Abstract
The roles of heat shock transcription factors (HSFs) under physiological conditions have recently become the focus of intense study. We generated avian cells lacking two heat-inducible HSFs, HSF1 and HSF3. In addition to complete loss of activation of heat shock genes under stress conditions, these cells exhibited a marked reduction in Hsp90α expression under normal growth conditions. Reduction in Hsp90α expression caused instability of a cyclin-dependent kinase, Cdc2, and cell cycle progression was blocked mainly at the G2 phase, but also at G1 phase even at mild heat shock temperatures. Restoration of Hsp90α expression rescued the temperature sensitivity without induction of Hsps. We demonstrated for the first time a molecular target affected by heat shock in vivo that causes cell cycle arrest in vertebrates and a novel mechanism of stress resistance controlled by vertebrate HSFs.
Keywords: Cdc2/cell cycle/G2 block/HSF/Hsp90
Introduction
The cell cycle is a collection of highly ordered processes that result in the duplication of a cell. Several cell cycle transitions are dependent on the activity of cyclin-dependent kinases, and inhibition of these kinases is a mechanism by which some checkpoint pathways such as the DNA damage checkpoint cause cell cycle arrest (Elledge, 1996). Various stresses such as heat shock and oxidative stress suppress cell growth by inhibiting cell cycle transitions and inducing cell death. However, molecular targets affected by these stresses and pathways, by which cell cycle transitions are inhibited, are unclear.
Heat shock proteins (Hsps) act as molecular chaperone by stabilizing intracellular proteins in normally growing cells. A number of cell cycle regulatory proteins have been demonstrated to use the Hsp90 chaperone complex, which is composed mainly of Hsp90 and p50Cdc37. Formation of active Weel1 tyrosine kinase requires interaction with Hsp90 in fission yeast (Aligue et al., 1994). Cdc37 in Drosophila and Hsp90 in fission yeast interact genetically with Cdc2 (Cutforth and Rubin, 1994; Munoz and Jimenez, 1999). Furthermore, mammalian Cdk4 is stabilized by Hsp90/Cdc37 (Stepanova et al., 1996). These observations suggest that molecular chaperones are involved in stress-induced cell cycle arrest and in re-entry into the cell cycle when cells are relieved from stress.
A high temperature stress is a common stress in nature. When cells are exposed to a high temperature stress, they induce a set of Hsps, which is called the heat shock response. The heat shock response is regulated mainly at the level of transcription by heat shock transcription factors (HSFs). In yeast, HSF is constitutively a trimer that binds to DNA and is essential for survival of normally growing cells (Sorger, 1991). In vertebrates, however, members of the HSF family are maintained in a cryptic monomer or dimer form in the absence of stress (Wu, 1995; Morimoto, 1998; Nakai, 1999). Upon heat shock, HSF1 and HSF3 undergo conformational changes associated with trimer formation and bind to DNA with high affinity, and subsequently induce Hsps. Exceptionally, HSF4 is a trimer that bind to DNA even in the absence of stress, like yeast HSF (Nakai et al., 1997; Tanabe et al., 1999). It is well known that cells pre-conditioned with sublethal heat shock can survive otherwise lethal heat stress, a phenomenon known as induced thermotolerance (Lindquist, 1986). HSFs are required for acquisition of the induced thermotolerance (Jedlicka et al., 1997; McMillan et al., 1998; Tanabe et al., 1998). HSFs are also suggested to promote cell death of spermatocytes exposed to high temperatures (Nakai et al., 2000).
Here we report an unexpected role for vertebrate HSFs. We showed that HSFs regulate expression of target genes in the absence of stress. One of these genes was Hsp90α. Reduction of Hsp90α by disruption of HSF genes caused instability of a cyclin-dependent kinase, Cdc2, against thermal stress. We show for the first time a molecule affected by thermal stress that induces cell cycle arrest in vertebrates. Loss of HSF function also makes cells hypersensitive to other stresses such as radiation and sodium arsenite. We provide a model system in which unstable proteins could be produced upon exposure even to mild stress conditions.
