Abstract
Invasion of the intestinal epithelium by Salmonella sp. requires a type III secretion system (TTSS) common in many bacterial pathogens. TTSS translocate effector proteins from bacteria into eukaryotic cells. These effectors manipulate cellular functions in order to benefit the pathogen. In the human and animal pathogen Salmonella typhimurium, the expression of genes encoding the secreted effector molecules Sip/Ssp ABCD, SigD, SptP and SopE requires both the AraC/XylS-like regulator InvF and the secretion chaperone SicA. In this work, an InvF binding site was identified in the promoter regions of three operons. SicA does not appear to affect InvF stability nor to bind DNA directly. However, SicA could be co-purified with InvF, suggesting that InvF and SicA interact with each other to activate transcription from the effector gene promoters. This is the first demonstration of a contact between a protein cofactor and an AraC/XylS family transcriptional regulator and, moreover, is the first direct evidence of an interaction between a transcriptional regulator and a TTSS chaperone. The regulation of effector genes described here for InvF and SicA may represent a new paradigm for regulation of virulence in a wide variety of pathogens.
Keywords: AraC/binding site/chaperone/regulation
Introduction
Salmonella species are Gram-negative, motile bacteria that cause diseases in humans ranging from a mild gastroenteritis (S.typhimurium, S.enteritidis) to a systemic disease that can result in death (S.typhi, S.paratyphi) (for a review see Darwin and Miller, 1999b). In a mouse model of oral infection, S.typhimurium can penetrate the intestinal epithelium and reach the deeper tissues of the liver and spleen. The invasion of the intestinal epithelium is an important initial step in pathogenesis by Salmonella as well as several other pathogenic Enterobacteriaceae. Much research has involved the use of in vitro tissue culture systems in order to identify genes involved in invasion of non-phagocytic cells. So far, the major contributing factors by which Salmonella spp. invade epithelial cells are encoded within a pathogenicity island known as Salmonella pathogenicity island 1 (SPI1) (Mills et al., 1995). The genes within this pathogenicity island are not found in Escherichia coli K-12 strains and appear to be specific for invasive Salmonella species. SPI1 encodes at least 30 proteins necessary for the production of a type III secretion system (TTSS), which secretes protein effectors out of the bacterium and translocates them into host cells (reviewed in Hueck, 1998). The effector molecules stimulate morphological changes of the eukaryotic cells, resulting in engulfment of the bacteria. Mutations in the TTSS genes result in a significant reduction of bacterial invasion in vitro (Galán and Curtiss, 1989; Galán et al., 1992; Stone et al., 1992; Behlau and Miller, 1993; Groisman and Ochman, 1993; Jones and Falkow, 1994; Kaniga et al., 1994) and a 100-fold attenuation in mice that have been infected by an oral route (Penheiter et al., 1997). In addition to invasion, the SPI1 TTSS appears to play a role in inducing apoptosis of macrophages (Hersh et al., 1999) and stimulating the transmigration of polymorphonuclear leukocytes (PMN) both in vitro and in vivo (McCormick et al., 1995; Galyov et al., 1997; Lee et al., 2000). Similar secretion systems are found in other pathogenic bacteria, such as species of Bordetella, Pseudomonas, Shigella, Yersinia and pathogenic E.coli, and are also required for virulence (reviewed in Hueck, 1998).
A central regulator of SPI1 expression is HilA (Bajaj et al., 1995). Currently, it is known that many environmental signals affect the expression of the SPI1 genes via HilA (Bajaj et al., 1996). HilA binds to a consensus sequence (HilA box) to activate the expression of the regulatory gene invF, the first gene of the large, putative SPI1 inv-spa-sic-sip/ssp operon (Lostroh et al., 2000). Genes encoding components of the TTSS are included within this and another HilA-dependent operon, prgHIJKorgAB (Behlau and Miller, 1993; Jones and Falkow, 1994). InvF, a member of the AraC/XylS family of transcriptional activators (for a review see Gallegos et al., 1997), and SicA (Salmonella invasion chaperone), a type III secretion chaperone specific for Sip/SspB and Sip/SspC (Salmonella invasion protein/Salmonella secreted protein) (Tucker and Galán, 2000), are required for the expression of several genes encoding proteins secreted by the TTSS and their cognate chaperones (Darwin and Miller, 1999a, 2000; Eichelberg and Galán, 1999). These include sigDE (Salmonella invasion gene), sopE (Salmonella outer protein), sicAsip/sspBCDA and sicPsptP (Salmonella tyrosine phosphatase). Genetic studies have suggested that SicA acts as a cofactor or inducer for InvF-dependent transcription activation of the sicA and sigD promoters (Darwin and Miller, 2000). However, it was not known whether or how SicA interacted with InvF, RNA polymerase, DNA or RNA. Prior to the suggestion that SicA may serve as a cofactor for InvF, a protein cofactor or co-activator had not been described for an AraC/XylS-family regulator. In fact, many AraC/XylS-like regulators have been assumed to act alone or to interact with small molecules such as sugars (Gallegos et al., 1997).
Type III chaperones typically interact with only one or two cognate effector molecules (Wattiau et al., 1996). Several of these chaperones have been suggested to participate in negative feedback regulation of virulence genes in Yersinia species (Bergman et al., 1991). How ever, no type III secretion chaperone has been directly associated with transcription regulation. In this work, promoter sequences that require both InvF and SicA for transcription activation were identified. In addition, evidence for a direct interaction between InvF and SicA is presented.
Results
Identification of an InvF/SicA-dependent consensus sequence for the sicA, sigD and sopE promoters
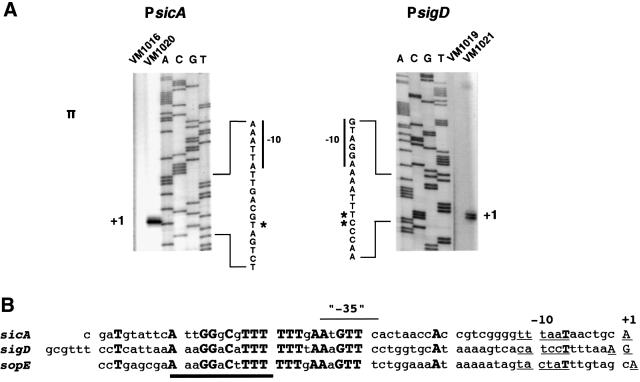
In order to identify an InvF/SicA consensus binding site, the starts of transcription (+1) sites of the sicA and sigD promoters were mapped by primer extension (see Materials and methods). RNA was purified from wild-type (wt) and ΔinvF Salmonella strains and E.coli containing either a sicA- or sigD-lacZYA fusion in plasmid pRW50 (1–2 copies per cell) (Figure 1A) (see Table I for primer pairs used to make each pRW50 derivative and Table II for primer sequences). The sicA-11 primer was used in primer extension analysis of sicA and the sigP2 primer was used in primer extension analysis of sigD. sicA and sigD transcripts were detectable from wt Salmonella, but no transcript was detectable from the ΔinvF mutant (data not shown). Escherichia coli that contained plasmids encoding invF and sicA produced abundant amounts of the sicA and sigD transcripts (Figure 1A, strains VM1020 and VM1021, respectively). The starts of transcription were the same in E.coli and Salmonella (the signal was much weaker in Salmonella and, therefore, is not shown in Figure 1A for clarity). The sigD promoter appeared to have two possible +1 sites (A or G), while the sicA promoter had a single distinct +1 site (A). The start of transcription for sopE was also mapped (A) from RNA isolated from wt Salmonella (L.Schechter and C.Lee, personal communication) (Figure 1B and data not shown).
Fig. 1. Primer extension analysis of the sicA, sigD and sopE promoters. (A) Primer extension products from the sicA and sigD promoters. The sequences indicated are of the antisense (bottom) strand. The asterisk indicates the start of transcription sites. See Table V for strain and plasmid descriptions. Shown are results from expression in E.coli; similar results were obtained in Salmonella. (B) Sequence alignment of the sicA, sigD and sopE promoters. Conserved sequences are indicated in bold and the starts of transcription sites (+1) are capitalized and underlined. The –10 hexamers are underlined. The minimal InvF binding domain (based on genetic analyses) is indicated by the heavy line.
