relative to untreated controls. In contrast, there was no difference between treated and untreated PrP0/0 retinas, albeit that the basal activity of the Erk pathway was higher in PrP0/0 than in wild-type retinas, similar to the results with cAMP/PKA (phospho-Elk detection, fold increase of 3.3 ± 0.4 in material from untreated and 2.7 ± 0.4 in material from PrR-treated retinas of PrP0/0 mice, relative to untreated wild-type controls).
Neuroprotection by the PrPc-binding peptide is mediated by activation of cAMP-dependent protein kinase
Rp-cAMPs, a competitive inhibitor of PKA, consistently blocked neuroprotection by PrR (Figure 5D). The stereoisomer Sp-cAMPs, an activator of PKA, blocked ANI-induced cell death by itself, consistent with the effect of forskolin (Figure 5E). In contrast, PD098059, an inhibitor of the Erk-activating enzyme MEK, potentiated the protective effect of the PrR peptide (Figure 5F). PD098059 consistently showed a protective effect against ANI-induced cell death, indicating that Erk activation in the immature retina has a pro-apoptotic effect, as shown by other experiments from our laboratory (C.B.L.Campos, P.-A.Bédard and R.Linden, unpublished results).
Effects of antibodies to PrPc upon cell death correlate with their action upon cAMP
Two polyclonal antisera raised in rabbits against either recombinant mouse PrPc or the NTX peptide prevented protection by the PrR peptide (Figure 6A), presumably either by competing for the same binding site or by steric hindrance. These results are consistent with the interpretation that interaction of PrPc and the PrR peptide induces neuroprotection.
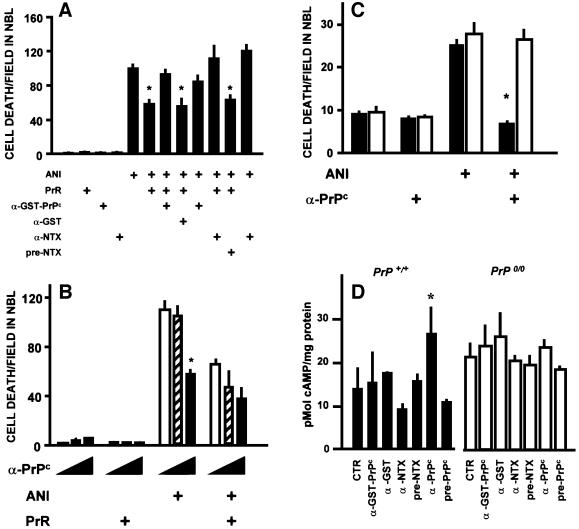
Fig. 6. Effects of antibodies raised against PrPc. (A) Both an antiserum to GST–PrPc (1:80) and an antiserum raised against the neurotoxic peptide (α-NTX) (1:50) prevented neuroprotection by the PrR peptide, while their respective controls, anti-GST and pre-immune serum, had no effect. Data are means ± SEM of triplicate experiments with retinal explants from neonatal rats. *P < 0.01 versus anisomycin alone. (B) A polyclonal antiserum raised against PrPc in PrP0/0 mice blocked anisomycin-induced cell death in the neuroblastic layer, and did not revert the neuroprotective effect of the PrR peptide. In each group of three bars, the concentration of the anti-PrPc antiserum was 0 (open bars), 20 (hatched bars) and 100 (filled bars) µg/ml, respectively. Data are means ± SEM of triplicate experiments with retinal explants from neonatal rats. *P < 0.01 versus no antiserum within the same group. (C) The anti-PrPc antibodies raised in knockout mice (same as in B) had a neuroprotective effect upon retinal explants from wild-type (filled bars), but not from PrP0/0 (open bars) mice. Data are means ± SEM of five replicates in each group. *P < 0.01 versus anisomycin alone among the same genotype. (D) Antibodies that induced neuroprotection increased the intracellular concentration of cAMP in wild-type, but not in PrP0/0 mouse retinal tissue. Results are means ± SEM of three experiments done in triplicate. *P < 0.01 versus control among the same genotype.