Results
Complete loss of heat shock response in cells deficient in HSF1 and HSF3
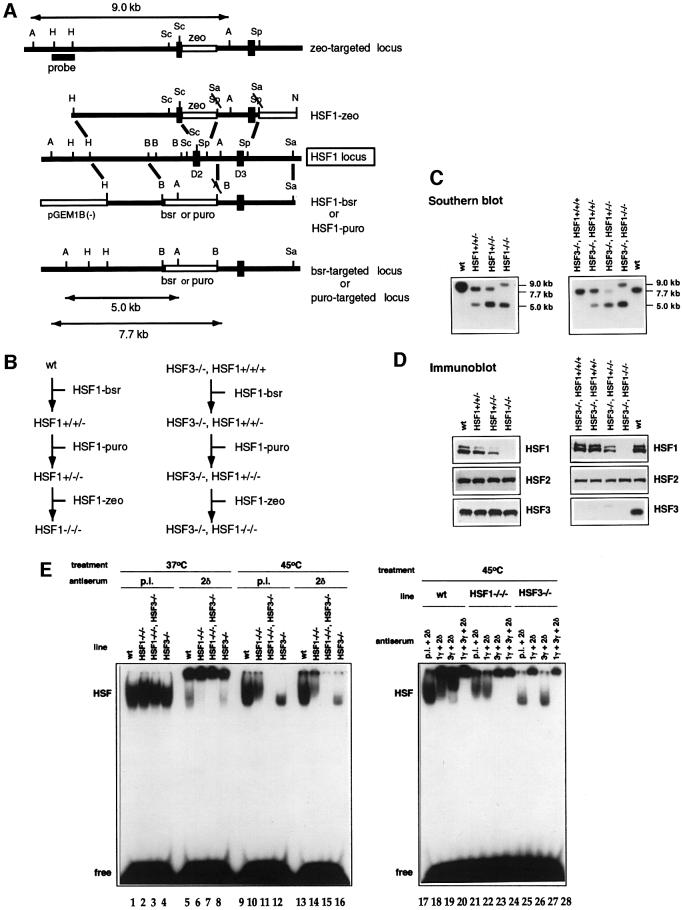
In avian cells, two heat shock-activated factors, HSF1 and HSF3, regulate induction of Hsps (Nakai et al., 1995; Tanabe et al., 1997). We previously mutated the HSF3 gene (Tanabe et al., 1998), and here we further generated chicken B lymphocyte DT40 cells deficient in both HSF1 and HSF3 by sequential transfection of targeting constructs and drug selection (Figure 1A–C). Western blot analysis showed no expression of HSF1 and HSF3 in cells mutated in all of the HSF1 and HSF3 gene alleles (Figure 1D). Heat shock-induced heat shock element-binding activity was composed exclusively of HSF1 and HSF3 (Figure 1E, left panel). HSF1 and HSF3 bind to the heat shock element independently (Figure 1E, right panel) (Nakai et al., 1995). Cells deficient in HSF1 and/or HSF3 grew normally (data not shown).
Fig. 1. Generation of DT40 cells deficient in HSF1 and cells deficient in both HSF1 and HSF3. (A) Restriction maps of the chicken HSF1 genomic fragment, targeting constructs and predicted structure of the targeted HSF1 allele. Restriction enzymes: A, ApaI; B, BamHI; H, HindIII; N, NotI; Sa, SalI; Sc, ScaI; Sp, SphI. Exons containing the DNA-binding domain (D2 and D3) are indicated by filled boxes. Open boxes indicate the blastcydin- or puromycin-containing genes under the control of the chicken β-actin promoter. A HindIII fragment of the HSF1 gene was used as a probe to distinguish between the wild-type and mutant HSF1 alleles on Southern blots. (B) Outline of the three-step transfection procedure to isolate HSF1 mutants. (C) Southern blot analysis of DT40 cell clones. Genomic DNA isolated from wild-type DT40 cells (wt), heterozygous HSF1 mutant cells (left) and homozygous HSF1 mutant cells deficient in HSF3 (right) were digested with ApaI and hybridized with the probe as described in (A). The predicted size of the ApaI fragment from the wild-type allele was 5.0 kb, and those of mutant alleles were 7.7 and 9.0 kb. The HSF1 gene was located on chromosome 2, which is present in three copies in DT40 cells. (D) Expression of HSF1, HSF3 and HSF2 proteins in wild-type DT40 cells, heterozygous HSF1 mutant cells (left) and homozygous HSF1 cells deficient in HSF3 (right). Western blotting of whole-cell extracts (30 µg protein) was performed using specific antisera. (E) Gel shift assay. Whole-cell extracts were prepared from the indicated cells with (lanes 9–28) and without (lanes 1–8) heat shock at 45°C for 1 h. The gel shift assay was performed in the presence of pre-immune serum (p.i.), αHSF1γ (1γ), αHSF2δ (2δ) or αHSF3γ (3γ).
