Abstract
TASK-1 belongs to the 2P domain K+ channel family and is the prototype of background K+ channels that set the resting membrane potential and tune action potential duration. Its activity is highly regulated by hormones and neurotransmitters. Although numerous auxiliary proteins have been described to modify biophysical, pharmacological and expression properties of different voltage- and Ca2+-sensitive K+ channels, none of them is known to modulate 2P domain K+ channel activity. We show here that p11 interacts specifically with the TASK-1 K+ channel. p11 is a subunit of annexin II, a cytoplasmic protein thought to bind and organize specialized membrane cytoskeleton compartments. This association with p11 requires the integrity of the last three C-terminal amino acids, Ser-Ser-Val, in TASK-1. Using series of C-terminal TASK-1 deletion mutants and several TASK-1–GFP chimeras, we demonstrate that association with p11 is essential for trafficking of TASK-1 to the plasma membrane. p11 association with the TASK-1 channel masks an endoplasmic reticulum retention signal identified as Lys-Arg-Arg that precedes the Ser-Ser-Val sequence.
Keywords: auxiliary protein/membrane trafficking/p11/2P domain K+ channels
Introduction
Potassium (K+) channels play an important role in setting the resting membrane potential of cells and regulating the slope and frequency of action potentials in excitable cells. Mammalian K+ channels are classified into three structural families, differentiated by the number of transmembrane segments (TMS) and pore domains (P) present in their α subunits: the voltage-dependent (Kv) channels (6TMS/1P), the inward rectifier (Kir) channels (2TMS/1P), and the background two pore-domain (K2P) channels (4TMS/2P). So far, 14 human K2P α subunits have been identified. They are classified into five subgroups based on their sequence homologies: (i) TWIK-1 (Lesage et al., 1996), TWIK-2 (Chavez et al., 1999; Patel et al., 2000) and KCNK7 (Salinas et al., 1999); (ii) TREK-1 (Fink et al., 1996; Patel et al., 1998), TREK-2 (Bang et al., 2000; Lesage et al., 2000) and TRAAK (Fink et al., 1998), which are mechanosensitive channels also activated by polyunsaturated fatty acids; (iii) TASK-1 (Duprat et al., 1997; Leonoudakis et al., 1998; Kim et al., 1999), TASK-3 (Kim et al., 2000; Rajan et al., 2000) and TASK-5 (Kim and Gnatenco, 2001); (iv) TASK-2 (Reyes et al., 1998), TALK-1 (Girard et al., 2001) and TALK-2 (also named TASK-4) (Decher et al., 2001; Girard et al., 2001); (v) THIK-1 (Rajan et al., 2001) and THIK-2 (Girard et al., 2001; Rajan et al., 2001).
TASK-1 was the first K2P channel identified that was able to produce a current presenting all background channel properties (Duprat et al., 1997). This channel is abundant in brain, heart, pancreas, placenta, lung, kidney, ovary, prostate and small intestine (Lesage and Lazdunski, 2000). TASK-1 channels are highly modulated by changes in extracellular pH around the physiological pH (pH 7.3) and lose most of their activity at pH values below 6.9–7.0 (Duprat et al., 1997). TASK-1 channel activity at physiological pH is drastically inhibited by a variety of hormones and neurotransmitters (Czirjak et al., 2000; Millar et al., 2000; Talley et al., 2000), presumably via activation of phospholipase C (Czirjak et al., 2001). This inhibition can result in large changes of the resting membrane potential, leading to important changes in cellular excitability (Millar et al., 2000). One particularly interesting example of such a control is the key role of TASK-like channels in glomerulosa cells, where they are essential players in angiotensin II-induced aldosterone secretion (Czirjak et al., 2000). TASK-1 has a close parent, TASK-3 (59.2% homology) (Kim et al., 2000; Rajan et al., 2000; Czirjak and Enyedi, 2002; Talley and Bayliss, 2002), which has very similar properties, particularly with respect to regulation with hormones and neurotransmitters. The TASK-1 channel is a major target of volatile general anaesthetics such as halothane or isoflurane (Patel et al., 1999; Talley and Bayliss, 2002); TASK-3 is also activated by this important class of drugs (Talley and Bayliss, 2002). TASK-1 and TASK-3 channels can be pharmacologically separated using anandamide, a specific blocker of TASK-1 channels (Maingret et al., 2001). Because of their role in the regulation of resting potentials, due to their regulation by small pH variations and by hormones and neurotransmitters, TASK-1 channels are probably involved in numerous physiological and physiopathological processes (Millar et al., 2000; Sirois et al., 2000; Talley et al., 2000; Washburn et al., 2002). One particularly interesting physiological function of TASK-1, linked to its high pH sensitivity and its presence in carotid bodies, is its role in respiratory control (Buckler et al., 2000; Patel and Honore, 2001). One interesting physiopathological function of TASK-1 is its contribution to the arrhythmogenic effect of platelet-activating factor in the heart (Barbuti et al., 2002).
K+ channels are known to interact with auxiliary subunits that modify their biophysical and/or pharmacological and/or expression properties (Rehm and Lazdunski, 1988; Rettig et al., 1994; Morales et al., 1995; Barhanin et al., 1998; Wible et al., 1998; An et al., 2000; Tinel et al., 2000; Attali, 2002). Mutations or deletions of these subunits can lead to a variety of desease states (Kuo et al., 2001; Attali, 2002; Warth and Barhanin, 2002).
To date, no associated subunit has been described for the K2P channel family, although the need for such subunits has been suggested for K2P channels that have not yet been functionally expressed despite much effort, such as KCNK7 (Salinas et al., 1999), THIK-2 (Girard et al., 2001; Rajan et al., 2001) and TASK-5 (Kim and Gnatenco, 2001).
This article reports the identification of the first K2P channel auxiliary protein, p11, isolated by a yeast two-hybrid system. Its interaction with the TASK-1 channel was confirmed by biochemical and immunological approaches. p11 is essential for membrane trafficking, and therefore for the functionality of TASK-1 channels. The last three amino acids of the TASK-1 C-terminal end are crucial for the association with p11. This association masks an endoplasmic reticulum (ER) retention signal that we have identified as the Lys-Arg-Arg sequence that normally prevents TASK-1 movement to the plasma membrane.
Results
Yeast two-hybrid system
We identified an auxiliary protein of the K+ channel TASK-1 by screening a human heart cDNA library with the yeast two-hybrid system. We constructed a bait with the cytoplasmic C-terminal region of TASK-1 (amino acids 242–394). Among 71 Leu+ clones from 5.4 × 106 yeast transformants, only 20 activated the green fluorescent protein (GFP) reporter gene and appeared green under UV light. Their sequence analysis revealed nine independent clones, all of them presented, in-frame with the B42 cassette of pJG4-5 vector, the open reading frame of the p11 protein (p11) also called calpactin I, annexin II light chain or S100A10 protein (Dooley et al., 1992).