Table I. Oligonucleotide pairs used for construction of lacZYA reporter fusions in pRW50.
| 5′ primer | 3′ primer | Size of fragment | Name of pRW50 derivative |
|---|---|---|---|
| spaS-EcoRI-3 | sicA-BamHI-1 | 404 | pHD11 |
| sicA-EcoRI-12 | sicA-BamHI-1 | 196 | pHD83 |
| sicA-EcoRI-13 | sicA-BamHI-1 | 173 | pHD84 |
| sicA-EcoRI-mut1 | sicA-BamHI-1 | 196 | pHD98 |
| sicA-EcoRI-mut2 | sicA-BamHI-1 | 196 | pHD96, pHD97 |
| sicA-EcoRI-mut3 | sicA-BamHI-1 | 196 | pHD99 |
| sicA-EcoRI-12 | sicA15 | 65 | pHD100 |
| M13reverse primer | sigP2 | 441 | pHD86 |
| sigD-EcoRI-3 | sigP2 | 157 | pHD87 |
| sigD-EcoRI-5 | sigP2 | 129 | pHD88 |
| sopE-EcoRI-4 | sopE-BamHI-4 | 95 | pHD95 |
| iacP-EcoRI-1 | sicP-BamHI-3 | ∼300 | pHD50 |
Table II. Oligonucleotide sequences used for construction of reporter fusions and primer extension reactions.
| Primer name | Sequence (5′ to 3′) |
|---|---|
| M13 Universala | AGCGGATAACAATTTCACACAGGA |
| spaS-EcoRI-3 | GGAATTCCGGGTTAAGCAGTTGGTTTTACGAGCGAGGGGCG |
| sicA-EcoRI-12 | GGAATTCCGATGTATTCATTGGGCG |
| sicA-EcoRI-13 | GGAATTCCTGAATGTTCACTAACCACCG |
| sicA-EcoRI-mut1b | GGAATTCCGATGTATTCBTTGGGCG |
| sicA-EcoRI-mut2c | GGAATTCCGATGTATTCATTHGGCG |
| sicA-EcoRI-mut3d | GGAATTCCGATGTATTCATTGGGDG |
| sicA-BamHI-1 | CGGGATCCCGGCGTGGCGCCTTCACTAACGGCATCC |
| sicA11 | ACATTATTTTGATAATCCATTACTTACTTCCTGTTAT |
| sicA15 | GCAGTTATTAAACCCCGACGG |
| sigD-EcoRI-3 | GGAATTCCGCGTTTCCTCATTAAAAAGGAC |
| sigD-EcoRI-4 | GGAATTCCTTAAAGTTCCTGGTGCATAAAAG |
| sigP2 | GTGAAGCTGAGTGATAGAAGCTCTGTATTTGC |
| sopE-EcoRI-4 | GGAATTCCTGAGCGAAAAGGACTTTTTTTGAA |
| sopE-BamHI-4 | CGGGATCCCGATGAATTAGAAAAATTCGGCTGATTC |
| iacP-EcoRI-1 | GGAATTCCCCTTTGCGGATATATGCCTGTTG |
| sicP-BamHI-3 | CGGGATCCCGCGTACCTTCATTATTCGCAGCC |
| sptP-XbaI | GCTCTAGACTGCAGGAATATGCTAAAGTATG |
| sptP-2 | GTCCCTTTAGCGCGATATCGAG |
aNew England Biolabs 24mer reverse sequencing primer (–48) #1233.
bB indicates that either a C, G or T nucleotide was incorporated at this position.
cH indicates that either an A, C or T nucleotide was incorporated at this position.
dD indicates that either an A, C or T nucleotide was incorporated at this position.
All of the promoters have potential σ70-dependent –10 hexamers ranging from 6 to 8 bp upstream of the +1 site. To identify a putative consensus activator binding site in the sicA, sigD and sopE promoters, the DNA sequences beginning with the –10 hexamers were aligned. A conserved region was identified in all three promoters, each with identical spacing from the +1 (Figure 1B). None of the promoters had a conspicuous –35 hexamer located 17 bp upstream of the –10 region. However, this was not surprising because many activator-dependent promoters lack this sequence (Busby and Ebright, 1994). The –35 regions in the sicA, sigD and sopE promoters share conserved nucleotides, suggesting that the putative activator binding site may overlap this region.
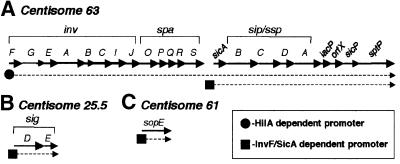
sptP, which encodes an effector protein secreted by the SPI1 TTSS (Fu and Galán, 1998b), was previously shown to be InvF and HilA dependent (Eichelberg and Galán, 1999), and is encoded downstream of the InvF/SicA-dependent sicAsip/sspBCDA operon. In the absence of InvF, sptP expression can be activated by HilA, suggesting that sptP can be expressed from a HilA-dependent promoter as well as from the InvF/SicA-dependent sicA promoter (Eichelberg and Galán, 1999). We constructed another sptP-lacZYA chromosome fusion (by insertion of pHD61) and found it to be, in addition to being InvF dependent, SicA dependent (data not shown). In contrast, a low-copy plasmid reporter, pHD50, containing sequence between iacP (encoded immediately downstream of sip/sspA) and sicP (the gene immediately upstream of sptP), failed to show hilA-, invF- or sicA-dependent regulation (data not shown). Moreover, a putative InvF consensus binding site was not found downstream of sicA or upstream of sptP. Taken together with previous data, it is likely that sptP expression is dependent on both a HilA-dependent promoter, i.e. the invF promoter (Bajaj et al., 1995; Lostroh et al., 2000), and the InvF/SicA-dependent sicA promoter, but not another promoter downstream of sicA. Based on these data, the HilA-dependent transcript would include the inv-spa-sicA-sip/ssp-iacP-orfX-sicP-sptP genes, while the InvF/SicA dependent transcript would encode sicA-sip/ssp-iacP-orfX-sicP-sptP genes (Figure 2A). Nevertheless, we can not rule out the existence of another promoter upstream of sptP that is InvF/SicA independent. In contrast to sptP (and sicAsip/sspBCDA), sigDE and sopE appear to be activated by an InvF/SicA-dependent promoter but not a HilA-dependent promoter (Figure 2B and C).
Fig. 2. Transcriptional organization of InvF/SicA regulated genes. Solid arrows indicate genes and dotted lines indicate either known or putative transcripts. The chromosomal location of each locus is indicated above. In the process of characterizing the region between iacP and sicP for an InvF/SicA-dependent promoter, we sequenced the region and found a small putative open reading frame, which we have designated orfX. This figure is not drawn to scale.
Deletion and point mutation analysis of the conserved sequences
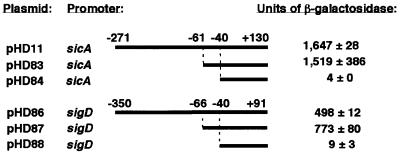
To determine the minimal sequence required for InvF/SicA-dependent activation of the sicA and sigD promoters, nested deletions of each promoter were constructed from the pRW50 fusions (‘long promoter’ constructs, pHD11 and pHD86) used for primer extension. Escherichia coli CC118λpir with plasmids encoding invF and sicA were transformed with each reporter plasmid. Deletion of sequences (from the 5′ end) of the long promoter constructs up to 3 or 8 bp upstream of the first conserved thymidine (T) (–58) (‘short promoter’ constructs, pHD83 and pHD87, respectively) from either the sicA or sigD promoter did not reduce β-galactosidase activity in E.coli when compared with the long promoter constructs (Figure 3). The slight increase in β-galactosidase activity for the sigD short promoter, pHD87, relative to the long sigD promoter construct, pHD86, was consistently observed. The significance of this observation is not clear, but raises the possibility that there is a sequence that has a negative impact on transcription upstream of the InvF/SicA activation site. Deletion of 21 or 26 bp from the 5′ end of the sicA or sigD short promoters, respectively, eliminated all but background levels of lacZYA expression (pHD84 and pHD88).
Fig. 3. Deletion analysis of the sicA and sigD promoters in E.coli. Strain CC118λpir containing invF (pHD9-1) and sicA (pHD30-2) was transformed with reporter plasmids containing either the wt (‘long’) promoters or nested deletions from the 5′ region of the long promoters. Nucleotide positions relative to +1 are indicated. Units of β-galactosidase activity (Miller units ± SD) are indicated on the right. The reporter vector pRW50 produces 0–1 Miller unit (data not shown). These values are representative of duplicate assays performed on different days.
To assess the behavior of the promoter fusions under native conditions, wt, invF and sicA S.typhimurium strains were transformed with the reporter fusions. As in E.coli, the expression of the short fusions was virtually identical to that of the long fusions for the sicA promoter (long versus short: 132 versus 108 Miller units, respectively) and sigD promoter (long versus short: 42 versus 49 Miller units, respectively). The expression of the short promoter constructs was complemented by invF and sicA in the invF and sicA mutants, respectively, as previously described for the long promoters (Table III) (Darwin and Miller, 2000). The fusions that were not activated in E.coli (pHD84 and pHD88) also exhibited background expression in S.typhimurium (5 Miller units for pHD84 and 20 Miller units for pHD88 in either wt or ΔinvF strains).
Table III. Complementation of ΔinvF and sicA::aphT mutations for the expression of lacZY(A) fusions to PsicA, PsigD and PsopE .
| Strain background |
Reporter |
Units of β-galactosidase activitya |
||
|---|---|---|---|---|
| + vector | + invFb | + sicAb | ||
| Wild type | Φ(sicA-lacZYA) (pHD83) | 170 ± 17 | 2509 ± 56 | 232 ± 17 |
| ΔinvF | Φ(sicA-lacZYA) (pHD83) | 4 ± 0.5 | 2547 ± 143 | 6 |
| sicA::aphT | Φ(sicA-lacZYA) (pHD83) | 5 | 7 ± 1 | 205 ± 11 |
| Wild type | Φ(sigD-lacZYA) (pHD87) | 40 ± 7 | 515 ± 40 | 43 ± 3 |
| ΔinvF | Φ(sigD-lacZYA) (pHD87) | 16 ± 0.5 | 460 ± 56 | 15 ± 0.5 |
| sicA::aphT | Φ(sigD-lacZYA) (pHD87) | 16 | 15 | 72 ± 0.5 |
| Wild type | Φ(sopE-lacZYA) (pHD95) | 34 ± 3 | 1451 ± 28 | 52 ± 4 |
| ΔinvF | Φ(sopE-lacZYA) (pHD95) | 9 | 1330 ± 68 | 8 |
| sicA::aphT | Φ(sopE-lacZYA) (pHD95) | 9 | 10 ± 0.5 | 53 ± 3 |
| Wild type | Φ(sopE-lacZY)/sopE– (chromosome) | 742 ± 93 | 1983 ± 394 | 804 ± 71 |
| ΔinvF | Φ(sopE-lacZY)/sopE– (chromosome) | 223 ± 42 | 1628 ± 149 | 156 ± 6 |
| sicA::aphT | Φ(sopE-lacZY)/sopE– (chromosome) | 155 ± 17 | 164 ± 5 | 669 ± 241 |
aUnits of β-galactosidase (± SD) represent the average of duplicate β-galactosidase assays performed on duplicate cultures and are representative of several assays performed on different days. If the SD was zero, it was not indicated in the table. Cultures were grown without aeration in screw-capped tubes at 37°C for 18 h.
bFor the pRW50-based reporters, invF and sicA were provided by pHD10-1 and pHD30-2, respectively, for complementation of ΔinvF and sicA::aphT. For the sopE-lacZY chromosomal reporter, invF and sicA were provided by pHD17 and pHD71, respectively, for complementation of ΔinvF and sicA::aphT.