In contrast, a third antiserum raised against PrPc in PrP0/0 mouse led to a 50% reduction of ANI-induced cell death in rat retina (Figure 6B). The neuroprotective antiserum had no effect upon the retinal tissue from PrP0/0 mice (Figure 6C), supporting the hypothesis that the protective effect depends on PrPc. The rabbit antisera that antagonized neuroprotection by the PrR peptide had no effect upon the intracellular concentration of cAMP in either wild-type or PrP0/0 tissue, whereas the neuroprotective mouse antiserum against PrPc induced an increase in the intracellular concentration of cAMP in wild-type, but not in PrP0/0 retinal tissue (Figure 6D). Thus, an agonist neuroprotective effect of antibodies to PrPc correlates with their ability to induce an increase in intracellular cAMP.
Discussion
This investigation showed that: (i) a peptide that binds residues 113–128 of the mouse (114–129 human) cellular prion protein (the PrR peptide) induces a neuroprotective response in the neuroblastic layer of the developing retina in vitro; (ii) this neuroprotective response depends on the presence of endogenous PrPc at the surface of the retinal cells; (iii) the PrR peptide activates both the cAMP/PKA and the Erk signaling pathways in the retina; (iv) neuroprotection by the PrR peptide is mediated by the activation of a cAMP-dependent protein kinase pathway; and (v) antibodies to PrPc that induce an increase in the concentration of intracellular cAMP also induce a PrPc-dependent neuroprotective response.
Both cAMP-dependent protein kinase and Erk signaling pathways were activated by the PrR peptide in wild-type retinas, but not in PrP0/0 retinas. We have shown previously that cAMP protects cells in the NBL, but not ganglion cells, from degeneration (Rehen et al., 1996; Varella et al., 1997, 1999; Silveira et al., 2002), which is consistent with neuroprotection restricted to the NBL in the current experiments. However, preliminary data show that the PrR peptide also protects from induced degeneration both photoreceptors subject to thapsigargin-induced cell death in older retinas and NIH-3T3 cells subject to cell death induced by blockade of protein synthesis (data not shown), indicating that a neuroprotective function of PrPc may be widespread. In contrast, activation of Erk was found associated with cell death in the NBL of retinal explants. Indeed, inhibition of PKA activity blocked the effect of the PrR peptide, whereas inhibition of the Erk pathway potentiated the PrPc-mediated neuroprotection. The balance between the pro-degenerative Erk and the neuroprotective PKA pathways may also explain why the PrR peptide led to a maximum effect of 50% on ANI-induced cell death, since concurrent inhibition of the Erk pathway potentiated neuroprotection by the PrR. The data suggest that PrPc may function as a trophic receptor, stimulation of which leads to a neuroprotective state through the engagement of a cAMP/PKA pathway. The neuroprotective effect of antibodies that increase the intracellular concentration of cAMP supports both the proposal that PrPc is a signaling molecule and the involvement of the cAMP/PKA pathway in the transduction of the PrPc-mediated signals.
It is, however, not clear how the GPI-anchored PrPc attached to the outer membrane leaflet may lead to the activation of adenylyl cyclase, which normally is regulated by G-proteins associated with the inner membrane leaflet. Various GPI-anchored surface proteins transduce signals for proliferation, secretion of cytokines, oxidative bursts and apoptosis in leukocytes and lymphoid cell lines (for a review, see Horejsi et al., 1998). Signaling was shown to involve tyrosine phosphorylation, mobilization of intracellular Ca2+ and, in a few instances, production of phosphoinositides and diacylglycerol, but no increases in cAMP have been reported following cross-linking of GPI-anchored proteins (Horejsi et al., 1998). Nevertheless, heterotrimeric G-proteins were shown associated with GPI-anchored proteins in low density detergent-insoluble membrane domains (Solomon et al., 1996, 1998; Lisanti et al., 1999; Oh and Schnitzer, 2001). It is likely that an association of PrPc with G-proteins within the membrane compartments that contain PrPc (Gorodinsky and Harris, 1995; Vey et al., 1996; Naslavsky et al., 1997) may explain the activation of adenylyl cyclase leading to downstream neuroprotection.
A recent study showed that antibody cross-linking of PrPc led to caveolin-1-dependent activation of the tyrosine kinase Fyn in a neuroectodermal cell line (Mouillet-Richard et al., 2000), but only when subject to a differentiation program, and restricted to neurites. Although that study indicated that PrPc may function as a signal transduction protein, the authors failed to obtain a morphological response downstream of Fyn. It remains to be tested whether tyrosine kinases are also associated with the PrPc-mediated neuroprotection. Prevention of cell death induced by overexpressed Bax has been also reported after overexpression of PrPc in primary human neurons, but the effect seems to depend strictly on the N-terminal domains of the relatively rare transmembrane forms of PrPc (Bounhar et al., 2001).