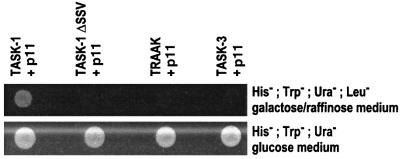
In order to confirm the interaction between these two proteins, the total coding sequence of p11 was subcloned and tested with the TASK-1 bait using a yeast two-hybrid system. Results indicate clearly that both proteins associate, because yeast grew on Leu+ glucose medium (where no association is necessary) as well as on Leu– galactose/raffinose medium (where an interaction is needed) (Figure 1, lane 1). When the last three amino acids of TASK-1 are deleted (TASK-1ΔSSV; Figure 2A), no channel activity is measurable. We tested whether this truncated form was capable of interacting with p11. Results indicate that yeast transformed with p11 and the TASK-1ΔSSV bait are only able to grow on Leu+ glucose medium. The lack of growth on Leu– galactose/raffinose medium indicates that p11 is unable to interact with TASK-1ΔSSV (Figure 1, lane 2). These results indicate that the last three amino acids of TASK-1 are crucial for the interaction of the channel with p11.
Fig. 1. p11 specifically interacts with human TASK-1 in the yeast two-hybrid system. Yeast were co-transfected with p11 pJG4-5 vector, GFP pGNG1 vector and different baits corresponding to the C-terminus of TASK-1, TASK-1ΔSSV, TRAAK and TASK-3 into pEG202 vector and plated on His–, Trp–, Ura–, Leu– galactose/raffinose medium or His–, Trp–, Ura– glucose medium.
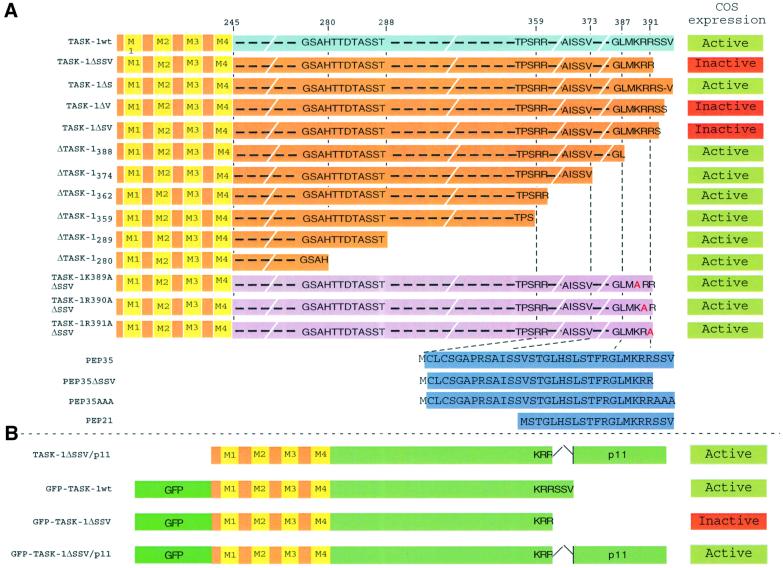
Fig. 2. (A and B) Sequences of mutants and peptides used for the identification of the p11 interaction and for the characterization of ER retention signal. Numbers indicate amino acid position in the human TASK-1wt sequence. For mutants named ΔTASK-1xxx, the number xxx corresponds to the position of the first amino acid deleted. A methionine residue was added at the N-terminus of PEP35, PEP21, PEP35ΔSSV and PEP35AAA sequences to allow their COS expression. In the COS expression column, active indicates that corresponding channels are able to produce a TASK-1 current after transfection in COS cells, and inactive indicates that they are not.
In order to determine whether or not the p11 interaction is specific for the TASK-1 channel, yeasts were transformed with p11 and other baits corresponding to the C-terminal regions of other K2P channels, such as TRAAK and TASK-3. In both cases, yeast grew only on Leu+ glucose medium (Figure 1, lanes 3 and 4). These data indicate that K2P channels that do not display the consensus SSV at their C-terminus cannot interact with p11.
RT–PCR experiments indicate that p11 is widely distributed and is expressed at detectable levels in all tissues tested, including brain, heart, skeletal muscle, pancreas, kidney, lung, liver, placenta, ovary, prostate, testis, colon, small intestine and spleen (data not shown).
In vitro protein–protein interaction assay: GST pull-down
The interaction of TASK-1 with p11 was confirmed by the glutatione S-transferase (GST) pull-down protocol (Figure 3A). Proteins corresponding to the C-terminal region of TASK-1 wild type (TASK-1wt) or TASK- 1ΔSSV were produced in Escherichia coli using the pGEX3a expression vector. The purified proteins were independently coupled on CNBr-activated Sepharose 4B and mixed with 35S-labelled p11 from in vitro translation experiments. [35S]p11 is only able to interact with the C-terminal region of TASK-1wt (Figure 3A, lanes 1 and 4). Purified GST protein is unable to compete with the p11 interaction, indicating that this association is specific for p11 (Figure 3A, lane 4).
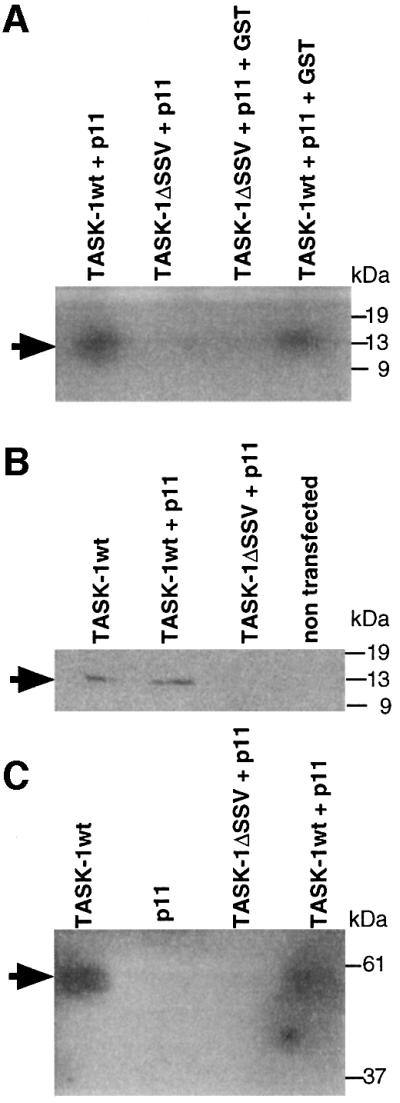
Fig. 3. p11 requires the presence of amino acids Ser-Ser-Val in the C-terminus of human TASK-1. (A) GST pull-down: GST–TASK-1 (lanes 1 and 4) or GST–TASK-1ΔSSV (lanes 2 and 3) were coupled to CNBr-activated Sepharose 4B and incubated with [35S]p11 in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of GST. Bound proteins were resolved on SDS–PAGE (5–16% polyacrylamide) followed by autoradiography. (B and C) Physical association between p11 and human TASK-1. T7-tagged TASK-1 or T7-tagged TASK- 1ΔSSV were transiently tranfected into COS cells in the presence or absence of p11. (B) TASK-1 was precipitated with T7-tag antibodies. Immunoprecipitated proteins were resolved on SDS–PAGE (5–16% polyacrylamide), blotted on to PVDF membrane and detected with p11 antibodies. (C) p11 was precipited with p11 antibodies, T7-TASK-1 was revealed by T7-tag antibodies.