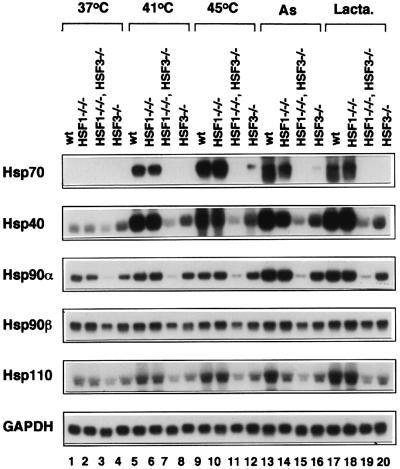
We examined mRNA levels of major Hsps in cells subjected to heat shock and other stresses (Figure 2). Although slight induction of Hsp70 mRNA was observed when HSF3-null cells were exposed to severe heat shock (Figure 2, lanes 12), its induction was completely blocked in cells deficient in both HSF1 and HSF3 (lanes 11). Similarly, induction of Hsp40, Hsp90α and Hsp110 was completely blocked in cells deficient in these two factors (Figure 2). These results demonstrated that HSF1 and HSF3 are the only factors among the HSF family members that regulate induction of Hsps in response to stresses such as heat shock, sodium arsenite and the proteasome inhibitor lactacystin.
Fig. 2. Complete loss of heat shock response in cells deficient in HSF1 and HSF3. Cells were exposed to heat shock at 41 or 43°C for 30 min, or treated with sodium arsenite (As, 100 µM) for 6 h or the proteasome inhibitor lactacystin (Lacta., 10 µM) for 4 h. Northern blot analysis was performed using each specific cDNA probe.
HSF1 and HSF3 control Hsp90α expression in the absence of stress
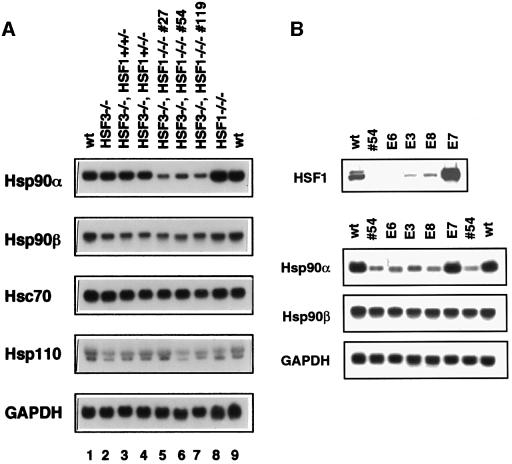
We then examined whether the two heat-responsive factors control expression of Hsps in normally growing cells. Remarkably, we observed a marked reduction in Hsp90α mRNA (∼25% of control level) in cells deficient in HSF1 and HSF3, and a slight decrease in Hsp90β expression (∼60%) in cells deficient in HSF3 (Figures 2 and 3A). Hsp90α mRNA was not reduced in cells deficient in either HSF1 or HSF3, suggesting a redundant role for the two factors in the constitutive expression of Hsp90α. Changes in levels of other constitutively expressed Hsps such as Hsc70 and Hsp110 were small (Figure 3A). The level of Hsp90α mRNA was restored to that in wild-type cells by reintroduction of HSF1 into cells deficient in HSF1 and HSF3 (Figure 3B).