Immortalized cell lines derived from Prnp–/– hippocampal cells were more sensitive to apoptosis induced by serum withdrawal than independent wild-type cell lines, whereas expression of either PrPc or Bcl-2 protected these cells from apoptosis (Kawahara et al., 1999). These data are also consistent with a cell-protective function of PrPc. In addition, PrP0/0 cerebellar cells are more sensitive than wild-type cells to oxidative stress (Brown et al., 1997b). This enhanced sensitivity was attributed to decreased superoxide dismutase activity associated with copper metabolism (Brown et al., 1997b; Brown and Besinger, 1998; Wong et al., 2000). Preliminary data from our laboratory show that the antioxidant ascorbic acid can prevent ANI-induced cell death in retinal explants (M.H.Varella, F.G.de Mello and R.Linden, unpublished results). Links between the resistance to oxidative stress mediated by PrPc (Wong et al., 2000) and the protective effect of PKA activation remain to be established.
If indeed PrPc transduces neuroprotective signals, then why do PrP0/0 mice show no obvious spontaneous neuronal death? One possibility is that the lack of PrPc from the earliest stages of embryogenesis may trigger compensatory responses in the intricate signaling network that feeds into the cell death execution mechanisms. For example, PrP0/0 retinas had a higher level of both PKA and Erk basal activities than wild type, showing that a variety of changes in signaling pathways within the retina accompany PrPc deletion. In contrast, a recent study showed that acute deletion of PrPc in adult mice does not lead to spontaneous neurodegeneration (Malluci et al., 2002), which might be taken as evidence against the relevance of PrPc-mediated neuroprotection, at least with respect to prion diseases. Notwithstanding this, it has not been reported whether neurons acutely depleted of PrPc may have become more sensitive to insults such as, for example, oxidative stress (Brown et al., 1997b). In addition, short-term compensatory changes in signal transduction pathways, such as the cAMP/PKA and Erk signaling pathways, have not been examined in the conditional knockout experiments (Malluci et al., 2002). Clearly, ruling out that the loss of PrPc function may be relevant for pathogenesis of prion diseases will require further experimentation.
The kinetics of cell loss in various models of neurodegenerative disease suggest that cell death in inherited neurodegenerations is a consequence of single catastrophic events imposed on an altered homeostatic state, instead of the traditional hypothesis of cumulative damage (Clarke et al., 2000). The present data are consistent with the view that the sensitivity to degeneration within the retinal tissue is controlled by a network of biochemical pathways (Linden et al., 1999). The data show that PrPc feeds into this network as a neuroprotective receptor, and suggest that engagement of PrPc helps maintain retinal cells in a steady state removed from the cell death execution pathways (cf. Clarke et al., 2000).
An antiserum to the PrR peptide also recognizes a PrPc-binding protein of 66 kDa on the surface of cells within the CNS (Martins et al., 1997). Cross-immunoreactivity of both the PrR peptide and the p66 PrPc-binding protein raises the possibility that the latter may be the natural trophic agent that leads to activation of PrPc. We recently have identified the p66 PrPc-binding protein as the stress-induced protein 1 (STI1), which contains a peptide domain with a hydropathy profile that corresponds to the PrR peptide used here as a probe (Zanata et al., 2002). The evidence for neuroprotection triggered either by STI1 or by the relevant peptide (Zanata et al., 2002) further support the neuroprotective signaling function of PrPc described here. Understanding PrPc-mediated neuroprotection is a major requirement to evaluate possible loss-of-function components in the pathogenesis of prion diseases.
Materials and methods
Animals
Lister hooded rats from our inbred colony, PrP0/0 mice from an inbred colony originally derived from mice of the Zurich knockout type kindly provided by Drs Charles Weissmann (Imperial College London) and Hans Kretzschmar (University of Munich), and wild-type control mice from an inbred C57BL6/J × 129 mixed lineage with a genetic background similar to the knockout animals were used in these experiments.