The absence of positive signal with the TASK-1ΔSSV construct (Figure 3A, lanes 2 and 3) also indicated that the three last amino acids of the C-terminal end of TASK-1 are crucial for the interaction with p11, confirming yeast two-hybrid system results.
Co-immunoprecipitation
The association between TASK-1wt and p11 was further demonstrated by co-immunoprecipitation of these two proteins in their native form after transient transfection in COS cells.
The T7 epitope was added to the N-terminus of TASK-1 and TASK-1ΔSSV channels. We first checked that the T7-tagged TASK-1wt channel displayed the same biophysical properties as the native channel (data not shown).
COS cells were transiently transfected with either TASK-1wt or TASK-1ΔSSV in the presence or absence of p11. After 2 days, cells were harvested and solubilized as described in Materials and methods. Solubilized proteins were immunoprecipitated with T7-tag antibodies. Immunoprecipitated proteins were loaded on to PAGE, blotted and the presence of p11 was detected with p11 antibodies (Figure 3B). p11 immunoprecipitated with TASK-1wt and not with TASK-1ΔSSV (Figure 3B, lanes 2 and 3). Interestingly, when only TASK-1wt is transfected in COS cells, p11 is immunoprecipitated (Figure 3B, lane 1), indicating that COS cells constitutively express p11.
We also used p11 antibodies for immunoprecipitation experiments (Figure 3C). Immunoprecipitated proteins were prepared as a western blot and probed with T7-tag antibodies. The TASK-1wt channel was detected in the absence or presence of exogenous p11 (Figure 3C, lanes 1 and 4). No signal was seen when p11 was transfected alone (Figure 3C, lane 2). As expected, the TASK-1ΔSSV channel was not immunoprecipitated by the p11 antibodies (Figure 3C, lane 3).
Effect of cytochalasin-D
p11 in the annexin II complex is known to bind to F-actin (Filipenko and Waisman, 2001). In order to test the effect of the microfilament disruption on TASK-1 channel activity, we used cytochalasin-D, a molecule that depolymerizes actin microfilaments (Cooper, 1987). TASK-1-transfected COS cells were incubated for 16 h at 37°C with 5 µg/ml cytochalasin-D. At this stage, COS cells have lost their normal shape, indicating an efficient action of the drug. However, the cytochalasin-D microfilament disruption did not significantly modify the TASK-1 current densities; values are 31.7 ± 10 pA/pF (n = 12) for control TASK-1-transfected cells and 29.4 ± 6.4 pA/pF (n = 10) for cytochalasin-D-treated cells.
Competition with C-terminal peptides of the TASK-1 channel
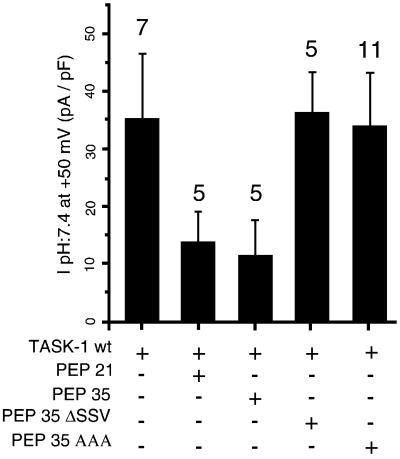
The presence of endogenous p11 in most, if not all, cells, and particularly in COS cells, makes difficult the analysis of the effects of p11 on TASK-1 channel activity. Thus, we decided to quench endogenous p11 expression using peptides corresponding to the C-terminal sequence of TASK-1, called PEP35 and PEP21 and corresponding to amino acids 359–394 and 374–395, respectively; a methionine residue was added to their N-terminus (Figure 2A). The corresponding nucleic sequences were introduced into a pIRES-GFP plasmid and co-expressed with TASK-1 channels in COS cells that endogenously express p11. Channel expression at the cell surface was indicated by the presence of the CD8 antigen. Co-expression of PEP21 or PEP35 with TASK-1 channels resulted in a strong decrease of current intensities [13.8 ± 2.8 pA/pF (n = 5) and 11.4 ± 5.9 pA/pF (n = 5) for PEP21 or PEP35, respectively] as compared with the TASK-1wt channel expressed alone [34.7 ± 11.4 pA/pF (n = 7)] (Figure 4). The transfection of a plasmid containing only GFP was without effect. Assuming that the GFP fluorescence signal is correlated with the peptide expression, since both proteins are produced by the same mRNA molecule, it appears that co-expression of either peptide with TASK-1 results in a decrease of channel activity. PEP35ΔSSV or PEP35AAA, constructs corresponding to PEP35 in which the terminal SSV sequence was deleted or replaced by three Ala residues (Figure 2A), respectively, were unable to decrease the TASK-1 current produced in COS cells (Figure 4). These latter results provide strong confirmation that the TASK-1 current inhibition is really due to competition between peptides and TASK-1 channels for association to p11 via the terminal SSV sequence.
Fig. 4. Effects of human TASK-1 C-terminal peptides on human TASK-1 channel activity. Current densities measured at +50 mV in physiological conditions (extracellular pH 7.4) on COS cells transfected with TASK-1wt alone or in combination with either PEP21, PEP35, PEP35ΔSSV or PEP35AAA. Numbers of measured cells are indicated above error bars. ‘+’ indicates the presence and ‘–’ the absence of the correspondant construct.
The C-terminal valine residue of TASK-1 is necessary for channel expression
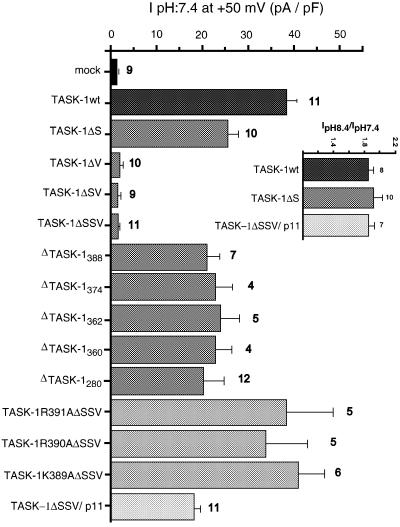
In whole-cell recordings performed on COS cells, the mutant TASK-1ΔSSV (Figure 2A) is unable to produce any current [1.4 ± 0.2 pA/pF (n = 5) versus 37.3 ± 4.9 pA/pF (n = 5) for the TASK-1wt, or 1.1 ± 0.2 pA/pF for mock cells] (Figure 5). To determine which of the three terminal amino acids is particularly necessary for TASK-1 channel activity, we constructed a series of TASK-1 deletion mutants (Figure 2A). Each mutant was transfected into COS cells. In control conditions (pH 7.4), the TASK-1wt and the TASK-1ΔS mutant generated similar levels of current [37.3 ± 4.9 pA/pF (n = 5) and 28.3 ± 5.3 pA/pF (n = 4), respectively], whereas the TASK-1ΔV mutant failed to produce any current [1.8 ± 0.2 pA/pF (n = 10)] (Figure 5). These data suggest that the single amino acid Val plays a crucial role in the regulation of TASK-1 expression. This result is corroborated by the absence of significant current recorded with the TASK-1ΔSV mutant [1.8 ± 0.2 pA/pF (n = 7)] (Figure 5).