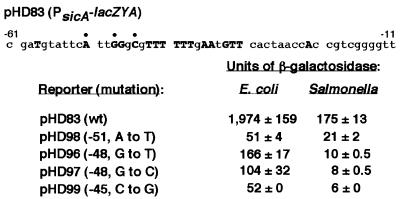
Deletion analysis began to define a region required for InvF/SicA-dependent transcription. To determine the importance of the conserved base pairs in this region, three nucleotides, conserved in all three promoters, were selected for site-directed mutagenesis of the sicA short promoter. Single base pair substitutions at any of the three positions (–51, –48, –45) significantly reduced activation of the sicA-lacZYA fusion in E.coli containing invF and sicA in multicopy (Figure 4). When these reporters, pHD96, pHD97, pHD98 and pHD99, were introduced into wt S.typhimurium, lacZYA expression was nearly abolished when compared with the wt sicA short promoter reporter pHD83 (Figure 4). Thus, these base pairs are likely to be an important part of the InvF/SicA consensus binding site.
Fig. 4. Point mutation analysis of the sicA promoter in E.coli (containing invF and sicA, on pHD9-1 and pHD30-2, respectively) and wt S.typhimurium. Mutations at three conserved nucleotides (indicated with bullets above) were introduced by site-directed mutagenesis. Positions and substitutions of each nucleotide are indicated next to the respective plasmid. Units of β-galactosidase (Miller units ± SD) are indicated on the right. These values are representative of duplicate assays performed on different days.
Expression of sopE requires both InvF and SicA
sopE encodes an invasion protein and is located on a cryptic prophage outside of SPI1 (Hardt et al., 1998b). Previous work has shown that sopE is InvF regulated (Eichelberg and Galán, 1999). In addition, a mutation in sicA has been shown to reduce the expression of sopE (Tucker and Galán, 2000). In this study, a putative InvF/SicA-dependent consensus site was identified upstream of sopE. In the work by Tucker and Galán (2000), a disruption mutation in sip/sspC could suppress the sicA defect for sopE-lacZY expression. This result suggested that in the absence of SicA and Sip/SspC, InvF could activate transcription of the sopE promoter. Because sicA and invF, but not invF alone, are required to activate transcription of the sicA and sigD promoters in E.coli and Salmonella, the results of the Tucker and Galán sopE study suggested that the sopE promoter may be regulated by a different mechanism. We attempted to reproduce their work by combining the same sicA::aphT mutation with a complete deletion of sip/sspC (Scherer et al., 2000) and measuring transcription from both a sopE-lacZYA plasmid reporter pHD95 (see Tables I and II for construct description) and a sigD-lacZYA chromosomal reporter. In both cases, the deletion in sip/sspC did not suppress the sicA::aphT mutation for activation of the sopE (49 ± 13 versus 11 ± 0 for wt versus sicAΔsspC strains, respectively) or sigD (59 ± 6 versus 3 ± 0.5 Miller units for wt versus sicAΔsspC strains, respectively) reporter fusions.
Similarly to the sicA- and sigD-lacZYA reporters, either the plasmid or chromosomal sopE reporter in an invF or sicA mutant could be fully complemented by invF or sicA, respectively (Table III). In addition, the sopE plasmid reporter could be activated 100-fold in E.coli CC118λpir containing invF and sicA (998 ± 53 versus 10.5 ± 0.5 without sicA), as was observed for the sicA- and sigD-lacZYA plasmid reporters (Darwin and Miller, 2000). Taken together, these results suggest that, like the sicA and sigD promoters, expression from the sopE promoter requires both InvF and SicA. Moreover, these results do not support the model of Tucker and Galán, which suggests that Sip/SspC is involved, directly or indirectly, in transcription of the sopE promoter.
InvF binds to DNA in vitro
The common feature among the AraC/XylS family members is the presence of two helix–turn–helix (HTH) motifs at the C-terminal domain of the protein (Gallegos et al., 1997). Although InvF has these two HTH motifs, it has not been shown to bind DNA. A filter binding assay was used to see whether InvF or SicA could bind to the sicA promoter (see Materials and methods). A 196 bp end-labeled sicA promoter fragment (the same fragment in pHD83) was incubated with InvF-His6, SicA-His6, or a mixture of both proteins that was partially purified by nickel–agarose affinity purification. InvF alone could bind to the sicA promoter in the absence of SicA (Figure 5). The binding of InvF-His6 appeared to be specific because it did not bind significantly to the lac promoter (Figure 5). The presence of SicA with InvF-His6 did not appear to increase binding of InvF to the sicA promoter (data not shown). In addition, SicA did not bind to DNA in this assay; however, we can not rule out the possibility that SicA plays a role in increasing the binding affinity of InvF to DNA or that SicA itself binds to DNA in vivo.
Fig. 5. InvF binds to the sicA promoter in a filter binding assay. A total of 15 000 c.p.m. of each 32P-labeled probe were incubated with no protein, SicA-His6 or InvF-His6 and filtered through nitrocellulose. The membranes were exposed to film to visualize the bound DNA. The membrane-retained c.p.m. were calculated as described in Materials and methods.
In addition to the wt sicA promoter, the mutant sicA promoters from pHD97 (–48, G to C) and pHD99 (–45, C to G) (called mut97 and mut99, respectively) were radioactively labeled and tested for binding to InvF-His6. InvF-His6 did not bind to the mutant sicA promoters as well as it bound to the wt sicA promoter (30 and 4% of wt promoter binding for mut97 and mut99 probes, respectively) (Figure 5). In fact, the binding of InvF-His6 to the mut99 probe was comparable to that of InvF-His6 to the control lac promoter probe (5% of wt promoter binding). Taken together, these results suggest that the base pairs at positions –48 and –45 of the sicA promoter are important for InvF binding.
SicA is not an RNA chaperone, nor does InvF require binding sites downstream of the +1 site of the sicA promoter
Recent work by Munson and Scott (2000) has shown that Rns, an AraC-like regulator in enterotoxigenic E.coli (ETEC), requires DNA binding sites downstream of +1 for transcription activation. All of the transcriptional fusions to lacZYA used in our work include the starts of transcription, untranslated and/or partial coding sequences. It was, therefore, possible that InvF required DNA binding sites downstream of the start site for transcription activation. In other studies, a mRNA signal has been shown to be used for the secretion of two type III secreted proteins (YopE and YopQ) in Yersinia enterocolitica (Anderson and Schneewind, 1997, 1999), suggesting that this signal interacts with a protein such as a chaperone or a component of the TTSS. Thus, it was possible that SicA recognized an RNA signal important for translation initiation or a structure that protected the mRNA from degradation. In the absence of the chaperone, lacZYA transcripts fused to a potential chaperone-dependent signal might not be translated or might be rapidly degraded by a 5′ exonuclease activity, making β-galactosidase activity undetectable. If the role for SicA were to regulate translation initiation or stabilize an RNA transcript via an RNA sequence, then it is unlikely that SicA is also directly involved in transcription activation. Therefore, in the absence of the RNA signal, SicA would not be needed to initiate translation or stabilize the transcript. Furthermore, if SicA were not required for transcription, InvF alone would be sufficient to produce a stable message if the hypothesized SicA–RNA interaction sequence were deleted.
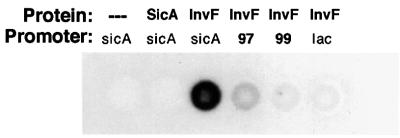
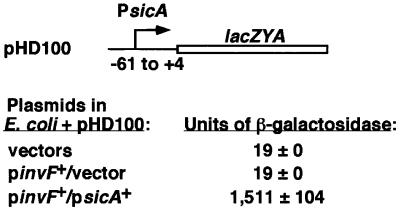
To test these possibilities, a sicA-lacZYA fusion in pRW50 that did not include sicA sequence downstream of +4 relative to the start of transcription was constructed (pHD100) (Figure 6). Escherichia coli CC118λpir containing pHG329 and pWKS130 (cloning vectors), pHG329 and pHD9-1 (invF+), or pHD30–2 (sicA+) and pHD9-1 (invF+) were transformed with this reporter plasmid. lacZYA expression was activated only in the presence of both invF and sicA (Figure 6). This result shows, in contrast to what is observed for Rns in E.coli, that there is no requirement for DNA binding sites downstream of +4 for transcription activation. In addition, this result suggests that detection of β-galactosidase does not require an interaction of SicA with an RNA sequence or structure present in the message. Most importantly, these results show that both InvF and SicA are required for transcription activation from the conserved sequence found between –61 and +4 of the sicA promoter.