Tissue culture
Rodents at postnatal days 1 and 2 were killed by decapitation, and their eyes rapidly removed. The retinas were dissected and pieces of ∼1 mm2 were cut in culture medium (BME, Gibco-BRL, with 5% fetal calf serum) and incubated at 37°C in 5% CO2 and 95% air in an orbital shaker at 80–90 r.p.m. The tissue was fixed by immersion in 2–4% paraformaldehyde in phosphate buffer pH 7.2 for at least 40 min, followed by 20% sucrose in the same buffer. The explants were oriented under a dissecting microscope in an aluminum chamber with OCT embedding medium, and 10 µm thick transverse sections were cut in a cryostat. Neonatal rat eyes were fixed in situ, and frozen sections were prepared similarly.
Immunohistochemistry and western blot
Sections were reacted with either monoclonal antibody 6H4 to amino acids 144–152 of human prion (Prionics) or polyclonal antibody N10 against prion (kindly provided by Dr S.B.Prusiner). Control sections were processed in parallel without the primary antibody. Permanent staining was obtained with an HRP-ABC kit (Vector) using diaminobenzidine as chromogen.
For western blots, retinas from either wild-type or PrP0/0 mice were dissected as described and extracts were prepared by direct tissue lysis in Laemmli buffer. Samples were resolved by SDS–PAGE and transferred to a nitrocellulose membrane. Blots were exposed to either an anti-PrPc antibody raised in PrP0/0 mouse (1:500; Lee et al., 2001) or monoclonal antibody 6H4 to amino acids 144–152 of human prion (Prionics) for 16 h at 4°C, washed three times with TBST (40 mM Tris pH 7.4, 120 mM NaCl and 0.05% Tween-20), followed by addition of peroxidase-labeled anti-mouse IgG. The reaction was developed using enhanced chemiluminescence (Amersham Pharmacia Co.). Loading controls were performed by western blot with an antibody to actin (Sigma, 1:200).
Treatments
Drugs were added at the beginning of a 24 h incubation period. ANI was stocked in water at 500 µg/ml and applied at 1 µg/ml. Forskolin (Calbiochem) was stored at 10 mM in dimethylsulfoxide (DMSO) and applied at 10 µM. We had determined that DMSO at up to 1% had no effect upon cell death in the retinal explants (data not shown). Peptides HVATKAPHHGPCRSSA (PrPc-binding peptide; PrR), KSRGHVHC HAPAPATS (scrambled; SCRA) and KTNMKHMAGAAAAGA VVGGLG (neurotoxic; NTX) were synthesized chemically and their purity was tested by HPLC (Neosystems, Immunograde). Stocks were prepared at 20 mg/ml in BME with HEPES 20 mM pH 7.2, and 0.8% DMSO for NTX.
PI-PLC (Sigma) was diluted at 4 µg/ml in BME with 12% glycerol (v/v), 2 mM Tris–HCl pH 8.0 and 2 mM EDTA, and explants were pre-incubated for 1 h at 37°C. Then the tissue was washed three times in 15 ml of BME and incubated with the drugs as above.
Antibodies used in functional tests included: a rabbit antiserum raised against the chemically synthetized NTX, produced by Neosystems (Strasbourg, France), and its pre-immune control; rabbit antisera raised against either GST or GST–moPrPc; and polyclonal antisera raised in PrP0/0 mouse against His6-moPrPc as well as pre-immune controls, all produced at the Ludwig Institute, SP. All antisera used in this study specifically recognize PrPc in western blots of protein extracts from rodent brains (data not shown).
Detection of cell death
Cell death was detected either as condensed, pyknotic profiles stained with neutral red, or with the TUNEL procedure, using either fluorescent Apoptag (Intergen) or permanent FraGel kits (Oncogene). We have shown previously that counts of anillin-stained condensed, hyperchromic profiles adequately represent cell death as confirmed with other methods such as TUNEL staining for apoptosis (Rehen et al., 1996; Varella et al., 1997). In each experiment, at least three microscopic fields delimited by an eyepiece graticule of 0.0148 mm2 were counted in 3–8 explants per group. Statistical analysis was performed by analysis of variance followed by planned comparisons using Duncan’s multiple range test, using SPSSPC software.