Fig. 5. p11 is involved in the production of the human TASK-1 current (main panel). Current densities produced by the human TASK-1 channel and its deletion mutants, and by p11 chimera constructed with ΔSSV channel. Currents were elicited every 10 s by 800 ms voltage ramps (from –130 to +80 mV) and were measured at +50 mV in physiological conditions (extracellular pH 7.4). The number of cells tested for each channel is indicated next to the error bars. Inset: activation of currents generated by TASK-1wt and TASK-1ΔS channels and TASK-1ΔSSV–p11 chimera after alkalinization of the extracellular solution (pH 8.4).
We then decided to construct a chimera, TASK- 1ΔSSV–p11, in which p11 was linked to the C-terminal end of the TASK-1ΔSSV mutant (Figure 2B). This chimera, although it lacks the three terminal amino acids and particularly the key Val residue, is able to generate the same level of current as that produced by the TASK-1ΔS mutant [17.8 ± 2.1 pA/pF (n = 9)] (Figure 5). Currents recorded with this chimera shared the same biophysical properties as those of TASK-1wt itself, i.e. no voltage or time dependency, and a linear current–voltage relationship in symmetrical K+ (data not shown). The sensitivity to external solution pH variations of TASK- 1ΔSSV–p11 is also the same as that of TASK-1ΔS and TASK-1wt. TASK-1ΔS, TASK-1ΔSSV–p11 and TASK- 1wt currents are similarly increased by an alkalinization (pH 8.4) of the external medium: 1.92 ± 0.11-fold increase measured at +50 mV (n = 10) for TASK-1ΔS, 1.86 ± 0.07-fold increase (n = 7) for TASK-1ΔSSV–p11 and 1.85 ± 0.06-fold increase (n = 8) for TASK-1wt (Figure 5, inset). TASK-1ΔS, TASK-1ΔSSV–p11 and TASK-1wt currents are nearly blocked by acidification (pH 6.4) of the external medium; IpH6.4/IpH7.4 current ratios are 0.12 ± 0.01 (n = 10) for TASK-1ΔS, 0.19 ± 0.05 (n = 6) for TASK-1ΔSSV–p11 and 0.10 ± 0.01 (n = 7) for TASK-1wt.
Our results clearly indicate that a covalent linkage of p11 to the core of the TASK-1 channel is able to restore the function of the TASK-1ΔSSV mutant.
p11 masks an ER signal
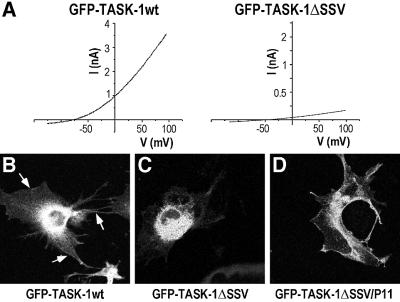
In order to analyse the molecular mechanism by which the association of p11 with the TASK-1 channel is essential for the generation of the K+ channel activity, we constructed another series of deletion mutants in the C-terminal of TASK-1 (Figure 2A). All of these mutants (ΔTASK-1280, ΔTASK-1360, ΔTASK-1362, ΔTASK-1374 and ΔTASK-1388) are able to generate a TASK-1-like current (Figures 2A and 5). Taken together, these results indicate that the sequence MKRR (see Figure 2A), which corresponds to the difference between the TASK-1ΔSSV mutant, which is inactive, and the ΔTASK-1388 mutant, which is active, is inhibitory for the expression of channel activity. It appears that this KRR sequence is similar to the RKR sequence previously described to be an ER retention signal for the inward rectifier channel Kir6.2 (Zerangue et al., 1999). We then immediately formed the hypothesis that the role of the association of p11 with TASK-1 might be to mask the potential ER retention signal MKRR, a masking necessary for normal trafficking to the plasma membrane and expression of the channel activity. To test this hypothesis we used several approaches. First, it was important to verify that the inactive form TASK-1ΔSSV is unable to reach the plasma membrane. To check this point we constructed chimeras between the GFP protein and the N-terminus of TASK-1wt or of TASK-1ΔSSV (Figure 2B). The GFP–TASK-1wt chimera is functionally active (Figure 6A, left trace) and the GFP–TASK-1ΔSSV chimera expressed in COS cells is functionally inactive (Figure 6A, right trace). Monitoring of GFP fluorescence with a confocal microscope indicated that GFP–TASK- 1wt was essentially expressed at the plasma membrane, as indicated by its uniform distribution with a strong fluorescence signal in the membrane areas (Figure 6B), while the GFP–TASK-1ΔSSV chimera appeared mainly in the intracellular part of the cell and was absent in the membrane sections (Figure 6C). These results definitively suggest that when p11 cannot associate with TASK-1, the KRR signal is accessible and the TASK-1 channel cannot be addressed to the plasma membrane. This view was corroborated by the triple fusion chimera GFP–TASK- 1ΔSSV–p11, where p11 was added to the GFP–TASK- 1ΔSSV construct. This addition restored the ability of mutated channels to reach the plasma membrane (Figure 6D).
Fig. 6. The SSV sequence is necessary for human TASK-1 trafficking to the plasma membrane. (A) Voltage–current curves for GFP– TASK-1wt and GFP–TASK-1ΔSSV, respectively, showing that GFP added at the N-terminus does not affect the channel properties. Representative confocal fluorescent images obtained with a 488 nm filter show that GFP–TASK-1wt chimera (B) is largely expressed in membrane section (indicated by arrows), that GFP–TASK-1ΔSSV chimera (C) is mainly present in intracellular structures and absent at the membrane section, and finally that fusing the p11 at the C-terminus of the GFP–TASK-1ΔSSV channel (GFP–TASK-1ΔSSV–p11) restores the ability of the protein complex to reach the plasma membrane (D).
In a second approach, we constructed different mutants in which each amino acid of the KRR sequence was independently replaced by an alanine. All of these mutants, called TASK-1K389AΔSSV, TASK-1R390AΔ SSV and TASK-1R391AΔSSV (Figure 2A), respectively, are active (Figure 5). The conclusion is that when one of the three charged amino acids is mutated, the ER retention signal becomes ineffective.
Discussion
This paper describes the identification of the first auxiliary protein for a K2P channel, called p11, which specifically associates with the TASK-1 channel.
p11, the small subunit of annexin II, is present in a large number of organisms, vertebrates, insects, nematodes and plants. p11 is a 97 amino acid protein that belongs to the S100 protein family; it is also called S100A10 (Drust and Creutz, 1988; Ayala-Sanmartin et al., 2001; Gerke and Moss, 2002). S100 is a multigenic family of small Ca2+-binding proteins of EF-hand type that are involved in the regulation of a number of physiological processes, such as protein phosphorylation, inflammatory response, cAMP signalling pathways, dynamics of cytoskeleton components, and cell proliferation and differentiation (for review, see Donato, 2001); most of them exist as dimers. The dimerization of the S100 proteins is a Ca2+-dependent process and is critical for their biological activitiy. p11 is an exception, since it does not require calcium to dimerize because it is constitutively in a ‘calcium binding’ state (Donato, 2001). Several members of the S100 protein family form heteromeric complexes with annexins: S100A11 with annexin I (Mailliard et al., 1996), S100A6 with annexin II (Tokumitsu et al., 1992), and p11 is often found tightly associated with the annexin II heavy chain, p36, to form a tetrameric complex (p11)2(p36)2 (Rety et al., 1999).
