Abstract
In eukaryotic cells, activation of cell surface receptors that couple to the phosphoinositide pathway evokes a biphasic increase in intracellular free Ca2+ concentration: an initial transient phase reflecting Ca2+ release from intracellular stores, followed by a plateau phase due to Ca2+ influx. A major component of this Ca2+ influx is store-dependent and often can be measured directly as the Ca2+ release-activated Ca2+ current (ICRAC). Under physiological conditions of weak intracellular Ca2+ buffering, respiring mitochondria play a central role in store-operated Ca2+ influx. They determine whether macroscopic ICRAC activates or not, to what extent and for how long. Here we describe an additional role for energized mitochondria: they reduce the amount of inositol 1,4,5-trisphosphate (InsP3) that is required to activate ICRAC. By increasing the sensitivity of store-operated influx to InsP3, respiring mitochondria will determine whether modest levels of stimulation are capable of evoking Ca2+ entry or not. Mitochondrial Ca2+ buffering therefore increases the dynamic range of concentrations over which the InsP3 is able to function as the physiological messenger that triggers the activation of store-operated Ca2+ influx.
Keywords: calcium influx/inositol 1,4,5-trisphosphate/mitochondria
Introduction
In non-excitable cells, the second messenger inositol 1,4,5-trisphosphate (InsP3) evokes Ca2+ release from intracellular stores followed by Ca2+ influx across the plasma membrane (Putney, 1986; Berridge, 1993). One major route for this Ca2+ entry is through store-operated Ca2+ channels (SOCs), which are activated by the process of emptying the intracellular Ca2+ stores (Parekh and Penner, 1997). Ca2+ influx through SOCs is required not only for refilling the intracellular stores but also for regulating a host of physiological processes including secretion, gene transcription and cell proliferation (Parekh and Penner, 1997).
Although several types of SOC have been described, the best characterized to date are the Ca2+ release-activated Ca2+ (CRAC) SOCs, which give rise to a highly selective whole-cell Ca2+ current called ICRAC (Hoth and Penner, 1992; Parekh and Penner, 1997). ICRAC can be measured directly using the whole-cell patch-clamp technique. Until recently, ICRAC was studied routinely in the presence of very high concentrations of Ca2+ chelators (mM concentrations of EGTA/BAPTA) in the recording pipette. Such strong Ca2+ buffering was used because the current could not be detected with weaker, more physiological levels of Ca2+ buffer (0.1 mM EGTA/BAPTA), and this inability to record ICRAC was attributed to Ca2+-dependent inactivation of the underlying CRAC channels. However, we and others have shown that this explanation cannot account for the inability to record ICRAC in weak Ca2+ buffer (Broad et al., 1999; Fierro and Parekh, 2000). We have found that SERCA pumps, which refill the stores, are very powerful in rat basophilic leukaemia-1 (RBL-1) cells (Fierro and Parekh, 1999) and that InsP3 is unable to deplete stores sufficiently for whole-cell (macroscopic) ICRAC to develop in weak Ca2+ buffer unless the SERCA pumps are inhibited (Fierro and Parekh, 2000; Bakowski and Parekh, 2001). Recently, we have demonstrated that ICRAC can be activated in weak buffer, even when SERCA pumps are active, provided Ca2+ uptake into mitochondria is functional (i.e. when mitochondria are in an energized state in the whole-cell configuration; Gilabert and Parekh, 2000). Furthermore, Ca2+-dependent slow inactivation of CRAC channels was reduced when mitochondrial Ca2+ uptake was operational. Hence mitochondria are important determinants of store-operated Ca2+ influx, determining whether macroscopic ICRAC activates in weak buffer, to what extent and for how long (Gilabert and Parekh, 2000).
Mitochondrial Ca2+ buffering has quite marked effects on Ca2+ release to submaximal concentrations of InsP3. In permeabilized hepatocytes, mitochondrial Ca2+ uptake suppresses the positive feedback actions of cytosolic Ca2+ on adjacent InsP3 receptors and this results in less Ca2+ release to a submaximal InsP3 concentration (Hajnoczky et al., 1999). A similar conclusion was reached by Boitier et al. (1999), who found that mitochondrial Ca2+ uptake reduced the rate of propagation of Ca2+ waves in astrocytes. In Xenopus oocytes, on the other hand, mitochondrial Ca2+ uptake increased the frequency of Ca2+ waves and this was thought to reflect a reduction in the extent of Ca2+-dependent inactivation of InsP3 receptors (Jouvaille et al., 1995).
Since many Ca2+-dependent processes are activated by modest increases in intracellular InsP3 levels, we have now investigated the effects of mitochondrial Ca2+ uptake on the ability of submaximal concentrations of InsP3 to activate ICRAC in weak Ca2+ buffer. We find that respiring mitochondria enhance the extent of ICRAC, thus enabling moderate levels of InsP3 to be more effective in triggering Ca2+ influx. In addition, the threshold concentration of InsP3 that triggers ICRAC is reduced in the presence of energized mitochondria. Mitochondrial Ca2+ uptake therefore increases the dynamic range over which InsP3 is an effective stimulus for Ca2+ entry and may determine the efficacy of relatively weak stimuli in promoting store-dependent Ca2+ influx.