Fig. 6. The sicA promoter does not require sequences downstream of +4 for transcription activation. Escherichia coli CC118λpir containing pHD100 along with one of the following plasmid pairs: cloning vectors pWKS130 and pHG329; pHD9-1 (invF+) and pHG329; or pHD9-1 (invF+)and pHD30-2 (sicA+). The sequence relative to the sicA start of transcription is indicated in this construct. Units of β-galactosidase (Miller units ± SD) are indicated on the right. These values are representative of duplicate assays performed on different days.
SicA does not affect the steady-state levels of InvF
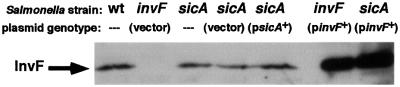
Although overexpressed and purified InvF-His6 was shown to bind DNA in vitro, it was still possible that SicA was required for the stability of InvF in vivo. IpgC, a homolog of SicA in Shigella, is a chaperone for the secreted invasion proteins IpaB and IpaC (Ménard et al., 1994). IpgC prevents the degradation and premature association of IpaB with IpaC in the bacterial cytoplasm. Like IpgC, SicA is a chaperone for Sip/SspB and Sip/SspC, homologs of IpaB and IpaC, respectively (Tucker and Galán, 2000). Because SicA is an intermolecular chaperone, it was possible that SicA was required for the stability or folding of InvF in addition to Sip/SspB and C. To determine whether SicA was required for the stability of InvF, polyclonal rabbit antibodies were raised against the InvF-His6 fusion protein (see Materials and methods). Cell pellets of wt, invF and sicA S.typhimurium strains were analyzed by western blotting on nitrocellulose membranes using the antibodies raised against InvF-His6. A sicA mutant produced wt levels of InvF and multicopy sicA did not increase the amount of InvF in the cells (Figure 7).
Fig. 7. SicA is not required for the stability of InvF in the cytoplasm. Immunoblot of whole cells of S.typhimurium wt, invF and sicA strains using rabbit polyclonal antibodies to InvF-His6. Strains and plasmids in each strain are indicated above each lane. Equivalent cell numbers as determined by A600 were loaded in each lane.
Previous work has shown that multicopy invF does not suppress a sicA mutation for the activation of sicA- and sigD-lacZYA expression (Darwin and Miller, 2000). However, it was not known whether InvF was produced or overproduced in a sicA mutant containing invF on a multicopy plasmid. The western blot in Figure 7 shows that a sicA mutant containing multicopy invF makes abundant amounts of InvF. Therefore, it does not appear that the reason why a sicA mutant can not activate transcription of the sicA, sigD or sopE promoters is due to reduced amounts of InvF. However, this experiment does not rule out the possibility that SicA is involved in changing the conformation of InvF to a state that allows InvF to activate transcription.
InvF interacts with SicA
Genetic studies have shown that SicA is required for transcription activation in conjunction with InvF (Darwin and Miller, 2000); however, it was not known whether InvF and SicA interacted directly with each other. Several attempts to determine whether InvF and SicA interact with each other in vivo were made using two-hybrid systems. An interaction between InvF and SicA was not observed using either a bacterial (Karimova et al., 1998) (Table IV) or yeast (Chien et al., 1991) two-hybrid system (data not shown). In the bacterial two-hybrid system, two domains (T25 and T18) of the Bordetella pertussis adenylate cyclase protein are fused to proteins whose putative interaction is being tested. Although the bacterial two-hybrid system did not show an InvF–SicA (Table IV) or an InvF–InvF interaction (data not shown), it did show that SicA fusion proteins (SicA–T18 and T25–SicA) could interact with each other. This result suggests that SicA dimerizes or forms higher order oligomers. Owing to the nature of this two-hybrid system, it was possible that dimerization of SicA fusion proteins sterically impaired the ability of either SicA–T18 or T25–SicA to interact with a third (T25–InvF or InvF–T18, respectively) fusion protein. A one-hybrid system was also used to test for the presence of an InvF–InvF interaction (Hu et al., 1990). Neither full-length InvF nor its N-terminal domain (non-conserved) demonstrated an InvF–InvF interaction (data not shown). However, it is possible that if InvF formed weak dimers, the dissociation constant between the InvF monomers would be too high to detect the interaction.
Table IV. Reconstitution of adenylate cyclase (Cya) activity by a SicA–SicA interaction.
| Test pair | Genotype of plasmids | Units of β-galactosidase activitya |
|---|---|---|
| pT18/pHD51 | vector/Cya′-SicA | 654 ± 20 |
| pHD53/pT25 | SicA-′Cya/vector | 516 ± 35 |
| pHD53/pHD51 | SicA-′Cya/Cya′-SicA | 1905 ± 197 |
| pHD52/pHD51 | InvF-′Cya/Cya′-SicA | 653 ± 16 |
| pHD53/pHD54 | SicA-′Cya/Cya′-InvF | 648 ± 35 |
| pT18-zip/pT25-zip | leucine zippers (positive control) | 7063 ± 138 |
aUnits of β-galactosidase (± SD) represent the average of duplicate β-galactosidase assays performed on duplicate cultures and are representative of assays carried out on different days. Cultures were grown in LB supplemented with Ap, Cm, 500 µM IPTG, and aerated on a roller drum at 26°C for 18 h.
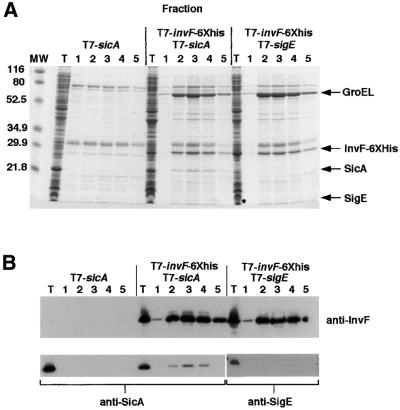
Because an interaction between InvF and SicA was not detected using a two-hybrid system, this interaction was also tested using a biochemical approach. An E.coli strain, BL21(DE3), encoding an inducible T7 polymerase gene was used to express the His-tagged invF fusion and sicA. Soluble cell lysates were mixed with nickel–agarose and washed extensively (see Materials and methods). When InvF-His6 was eluted from the nickel–agarose column, native SicA co-eluted in the same fractions. SicA in the eluted fractions was visible on a Coomassie Blue-stained gel (Figure 8A) and was confirmed to be SicA by western blotting using polyclonal antibodies to SicA (Figure 8B). Two negative controls were performed: BL21(DE3) producing native SicA and containing the His tag fusion vector pET24b(+); and BL21(DE3) making InvF-His6 and a different Salmonella-specific type III secretion chaperone, SigE (Hong and Miller, 1998; Darwin et al., 2001). Native SicA did not bind the nickel–agarose column in the absence of InvF-His6, nor did SigE bind to InvF-His6 (Figure 8). In a separate study, SicA-His6 was also shown not to bind SigD or SigE, a TTSS effector and its cognate chaperone, respectively (Darwin et al., 2001). These results suggest that SicA can specifically interact with InvF, even under the relatively stringent conditions of a protein purification column.
Fig. 8. SicA binds to InvF-His6. Escherichia coli strains were grown and harvested as described in Materials and methods. (A) A 12.5% Coomassie Blue-stained gel. InvF-His6, SicA and SigE are indicated on the right. SigE in the total protein sample (T) is noted with an asterisk. Lanes 1–5 represent consecutive fractions eluted from the nickel–agarose columns. Molecular weight (MW) standards are indicated on the left. (B) Immunoblot analysis of the same fractions presented in (A). Top panel: polyclonal antibodies to InvF-His6 were used. Bottom panel: antibodies to SicA-His6 or MBP-SigE were used as indicated in the figure.
In addition to SicA, another larger protein consistently eluted from the nickel–agarose columns along with InvF-His6 (Figure 8A). The sequence of the first 12 amino acids of this protein was determined and it was identical to that of the essential E.coli chaperonin GroEL (Hsp60). GroEL binds to newly synthesized polypeptides and is required for proper folding of the protein (Mayhew and Hartl, 1996). Because GroEL is required for folding many cytoplasmic proteins, it is possible that InvF, especially when overexpressed, interacts with GroEL. Because GroEL has not been suggested to bind to DNA, it is not clear whether GroEL serves a direct role in transcription regulation or if it is simply stabilizing or folding overexpressed InvF-His6 into its proper conformation. Whether or not GroEL binds to InvF under normal conditions, i.e. when not overexpressed, this experiment suggests for the first time that the type III secretion chaperone SicA can interact directly with the transcriptional activator InvF.
Discussion
Many environmental signals are known to stimulate or repress expression of the Salmonella invasion regulon (for a review see Lucas and Lee, 2000). These signals are transduced by an unknown mechanism to HilA, which then directly activates invF (Lostroh et al., 2000). In this study, we examined the role of InvF and SicA in the regulation of a specific subset of invasion genes encoding secreted effector proteins and their cognate chaperones. A consensus sequence, the InvF binding site, was identified in three chromosomally unlinked invasion loci. The sicA promoter controls the expression of the sicAsip/sspBCDA and, most likely, the sicPsptP genes. Sip/SspB and C have been shown to be required for the translocation of several other proteins, including SigD, SopE, and SptP (Wood et al., 1996; Collazo and Galán, 1997; Galyov et al., 1997; Fu and Galán, 1998b). SipB has been assigned several functions from effector translocator to stimulator of bacterial induced apoptosis (Kaniga et al., 1995b; Collazo and Galán, 1996; Hersh et al., 1999). SipC has been shown to insert into epithelial cell plasma membranes (Scherer et al., 2000) and is also important for effector translocation into eukaryotic cells (Collazo and Galán, 1997). SipA has been shown to stimulate the transmigration of PMN across polarized monolayers in an in vitro system (Lee et al., 2000), suggesting a role for SipA in causing disease. SigD was identified as a protein required for the efficient invasion of S.typhimurium into epithelial cells in vitro (Hong and Miller, 1998) and has been shown to contribute to diarrhea in a calf model of infection by Salmonella dublin (Galyov et al., 1997). SopE was also found to stimulate membrane ruffling of cultured epithelial cells by S.typhimurium (Hardt et al., 1998a) and S.dublin (Wood et al., 1996) by stimulating rho GTPases. SptP appears to be an antagonist to SopE (Fu and Galán, 1999), raising the intriguing question: why are two proteins that have opposing effects on the eukaryotic cell co-regulated? It is not known how much SopE is made compared with SptP or how efficiently each is translocated into host cells; therefore, it is possible that a certain amount of SptP must accumulate before antagonizing the effects of SopE on membrane ruffling in the eukaryotic host cell.