We attempted to co-immunoprecipitate p36 with the TASK-1–p11 complex, but we were not able to show this interaction. This is clearly not an indication that these three proteins are not associated. The association might exist, but the physical interaction between p11 and p36 could not be strong enough, when p11 is also associated with the channel, to resist standard immunoprecipitation conditions, which require detergent solubilization of plasma membrane components. p36 would dissociate from the complex under such conditions. Very recently, it was reported that p11 is also capable of interacting with the TTX-insensitive, voltage-dependent Na+ channel Nav1.8 (Okuse et al., 2002). However, no evidence was presented in this paper that p11 associates with p36.
p11, and more generally annexin II, has been implicated in numerous biological processes, such as regulation of membrane organization, membrane cytoskeleton linkage, membrane trafficking, endocytosis (Emans et al., 1993; Mayorga et al., 1994; Gerke and Moss, 2002) and exocytosis (Ali et al., 1989; Gerke and Moss, 2002). Annexin II is believed to bind to the cytoplasmic domain of membrane rafts to stabilize these domains and provide a link with the cortical actin cytoskeleton (Gerke and Moss, 2002). It has been proposed that one of the numerous roles of annexin II could be to regulate ion channel activity (Gerke and Moss, 2002).
Our work indicates that the annexin II subunit p11 associates with the TASK-1 channel, and that this association requires the presence of the C-terminal sequence SSV of TASK-1. Of these three amino acids, the valine residue plays the crucial role, since all mutants in which this amino acid was deleted (TASK-1ΔV, TASK-1ΔSV and TASK-1ΔSSV) were unable to generate any K+ current after transient transfection in COS cells. This loss of function was reversed by the covalent addition of p11 to the TASK-1 channel amputated from its SSV sequence. The chimera TASK-1ΔSSV–p11 was able to reach the plasma membrane and to generate a background K+ current.
Our work also led to the identification of an intracellular retention signal that would normally segregate the TASK-1 channel, presumably in the ER. This retention signal corresponds to the sequence KRR, which is located immediately upstream of the key SSV sequence. When this KRR sequence is deleted or mutated, all of the mutants that lack the C-terminal SSV or the key Val residue that serve to bind p11 are then able to generate a TASK-1 current without this interaction that would have been necessary otherwise.
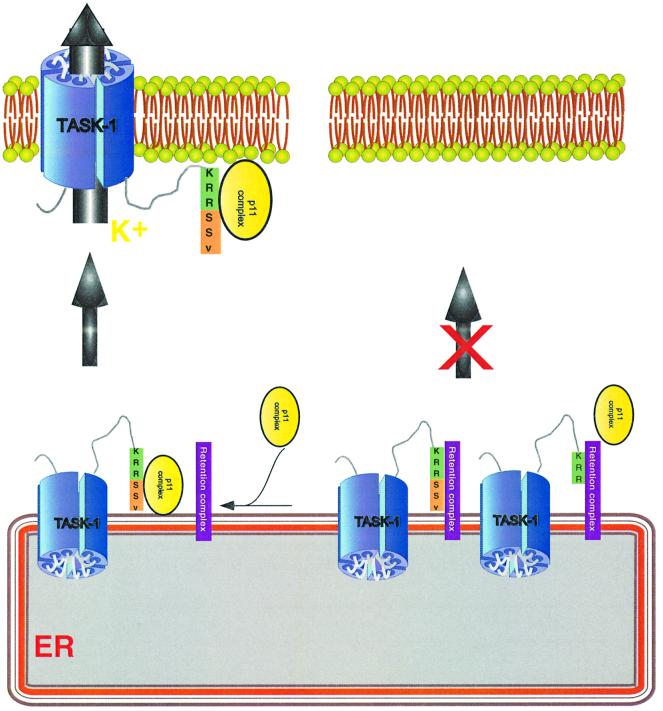
Figure 7 summarizes our view of how p11 binds to TASK-1, and suggests that the role of this association is to mask the ER retention signal KRR. In this model, when p11 is unable to bind to the channel, after C-terminal deletion, as in TASK-1ΔSSV, TASK-1ΔSV and TASK- 1ΔV mutants, the KRR sequence is evidently freely accessible and the TASK-1 channel is retained inside the ER. When p11 binds to the TASK-1wt channel, we suggest that the retention sequence is hidden and that the channel can then reach the plasma membrane. This hypothesis is supported by results obtained with GFP– TASK-1 and GFP–TASK-1ΔSSV chimera constructs, which indicate that when the C-terminal sequence SSV is absent, channels are indeed retained in intracellular compartments.
Fig. 7. Model of the interaction of human TASK-1 with p11. When the SSV sequence is present, the p11 complex can associate with the C-terminus of TASK-1. The KRR sequence is then hidden, preventing the retention complex from retaining TASK-1 channels inside the ER; channels can then be addressed to the cell membrane (left part of the figure). When the SSV sequence is missing or when p11 is absent, the p11–TASK-1 complex cannot form and the retention complex can bind to the KRR sequence, and then TASK-1 channels cannot reach the plasma membrane (right-hand side). ER, endoplasmic reticulum.
TASK-1 and TASK-3 clearly have homologous sequences, and similar biophysical and regulation properties (Kim et al., 2000; Lesage and Lazdunski, 2000; Rajan et al., 2000). Therefore, it was important to determine whether conclusions reached with TASK-1 would also hold for TASK-3. The C-terminal sequence of TASK-3 is Lys-Arg-Arg-Lys-Ser-Val, but we have shown that TASK-3 does not interact with the auxiliary protein p11. In addition, deletion of the last three C-terminal amino acids Lys-Ser-Val of TASK-3 diminished, but did not abolish, the K+ current measured in COS cells (data not shown). Contrary to those of TASK-1, the three amino acids are not crucial for the TASK-3 channel activity.
Are there other proteins with either the KRR or the SSV sequences, or both? To answer this question we screened in silico data libraries using the last 25 amino acids of TASK-1 as a probe, and we were able to identify only TASK-1 and TASK-3 channels. The KRR motif resembles the RKR motif identified in the Kir6.2 channel and SUR1 receptor (Zerangue et al., 1999), and the RRR motif present in the NMDA receptor (Standley et al., 2000). Similar polyarginine motifs have been identified in the GABAB receptor (Margeta-Mitrovic et al., 2000) and CFTR channel (Chang et al., 1999). It seems that a triplet of basic amino acids represents a widely distributed ER retention signal in membrane proteins (Ma and Jan, 2002). The SSV sequence resembles a type I PDZ consensus binding sequence S/T-X-V/L/I (where X is a non-specified amino acid). Many proteins including ionic channels, like Shaker K+ channels or voltage-gated Na+ channels, have such a C-terminal PDZ binding sequence (Harris and Lim, 2001; Hung and Sheng, 2002), but none of them has the exact SSV sequence that is specific for the p11 interaction.