Results
A range of InsP3 concentrations fail to activate ICRAC consistently in weak Ca2+ buffer
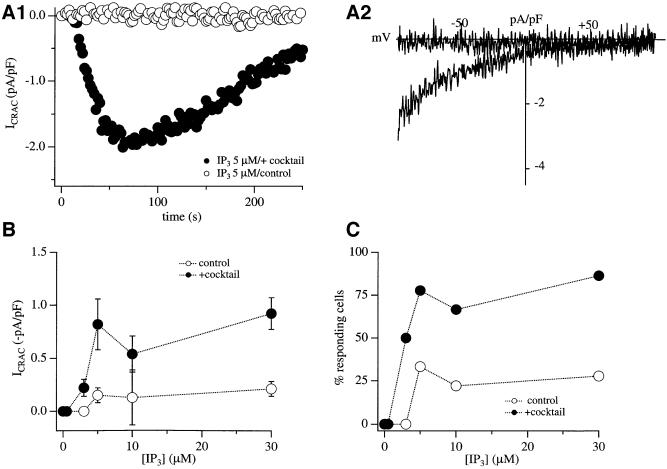
When RBL-1 cells are dialysed with a patch pipette containing strong Ca2+ buffer (10 mM EGTA) and a maximally effective concentration of InsP3 (30 µM), virtually all cells respond by generating a relatively large store-operated Ca2+ current (Fierro and Parekh, 2000; Gilabert and Parekh, 2000; Bakowski and Parekh, 2001). However, if this experiment is repeated in the presence of more physiological levels of intracellular Ca2+ buffering (0.1 mM EGTA), the same concentration of InsP3 is largely ineffective. The majority of cells (>75%) fail to develop any detectable ICRAC and, for those that do, the current is substantially smaller than in strong buffer. To examine whether lower, and presumably more physiological, concentrations of InsP3 were similarly ineffective in weak Ca2+ buffer, we dialysed RBL-1 cells with different concentrations of InsP3 (in 0.1 mM EGTA). In Figure 1A1, the time course of ICRAC in response to 5 µM InsP3, a concentration that is just maximal in strong buffer (Parekh et al., 1997), is depicted (open circles in Figure 1A1). Figure 1A2 shows the current–voltage relationship, taken at 100 s. InsP3 at 5 µM failed to evoke any detectable ICRAC in this cell. Averaged data from several cells, exposed to different concentrations of InsP3, are shown in Figure 1B, and the fraction of responding cells is depicted in Figure 1C. In Figure 1B, cells that responded have been pooled together with those that did not. Concentrations of InsP3 ≤3 µM consistently failed to evoke any discernible current. These concentrations nevertheless do release Ca2+ from the stores (Parekh et al., 1997). For higher InsP3 concentrations, ICRAC was very small and only ∼25% of the cells responded. For those cells that did respond, ICRAC was still several fold smaller than the amplitude in strong buffer (–0.51 pA/pF compared with –2.85 pA/pF in strong buffer for 30 µM InsP3). Hence, over a range of concentrations, InsP3 is a very weak activator of ICRAC in the presence of weak intracellular Ca2+ buffer.
Fig. 1. Energized mitochondria increase the ability of InsP3 to activate ICRAC in weak intracellular Ca2+ buffer. (A1) Time course of ICRAC to 5 µM InsP3 in the absence (open circles) and presence (filled circles) of mitochondrial cocktail solution. (A2) Current–voltage relationships for the recordings shown in (A1), taken after 100 s. (B) Amplitude of ICRAC (measured at –80 mV from the voltage ramps and normalized for cell capacitance) is plotted against InsP3 concentration included in the recording pipette. Open and filled circles correspond to experiments in the absence and presence of the mitochondrial cocktail solution, respectively. (C) The percentage of responding cells is plotted against InsP3 concentration. Note that, at 3 µM InsP3, no cell responds in the absence of the mitochondrial cocktail whereas 50% do so when the cocktail is present.
InsP3 still fails to activate ICRAC consistently when K+ is the major intracellular cation
In the preceding experiments, we used Cs+ as the dominant cation in our pipette solution. For example, RBL cells express a GTP-dependent K+ conductance (McCloskey and Cahalan, 1990), and GTP is a component of the intracellular cocktail used to maintain mitochondria in an energized state (Gilabert and Parekh, 2000; see below). The GTP-dependent K+ current is not permeable to Cs+, and, therefore, in the presence of Cs+, it would not contaminate our recordings. However, under physiological conditions, K+ is the major intracellular cation. It is possible that counter-movement of K+ is required in order to sustain Ca2+ release from the stores, and Cs+ might be unable to substitute for K+ in this action.
Both InsP3-gated channels and ryanodine receptors are non-selective cation channels permeable to monovalent and divalent cations, with a higher permeability for divalents (Bezprozvanny and Ehrlich, 1995). The selectivity profile is believed to be very similar for these two Ca2+ release channels, which is not unexpected as there is significant homology between the genes encoding these proteins. Although there is little information on the relative permeabilities of Cs+ and K+ for the InsP3 receptors, Cs+ is quite permeable through ryanodine-sensitive channels. It is more permeable than K+ in the channels from skeletal muscle (Smith et al., 1988) and is 0.61 times that of K+ in cardiac muscle (Lindsay et al., 1991). Nevertheless, we compared the ability of 30 µM InsP3 to activate ICRAC in weak buffer in the presence of either Cs+ or K+. With Cs+, the mean ICRAC was –0.34 ± 0.04 pA/pF and in K+ it was –0.41 ± 0.05 pA/pF (six cells for each condition), and the amplitudes were not significantly different (p >0.3). These results are entirely consistent both with our previous findings in which carbachol, acting via an increase in InsP3, evoked robust Ca2+ release after cells had been dialysed with Cs+ (see Figure 3 of Parekh et al., 1997), and with a recent report by Hermosura et al. (2000). These authors showed that the size of the carbachol-evoked transient in RBL cells was very similar in intact cells (where K+ is the major intracellular cation) and after extensive dialysis of the cytosol with Cs+ (Figure 4 of Hermosura et al., 2000).