One common denominator of the InvF/SicA-regulated operons is that they are all required for invasion. Many other proteins, including AvrA (Hardt and Galán, 1997), SopD (Jones et al., 1998), SopE2 (Bakshi et al., 2000), SlrP (Miao and Miller, 2000) and SspH1 (Miao et al., 1999), have been shown to be secreted by the SPI1 TTSS. The genes encoding these proteins (with the exception of sopE2) have not been implicated in invasion nor do any of these genes possess an InvF binding site. Therefore, it is not surprising that the expression of avrA (Eichelberg and Galán, 1999) and sopD (our unpublished results) is not InvF/SicA dependent. Reporter fusions to the other genes encoding secreted effectors that do not have the InvF binding site will have to be constructed in order to determine whether they are also InvF/SicA independent. At this point, it is clear that not all genes encoding proteins secreted by the type III system are regulated by InvF and SicA. Interestingly, slrP (Miao and Miller, 2000), sspH1 (Miao et al., 1999), sopD (our unpublished results) and avrA (Eichelberg and Galán, 1999) are not part of the hilA regulon either.
InvF is a member of the growing AraC/XylS family of transcriptional regulators (Gallegos et al., 1997). The hallmark of this family of regulators is the presence of HTH motifs at their C-terminal domains. AraC contains an N-terminal domain that allows it to form dimers; the AraC dimer can bind to different regulatory sequences depending on its conformation (Soisson et al., 1997). Unlike AraC, InvF does not appear to bind multiple DNA sites. The InvF binding site lacks direct and inverted repeats, and does not require any distal binding sites to stimulate transcription. In several aspects, the InvF binding site resembles those sites that are bound by monomer AraC/XylS family members, such as SoxS or MarA (Gallegos et al., 1997; Rhee et al., 1998). SoxS and MarA binding sites generally span no more than 20 bp and are believed to be bound by monomeric proteins. Because bacterial one- and two-hybrid systems using the entire InvF protein or just the N-terminal domain (lacking the putative DNA binding domain) have not shown that InvF dimerizes, it seems likely that InvF, like SoxS or MarA, binds to DNA as a monomer. Nevertheless, these negative results do not yet rule out the possibility that InvF multimerizes.
The crystal structure of MarA bound to DNA revealed that two HTH motifs of MarA interact with two tandem major grooves on one face of a DNA molecule (Rhee et al., 1998). Based on this model, one could propose potential major groove interactions of the InvF binding site of the sicA promoter by a monomer of InvF: three conserved nucleotides (–48, –47 and –45), two of which have been shown to be important for activation by InvF, could be part of one major groove making contacts with the C-terminal HTH (relative to the other HTH in InvF). The following conserved region consists of six T nucleotides (–44 to –39), perhaps providing flexibility of the binding site. This sequence is followed by five more conserved nucleotides (–37, –36, –34, –33, –32) near or in the –35 region of these promoters. This set of conserved base pairs starts ∼10 bp from the first conserved region and is partially (–37, –36,–34) included in the major groove adjacent to the first major groove. This may suggest that these base pairs interact with the N-terminal HTH motif of InvF.
Although it is possible that InvF alone can bind DNA, it is not sufficient for activation of the sicA, sigD and sopE promoters. In addition to InvF, the type III secretion chaperone SicA is required for transcription activation of invasion effector genes. Several type III secretion chaperones have been implicated to participate in negative feedback regulation of virulence genes in Yersinia sp. (Bergman et al., 1991). However, none has been shown to directly regulate transcription. Moreover, none of these chaperones has been suggested to, directly or indirectly, activate transcription. Previous work has provided genetic evidence that sicA is required for the activation of two operons encoding secreted proteins (sicAsip/sspBCDA and sigDE) (Darwin and Miller, 2000). In this work, a third invasion gene promoter (sopE) was shown to require both proteins in S.typhimurium and an E.coli K-12 strain. All three of these genetically unlinked promoters have a highly conserved sequence upstream of the start of transcription, which appears to be sufficient for transcription activation.
Our work also demonstrates that SicA interacts with InvF, the first evidence of an interaction between a member of the AraC/XylS family of regulators and a type III secretion chaperone. Unlike some chaperones, such as those involved in heat shock (Mayhew and Hartl, 1996), type III secretion chaperones are usually associated with only one or two cognate effector molecules. These chaperones have been shown to protect effector molecules from degradation as well as prevent the inappropriate association of effectors prior to secretion out of the bacterium (Ménard et al., 1994; Fu and Galán, 1998a; Tucker and Galán, 2000; Darwin et al., 2001). Interestingly, SicA is not required to protect InvF from degradation and is not required for InvF binding to DNA in vitro, nor does SicA appear to bind DNA directly. Therefore, it appears that SicA either changes the conformation or binding specificity of InvF, allowing it to activate transcription, or SicA is itself directly interacting with RNA polymerase in order to stimulate transcription. Further characterization of the InvF–SicA interaction will require the purification of significant amounts of active InvF. This work is ongoing but, as is the case for many AraC-like regulators, this is proving difficult.
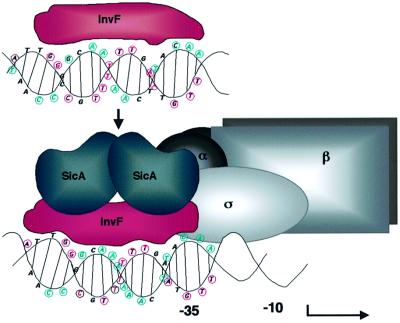
From the results of this work, we present a model for how InvF and SicA may activate transcription (Figure 9). In the absence of SicA, InvF is predicted to bind to DNA, but can not activate transcription. Once SicA is produced, it binds to InvF, stimulating transcription, perhaps by changing the conformation of InvF and/or DNA, or interacting with RNA polymerase. It is also possible that if SicA changes the conformation of InvF, this change results in InvF interacting with σ70 or other domains of RNA polymerase (e.g. the α-C-terminal domain or region 4 of σ70) (Busby and Ebright, 1994). Another scenario may be that interactions of SicA with InvF increase the binding affinity of InvF to DNA, although we did not observe this in vitro.
Fig. 9. Hypothetical model for activation of transcription of the sicA, sigD and sopE promoters. InvF binds to DNA in the absence of SicA, but can not activate transcription. Conserved base pairs are circled and colored in magenta for one strand and in cyan for the other. Based on the MarA model, it is likely that two HTH motifs of InvF (in red) interact with two consecutive major grooves on one face of the DNA molecule. Once SicA (in blue) is made, it interacts with InvF, but it is not known whether SicA binds as a dimer or monomer. The binding of SicA to InvF may create a bend in the DNA in a region where there are six conserved A–T base pairs susceptible to this conformation. This interaction results in the activation of transcription by an unknown mechanism, but may involve contacts between InvF, SicA or both proteins with subunits of RNA polymerase (in gray). This figure is not drawn to scale.
InvF represents a unique member of the AraC/XylS family of regulators because it interacts with another protein required for transcription activation. Nearly all, if not all, of the Gram-negative bacteria that possess TTSSs have AraC/XylS homologs involved in expression of the TTSS genes. In SPI1 alone, there are three known AraC/XylS homologs with different functions (Rakeman et al., 1999; Schechter et al., 1999). Most of these regulators have not been extensively characterized; therefore, it is possible that these homologs also require type III chaperones for transcription regulation.
SicA is an interesting type III chaperone in that it interacts with two secreted proteins (Tucker and Galán, 2000) and a transcriptional activator. An intriguing possibility is that SicA contacts RNA polymerase in order to activate transcription. It is also compelling to speculate that SicA may participate in the targeting of proteins destined for type III secretion by somehow ‘docking’ the transcription machinery to or near the secretion apparatus. Because transcription and translation are typically coupled in prokaryotes, this would also imply that the translation machinery could be brought to the secretion apparatus. This model would suggest that, in addition to interacting with effectors of invasion and apoptosis (Sip/SspB and Sip/SspC) and a regulator (InvF), SicA might also interact with a component of the secretion machinery. It has been proposed that Y.enterocolitica yop (Yersinia outer protein) mRNA signals recognize components of the TTSS, coupling translation of yop genes and secretion of Yops (Anderson and Schneewind, 1997, 1999). Perhaps SicA acts by targeting the transcription and translation machinery near or at the TTSS rather than coupling translation and secretion via a mRNA signal.
It is important to note that, unlike a Yersinia secretion mutant, a S.typhimurium secretion mutant can still make effector proteins that remain within the bacterium (Kaniga et al., 1995a). Therefore, it appears that the SPI1 TTSS and the Yersinia virulence plasmid TTSS have, so far, different ways of regulating expression and translation of their effector genes. Nevertheless, it seems likely that multiple mechanisms of transcription, translation and secretion regulation occur in any single bacterial species. Future work will determine how other S.typhimurium effector genes that are not InvF or SicA dependent are regulated and how all secreted proteins, InvF/SicA dependent or not, are targeted for secretion.