Fig. 3. Reduction of Hsp90α expression in normally growing cells deficient in HSF1 and HSF3. (A) Expression of Hsp mRNAs in normally growing cells at 37°C. Total RNAs were isolated from three independently isolated cell lines deficient in both HSF1 and HSF3 (#27, #54 and #119) as well as from wild-type cells, HSF1-null cells, HSF3-null cells and heterozygous HSF1 mutant cells deficient in HSF3. Northern blot analysis was performed. (B) Clone #54 was stably transfected with a HSF1 expression vector. The levels of HSF1 protein and mRNAs of Hsp90α, Hsp90β and GAPDH were examined in four clones.
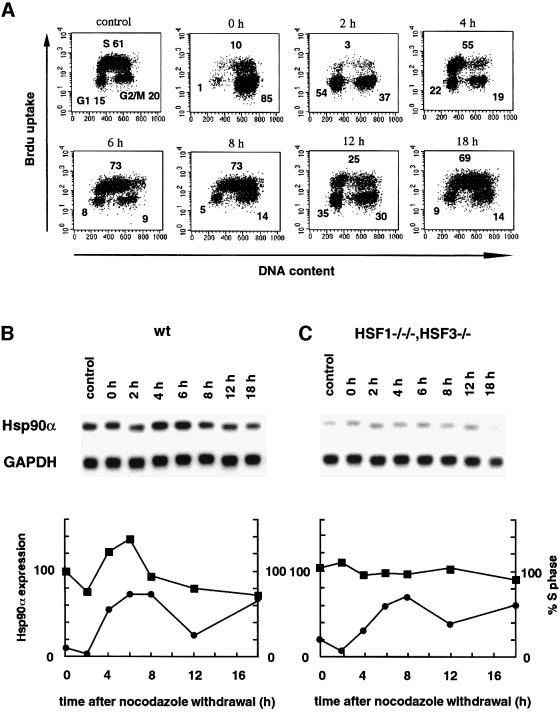
To test whether Hsp90α expression is actually regulated through HSFs in normally growing cells, its mRNA level was examined during cell cycle transition. Wild-type cells were synchronized at mitosis in the presence of nocodazole. At 4–6 h after washout of nocodazole, cells were moved to early S phase and the Hsp90α mRNA level reached a peak, and then declined rapidly (Figure 4A and B). Induction of Hsp90α expression in early S phase was also observed in other cell types (Jerome et al., 1993). In contrast, in cells deficient in HSF1 and HSF3, the level of Hsp90α mRNA was constant during cell cycle transition (Figure 4C). These results indicated that HSFs regulate Hsp90α expression in the absence of stress.
Fig. 4. Cell cycle-dependent regulation of Hsp90α expression. Wild-type DT40 cells and cells deficient in HSF1 and HSF3 were treated with nocodazole (0.5 µg/ml) for 7 h. After washing with PBS, cells were maintained in complete medium for 18 h. At the indicated time points, the cell cycle was analyzed using a FACScan flow cytometer as described in Materials and methods. Fluorescence data are displayed as dot plots in wild-type cells (A), and the proportions of cells at S phase are shown (B and C). The proportions of cells at G1, S and G2/M phases are indicated in each panel in (A). Profiles of Hsp90α expression in wild-type cells (B) and cells deficient in HSF1 and HSF3 (C) were analysed. The signals of Hsp90α were quantified by Phosphoanalyst (Bio-Rad), and these relative levels are shown after normalizing relative to GAPDH signals (squares). The proportions of cells at S phase are shown as circles.
Cells deficient in HSF1 and HSF3 are hypersensitive to high temperatures
Specific reduction of Hsp90α expression in vertebrate cells deficient in heat-responsive factors was unexpected. Experiments conducted using yeast cells yielded interesting results (Zarzov et al., 1997; Morano et al., 1999). Yeast HSF has two separate transcriptional activation domains. Yeast cells expressing a truncated form of HSF, in which the C-terminal activation domain was deleted, were viable, but were temperature sensitive due to the reduction in Hsp90α expression (Morano et al., 1999). Hsp90 is essential for cell growth at high temperature (Borkovich et al., 1989).