Respiring mitochondria potentiate ICRAC and reduce the threshold for activation by InsP3
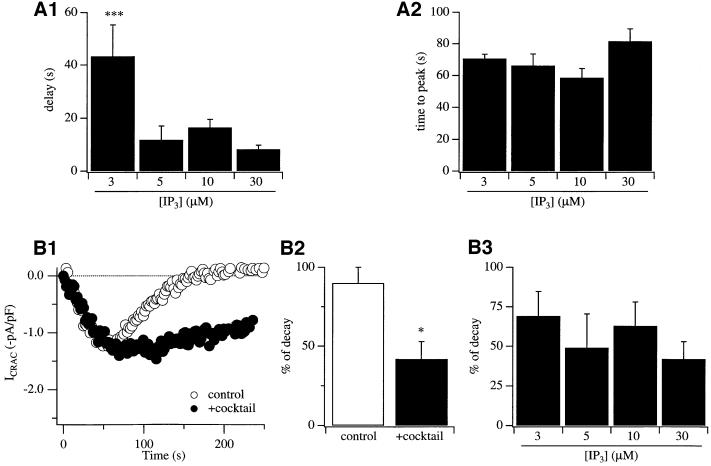
We have found that mitochondrial Ca2+ uptake is essential in order for 30 µM InsP3 to activate ICRAC in weak Ca2+ buffer (Gilabert and Parekh, 2000). Whereas most cells fail to respond to InsP3 in weak buffer, and for those that do the current is very small (see above), dialysis with a solution designed to maintain mitochondria in an energized state (mitochondrial cocktail solution, see Materials and methods) results in virtually all cells responding to produce a robust ICRAC. The effects of the cocktail are due entirely to an action on mitochondria because manoeuvres that suppress mitochondrial Ca2+ uptake abolish the enhancing effects of the cocktail (Gilabert and Parekh, 2000). To investigate whether mitochondrial Ca2+ uptake similarly potentiates ICRAC to more moderate levels of InsP3, we dialysed cells with different concentrations of InsP3 in weak Ca2+ buffer but now also in the presence of the mitochondrial cocktail. The results were dramatic. Whereas 5 µM InsP3 generally failed to activate ICRAC in weak Ca2+ buffer (Figure 1A1, open circles), it evoked a sizeable current in the presence of the cocktail (Figure 1A2, filled circles). The I–V relationship is shown in Figure 1A2. The current is inwardly rectifying, voltage-independent (over 50 ms) and reverses at potentials >50 mV, which are the hallmarks of ICRAC (Parekh and Penner, 1997). The extent of ICRAC to different InsP3 concentrations in the absence (open circles) and presence (filled circles) of the mitochondrial cocktail solution is compared in Figure 1B, where data from responders and non-responders have been pooled together. The percentage of responding cells for each condition is shown in Figure 1C. Kinetic features of the current for the two different conditions are summarized in Figure 2A1 and A2. Several striking differences are apparent in the presence of energized mitochondria. (i) The size of ICRAC is potentiated over the range of concentrations of InsP3 that evoke a response (5–30 µM; Figure 1B). (ii) The fraction of cells that respond over this concentration range increases substantially in the presence of respiring mitochondria (Figure 1C). (iii) Whereas 3 µM InsP3 consistently fails to evoke ICRAC and is hence a subthreshold concentration in the absence of mitochondrial cocktail, around half of the cells respond to this dose of InsP3 when mitochondria are energized (Figure 1B and C). (iv) The only kinetic parameter that changes with InsP3 concentration is the delay before ICRAC starts to acti vate (Figure 2A1). However, once the current is initiated, then it develops at the same rate (Figure 2A2). (v) If we consider the amplitude of ICRAC in the presence of cocktail for responding cells only, then this was quite similar for all InsP3 concentrations that evoked a response [–0.44 ± 0.08 (n = 5), –1.06 ± 0.25 (n = 7), –0.81 ± 0.16 (n = 6) and –1.18 ± 0.16 (n = 21) pA/pF for 3, 5, 10 and 30 µM InsP3, respectively; the only significant difference was between 3 and 30 µM InsP3]. Collectively, these results indicate that the threshold concentration of InsP3 required to evoke ICRAC is reduced in the presence of energized mitochondria. Mitochondrial Ca2+ uptake therefore increases the sensitivity of store-operated Ca2+ influx to InsP3. However, lowering the InsP3 concentration further (0.5 µM) failed to activate ICRAC in weak buffer, even in the presence of the mitochondrial cocktail.