Materials and methods
Bacterial strains and plasmids
See Table V for bacterial strains and plasmids used in this study. PCRs were carried out using the high-fidelity polymerase Pfu (Stratagen). All plasmids with PCR amplified genes were sequenced. Electroporation of plasmids into bacteria was carried out as previously described (Sambrook et al., 1989). Plasmids that were purified from E.coli were passaged through a restriction-minus (hsd) S.typhimurium LT2 strain (LB5000) (Sanderson and Stocker, 1987) prior to electroporation into other S.typhimurium strains. For transductions, P22 HT int lysates were harvested and used as described previously (Maloy et al., 1996).
Table V. Bacterial strains and plasmids used in this work.
| Strain | Genotype | Source/reference |
|---|---|---|
| S.typhimurium | ||
| SL1344 derivatives | ||
| SL1344 | wt | Hoiseth and Stocker (1981) |
| SVM473 | Cmr; Φ(sigD-lacZYA)/sigDE+ | Darwin and Miller (1999a) |
| SVM579 | ΔinvF (in-frame deletion of 465 bp) | Darwin and Miller (1999a) |
| SVM609 | Cmr; ΔinvF, sigD-lacZYA/sigDE+ | Darwin and Miller (1999a) |
| SVM687 | Kmr; sicA::aphT | Darwin and Miller (2000) |
| GG5 | Ampr; sopE-lacZY/sopE– | gift from Catherine Lee |
| SVM935 | sicA::aphT ΔsspC sigD-lacZYA/sigDE+ | this work |
| 14028s derivatives | ||
| 14028s | wt | ATCCa |
| CAS108 | ΔsspC phoN::Tn10dCm | Scherer et al. (2000) |
| SVM933 | CAS108 with sicA::aphT | this work |
| LT2 strain | ||
| LB5000 | LT2, flaA66, metA22, trp-2, rpsL, xyl-401, ilv-452, leu, hsd, mod+ | Sanderson and Stocker (1987) |
| |
||
| E.coli strains | ||
| DH5α | F– p80ΔlacZΔM15 Δ(lacZYA-argF)U169 deoP recA1 endA1 hsdR17 (rk–mk–) | Gibco-BRL |
| DHP1 | F– cya glnV44 (AS) recA1 endA1 gyrA96 (Nalr) thi1 hsdR17 spoT1 rfbD1 | Karimova et al. (1998) |
| BL21(DE3) | F– ompT gal [dcm] [lon] hsdSB (rB–mB–) λ prophage carrying T7 polymerase | Studier et al. (1990) |
| CC118λpir | araD139 Δ(ara,leu)7697 ΔlacX74 phoA20 galE galK thi rpsE rpoB argEam recA1 λpir | Herrero et al. (1990) |
| VM1016 | Tetr, Kmr, Apr; CC118λpir pRW50, pHD9-1, pHG329 | this work |
| VM1020 | Tetr, Kmr, Apr; CC118λpir pHD11, pHD9-1, pHD30-2 | this work |
| VM1019 | Tetr, Kmr, Apr; CC118λpir pRW50, pHD9-1, pHD30-2 | this work |
| VM1021 | Tetr, Kmr, Apr; CC118λpir pHD34, pHD9-1, pHD30-2 | this work |
| Plasmids | ||
| pAJD107 | Apr; medium copy-number cloning vector | A.J.Darwin (Darwin and Miller, 2001) |
| pHG329 | Apr; medium copy-number cloning vector | Stewart et al. (1986) |
| pWKS130 | Knr; low copy-number cloning vector | Wang and Kushner (1991) |
| pWSK29/pWKS30 | Apr; low copy-number cloning vector | Wang and Kushner (1991) |
| pFUSE | Cmr;MobRP4 oriR6K, polylinker upstream of promoterless lacZYA | Bäumler et al. (1996) |
| pHD9-1 | Knr; 1.7 kb PstI fragment containing invF in pWKS130 | Darwin and Miller (1999a) |
| pHD10-1 | Apr; 1.7 kb PstI fragment containing invF in pHG329 | Darwin and Miller (1999a) |
| pHD17 | Cmr; pACYC184 with a 1.7 kb HindIII fragment containing invF from pHD10-1 | Darwin and Miller (1999a) |
| pHD30-2 | Apr; 0.9 kb sicA fragment in pHG329 | Darwin and Miller (2000) |
| pHD50 | Tcr; pRW50 with ∼300 bp fragment containing the iacP to sicP intergenic region | this work |
| pHD57 | Apr; pWKS30 with a 0.9 kb EcoRI–HindIII fragment from pHD30-2 containing sicA | this work |
| pHD61 | Cmr; pFUSE containing an ∼550 bp fragment fusing the first 535 bp of sptP to lacZYA | this work |
| pHD71 | Cmr; 0.9 kb SalI–XbaI fragment containing sicA in pACYC184 | this work |
| pRW50 | Tcr; low copy transcriptional reporter fusion vector | Lodge et al. (1992) |
| pHD11 | Tcr; Φ(sicA-lacZYA) in pRW50 (–271 to +130) | Darwin and Miller (1999a) |
| pHD83 | Tcr; Φ(sicA-lacZYA) in pRW50 (–61 to +130) | this work |
| pHD84 | Tcr; Φ(sicA-lacZYA) in pRW50 (–35 to +130) | this work |
| pHD98 | Tcr; same as pHD83 but with an A to T mutation at –51 | this work |
| pHD96 | Tcr; same as pHD83 but with a G to T mutation at –48 | this work |
| pHD97 | Tcr; same as pHD83 but with a G to C mutation at –48 | this work |
| pHD99 | Tcr; same as pHD83 but with a C to G mutation at –45 | this work |
| pHD86 | Tcr; Φ(sigD-lacZYA) in pRW50 (–350 to +91) | this work |
| pHD87 | Tcr; Φ(sigD-lacZYA) in pRW50 (–66 to +91) | this work |
| pHD88 | Tcr; Φ(sigD-lacZYA) in pRW50 (–37 to +91) | this work |
| pHH10 | Apr; 4.1-kb EcoRI sigDE fragment cloned into pHG329 | Hong and Miller (1998) |
| pAJDsicA61 | Apr; pAJD107 with a 61 bp PCR sicA fragment from –61 to +4 | this work |
| pHD100 | Tcr; EcoRI–HindIII fragment from pAJDsicA61 containing the sicA promoter from –61 to +4 cloned into pRW50 | this work |
| pT25 | Cmr; encodes the N-terminal domain of B.pertussis adenylate cyclase | Karimova et al. (1998) |
| pHD51 | Cmr; pT25 with a PstI–KpnI sicA PCR fragment | this work |
| pHD54 | Cmr; pT25 with a PstI–KpnI invF PCR fragment | this work |
| pT18 | Apr; encodes the C-terminal domain of B.pertussis adenylate cyclase adenylate cyclase | Karimova et al. (1998) |
| pHD52 | Apr; pT18 with a KpnI–XhoI invF PCR fragment | this work |
| pHD53 | Apr; pT18 with a KpnI–XhoI sicA PCR fragment | this work |
| pHD101 | Apr; pWSK29 with a 1.27 kb KpnI fragment containing sigE downstream of a T7 promoter | this work |
aAmerican Type Culture Collection.
To construct the sicA::aphTΔsspC double mutant, the sicA::aphT mutation was transduced from the SL1344 strain background into strain CAS108 (14028s phoN ΔsspC) (Scherer et al., 2000). Because sicA and sspC are linked, PCR was used to check that the ΔsspC deletion was not crossed out of the chromosome during the transduction. Primers to sspB and sspD (which flank sspC) were used to amplify DNA between sspB and sspD in the wt and ΔsspC strains, and six transductants. Kanr transductants missing sspC were then transformed with the plasmid reporter pHD95 (sopE). To make the SL1344 sicA::aphT ΔsspC sigD-lacZYA/sigDE+ strain, a P22 HT lysate was made from strain 14028s sicA::aphT ΔsspC and Kanr (from sicA::aphT) was transduced into SL1344 sigD-lacZYA/sigDE+. As above, because sicA and sspC are linked, Kanr transductants were checked for the sspC deletion by PCR.
Growth conditions
Salmonella typhimurium and E.coli strains were grown in Luria–Bertani (LB) Miller Broth (Difco) at 37°C with aeration on a roller drum or without aeration in standing cultures, depending on the assay. Evans Blue uranine (EBU) agar was made as described previously (Maloy et al., 1996). Antibiotics were used at the following final concentrations: ampicillin (Ap), 100–200 µg/ml; chloramphenicol (Cm), 25 µg/ml; kanamycin (Kn), 100 µg/ml; tetracycline (Tc), 15 µg/ml. For the detection of β-galactosidase activity, solid medium was supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) at 40 µg/ml. For induction of the T7 polymerase gene in BL21(DE3), isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 100 µM.
Purification of RNA and primer extension
RNA was purified from Salmonella (strains SL1344 pHH10, SL1344 pHD30-2, SVM579 pHH10 and SVM579 pHD30-2) or E.coli (strains VM1016, VM1020, VM1019 and VM1021) using Trizol reagent (Lifetechnologies). [32P]ATP labeling of the sicA and sigD primers and primer extension were performed as described previously (Ausubel et al., 1992). For the sicA promoter, oligonucleotide sicA-11 was used (Table II). For the sigD promoter, primer sigP2 was used (Table II). Sequencing reactions (see below) were performed with the same primers used for primer extension.