Binding and competition assays
PrR peptide conjugated to bovine serum albumin (BSA) was biotinylated with Sulfo-NHS-LC-biotin (Pierce). Increasing amounts (2.5–500 ng) of either the biotinylated conjugate or biotinylated BSA, as a non-specific binding control, were incubated for 90 min at room temperature with 0.25 µg of either heterologous expressed wild-type His6-moPrPc or His6-moPrPc with amino acids 105–128 deleted (His6-moPrPcΔ105–128), immobilized on an ELISA plate (Covalink). Following two washes with phosphate-buffered saline (PBS) and incubation with avidin–peroxidase (Sigma) 1:1000, 1 h at room temperature in the dark, the reaction was developed with o-phenylnedramine-2HCl (OPD) (Sigma). Absorbance was measured in a Benchmark microplate reader (Bio-Rad) at 490 nm.
For the competition assay, increasing amounts of either scrambled (6.25, 31.25 or 62.5 µg) or non-biotinylated PrPc-binding (6.25, 31.25, 62.5 or 75 µg) peptides were pre-incubated with 0.25 µg of His6-PrPc for 90 min at room temperature. Then, 0.125 µg of the biotinylated conjugate was added, and incubated for a further 90 min at room temperature. The neurotoxic peptide (0.31 or 1.25 µg) was pre-incubated with the biotinylated conjugate for 90 min before adding it to adsorbed His6-PrPc. After two washes with PBS pH 7.4, samples were developed as described above.
Second messenger and kinase assays
Treatments. Retinas of P2-3 mice were pre-incubated with 100 µM IBMX for 1 h at 37°C, and then treated with 80 µM of either PrR or scrambled peptides for 10 min. Controls were kept in Dulbecco’s modified Eagle’s medium (DMEM).
cAMP. The tissue was washed with PBS pH 7.4 and homogenized with ice-cold 5% trichloroacetic acid. The homogenates were centrifuged at 4°C at 4000 g for 15 min, and the supernatants were extracted three times with ether acidified with 0.1 M HCl, and the aqueous phase dried in a speed-vac concentrator (Savant). The residues were dissolved in Tris-EDTA buffer pH 7.5. The concentration of cAMP per mg of protein was determined with a DPC kit (Diagnostic Products Corporation, Los Angeles, CA), according to the manufacturer’s instructions.
cAMP-dependent protein kinase activity. The tissue was washed with PBS pH 7.4 and homogenized with ice-cold extraction buffer (5 mM EDTA, 50 mM Tris pH 7.5). Cellular debris was removed by centrifugation for 2 min at 4000 g, and activity was determined using a protein kinase A assay system (Gibco-BRL).
p44/42 MAPK activity. The retinas were rinsed once with ice-cold PBS, lysed with ice-cold lysis buffer [20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 µg/ml leupeptin and 1 mM phenylmethylsulfonyl fluoride (PMSF)] and kept on ice for 5 min, then sonicated four times for 5 s on ice. The samples were centrifuged for 10 min at 4°C and the supernatant was transferred to a fresh tube. Approximately 200 µg of total protein in each sample was used to immunoprecipitate the active MAPK in the cell extract using an immobilized phospho-p44/42 MAP kinase monoclonal antibody (New England Biolabs non-radioactive kit). MAPK activity was evaluated by incubation with Elk-1 substrate, followed by eletrophoresis and western blotting with anti-phospho-Elk-1 (NEB). Phospho-MAPK was detected by western blotting using specific antibodies (NEB). Loading control was performed by western blotting of the supernatant with an antibody to actin (Sigma, 1:200).
Acknowledgments
Acknowledgements
We thank José Nilson dos Santos, José Francisco Tiburcio and Gildo de Souza for technical assistance, and Marilene Hohmuth Lopes for His6-PrPc preparations and help with binding experiments. This investigation was supported by grants and fellowships from FAPESP (99/07124-8), FAPERJ, CNPq and PRONEX-MCT.