Fig. 2. Effects of mitochondrial Ca2+ buffering on the kinetics of ICRAC under conditions where SERCA pumps are active. The delay before ICRAC activates (A1) and the time to peak (A2) are plotted against InsP3 concentration for cells with energized mitochondria. The time to peak was corrected for the delay. (B1) The time course of ICRAC is shown for a cell dialysed with 30 µM InsP3 in weak Ca2+ buffer in the absence (open circles) and presence (filled circles) of mitochondrial cocktail. Note that the amplitude of the current was similar for the two cells, but ICRAC declined much more quickly when mitochondria were not energized. (B2) The extent of decline of ICRAC is compared for cells dialysed in the absence (open bar) and presence (filled bar) of mitochondrial cocktail. (B3) The extent of decline of ICRAC in the presence of cocktail is plotted against the different InsP3 concentrations. *p <0.05 and ***p <0.001.
The relationship between InsP3 concentration and extent of ICRAC is highly non-linear in weak Ca2+ buffer
We had reported previously that the relationship between InsP3 concentration and the amplitude of ICRAC was highly non-linear in strong Ca2+ buffer, with a Hill coefficient of 12 (Parekh et al., 1997; Glitsch and Parekh, 2000). This supralinear relationship still held in the presence of moderate Ca2+ buffering (Glitsch and Parekh, 2000). However, it was not clear whether the steep relationship was also valid in the presence of weak Ca2+ buffer because the current could not be measured consistently under those conditions. Since we can record robust ICRAC in weak Ca2+ buffer provided mitochondria are energized, we have been able to address this important issue directly. As shown in Figure 1B, concentrations of InsP3 <3 µM fail to evoke any detectable ICRAC, whereas 5 µM InsP3 generates maximal current. Fitting this dose–response curve with a modified Hill equation yielded a Hill coefficient of 17. Because the relationship is so steep, the Hill coefficient (derived from the fit) is only an approximation. However, the key point is that ICRAC is related supralinearly to InsP3 concentration in the presence of physiological levels of intracellular Ca2+ buffering and energized mitochondria. Once the threshold concentration of InsP3 is exceeded, then only small further increases in InsP3 will result in maximal activation of Ca2+ influx.
Kinetics of decay of ICRAC in the presence of energized mitochondria
Mitochondrial Ca2+ uptake reduces both the rate and extent of Ca2+-dependent slow inactivation of CRAC channels in RBL cells (Gilabert and Parekh, 2000) and Jurkat T lymphocytes (Hoth et al., 2000). In order to isolate this inactivation mechanism, Ca2+-dependent store refilling by SERCA pumps had to be suppressed. Because SERCA pumps are very powerful in RBL cells, we wanted to see whether mitochondria could prolong the duration of ICRAC even when these pumps were active. To this end, we compared the time course of ICRAC following activation by InsP3 in weak Ca2+ buffer in the absence and presence of the mitochondrial cocktail. Most cells failed to generate ICRAC to InsP3 in the absence of cocktail, whereas for the minority that did so the current was transient. Figure 2B1 shows a typical response to InsP3 in the absence of cocktail (open circles, control) and the extent of decline of the current is summarized in the histogram of Figure 2B2. ICRAC declined almost completely within 200 s. However, in the presence of cocktail, the current decayed much more slowly such that the amplitude of ICRAC at times >100 s was significantly larger than the case when mitochondria were not maintained in an energized state (Figure 2B1 and B2). Therefore, under conditions where SERCA pump activity is maintained, mitochondrial Ca2+ buffering is still an important factor that prolongs the time course of ICRAC. There was some variability in the extent of decay for ICRAC in the presence of energized mitochondria between different cells (see also Gilabert and Parekh, 2000), but overall the decay was not significantly different between the various InsP3 concentrations (Figure 2B3).
The facilitatory effects of mitochondria are suppressed by moderate concentrations of Ca2+chelator
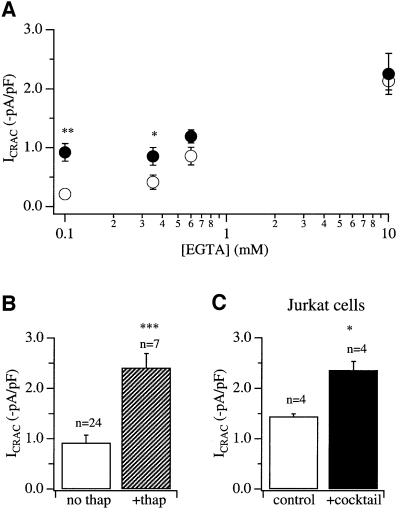
Thin-section electron microscopic studies on RBL-2H3 cells have revealed that a small fraction of the mitochondrial surface is closely apposed to the endoplasmic reticulum (ER) membrane (Hajnoczky et al., 2000). This suggests that Ca2+ released by InsP3 receptors on the ER might need to diffuse, at least over short distances, in the cytosol before they are taken up by mitochondria. To see whether a slow Ca2+ chelator could intercept the diffusing Ca2+ ions and what concentration of this chelator suppressed the facilitatory effects of mitochondrial Ca2+ uptake, we constructed dose–response curves to EGTA in the absence and presence of the mitochondrial cocktail solution. Results are summarized in Figure 3A. For control (non-cocktail-treated) cells, 30 µM InsP3 failed to evoke any ICRAC in 0.1 mM EGTA. EGTA at 0.35 mM was at around the threshold concentration for detection (3/9 cells failed to respond). In the presence of 0.6 mM EGTA, all cells responded and ICRAC was ∼50% of the maximum, the maximum being obtained in the presence of 10 mM EGTA. These results are very similar to our previous findings (Glitsch and Parekh, 2000). The presence of the cocktail significantly potentiated ICRAC in the presence of weak to moderate concentrations of EGTA (<0.6 mM EGTA; Figure 3A) and virtually all cells responded under these conditions (90% in 0.1 mM and 100% in 0.35 mM EGTA). In 0.6 mM EGTA, the current was enhanced slightly by cocktail but this was not significant (Figure 3A). No facilitatory effects of the cocktail were seen in 10 mM EGTA. These results are in good agreement with those reported by Csordas et al. (1999) who found that 0.6 mM EGTA suppressed the increase in intramitochondrial Ca2+ following InsP3-mediated Ca2+ release in permeabilized RBL-2H3 cells. Because slow buffers such as EGTA are unable to reduce Ca2+ levels at distances <20 nm from open Ca2+-permeable channels such as InsP3-gated channels (Neher, 1998), our results indicate that a molecular distance significantly larger than 20 nm separates the Ca2+ release and uptake sites on the ER and mitochondria, respectively. This is entirely consistent with the findings of Csordas et al. (1999), who calculated an average distance of 100 nm between InsP3 receptors and mitochondrial Ca2+ uptake sites in permeabilized RBL-2H3 cells.