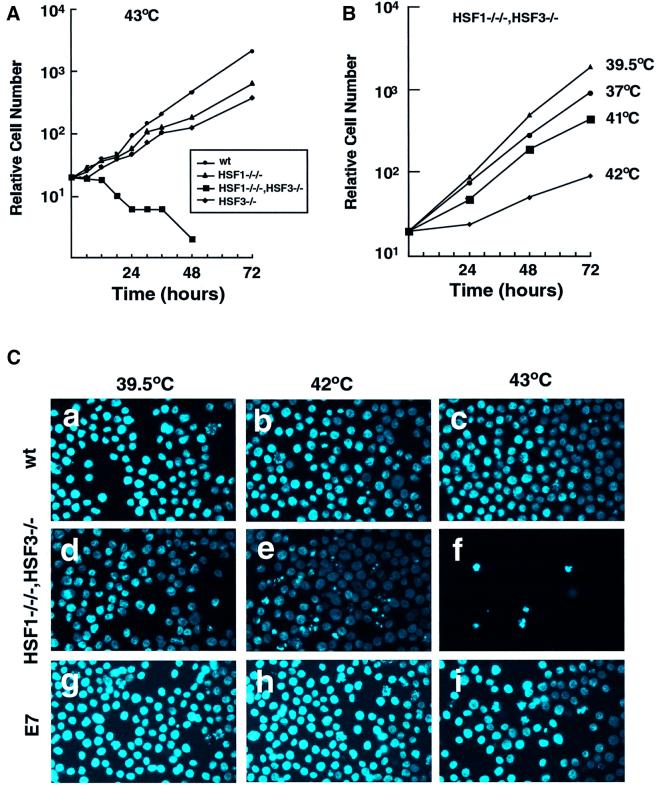
We tested whether DT40 cells deficient in HSFs were able to grow at higher temperatures. When cells were maintained at 43°C, wild-type cells and cells deficient in HSF1 or HSF3 grew exponentially (Figure 5A). In marked contrast, cells deficient in both HSF1 and HSF3 died. DT40 cells grow at the maximal rate at the physiological temperature of 39.5°C (Aschoff and Paul, 1973), and grow even at 45°C. The growth rate of cells deficient in HSF1 and HSF3 was markedly reduced even at mild heat shock temperatures such as 42°C (Figure 5B). Microscopic examination showed many dead cells in which the nuclei were fragmented at 42°C (Figure 5C). Dead cells were observed even at 41°C, which was only 1.5°C higher than the physiological temperature (data not shown). These results indicated that vertebrate HSFs are quite important to protect cells from a single continuous thermal stress.
Fig. 5. Sensitivity to higher temperatures. (A) Growth curves of wild-type cells and cells deficient in HSF1 and/or HSF3 at 43°C. Aliquots of 1 × 104 cells maintained at 37°C were inoculated into 35 mm dishes and the numbers of surviving cells were counted after Trypan blue staining for 72 h at 43°C. (B) Growth curves of cells deficient in both HSF1 and HSF3 at various temperatures. (C) Wild-type cells, cells deficient in HSF1 and HSF3 (#54), and HSF1-restored cells in the #54 line (E7, see Figure 3B) maintained at 39.5°C were incubated at 42 or 43°C for 24 h. The nuclei were stained with DAPI.
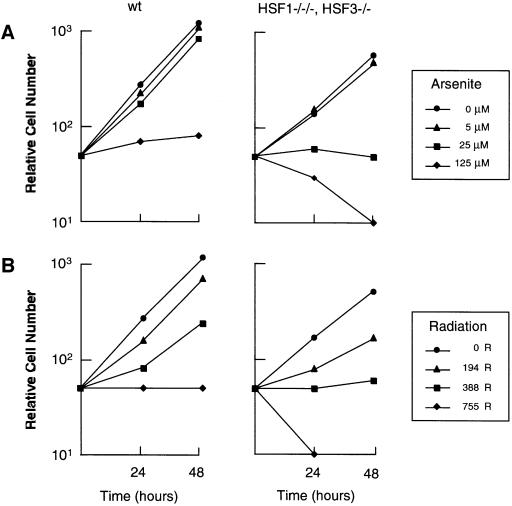
We next examined whether cells deficient in HSF1 and HSF3 were more sensitive to other stresses than wild-type cells. Wild-type cells grew exponentially in the presence of sodium arsenite at a concentration of 25 µM until 48 h, whereas cells deficient in HSF1 and HSF3 never grew at that concentration (Figure 6A). Similarly, cells deficient in HSF1 and HSF3 were more sensitive to exposure to radiation than wild-type cells (Figure 6B). These results suggest that HSFs are necessary for cell growth in the presence of various stresses.