Recent work suggests that the TASK-1 channel is probably inhibited by hormones and neurotransmitters via a phospholipase C-induced breakdown of polyphosphoinositides in the plasma membrane (Czirjak et al., 2001). Interestingly, annexin II associates preferentially with negatively charged phospholipids, such as polyphosphoinositides, when it binds to the plasma membrane (Drust and Creutz, 1988; Ayala-Sanmartin et al., 2001). There fore, the function of p11, in addition to its masking effect, could be to address TASK-1 channels in specific membrane areas that are enriched in negatively charged phospholipids. This localization would be important if, as suggested, the regulation by hormones and neurotransmitters involves a breakdown of PIP2 in the close vicinity of the channel.
Since the submission of this paper, Okuse et al. (2002) have proposed that p11 is a regulator of the TTX-insensitive Na+ channel, Nav1.8 (SCN10a), which is abundant in nociceptive neurons. p11 was shown to facilitate Nav1.8 trafficking to the plasma membrane, as it does for the TASK-1 channel. The binding process of p11 to the Nav1.8 channel has not been analysed in detail, but it clearly differs from that to TASK-1, since the Nav1.8 structure does not contain an SSV terminal sequence. p11 might well bind both TASK-1 and Nav1.8, as both are present in DRG neurons (N.Voilley, unpublished results). In this case, p11 would require two different sequences to recognize these two different channels. No KRR motif is found in the Nav1.8 channel. However, this channel possesses at least two retention consensus motifs: a RRR motif at position 495 and a RPR motif at position 460 (both are located in a cytoplasmic loop). In addition, it also possesses in its N-terminus several KXR motifs that resemble the RXR motif described for KATP channels (Zerangue et al., 1999). It would be very interesting to determine whether p11 also acts on this particular Na+ channel by masking one of these retention sequences.
Our data and those of Okuse et al. (2002) clearly show that p11 is an important component for the trafficking to the plasma membrane of different ionic channels, and is thus an important regulator of cell excitability.
Materials and methods
Plasmids and DNA constructs
The C-terminal end of the TASK-1 channel (amino acids 242–394) was used as bait in a yeast two-hybrid system (Grow ’n Glow system; Molecular Biologishe Tecnologie). The coding sequence corresponding to the last 152 amino acids of TASK-1, obtained by PCR, was subcloned in-frame with the LexA DNA binding domain into the yeast two-hybrid expression vector pEG202.
The C-terminal coding sequence of TASK-1 and TASK-1ΔSSV (corresponding to the deletion of the three last amino acids SSV) were also subcloned in-frame with the GST into the pGEX 3a vector (Amersham Pharmacia).
Deletion mutants of TASK-1 were obtained by PCR as described elsewhere (Yon and Fried, 1989). All constructs, including chimeras between p11 and TASK-1wt and mutant channels, were subcloned in pIRES-CD8 vector (Fink et al., 1998). Chimeras between GFP and TASK-1wt or TASK-1ΔSSV channels were generated using PCR and subcloned in pCI vector (Promega); GFP was added at the N-terminus of TASK-1 channels.
The integrity of each construct was checked by sequencing.
Yeast two-hybrid screening
The Saccharomyces cerevisiae strain EGY48 (MATα, trp1, his3, ura3, leu2::6 LexAop-LEU2) was transformed with the plasmid pEG202 containing the TASK-1 C-terminus, the plasmid pGNG1 containing the GFP reporter gene and the Match-maker human heart cDNA library (Clontech) by the lithium acetate method according to the manufacturer’s instructions (Grow ’n Glow system). Positive clones were selected on His–, Leu–, Trp–, Ura– SD medium (yeast nitrogen 0.23%, ammonium sulfate 0.67%, d-raffinose 2.67%, galactose 4%, glycerol 2.67%). All putative positive clones were exposed to standard UV light and only green clones were retained for further studies.
In vitro protein–protein interaction assay: GST pull-down
Cultures of E.coli (BL21) were transformed with pGEX recombinant vectors. Fusion proteins were purified and coupled on CNBr-activated Sepharose 4B according to the manufacturer’s protocol (Amersham Biosciences).
[35S]methionine (ICN)-labelled p11 was produced by in vitro translation (Speedread lysate; Novagen). One-fifth of the total reaction was incubated with the TASK-1-C-terminus–Sepharose 4B (or TASK- 1ΔSSV-C-terminus–Sepharose 4B) at 4°C for 15 h. Unbound proteins were removed by three washing steps with a cold buffer containing 140 mM NaCl, 50 mM Tris–HCl pH 7.5. Specifically bound proteins were eluted by boiling the samples in SDS–PAGE loading buffer and resolved on a 5–16% polyacrylamide gel. Gels were then dried and exposed to X-OMAT film (Kodak).
Co-immunoprecipitation
T7-tagged TASK-1 and T7-tagged TASK-1ΔSSV were prepared by PCR and subcloned into the pCI vector (Promega); full-length p11 was also subcloned into the pCI vector. Each construct was checked by sequencing.
COS cells were grown and transiently transfected as described previously (Tinel et al., 1998) with 2.5 µg (or 5 µg in the co-expression experiments) of pCI-T7-tagged TASK-1 (or pCI-T7-tagged TASK- 1ΔSSV) and pCI-p11 per 100 mm dish.
After 48 h of culture, cells were scraped, solubilized for 2 h at 4°C in solubilization buffer containing 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% Na deoxycholate, 10 mM Tris–HCl pH 8.0 and centrifuged at 100 000 g for 30 min. Supernatants were immunoprecipitated with T7-tag monoclonal antibodies (dilution 1/500; Novagen) or with p11 monoclonal antibodies (clone LC148, dilution 1/500; ICN) immobilized on protein A–Sepharose gel (Amersham Biosciences). After three washing steps with the solubilization buffer, immunoprecipitated proteins were eluted by boiling with the PAGE loading buffer, resolved on a 5–16% polyacrylamide gel and blotted on to PVDF membrane (Hybond-P; Amersham Biosciences). Western blot of proteins immunoprecipitated with the p11 antibodies was incubated with T7-tag antibodies (dilution 1/10 000), and western blot of proteins immunoprecipitated with T7-tag antibodies was incubated with p11 antibodies (dilution 1/10 000), 15 h at 4°C. Primary antibodies were then removed, blots were washed three times for 10 min with a phosphate-buffered saline containing 0.1% Tween-20. After the washing steps, peroxidase-conjugated anti-mouse secondary antibodies (dilution 1/20 000; Jackson Immunoresearch) were added for 2 h at 4°C. Bands were revealed with the supersignal WestPico luminescent detection system (Pierce).
Electrophysiology
Culture and transfection of COS cells (DEAE–dextran method) have been described in detail elsewhere (Lesage et al., 2000). Recordings using the whole-cell configuration of the patch–clamp technique were obtained at room temperature (20–22°C) with a RK400 patch–clamp amplifier (Bio-Logic, Claix, France). The pipette (internal) solution was 150 mM KCl, 2 mM MgCl2, 5 mM EGTA, 10 mM HEPES pH 7.2 with KOH. The external solution contained 150 mM NaCl, 5 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM HEPES pH 7.4 with NaOH. Cells were continuously perfused with a microperfusion system during the course of the experiment (0.2 ml/min). Data analysis was performed using pClamp software. Results are expressed as mean ± SEM, with n indicating the number of cells tested.