Fig. 3. Moderate concentrations of the slow Ca2+ chelator EGTA suppress the potentiating effects of mitochondrial cocktail on ICRAC. (A) Amplitude of ICRAC is plotted against EGTA concentration in the absence (open circles) and presence (filled circles) of mitochondrial cocktail. ICRAC was potentiated by the cocktail for EGTA concentrations <0.6 mM. (B) In the presence of respiring mitochondria, 30 µM InsP3 and 0.1 mM EGTA, inhibition of SERCA pumps by thapsigargin (2 µM) results in a further increase in the amplitude of ICRAC. (C) Energized mitochondria potentiate the size of ICRAC when Jurkat T cells are dialysed with InsP3 (30 µM) and 0.1 mM EGTA. *p <0.05 and ***p <0.001.
SERCA pumps can compete with mitochondria for removal of cytosolic Ca2+
Inspection of Figures 1B and 3A reveals that, in energized mitochondria and weak Ca2+ buffer, ICRAC is generally around –1 pA/pF. In strong buffer, the current is almost three times larger. This difference in current amplitudes could arise from some vestigial Ca2+-dependent inactivation of the CRAC channels in weak buffer such that the current size is reduced. Alternatively, it could reflect some Ca2+-dependent store refilling, implying that not all of the Ca2+ released by InsP3 is taken up by mitochondria but that some of this Ca2+ is resequestrated into the stores. This latter scenario would constitute a form of physiological antagonism between two major Ca2+ clearance mechanisms in RBL cells, with mitochondria enhancing depletion of InsP3-sensitive Ca2+ stores and hence activation of ICRAC, and SERCA pumps promoting store refilling and therefore a reduction in the extent of activation of ICRAC. To distinguish between these possibilities, we compared the size of ICRAC between cells dialysed with InsP3 + weak Ca2+ buffer + mitochondrial cocktail in the absence and presence of thapsigargin. Results are summarized in the histogram of Figure 3B. Inclusion of thapsigargin resulted in an almost 3-fold increase in the size of the current. The amplitude of ICRAC now was similar to that seen in strong buffer (InsP3 + 10 mM EGTA; Figure 3A). Hence, SERCA pumps can still resequestrate sufficient Ca2+ even in the presence of energized mitochondria such that ICRAC cannot activate to its maximum extent. This would be consistent with the notion that mitochondria and SERCA pumps compete for Ca2+ and that this impacts upon the extent of store depletion and subsequent activation of ICRAC.
Energized mitochondria potentiate ICRAC in weak Ca2+ buffer in Jurkat T lymphocytes
In Jurkat T lymphocytes, like RBL-1 cells, we have found that macroscopic ICRAC can be activated only weakly following dialysis with InsP3 and 0.1 mM EGTA (Fierro et al., 2000) We therefore investigated whether energized mitochondria could enhance the size of the current, as is the case in RBL cells. As shown in Figure 3C, the amplitude of ICRAC was significantly larger in the presence of the mitochondrial cocktail. These results complement the reports by Hoth et al. (1997, 2000), who found that mitochondrial Ca2+ buffering reduced Ca2+-dependent slow inactivation of CRAC channels in the Jurkat cell line. Like RBL cells, energized mitochondria in Jurkat T lymphocytes seem to increase the size of ICRAC following dialysis with InsP3 in weak Ca2+ buffer.