Fig. 6. Sensitivity to sodium arsenite and radiation. (A) Growth curves of wild-type cells and cells deficient in HSF1 and HSF3 in the presence of sodium arsenite at the indicated concentrations. Aliquots of 1 × 104 cells were inoculated into 35 mm dishes and the numbers of surviving cells were counted after Trypan blue staining for 48 h. (B) Aliquots of 1 × 104 cells were exposed to the indicated doses of radiation and inoculated into 35 mm dishes. The numbers of surviving cells were counted as in (A).
Cdc2 is a molecular target affected by high temperatures
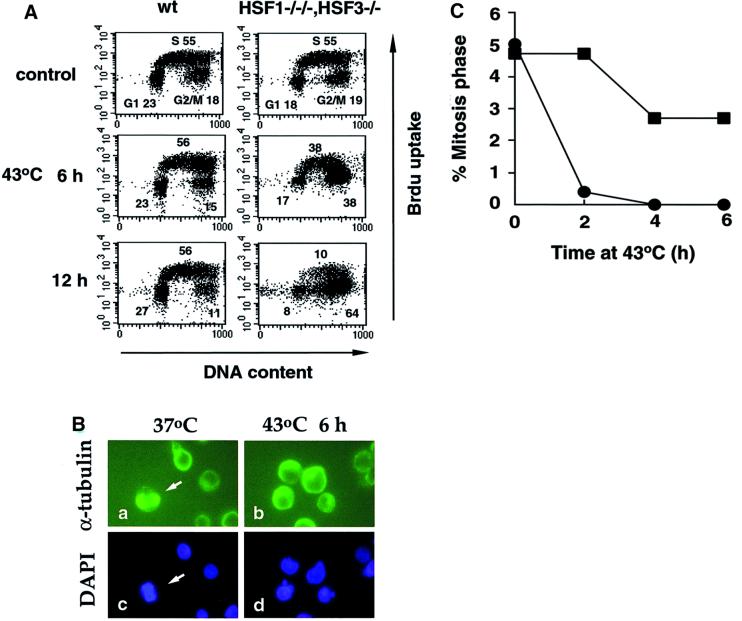
We examined stages of the cell cycle affected by heat shock. In wild-type cells, the cell cycle continued to progress at 43°C (Figure 7A), and even at 45°C (data not shown). In contrast, in cells deficient in HSF1 and HSF3, heat shock caused a marked increase in the population of cells in the G2/M phase at 43°C after 12 h (Figure 7A). Cells in the G1 phase were still observed after 12 h, also suggesting G1 block. These results were consistent with those reported previously in yeast cells having a mutant HSF (Zarzov et al., 1997; Morano et al., 1999). To distinguish cells at G2 phase from those at M phase, we examined microtubule spindle formation and chromatin condensation by staining α-tubulin and DNA (Figure 7B, arrows). Cells in the mitotic phase were observed in 5% of normally growing cells, but disappeared completely at 43°C by 4 h in cells deficient in HSF1 and HSF3 (Figure 7C). These results revealed G2 block of the cell cycle.
Fig. 7. Cell cycle block at higher temperatures. (A) Cell cycle analysis of wild-type cells (left) and cells deficient in HSF1 and HSF3 (right) incubated at 43°C for 12 h. Flow cytometric analysis was performed. The proportions of cells at G1, S and G2/M phases are indicated in each panel. (B) Cells deficient in HSF1 and HSF3 maintained at 39.5°C and these cells incubated at 43°C for 6 h were stained with anti-α-tubulin antibody and with DAPI. Arrows indicate cells at mitosis. (C) Wild-type cells (squares) and cells deficient in HSF1 and HSF3 (circles) maintained at 39.5°C were incubated at 43°C for 6 h. After staining with anti-α-tubulin antibody and DAPI, the proportions of mitotic cells were shown.