GFP fluorescence
COS cells were seeded at 100 000 cells per 35-mm dish 24 h before the transfection. Cells were transfected with 1 µg of plasmid containing GFP chimeras using the DEAE–dextran method. After 48 h of culture, cells were dissociated and plated on to polylysine treated 12-mm glass coverslips. Twenty-four hours after plating, cells were fixed for 15 min with a 4% (w/v) paraformaldehyde/phosphate saline buffer.
Transfected cells were visualized on a Leica TCSSP confocal microscope. Enhanced GFP was excited at 488 nm using a mixed gas argon/krypton laser; the emitted fluorescence was monitored at wavelength of 500–600 nm.
Acknowledgments
Acknowledgements
We are grateful to Dr M.Franco and C.Favard for their confocal expertise, and to Dr A.Patel for reading the manuscript. We also thank N.Leroudier, M.Jodar and F.Aguila for excellent technical assistance, and V.Lopez for secretarial assistance. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Association Francaise contre les Myopathies (AFM), the Ministère de la Recherche, the Paul Hamel Institute and the Conseil Régional PACA.
References
- Ali S.M., Geisow,M.J. and Burgoyne,R.D. (1989) A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature, 340, 313–315. [DOI] [PubMed] [Google Scholar]
- An W.F. et al. (2000) Modulation of A-type potassium channels by a family of calcium sensors. Nature, 403, 553–556. [DOI] [PubMed] [Google Scholar]
- Attali B. (2002) Human congenital long QT syndrome: more than previously thought? Trends Pharmacol. Sci., 23, 249–251. [DOI] [PubMed] [Google Scholar]
- Ayala-Sanmartin J., Henry,J.P. and Pradel,L.A. (2001) Cholesterol regulates membrane binding and aggregation by annexin 2 at submicromolar Ca2+ concentration. Biochim. Biophys. Acta, 1510, 18–28. [DOI] [PubMed] [Google Scholar]
- Bang H., Kim,Y. and Kim,D. (2000) TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J. Biol. Chem., 275, 17412–17419. [DOI] [PubMed] [Google Scholar]
- Barbuti A., Ishii,S., Shimizu,T., Robinson,R.B. and Feinmark,S.J. (2002) Block of the background K+ channel TASK-1 contributes to arrhythmogenic effects of platelet-activating factor. Am. J. Physiol. Heart Circ. Physiol., 282, H2024–H2030. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Attali,B. and Lazdunski,M. (1998) IKs, a slow and intriguing cardiac K+ channel and its associated long QT diseases. Trends Cardiovasc. Med., 8, 207–214. [DOI] [PubMed] [Google Scholar]
- Buckler K.J., Williams,B.A. and Honore,E. (2000) An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol., 525, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.B., Cui,L., Hou,Y.X., Jensen,T.J., Aleksandrov,A.A., Mengos,A. and Riordan,J.R. (1999) Removal of multiple arginine-framed trafficking signals overcomes misprocessing of delta F508 CFTR present in most patients with cystic fibrosis. Mol. Cell, 4, 137–142. [DOI] [PubMed] [Google Scholar]
- Chavez R.A., Gray,A.T., Zhao,B.B., Kindler,C.H., Mazurek,M.J., Mehta,Y., Forsayeth,J.R. and Yost,C.S. (1999) TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. J. Biol. Chem., 274, 7887–7892. [DOI] [PubMed] [Google Scholar]
- Cooper J.A. (1987) Effects of cytochalasin and phalloidin on actin. J. Cell Biol., 105, 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirjak G. and Enyedi,P. (2002) Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J. Biol. Chem., 277, 5426–5432. [DOI] [PubMed] [Google Scholar]
- Czirjak G., Fischer,T., Spat,A., Lesage,F. and Enyedi,P. (2000) TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol. Endocrinol., 14, 863–874. [DOI] [PubMed] [Google Scholar]
- Czirjak G., Petheo,G.L., Spat,A. and Enyedi,P. (2001) Inhibition of TASK-1 potassium channel by phospholipase C. Am. J. Physiol. Cell Physiol., 281, C700–C708. [DOI] [PubMed] [Google Scholar]
- Decher N., Maier,M., Dittrich,W., Gassenhuber,J., Bruggemann,A., Busch,A.E. and Steinmeyer,K. (2001) Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett., 492, 84–89. [DOI] [PubMed] [Google Scholar]
- Donato R. (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol., 33, 637–668. [DOI] [PubMed] [Google Scholar]
- Dooley T.P., Weiland,K.L. and Simon,M. (1992) cDNA sequence of human p11 calpactin I light chain. Genomics, 13, 866–868. [DOI] [PubMed] [Google Scholar]
- Drust D.S. and Creutz,C.E. (1988) Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature, 331, 88–91. [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage,F., Fink,M., Reyes,R., Heurteaux,C. and Lazdunski,M. (1997) TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J., 16, 5464–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N., Gorvel,J.P., Walter,C., Gerke,V., Kellner,R., Griffiths,G. and Gruenberg,J. (1993) Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol., 120, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko N.R. and Waisman,D.M. (2001) The C terminus of annexin II mediates binding to F-actin. J. Biol. Chem., 276, 5310–5315. [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat,F., Lesage,F., Reyes,R., Romey,G., Heurteaux,C. and Lazdunski,M. (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J., 15, 6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M., Lesage,F., Duprat,F., Heurteaux,C., Reyes,R., Fosset,M. and Lazdunski,M. (1998) A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acid. EMBO J., 17, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V. and Moss,S.E. (2002) Annexins: from structure to function. Physiol. Rev., 82, 331–371. [DOI] [PubMed] [Google Scholar]
- Girard C., Duprat,F., Terrenoire,C., Tinel,N., Fosset,M., Romey,G., Lazdunski,M. and Lesage,F. (2001) Genomic and functional characteristics of novel human pancreatic 2P domain K+ channels. Biochem. Biophys. Res. Commun., 282, 249–256. [DOI] [PubMed] [Google Scholar]
- Harder T. and Gerke,V. (1993) The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J. Cell Biol., 123, 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B.Z. and Lim,W.A. (2001) Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci., 114, 3219–3231. [DOI] [PubMed] [Google Scholar]
- Hung A.Y. and Sheng,M. (2002) PDZ domains: structural modules for protein complex assembly. J. Biol. Chem., 277, 5699–5702. [DOI] [PubMed] [Google Scholar]
- Kim D. and Gnatenco,C. (2001) TASK-5, a new member of the tandem-pore K+ channel family. Biochem. Biophys. Res. Commun., 284, 923–930. [DOI] [PubMed] [Google Scholar]
- Kim Y., Bang,H. and Kim,D. (1999) TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am. J. Physiol., 277, H1669–H1678. [DOI] [PubMed] [Google Scholar]
- Kim Y., Bang,H. and Kim,D. (2000) TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem., 275, 9340–9347. [DOI] [PubMed] [Google Scholar]