Purification of His-tagged proteins and production of polyclonal antibodies
Oligonucleotide primers (Lifetechnologies) were used to amplify invF and sicA by PCR using Pfu polymerase (Stratagene). PCR products were digested with EcoRI and XhoI, and cloned into pET24+ (for sicA) or pET24b(+) (for invF). The resulting plasmids, pHD40-1 and pHD48 for sicA and invF, respectively, were sequenced to ensure that the fusions were in-frame and that the sicA and invF coding regions were unaltered. For making polyclonal antibodies, SicA-His6 was purified under native conditions and InvF-His6 was purified under denaturing conditions as described in the QIAexpressionist manual (Qiagen). The His-tagged proteins were separated from contaminating proteins by running the most concentrated fractions on a 3 mm 12.5% SDS–PAGE gel (Ausubel et al., 1992). The gels were stained in a solution of 0.05% Coomassie Blue in water to visualize the proteins and the His-tagged fusion proteins were sliced from the gels using a clean razor blade (Harlow and Lane, 1988). The gel slices were lyophilized and used for immunization of Elite New Zealand white rabbits by Covance (Denver, PA).
Co-purification of SicA with InvF-His6
invF was cloned into plasmid pET24b(+) (Novagen), resulting in plasmid pHD48 as described above. This plasmid with pHD57 (T7-sicA) was able to activate transcription of the sicA promoter in the lac-minus E.coli strain ER2566, which encodes an inducible T7 polymerase gene (data not shown) (Chong et al., 1997). For purification of soluble InvF-His6, BL21(DE3) containing pHD48 was transformed with plasmid pHD57 (T7-sicA) or pHD101 (T7-sigE). In addition, BL21(DE3) with pET24b(+) was transformed with pHD57 as an additional negative control. Although the majority of InvF-His6 was insoluble, a portion of the fusion protein was soluble when purified as follows: 25 ml overnight cultures were subcultured into 1 l of LB broth with Kn and Ap and grown for 2 h at 37°C with aeration. At 2 h, the cultures were moved to a 12°C water bath and incubated for 20 min with shaking before adding 1 ml of 100 mM IPTG (final concentration 100 µM). The cultures were incubated with shaking at 12°C overnight (∼20 h). Cells were harvested by centrifugation (10 min, 6000 g) and pellets were stored at –20°C. Pellets were frozen and thawed twice before resuspension in 10 ml of lysis buffer supplemented with a Complete Mini protease inhibitor tablet (Roche/Boehringer Manheim). Cell lysates and fractions were prepared under native conditions as described in the QIAexpressionist manual with one exception: the column washes were increased from two 4-ml washes to three 10-ml washes.
Proteins were analyzed by either Coomassie Blue staining (Ausubel et al., 1992) or immunoblotting using the ECL Western Blotting Detection System (Amersham Pharmacia Biotech) as described previously.
Radioactive DNA probes and filter binding assays
Promoter fragments were isolated by PCR amplification using Pfu (Stratagene). For the sicA promoter, primers sicA-EcoRI-12 and sicA-BamHI-1 (Table II) were used. For the mutant sicA promoters, the same sequence as the sicA-EcoRI-12 primer was used, but with the appropriate base changes found in pHD97 and pHD99. To amplify the lac promoter from chromosomal DNA purified from E.coli S17-1λpir, primers lacP1-EcoRI (5′-GGAATTCGGCGCCCAATACGCAAACCGCC-3′) and lacP2 (5′-CGAGCTCGTCCACACAACATACGAGCCGGAAGC-3′) were used. Each promoter had an EcoRI site engineered at the 5′ end. PCR products were purified using QIAEX II (Qiagen) and digested overnight with EcoRI. The digested fragments were purified again with QIAEX II, eluted in 50 µl of water, and 10 µl of each fragment were labeled with [32P]dATP and [32P]dTTP (Amersham Pharmacia Biotech) using Klenow (New England Biolabs) (Ausubel et al., 1992). Each probe was purified using DyeEx spin columns (Qiagen). The final amount (µg/ml) of DNA was determined by ethidium bromide dot quantitation (Ausubel et al., 1992). The counts per minute (c.p.m.) of each probe were measured by diluting in TE (10 mM Tris pH 8, 1 mM EDTA) and measuring the c.p.m. in a scintillation counter. For final use, each probe was diluted in TE to 15 000 c.p.m./µl, where 15 000 c.p.m. represented 0.2–0.6 ng/µl DNA.
Filter binding assays were performed based on methods described previously (McEntee et al., 1980; Ausubel et al., 1992). Briefly, to prepare nitrocellulose filters for the assay, 0.45 µm pure nitrocellulose membranes (Schleicher and Schuell BA85) were soaked for 20 min in 0.5 M KOH, rinsed extensively for 10 min in deionized water, and soaked in 0.1 M Tris–HCl pH 7.4 for 45 min. Native InvF-His6 used in these assays was purified as described above and SicA-His6 was purified under native conditions as described in the QIAexpressionist manual. All proteins used were dialyzed in 50 mM sodium phosphate, monobasic, 10 mM Tris–HCl and 100 mM NaCl. The pH was adjusted to 6.5. The proteins were diluted in 50% glycerol in 0.5× dialysis buffer to a final concentration of 1 µg/µl. Binding reactions (50 µl) were carried out by incubating protein (5 µg, a non-saturating amount), 20 mM Tris–HCl pH 8, 2 mM dithiothreitol, 10 mM MgCl2, 50 µg/ml bovine serum albumin, 100 µM EDTA, 10 µM MnCl2, 200 mM NaCl and 3 µg of poly(dI–dC). After 20 min at room temperature, 15 000 c.p.m. of labeled DNA (0.2–0.6 ng/µl) were added, gently tapped to mix, and incubated at room temperature for 30 min. The prepared nitrocellulose membrane and two sheets of Whatman 3 mm paper soaked in 0.1 M Tris–HCl pH 7.4 were mounted on a Millipore Milliblot system and the samples were slowly vacuumed through the membrane. Each well was washed twice with 200 µl of 1× binding buffer. Filters were wrapped in plastic wrap and exposed to Hyperfilm (Amersham) overnight at –80°C or room temperature. After exposure to film, each spot on the nitrocellulose membrane was excised and placed in a scintillation vial with scintillation fluid (Fisher Scintiverse) and the c.p.m. on each membrane piece were determined.
Enzyme assays
β-galactosidase assays were performed and values calculated as described previously (Miller, 1972).
Sequence analysis
Nucleotide (except for the primer extension experiments) and protein sequencing (N-terminal) were performed by the Washington University Protein and Nucleic Acid Chemistry Laboratory (St Louis, MO). For the primer extension experiments, sequencing was performed using the dideoxy chain termination method (Sanger sequencing) with Sequenase (USB). Sequence analyses (homologies, mapping, etc.) were performed using the Wisconsin Sequence Analysis Package by the Genetics Computer Group, Inc. (GCG).
Acknowledgments
Acknowledgements
We thank Michael Caparon and Andrew Darwin for critically reviewing this manuscript. We are grateful to Vic DiRita and Rosa Yu for tips on purifying soluble InvF-His6, and to Susan Egan for insight on the analysis of the InvF binding site. We also thank Sam Miller for the sspC deletion mutant, and are indebted to Cathy Lee and her laboratory for primer extension data for the sopE promoter and the sopE–lacZY chromosomal fusion used in this work. We are also grateful to Katherine King for assistance on the filter binding assays.
References
- Anderson D.M. and Schneewind,O. (1997) A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science, 278, 1140–1142. [DOI] [PubMed] [Google Scholar]
- Anderson D.M. and Schneewind,O. (1999) Yersinia enterocolitica type III secretion: a mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol., 31, 1139–1148. [DOI] [PubMed] [Google Scholar]
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (eds) (1992) Short Protocols in Molecular Biology. Greene Publishing Associates, New York, NY.