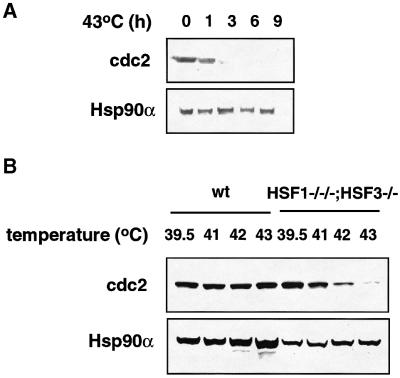
Activation of the cyclin-dependent kinase Cdc2 is necessary for entry into M phase. Cdc2 protein level is constant during cell cycle progression in normally growing cells (Townsley et al., 1998). Unexpectedly, the level of Cdc2 protein was reduced markedly by 3 h at 43°C in cells deficient in HSF1 and HSF3 (Figure 8A). This result is consistent with the profile of disappearance of cells undergoing mitosis at 43°C (Figure 7B). Cdc2 protein levels were analyzed further in cells heat shocked at various temperature for 9 h. The levels were markedly decreased in cells deficient in both HSF1 and HSF3 even by mild heat shock such as 42°C (Figure 8B), which is consistent with the reduced growth at this temperature (Figure 5B). These results suggested that Cdc2 is a molecular target affected by heat shock that causes a G2 block of the cell cycle.
Fig. 8. Stability of Cdc2. (A) Cells maintained at 39.5°C were incubated at 43°C for the indicated times, and were immediately suspended in Laemmli’s SDS sample buffer and sonicated. Western blot analysis was performed using antibodies specific for Cdc2 and Hsp90α. (B) Cells were incubated at the indicated temperatures for 9 h, and Cdc2 protein levels were estimated by western blot analysis.
Heat shock response is not necessary for cell cycle transition at high temperatures
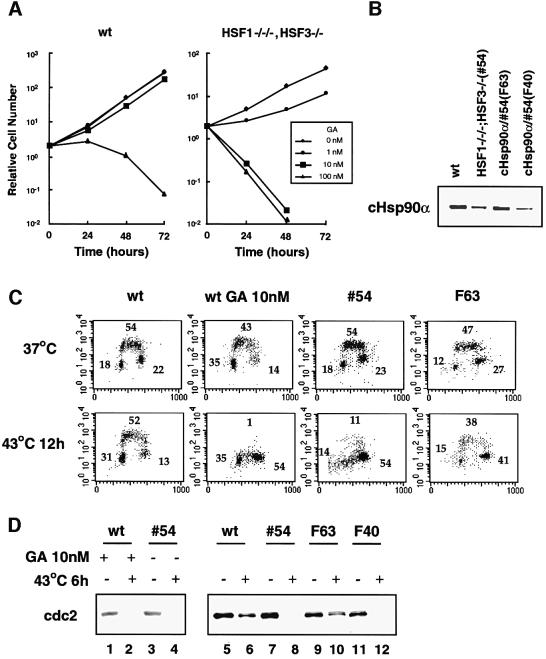
To confirm the effect of Hsp90α on Cdc2 stability under stress conditions, we analyzed cells treated with the Hsp90-specific inhibitor geldanamycin (GA) and Hsp90α-restored cells deficient in HSF1 and HSF3. Wild-type cells grew well in the presence of 10 nM GA, whereas cells deficient in HSF1 and HSF3 were sensitive to GA at 10 nM (Figure 9A). The cell cycle of wild-type cells treated with 10 nM GA was blocked at the G2/M phase as well as at G1 at 43°C (Figure 9C). Cdc2 in wild-type cells was unstable in the presence of GA under heat shock conditions (Figure 9D, lane 2). Furthermore, Hsp90α-restored cells deficient in HSF1 and HSF3 (Figure 9B, clone F63) did not show a cell cycle block at 43°C (Figure 9C). Cdc2 in F63 cells was stabilized even at 43°C due to the restoration of Hsp90α (Figure 8D, lane 10). These results indicated that Hsp90α is necessary for the stability of Cdc2 and, subsequently, cell cycle transition. These results also demonstrated that the heat shock response is not necessary for cell growth at high temperatures.