References
- Aguzzi A. and Weissmann,C. (1997) Prion research: the next frontiers. Nature, 389, 795–798. [DOI] [PubMed] [Google Scholar]
- Aronoff-Spencer E. et al. (2000) Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry, 39, 13760–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet D., Dery,O., Frobert,Y., Grassi,J. and Couraud,J.Y. (1995) Is hydropathic complementarity involved in antigen–antibody binding? Mol. Immunol., 32, 303–308. [DOI] [PubMed] [Google Scholar]
- Bost K.L., Smith,E.M. and Blalock,J.E. (1985) Similarity between the corticotropin (ACTH) receptor and a peptide encoded by an RNA that is complementary to ACTH mRNA. Proc. Natl Acad. Sci. USA, 82, 1372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounhar Y., Zhang,Y., Goodyear,C. and LeBlanc,A. (2001) Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem., 276, 39145–39149. [DOI] [PubMed] [Google Scholar]
- Brentani R.R. (1988) Biological implications of complementary hydropathy of amino acids. J. Theor. Biol., 135, 495–499. [DOI] [PubMed] [Google Scholar]
- Brown D.R. (1999) Prion protein peptide neurotoxicity can be mediated by astrocytes. J. Neurochem., 73, 1105–1113. [DOI] [PubMed] [Google Scholar]
- Brown D.R. (2001) Prion and prejudice: normal protein and the synapse. Trends Neurosci., 24, 85–90. [DOI] [PubMed] [Google Scholar]
- Brown D.R. and Besinger,A. (1998) Prion protein expression and superoxide dismutase activity. Biochem. J., 334, 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R. et al. (1997a) The cellular prion protein binds copper in vivo. Nature, 390, 684–687. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Schulz-Schaeffer,W.J., Schmidt,B. and Kretzschmar,H.A. (1997b) Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp. Neurol., 146, 104–111. [DOI] [PubMed] [Google Scholar]
- Bueler H., Fischer,M., Lang,Y., Bluethmann,H., Lipp,H.P., DeArmond, S.J., Prusiner,S.B., Aguet,M. and Weissmann,C. (1992) Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature, 356, 577–582. [DOI] [PubMed] [Google Scholar]
- Clarke G., Collins,R.A., Leavitt,B.R., Andrews,D.F., Hayden,M.R., Lumsden,C.J. and McInnes,R.R. (2000) A one-hit model of cell death in inherited neuronal degenerations. Nature, 406, 195–199. [DOI] [PubMed] [Google Scholar]
- Colling S.B., Collinge,J. and Jefferys,J.G. (1996) Hippocampal slices from prion protein null mice: disrupted Ca(2+)-activated K+ currents. Neurosci. Lett., 209, 49–52. [DOI] [PubMed] [Google Scholar]
- Collinge J., Whittington,M.A., Sidle,K.C., Smith,C.J., Palmer,M.S., Clarke,A.R. and Jefferys,J.G. (1994) Prion protein is necessary for normal synaptic function. Nature, 370, 295–297. [DOI] [PubMed] [Google Scholar]
- Ettaiche M., Pichot,R., Vincent,J.P. and Chabry,J. (2000) In vivo cytotoxicity of the prion protein fragment 106–126. J. Biol. Chem., 275, 36487–36489. [DOI] [PubMed] [Google Scholar]
- Forloni G., Angeretti,N., Chiesa,R., Monzani,E., Salmona,M., Bugiani,O. and Tagliavini,F. (1993) Neurotoxicity of a prion protein fragment. Nature, 362, 543–546. [DOI] [PubMed] [Google Scholar]
- Gambetti P. et al. (1999) Inherited prion diseases. In Prusiner,S.B. (ed.), Prion Biology and Diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 509–584.
- Giese A., Brown,D.R., Groschup,M.H., Feldmann,C., Haist,I. and Kretzschmar,H.A. (1998) Role of microglia in neuronal cell death in prion disease. Brain Pathol., 8, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodinsky A. and Harris,D.A. (1995) Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J. Cell Biol., 129, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner E. et al. (2000) Cellular prion protein binds laminin and mediates neuritogenesis. Brain Res. Mol. Brain Res., 76, 85–92. [DOI] [PubMed] [Google Scholar]
- Herms J.W., Kretzchmar,H.A., Titz,S. and Keller,B.U. (1995) Patch-clamp analysis of synaptic transmission to cerebellar Purkinje cells of prion protein knockout mice. Eur. J. Neurosci., 7, 2508–2512. [DOI] [PubMed] [Google Scholar]
- Horejsi V., Cebecauer,M., Cerny,J., Brdicka,T., Angelisova,P. and Drbal,K. (1998) Signal transduction in leucocytes via GPI-anchored proteins: an experimental artefact or an aspect of immunoreceptor function? Immunol. Lett., 63, 63–73. [DOI] [PubMed] [Google Scholar]