Discussion
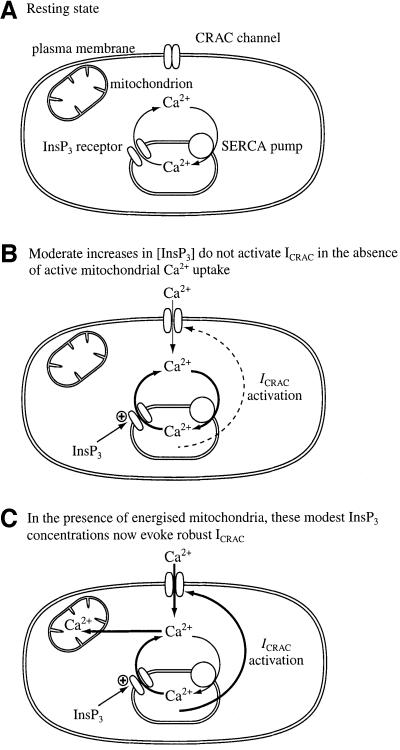
Our new findings demonstrate that energized mitochondria determine whether macroscopic ICRAC activates over a range of stimulus intensities. By reducing the threshold concentration of InsP3 required to evoke ICRAC, mitochondria increase the dynamic range over which this ubiquitous second messenger is able to control, via store depletion, the extent of Ca2+ influx. A cartoon summarizing this effect is shown in Figure 4 (see legend for explanation). The increased sensitivity to lower levels of InsP3 may be an important factor that helps determine whether weak/moderate stimulation of cell surface receptors can promote store-operated Ca2+ entry. It is intriguing to consider that regulation of mitochondrial Ca2+ uptake and/or spatial distribution of mitochondria relative to the ER might be a novel mechanism to dictate whether weak/moderate receptor stimulation evokes Ca2+ influx or not.
Fig. 4. Cartoon summary of the role of mitochondria in activation of ICRAC for moderate increases in InsP3. (A) The resting situation, where most CRAC channels are closed. The stores are sufficiently full that ICRAC is deactivated and Ca2+ that leaks out of the stores is taken back up by the SERCA pumps. (B) Moderate increases in InsP3 evoke substantial Ca2+ release from the stores but sufficient Ca2+ is resequestrated by the pumps so that the threshold for macroscopic activation of ICRAC is not reached. The current therefore does not activate to detectable levels. (C) In the presence of energized mitochondria, these moderate concentrations of InsP3 are now able to activate macroscopic ICRAC. Mitochondria take up some of the Ca2+ that has been released by InsP3. This will result in less Ca2+ being available for the SERCA pumps, resulting in a reduction in net re-uptake. In addition, mitochondrial Ca2+ buffering may reduce the extent of Ca2+-dependent inactivation of the InsP3 receptors, thereby enabling greater Ca2+ release and hence store depletion. Mitochondrial Ca2+ uptake will also reduce the rate and extent of Ca2+-dependent slow inactivation, and this will increase the size of ICRAC as well as prolong its duration (Gilabert and Parekh, 2000).
Because relatively high concentrations of InsP3 or receptor engagement are required to activate ICRAC, it has been proposed that additional Ca2+ entry pathways may be involved during weaker levels of stimulation (Shuttleworth, 1999). Our findings, demonstrating that energized mitochondrial Ca2+ uptake can reduce the amount of InsP3 that is necessary to evoke ICRAC, suggest instead that ICRAC can be activated by lower InsP3 concentrations when care is taken to maintain mitochondria in an energized state. It is likely that addition to our pipette solution of further components that support respiring mitochondria may result in greater Ca2+ uptake by this organelle and hence a further reduction in the InsP3 concentration required to activate ICRAC.
In RBL-1 cells, like certain other non-excitable cells, low concentrations of InsP3 can trigger Ca2+ release and reduce the amount of Ca2+ within the stores without evoking any Ca2+ influx (Parekh et al., 1997; Hartmann and Verkhratsky, 1998; Liu et al., 1998). Growing evidence from RBL-1 cells points towards a specialized subcompartment of the ER that is involved specifically in the activation of ICRAC (Parekh et al., 1997; Broad et al., 1999; Krause et al., 1999). This store seems harder to deplete since somewhat higher concentrations of InsP3 are required to activate Ca2+ influx than Ca2+ release. It has been suggested that low levels of InsP3 fail to access these stores because the InsP3 5-phosphatase breaks down these concentrations very efficiently (Hermosura et al., 2000). Only high concentrations of InsP3, which are well above the KM of the phosphatase, can mobilize the stores and thus activate ICRAC. An alternative explanation is that low concentrations of InsP3 do access these specialized stores but that the SERCA pumps are so active that they prevent the Ca2+ released by these low InsP3 concentrations from depleting stores sufficiently for ICRAC to activate. Energized mitochondria reduce the threshold concentration of InsP3 that is required to evoke ICRAC. Because the activity of the 5-phosphatase is not thought to be Ca2+ dependent (Shears, 1992) and presumably, therefore, would not be affected by mitochondrial Ca2+ uptake, this indicates that low concentrations of InsP3 (3 µM) probably do in fact mobilize these specialized stores and hence that metabolism of InsP3 is unlikely to be the main factor preventing these InsP3 concentrations from evoking the current. Instead, enhanced mitochondrial Ca2+ uptake would facilitate store depletion by reducing both possible Ca2+-dependent inactivation of InsP3 receptors and Ca2+ uptake via SERCA pumps.