- Kuo H.C. et al. (2001) A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell, 107, 801–813. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D., Gray,A.T., Winegar,B.D., Kindler,C.H., Harada,M., Taylor,D.M., Chavez,R.A., Forsayeth,J.R. and Yost,C.S. (1998) An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J. Neurosci., 18, 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F. and Lazdunski,M. (2000) Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Renal Physiol., 279, F793–F801. [DOI] [PubMed] [Google Scholar]
- Lesage F., Guillemare,E., Fink,M., Duprat,F., Lazdunski,M., Romey,G. and Barhanin,J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J., 15, 1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Terrenoire,C., Romey,G. and Lazdunski,M. (2000) Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids and Gs, Gi and Gq protein-coupled receptors. J. Biol. Chem., 275, 28398–28405. [DOI] [PubMed] [Google Scholar]
- Ma D. and Jan,L.Y. (2002) ER transport signals and trafficking of potassium channels and receptors. Curr. Opin. Neurobiol., 12, 287–292. [DOI] [PubMed] [Google Scholar]
- Mailliard W.S., Haigler,H.T. and Schlaepfer,D.D. (1996) Calcium-dependent binding of S100C to the N-terminal domain of annexin I. J. Biol. Chem., 271, 719–725. [DOI] [PubMed] [Google Scholar]
- Maingret F., Patel,A.J., Lazdunski,M. and Honore,E. (2001) The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J., 20, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M., Jan,Y.N. and Jan,L.Y. (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron, 27, 97–106. [DOI] [PubMed] [Google Scholar]
- Mayorga L.S., Beron,W., Sarrouf,M.N., Colombo,M.I., Creutz,C. and Stahl,P.D. (1994) Calcium-dependent fusion among endosomes. J. Biol. Chem., 269, 30927–30934. [PubMed] [Google Scholar]
- Millar J.A., Barratt,L., Southan,A.P., Page,K.M., Fyffe,R.E., Robertson,B. and Mathie,A. (2000) A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc. Natl Acad. Sci. USA, 97, 3614–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M.J., Castellino,R.C., Crews,A.L., Rasmusson,R.L. and Strauss,H.C. (1995) A novel β subunit increases rate of inactivation of specific voltage-gated potassium channel α subunits. J. Biol. Chem., 270, 6272–6277. [DOI] [PubMed] [Google Scholar]
- Okuse K., Malik-Hall,M., Baker,M.D., Poon,W.Y., Kong,H., Chao,M.V. and Wood,J.N. (2002) Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature, 417, 653–656. [DOI] [PubMed] [Google Scholar]
- Patel A.J. and Honore,E. (2001) Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci., 24, 339–346. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Maingret,F., Lesage,F., Fink,M., Duprat,F. and Lazdunski,M. (1998) A mammalian two pore domain mechano-gated S-type K+ channel. EMBO J., 17, 4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Lesage,F., Fink,M., Romey,G. and Lazdunski,M. (1999) Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci., 2, 422–426. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Maingret,F., Magnone,V., Fosset,M., Lazdunski,M. and Honore,E. (2000) TWIK-2, an inactivating 2P domain K+ channel. J. Biol. Chem., 275, 28722–28730. [DOI] [PubMed] [Google Scholar]
- Rajan S., Wischmeyer,E., Xin Liu,G., Preisig-Muller,R., Daut,J., Karschin,A. and Derst,C. (2000) TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J. Biol. Chem., 275, 16650–16657. [DOI] [PubMed] [Google Scholar]
- Rajan S., Wischmeyer,E., Karschin,C., Preisig-Muller,R., Grzeschik,K.H., Daut,J., Karschin,A. and Derst,C. (2001) THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J. Biol. Chem., 276, 7302–7311. [DOI] [PubMed] [Google Scholar]
- Rehm H. and Lazdunski,M. (1988) Purification and subunit structure of a putative K+-channel protein identified by its binding properties for dendrotoxin I. Proc. Natl Acad. Sci. USA, 85, 4919–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J., Heinemann,S.H., Wunder,F., Lorra,C., Parcej,D.N., Dolly,J.O. and Pongs,O. (1994) Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature, 369, 289–294. [DOI] [PubMed] [Google Scholar]
- Rety S., Sopkova,J., Renouard,M., Osterloh,D., Gerke,V., Tabaries,S., Russo-Marie,F. and Lewit-Bentley,A. (1999) The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat. Struct. Biol., 6, 89–95. [DOI] [PubMed] [Google Scholar]
- Reyes R., Duprat,F., Lesage,F., Fink,M., Farman,N. and Lazdunski,M. (1998) Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem., 273, 30863–30869. [DOI] [PubMed] [Google Scholar]
- Salinas M., Reyes,R., Lesage,F., Fosset,M., Heurteaux,C., Romey,G. and Lazdunski,M. (1999) Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J. Biol. Chem., 274, 11751–11760. [DOI] [PubMed] [Google Scholar]
- Sirois J.E., Lei,Q., Talley,E.M., Lynch,C.,III and Bayliss,D.A. (2000) The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J. Neurosci., 20, 6347–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley S., Roche,K.W., McCallum,J., Sans,N. and Wenthold,R.J. (2000) PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron, 28, 887–898. [DOI] [PubMed] [Google Scholar]
- Talley E.M. and Bayliss,D.A. (2002) Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J. Biol. Chem., 277, 17733–17742. [DOI] [PubMed] [Google Scholar]
- Talley E.M., Lei,Q., Sirois,J.E. and Bayliss,D.A. (2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron, 25, 399–410. [DOI] [PubMed] [Google Scholar]
- Tinel N., Lauritzen,I., Chouabe,C., Lazdunski,M. and Borsotto,M. (1998) The KCNQ2 potassium channel: splice variants, functional and developmental expression. Brain localization and comparison with KCNQ3. FEBS Lett., 438, 171–176. [DOI] [PubMed] [Google Scholar]
- Tinel N., Diochot,S., Borsotto,M., Lazdunski,M. and Barhanin,J. (2000) KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J., 19, 6326–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu H., Mizutani,A., Minami,H., Kobayashi,R. and Hidaka,H. (1992) A calcyclin-associated protein is a newly identified member of the Ca2+/phospholipid-binding proteins, annexin family. J. Biol. Chem., 267, 8919–8924. [PubMed] [Google Scholar]
- Warth R. and Barhanin,J. (2002) The multifaceted phenotype of the knockout mouse for the KCNE1 potassium channel gene. Am. J. Physiol. Regul. Integr. Comp. Physiol., 282, R639–R648. [DOI] [PubMed] [Google Scholar]
- Washburn C.P., Sirois,J.E., Talley,E.M., Guyenet,P.G. and Bayliss,D.A. (2002) Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J. Neurosci., 22, 1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible B.A., Yang,Q., Kuryshev,Y.A., Accili,E.A. and Brown,A.M. (1998) Cloning and expression of a novel K+ channel regulatory protein, KChAP. J. Biol. Chem., 273, 11745–11751. [DOI] [PubMed] [Google Scholar]
- Yon J. and Fried,M. (1989) Precise gene fusion by PCR. Nucleic Acids Res., 17, 4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N., Schwappach,B., Jan,Y.N. and Jan,L.Y. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron, 22, 537–548. [DOI] [PubMed] [Google Scholar]