- Kawahara C. et al. (1999) Prion prevents neuronal cell-line death. Nature, 400, 226–227. [DOI] [PubMed] [Google Scholar]
- Kurschner C. and Morgan,J.I. (1995) The cellular prion protein (PrP) selectively binds to Bcl-2 in the yeast two-hybrid system. Brain Res. Mol. Brain Res., 30, 165–168. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Magalhaes,A.C., Zanata,S.M., Brentani,R.R., Martins,V.R. and Prado,M.A. (2001) Internalization of mammalian fluorescent cellular prion protein and N-terminal deletion mutants in living cells. J. Neurochem., 79, 79–87. [DOI] [PubMed] [Google Scholar]
- Linden R. (2000) The anti-death league: associative control of apoptosis in developing retinal tissue. Brain Res. Rev., 32, 146–158. [DOI] [PubMed] [Google Scholar]
- Linden R., Rehen,S.K. and Chiarini,L.B. (1999) Apoptosis in developing retinal tissue. Progr. Retinal Eye Res., 18, 133–165. [DOI] [PubMed] [Google Scholar]
- Lisanti M.P., Sargiacomo,M. and Scherer,P.E. (1999) Purification of caveolae-derived membrane microdomains containing lipid-anchored signaling molecules, such as GPI-anchored proteins, H-Ras, Src-family tyrosine kinases, eNOS and G-protein α-, β- and γ-subunits. Methods Mol. Biol., 116, 51–60. [DOI] [PubMed] [Google Scholar]
- Lledo P.M., Tremblay,P., DeArmond,S.J., Prusiner,S.B. and Nicoll,R.A. (1996) Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc. Natl Acad. Sci. USA, 93, 2403–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malluci G.R., Ratté,S., Asante,E.A., Linehan,J., Gowland,I., Jefferys, J.G.R. and Collinge,J. (2002) Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J., 21, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V.R., Graner,E., Garcia-Abreu,J., Souza,S.J., Mercadante,A.F., Veiga,S.S., Zanata,S.M., Moura Neto,V. and Brentani,R.R. (1997) Complementary hydropathy identifies a cellular prion receptor. Nat. Med., 3, 1376–1382. [DOI] [PubMed] [Google Scholar]
- Moore R.C. et al. (1999) Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol., 292, 797–817. [DOI] [PubMed] [Google Scholar]
- Mouillet-Richard S., Ermonval,M., Chebassier,C., Laplanche,J.L., Lehmann,S., Launay,J.M. and Kellermann,O. (2000) Signal transduction through prion protein. Science, 289, 1925–1928. [DOI] [PubMed] [Google Scholar]
- Namura S., Zhu,J., Fink,K., Endres,M., Srinivasan,A., Tomaselli,K.J., Yuan,J. and Moskowitz,M.A. (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J. Neurosci., 18, 3659–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N., Stein,R., Yanai,A., Friedlander,G. and Taraboulos,A. (1997) Characterization of detergent-insoluble complexes containing the cellular prion and its scrapie isoform. J. Biol. Chem., 272, 6324–6331. [DOI] [PubMed] [Google Scholar]
- Oh P. and Schnitzer,J.E. (2001) Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas g(i) and g(s) target lipid rafts by default. Mol. Biol. Cell, 12, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. (1998). Prions. Proc. Natl Acad. Sci. USA, 95, 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehen S.K., Varella,M.H., Freitas,F.G., Moraes,M.O. and Linden,R. (1996) Contrasting effects of protein synthesis inhibition and of cyclic AMP on apoptosis in the developing retina. Development, 122, 1439–1448. [DOI] [PubMed] [Google Scholar]
- Rehen S.K., Diniz,D.M., Madeira,L.F., Brito,L.R.G. and Linden,R. (1999) Selective sensitivity of early postmitotic cells to apoptosis induced by inhibition of protein synthesis. Eur. J. Neurosci., 11, 349–356. [DOI] [PubMed] [Google Scholar]
- Rieger R., Edenhofer,F., Lasmezas,C.I. and Weiss,S. (1997) The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nature Med., 3, 1383–1388. [DOI] [PubMed] [Google Scholar]
- Roesler R., Walz,R., Quevedo,J., de Paris,F., Zanata,S.M., Graner,E., Izquierdo,I., Martins,V.M. and Brentani,R.R. (1999) Normal inhibitory avoidance learning and anxiety, but increased locomotor activity in mice devoid of PrP(C). Mol. Brain Res., 71, 349–353. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. et al. (1996) Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature, 380, 528–531. [DOI] [PubMed] [Google Scholar]