- Bajaj V., Hwang,C. and Lee,C.A. (1995) hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol., 18, 715–727. [DOI] [PubMed] [Google Scholar]
- Bajaj V., Lucas,R.L., Hwang,C. and Lee,C.A. (1996) Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol., 22, 703–714. [DOI] [PubMed] [Google Scholar]
- Bakshi C.S., Singh,V.P., Wood,M.W., Jones,P.W., Wallis,T.S. and Galyov,E.E. (2000) Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol., 182, 2341–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler A.J., Tsolis,R.M., van der Veldon,A.W.M., Stojiljkovic,I., Anic,S. and Heffron,F. (1996) Identification of a new iron regulated locus of Salmonella typhi. Gene, 183, 207–213. [DOI] [PubMed] [Google Scholar]
- Behlau I. and Miller,S.I. (1993) A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J. Bacteriol., 175, 4475–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman T., Håkansson,S., Forsberg,A., Norlander,L., Marcellaro,A., Backman,A., Bolin,I. and Wolf-Watz,H. (1991) Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol., 173, 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S. and Ebright,R.H. (1994) Promoter structure, promoter recognition and transcription activation in prokaryotes. Cell, 79, 743–746. [DOI] [PubMed] [Google Scholar]
- Chien C.T., Bartel,P.L., Sternglanz,R. and Fields,S. (1991) The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl Acad. Sci. USA, 88, 9578–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S. et al. (1997) Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene, 192, 271–281. [DOI] [PubMed] [Google Scholar]
- Collazo C.M. and Galán,J.E. (1996) Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect. Immun., 64, 3524–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo C.M. and Galán,J.E. (1997) The invasion-associated type III system of Salmonella typhimurium directs the translocation of the Sip proteins into the host cell. Mol. Microbiol., 24, 747–756. [DOI] [PubMed] [Google Scholar]
- Darwin K.H. and Miller,V.L. (1999a) InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J. Bacteriol., 181, 4949–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin K.H. and Miller,V.L. (1999b) Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev., 12, 405–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin K.H. and Miller,V.L. (2000) The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol. Microbiol., 35, 949–959. [DOI] [PubMed] [Google Scholar]
- Darwin A.J. and Miller,V.L. (2001) The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol. Microbiol., 39, 429–445. [DOI] [PubMed] [Google Scholar]
- Darwin K.H., Robinson,L.S. and Miller,V.L. (2001) SigE is a chaperone for the Salmonella typhimurium invasion effector SigD. J. Bacteriol., 183, 1452–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberg K. and Galán,J.E. (1999) Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect. Immun., 67, 4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. and Galán,J.E. (1998a) Identification of a specific chaperone for SptP, a substrate of the centisome 63 type III secretion system of Salmonella typhimurium. J. Bacteriol., 180, 3393–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. and Galán,J.E. (1998b) The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol. Microbiol., 27, 359–368. [DOI] [PubMed] [Google Scholar]
- Fu Y. and Galán,J.E. (1999) A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature, 401, 293–297. [DOI] [PubMed] [Google Scholar]
- Galán J.E. and Curtiss,R.,III (1989) Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl Acad. Sci. USA, 86, 6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J.E., Ginocchio,C. and Costeas,P. (1992) Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J. Bacteriol., 174, 4338–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos M.-T., Schleif,R., Bairoch,A., Hofman,K. and Ramos,J.L. (1997) AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev., 61, 393–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galyov E.E., Wood,M.W., Rosqvust,R., Mullan,P.B., Watson,P.R., Hedges,S. and Wallis,T.S. (1997) A secreted effector protein of Salmonella dublin is translocated into eucaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol., 25, 903–912. [DOI] [PubMed] [Google Scholar]
- Groisman E.A. and Ochman,H. (1993) Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J., 12, 3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt W.-D. and Galán,J.E. (1997) A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl Acad. Sci. USA, 94, 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt W.-D., Chen,L.-M., Schuebel,K.E., Bustelo,X.R. and Galán,J.E. (1998a) S. typhimurium encodes an activator of rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell, 93, 815–826. [DOI] [PubMed] [Google Scholar]
- Hardt W.-D., Urlaub,H. and Galán,J.E. (1998b) A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic prophage. Proc. Natl Acad. Sci. USA, 95, 2574–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 63–69.
- Herrero M., de Lorenzo,V. and Timmis,K.N. (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J. Bacteriol., 172, 6557–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh D., Monack,D.M., Smith,M.R., Ghori,N., Falkow,S. and Zychlinsky,A. (1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA, 96, 2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S.K. and Stocker,B.A.D. (1981) Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature, 291, 238–239. [DOI] [PubMed] [Google Scholar]
- Hong K.H. and Miller,V.L. (1998) Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J. Bacteriol., 180, 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.C., O’Shea,E.K., Kim,P.S. and Sauer,R.T. (1990) Sequence requirements for coiled-coils: analysis with λ repressor–GCN4 leucine zipper fusions. Science, 250, 1400–1403. [DOI] [PubMed] [Google Scholar]
- Hueck C.J. (1998) Type III protein secretion in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev., 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.D. and Falkow,S. (1994) Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect. Immun., 62, 3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.A., Wood,M.W., Mullan,P.B., Watson,P.R., Wallis,T.S. and Galyov,E.E. (1998) Secreted effector proteins of Salmonella dublin act in concert to induce enteritis. Infect. Immun., 66, 5799–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga K., Bossio,J.C. and Galán,J.E. (1994) The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol. Microbiol., 13, 555–568. [DOI] [PubMed] [Google Scholar]
- Kaniga K., Trollinger,D. and Galán,J.E. (1995a) Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J. Bacteriol., 177, 7078–7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga K., Tucker,S., Trollinger,D. and Galán,J.E. (1995b) Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol., 177, 3965–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimova G., Pidoux,J., Ullmann,A. and Ladant,D. (1998) A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl Acad. Sci. USA, 95, 5752–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.A., Silva,M., Siber,A.M., Kelly,A.J., Galyov,E. and McCormick,B.A. (2000) A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl Acad. Sci. USA, 97, 12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge J., Fear,J., Busby,S., Gunasekaran,P. and Kamini,N.R. (1992) Broad host range plasmids carrying the Escherichia coli lactose and galactose operons. FEMS Microbiol. Lett., 95, 271–276. [DOI] [PubMed] [Google Scholar]
- Lostroh C.P., Bajaj,V. and Lee,C.A. (2000) The cis requirements for transcription activation by HilA, a virulence determinant encoded on SPI-1. Mol. Microbiol., 37, 300–315. [DOI] [PubMed] [Google Scholar]
- Lucas R.L. and Lee,C.A. (2000) Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol. Microbiol., 36, 1024–1033. [DOI] [PubMed] [Google Scholar]
- Maloy S.R., Stewart,V.J. and Taylor,R.K. (1996) Genetic Analysis of Pathogenic Bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mayhew M. and Hartl,F.-U. (1996) Molecular chaperone proteins. In Neidhardt,F.C. et al. (eds), Escherichia coli and Salmonella: Cellular and Molecular Biology, Vol. I. ASM Press, Washington, DC, pp. 922–937.
- McCormick B.A., Miller,S.I., Carnes,D. and Madara,J.L. (1995) Transepithelial signaling to neutrophils by Salmonellae: a novel virulence mechanism for gastroenteritis. Infect. Immun., 63, 2302–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock,G.M. and Lehman,I.R. (1980) recA protein-catalyzed strand assimilation: stimulation by Escherichia coli single-stranded DNA-binding protein. Proc. Natl Acad. Sci. USA, 77, 857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard R., Sansonetti,P., Parsot,C. and Vasselon,T. (1994) Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell, 79, 515–525. [DOI] [PubMed] [Google Scholar]
- Miao E.A. and Miller,S.I. (2000) A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl Acad. Sci. USA, 97, 7539–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao E.A., Scherer,C.A., Tsolis,R.M., Kingsley,R.A., Adams,L.G., Bäumler,A.J. and Miller,S.I. (1999) Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol., 34, 850–864. [DOI] [PubMed] [Google Scholar]
- Miller J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mills D.M., Bajaj,V. and Lee,C.A. (1995) A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol., 15, 749–759. [DOI] [PubMed] [Google Scholar]
- Munson G.P. and Scott,J.R. (2000) Rns, a virulence regulator within the AraC family, requires binding sites upstream and downstream of its own promoter to function as an activator. Mol. Microbiol., 36, 1391–1402. [DOI] [PubMed] [Google Scholar]
- Penheiter K.L., Mathur,N., Giles,D., Fahlen,T. and Jones,B.D. (1997) Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer’s patches. Mol. Microbiol., 24, 697–709. [DOI] [PubMed] [Google Scholar]
- Rakeman J., Bonifield,H.R. and Miller,S.I. (1999) A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J. Bacteriol., 181, 3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S., Martin,R.G., Rosner,J.L. and Davies,D.R. (1998) A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl Acad. Sci. USA, 95, 10413–10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sanderson K.E. and Stocker,B.A.D. (1987) Salmonella typhimurium strains used in genetic analysis. In Neidhardt,F.C. et al. (eds), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology, Washington, DC, pp. 1220–1224.
- Schechter L.M., Damrauer,S.M. and Lee,C.A. (1999) Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol. Microbiol., 32, 629–642. [DOI] [PubMed] [Google Scholar]
- Scherer C.A., Cooper,E. and Miller,S.I. (2000) The Salmonella type III secretion translocon protein SspC is inserted into epithelial cell plasma membrane upon infection. Mol. Microbiol., 37, 1–14. [DOI] [PubMed] [Google Scholar]
- Soisson S.M., MacDougall-Shackleton,B., Schleif,R. and Wolberger,C. (1997) Structural basis for ligand-regulated oligomerization of AraC. Science, 276, 421–425. [DOI] [PubMed] [Google Scholar]
- Stewart G.S.A.B., Lubinsky-Mink,S., Jackson,C.G., Cassel,A. and Kuhn,J. (1986) pHG165: A pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid, 15, 172–181. [DOI] [PubMed] [Google Scholar]
- Stone B.J., Garcia,C.M., Badger,J.L., Hassett,T., Smith,R.I.F. and Miller,V.L. (1992) Identification of novel loci affecting entry of Salmonella enteritidis into eukaryotic cells. J. Bacteriol., 174, 3945–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F., Rosenberg,A., Dunn,J. and Dubendorf,J. (1990) Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol., 185, 60–89. [DOI] [PubMed] [Google Scholar]
- Tucker S.C. and Galán,J.E. (2000) Complex function for SicA, a Salmonella enterica serovar typhimurium type III secretion-associated chaperone. J. Bacteriol., 182, 2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.F. and Kushner,S.R. (1991) Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene, 100, 195–199. [PubMed] [Google Scholar]
- Wattiau P., Woestyn,S. and Cornelis,G.R. (1996) Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol., 20, 255–262. [DOI] [PubMed] [Google Scholar]
- Wood M.W., Rosqvist,R., Mullan,P.B., Edwards,M.H. and Galyov,E.E. (1996) SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol., 22, 327–338. [DOI] [PubMed] [Google Scholar]