Our results also suggest that mitochondrial Ca2+ uptake and Ca2+ ATPases of the ER, two major Ca2+ removal mechanisms in these and other cells (Herrington et al., 1996; Tinel et al., 1999), can functionally antagonize one another. In the absence of energized mitochondria, ICRAC is generally not activated by InsP3 in weak Ca2+ buffer unless SERCA pumps are blocked (Fierro and Parekh, 2000; Gilabert and Parekh, 2000). This indicates that a sizeable fraction of the Ca2+ released by InsP3 is resequestrated into the stores such that the intraluminal Ca2+ content does not fall sufficiently for macroscopic ICRAC to activate. On the other hand, in the presence of energized mitochondria and active SERCA pumps, enough Ca2+ is taken up by the mitochondria (and hence away from the pumps) so that stores are depleted to an extent that macroscopic ICRAC activates, albeit to a submaximal level (Gilabert and Parekh, 2000). This dynamic interplay between SERCA pumps and mitochondrial Ca2+ uptake sites might require close apposition between the two Ca2+ removal mechanisms so that effective competition can take place. Recent morphological evidence strongly suggests that this is the case in RBL cells. Csordas and Hajnoczky (2001) have found that almost every mitochondrion has a region that is positioned very close to the SERCA pumps of the ER. It is likely therefore that at these mitochondria–ER junctions, competition between the two organelles for removing Ca2+ would be particularly strong.
At moderate InsP3 concentrations (nM to low µM range), Ca2+ inactivation of InsP3 receptors becomes more prominent (Mak et al., 1998). Through their effects on buffering cytosolic Ca2+, mitochondria might reduce this inactivation process and thereby promote further Ca2+ release from the stores. If the pumps are now inhibited, then the amplitude of ICRAC increases to the maximal extent. Hence, in respiring mitochondria, some of the released Ca2+ is still taken back up into the InsP3-sensitive stores. Coordinated regulation of the Ca2+ transport capacities of these two organelles would therefore have quite marked effects on store depletion and subsequent Ca2+ influx.
Mitochondrial Ca2+ buffering prolongs the time course of ICRAC even when the powerful SERCA pumps are active (Figure 2B), and therefore it is an important factor that helps determine the extent of Ca2+ influx under conditions where other Ca2+ removal mechanisms are still operational. Under physiological conditions, therefore, mitochondria are powerful intracellular Ca2+ buffering organelles that help prolong the duration of Ca2+ influx.
Our results demonstrate that mitochondria are key orchestrators of store-operated Ca2+ entry in RBL cells. Mitochondria are involved in three crucial aspects of Ca2+ influx: (i) they determine whether macroscopic ICRAC activates or not (Gilabert and Parekh, 2000); (ii) they help set the time course of ICRAC following its activation; and (iii) they reduce the levels of intracellular InsP3 required to activate the current. By sensitizing cells to lower InsP3, mitochondria may determine whether relatively weak stimuli are capable of evoking Ca2+ influx or not.
Materials and methods
Cell culture
RBL-1 cells and Jurkat T lymphocytes, which were bought from Cell Bank at the Sir William Dunn School of Pathology, Oxford University, were cultured as previously described (Fierro and Parekh, 2000; Fierro et al., 2000).
Electrophysiology
Patch-clamp experiments were conducted in the tight-seal whole-cell configuration at room temperature (20–23°C) as previously described (Parekh et al., 1997; Fierro and Parekh, 1999). Sylgard-coated, fire-polished pipettes had DC resistances of 2.5–4 MΩ when filled with standard internal solution that contained 145 mM Cs glutamate, 8 mM NaCl, 1 mM MgCl2, 0.1 mM EGTA, 2 mM Mg-ATP and 10 mM HEPES pH 7.2 with CsOH. A correction of +10 mV was applied for the subsequent liquid junction potential that arose from this glutamate-based internal solution. In some experiments, Cs+ was replaced with K+ (see text). Mitochondrial cocktail contained 2 mM pyruvic acid, 2 mM malic acid, 1 mM NaH2PO4, 0.5 mM cAMP, 0.5 mM GTP and 0.5 mM MgCl2. Extracellular solution contained 145 mM NaCl, 2.8 mM KCl, 10 mM CaCl2, 2 mM MgCl2, 10 mM CsCl, 10 mM glucose and 10 mM HEPES pH 7.4 with NaOH. ICRAC was measured by applying voltage ramps (–100 to +100 mV in 50 ms) at 0.5 Hz from a holding potential of 0 mV as previously described (Parekh et al., 1997). Currents were filtered using an eight-pole Bessel filter at 2.5 kHz and digitized at 100 µs. Currents were normalized by dividing the amplitudes (measured from the voltage ramps at –80 mV) by the cell capacitance. Capacitative currents were compensated before each ramp by using the automatic compensation of the EPC 9-2 amplifier. All leak currents were subtracted by averaging the first few ramp currents (usually two), and then subtracting this from all subsequent currents.
Data are presented as the mean ± SEM, and statistical analysis was carried out using both Student’s t and Mann–Whitney non-parametric tests. Thapsigargin was purchased from Alomone Laboratories. All other chemicals were from Sigma.
Acknowledgments
Acknowledgements
J.A.G. is supported by a Marie Curie EU Postdoctoral Fellowship. D.B. holds a British Heart Foundation Prize Studentship. A.B.P. is a Lister Institute Research Fellow. Early stages of this work was supported by the Wellcome Trust (Career Development Fellowship award to A.B.P.).