- Samaia H.B. and Brentani,R.R. (1998) Can loss-of-function prion-related diseases exist? Mol. Psychiatry, 3, 196–197. [DOI] [PubMed] [Google Scholar]
- Shearman L.P., Zeitzer,J. and Weaver,D.R. (1997) Widespread expression of functional D1-dopamine receptors in fetal rat brain. Dev. Brain Res., 102, 105–115. [DOI] [PubMed] [Google Scholar]
- Shmerling D. et al. (1998) Expression of amino terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell, 93, 203–214. [DOI] [PubMed] [Google Scholar]
- Silveira M.S., Costa,M.R., Bozza,M. and Linden,R. (2002) Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J. Biol. Chem., 277, 16075–16080. [DOI] [PubMed] [Google Scholar]
- Solomon K.R., Rudd,C.E. and Finberg,R.W. (1996) The association between glysosylphosphatidylinositol-anchored proteins and heterotrimeric G protein α subunits in lymphocytes. Proc. Natl Acad. Sci. USA, 93, 6053–6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon K.R., Kurt-Jones,E.A., Saladino,R.A., Stack,A.M., Dunn,I.F., Ferretti,M., Golenbock,D., Fleisher,G.R. and Finberg,R.W. (1998) Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. J. Clin. Invest., 102, 2019–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboulos A., Raeber,A.J., Borchelt,D.R., Serban,D. and Prusiner,S.B. (1992) Synthesis and trafficking of prion protein in cultured cells. Mol. Biol. Cell, 3, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler I. et al. (1996) Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature, 380, 639–642. [DOI] [PubMed] [Google Scholar]
- Varella M.H., Correa,D.F., Campos,C.B.L., Chiarini,L.B. and Linden,R. (1997) Protein kinases selectively modulate apoptosis in the developing retina in vitro. Neurochem. Int., 31, 217–227. [DOI] [PubMed] [Google Scholar]
- Varella M.H., De Mello,F.G. and Linden,R. (1999) Evidence for an anti-apoptotic role of dopamine in developing retinal tissue. J. Neurochem., 73, 485–492. [DOI] [PubMed] [Google Scholar]
- Vey M., Pilkuhn,S., Wille,H., Nixon,R., DeArmond,S.J., Smart,E.A., Anderson,R.G.W., Taraboulos,A. and Prusiner,S.B. (1996) Sub cellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl Acad. Sci. USA, 96, 14945–14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz R., Amaral,O.B., Rockenbach,I.C., Roesler,R., Izquierdo,I., Cavalheiro,E.A., Martins,V.M. and Brentani,R.R. (1999) Increased sensitivity to seizures in mice lacking cellular prion protein. Epilepsia, 40, 1679–1682. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Raeber,A.J., Shmerling,D., Aguzzi,A. and Manson,J.C. (1999). Knockouts, transgenics and transplants in prion research. In Prusiner,S.B. (ed.), Prion Biology and Diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 273–306.
- Whittal R.M., Ball,H.L., Cohen,F.E., Burlingame,A.L., Prusiner,S.B. and Baldwin,M.A. (2000) Copper binding to octarepeat peptides of the prion protein monitored by mass spectrometry. Protein Sci., 9, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will R.G., Alpers,M.P., Dormont,D., Schonberger,L.B. and Tateishi,J. (1999). Infectious and sporadic prion diseases. In Prusiner,S.B. (ed.), Prion Biology and Diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 465–508.
- Wong B.S., Pan,T., Liu,T., Li,R., Petersen,R.B., Jones,I.M., Gambetti,P., Brown,D.R. and Sy,M.S. (2000) Prion disease: a loss of antioxidant function? Biochem. Biophys. Res. Commun., 275, 249–252. [DOI] [PubMed] [Google Scholar]
- Wong B.S. et al. (2001) Induction of ho-1 and nos in doppel-expressing mice devoid of PrP: implications for doppel function. Mol. Cell. Neurosci., 17, 768–775. [DOI] [PubMed] [Google Scholar]
- Yehiely F., Bamborough,P., Da Costa,M., Perry,B.J., Thinakaran,G., Cohen,F.E., Carlson,G.A. and Prusiner,S.B. (1997) Identification of candidate proteins binding to prion protein. Neurobiol. Dis., 3, 339–355. [DOI] [PubMed] [Google Scholar]
- Zanata M. et al. (2002) Stress inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J., 21, 3307–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]