References
- Bakowski D. and Parekh,A.B. (2001) Sarcoplasmic/endoplasmic-reticulum-Ca2+-ATPase-mediated Ca2+ reuptake and not Ins(1,4,5)P3 receptor inactivation, prevents the activation of macroscopic Ca2+ release-activated Ca2+ current in the presence of physiological Ca2+ buffer in rat basophilic leukaemia-1 cells. Biochem. J., 353, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. and Ehrlich,B.E. (1995) The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol., 145, 205–216. [DOI] [PubMed] [Google Scholar]
- Boitier E., Rea,R. and Duchen,M.R. (1999) Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol., 145, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad L.M., Armstrong,D.L. and Putney,J.W. (1999) Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback inhibition of calcium release-activated calcium current (ICRAC). J. Biol. Chem., 274, 32881–32888. [DOI] [PubMed] [Google Scholar]
- Csordas G. and Hajnoczky,G. (2001) Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium, 29, 249–262. [DOI] [PubMed] [Google Scholar]
- Csordas G., Thomas,A.P. and Hajnoczky,G. (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J., 18, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (1999) On the characterisation of the mechanism underlying passive activation of the Ca2+ release-activated Ca2+ current ICRAC. J. Physiol. (Lond.), 520, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (2000) Substantial depletion of the intracellular calcium stores is required for macroscopic activation of ICRAC in RBL-1 cells. J. Physiol. (Lond.), 522, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L., Lund,P.E. and Parekh,A.B. (2000) Comparison of the activation of the Ca2+ release-activated Ca2+ current ICRAC to InsP3 in Jurkat T-lymphocytes, pulmonary artery endothelia and RBL-1 cells. Pflugers Arch., 440, 580–587. [DOI] [PubMed] [Google Scholar]
- Gilabert J.A. and Parekh,A.B. (2000) Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J., 19, 6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M.D. and Parekh,A.B. (2000) Ca2+ store dynamics determines the pattern of activation of the store-operated Ca2+ current ICRAC in response to InsP3 in rat basophilic leukemia cells. J. Physiol. (Lond.), 523, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G., Hager,R. and Thomas,A.P. (1999) Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate-receptors by Ca2+. J. Biol. Chem., 274, 14157–14162. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Csordas,G., Madesh,M. and Pacher,P. (2000) The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J. Physiol. (Lond.), 529, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J. and Verkhratsky,A. (1998) Relations between intracellular Ca2+ stores and store-operated Ca2+ entry in primary cultured human glioblastoma cells. J. Physiol. (Lond.), 513, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura M.C., Takeuchi,H., Fleig,A., Riley,A.M., Potter,B.V., Hirata,M. and Penenr,R. (2000) InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature, 408, 735–740. [DOI] [PubMed] [Google Scholar]
- Herrington J., Park,Y.B., Babcock,D.F. and Hille,B. (1996) Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron, 16, 219–228. [DOI] [PubMed] [Google Scholar]
- Hoth M. and Penner,R. (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature, 355, 353–356. [DOI] [PubMed] [Google Scholar]
- Hoth M., Fanger,C.M. and Lewis,R.S. (1997) Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol., 137, 633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Button,D.C. and Lewis,R.S. (2000) Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl Acad. Sci. USA, 97, 10607–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvaille L.S., Ichas,F., Holmuhamedov,E.L., Camacho,P. and Lechleiter,J.D. (1995) Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature, 377, 438–411. [DOI] [PubMed] [Google Scholar]
- Krause E., Schmid,A., Gonzalez,A. and Schulz,I. (1999) Low cytoplasmic [Ca2+] activates ICRAC independently of global Ca2+ store depletion in RBL-1 cells. J. Biol. Chem., 274, 36957–36962. [DOI] [PubMed] [Google Scholar]
- Lindsay A.R., Manning,S.D. and Williams,A.J. (1991) Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J. Physiol. (Lond.), 439, 463–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.-Q., Bunnell,S.C., Gurniak,C.B. and Berg,L.J. (1998) T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med., 187, 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.O., McBride,S. and Foskett,J.K. (1998) Inositol 1,4,5-tris-phosphate activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl Acad. Sci. USA, 95, 15821–15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M.A. and Cahalan,M.D. (1990) G protein control of potassium channel activity in a mast cell line. J. Gen. Physiol., 95, 205–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. (1998) Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron, 20, 389–399. [DOI] [PubMed] [Google Scholar]
- Parekh A.B. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Parekh A.B., Fleig,A. and Penner,R. (1997) The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell, 89, 973–980. [DOI] [PubMed] [Google Scholar]
- Putney J.W. (1986) A model for receptor-regulated calcium entry. Cell Calcium, 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Shears S.B. (1992) Metabolism of inositol phosphates. Adv. Second Messenger Phosphoprotein Res., 26, 63–92. [PubMed] [Google Scholar]
- Shuttleworth T.J. (1999) What drives calcium entry during [Ca2+]i oscillations?—Challenging the capacitative model. Cell Calcium, 25, 237–246. [DOI] [PubMed] [Google Scholar]
- Smith J.S., Imagawa,T., Ma,J., Fill,M., Campbell,K.P. and Coronado,R. (1988) Purified ryanodine receptor from rabbit skeletal muscle is the calcium release channel of sarcoplasmic reticulum. J. Gen. Physiol., 92, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Geramisenko, O.V., Tepikin,A.V. and Petersen,O.H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]