Fig. 9. Hsp90α is necessary for cell cycle progression and Cdc2 stability. (A) Cells were maintained in the presence of GA (0–100 nM). The numbers of cells were counted for 3 days. (B) Establishment of cells deficient in HSF1 and HSF3 in which cHsp90α expression was restored. The protein levels of Hsp90α in two clones (F63 and F40) are shown. (C) Cell cycle analysis of wild-type cells, wild-type cells treated with 10 nM GA, cells deficient in HSF1 and HSF3 (#54), and the Hsp90α-restored clone F63 in the presence or absence of heat shock at 43°C for 12 h. (D) Cell extracts were prepared from wild-type cells, cells deficient in HSF1 and HSF3, and Hsp90α-restored cells treated or not with GA (10 nM), and western blot analysis was performed.
Discussion
In budding yeasts, HSF is a trimer that binds to DNA and is essential for survival in the absence of stress (Sorger and Pelham, 1988; Wiederrecht et al., 1988), suggesting that yeast HSF regulates target genes in normally growing cells. Recently, it was shown that Hsp90α expression was reduced specifically in a HSF mutant of the yeast Saccharomyces cerevisiae (Zarzov et al., 1997) or a deletion mutant lacking an N-terminal activation domain (Morano et al., 1999). These results suggested that yeast HSF regulates constitutive expression of Hsp90α. However, this result left the possibility that the reduction in Hsp90α expression was an artificial effect due to the presence of non-physiological HSF. In contrast to HSF in budding yeasts, biochemical analysis showed that vertebrate HSF1 and HSF3 are a monomer and a dimer, respectively, which do not bind to DNA in the absence of stress (Baler et al., 1993; Sarge et al., 1993; Nakai et al., 1995). We thought from these results that HSF1 and HSF3 are not functional in the absence of stress, unlike yeast HSF. An unexpected observation in this report is that lack of both HSF1 and HSF3 results in the specific reduction of Hsp90α expression. Taken together, our results suggested an evolutionarily conserved fundamental role for HSF in the absence of stress. Not only yeast HSF, but also vertebrate HSFs regulate constitutive expression especially of Hsp90α in normally growing cells.
It was shown that growth of yeast cells at higher temperatures depends on the concentration of Hsp90 (Borkovich et al., 1989). As HSFs can regulate the level of Hsp90α expression, vertebrate HSFs should determine the temperatures at which cells can survive by regulat ing Hsp90α expression in normally growing cells. Remarkably, restoration of Hsp90α in DT40 cells deficient in HSF1 and HSF3 was sufficient for them to survive at 43°C without induction of Hsps (Figure 9). Thus, we would like to emphasize that induction of Hsps through activation of HSFs is not very involved in cell cycle arrest or cell death caused by a single continuous heat shock. Cells acquire resistance to lethal heat shock when they are pre-exposed to sublethal heat, which is known as induced thermotolerance. Induction of Hsps through activation of HSFs is necessary for acquisition of this induced thermotolerance (Jedlicka et al., 1997; McMillan et al., 1998; Tanabe et al., 1998). In this report, we identified a novel role for HSFs different from that necessary for induced thermotolerance. As continuous temperature stress is common in nature, this novel role of HSFs should be very significant for cell survival.
When cells are exposed to heat stress, cell cycle transition is blocked and cell death is induced. These two phenomena may be induced by independent mechanisms. Growth arrest in yeast cells with truncated HSF at higher temperatures was shown to be reversible (Morano et al., 1999), whereas DT40 cells deficient in HSF1 and HSF3 exposed to higher temperatures died. This difference in reversibility may have been due to the absence of typical apoptotic mechanisms in yeast cells (Fröhlich and Madeo, 2000).
Hsp90 regulates many signaling molecules such as pp60v-src, Raf, transcription factors such as steroid hormone receptors and HSF. The expression level of Hsp90 varies depending on cells and tissues (Vamvakopoulos, 1993) and on growth conditions (Figure 4) (Jerome et al., 1993). Interestingly, we could not isolate any stable cell line that had more than 1.5 times the normal Hsp90α level (data not shown). It was shown that even a 1.2-fold increase in Hsp90 in the human monoblastoid cell line U937 caused a marked increase in apoptosis following induction with tumor necrosis factor-α and cycloheximide (Galea-Lauri et al., 1996). These results suggest that even small changes in the Hsp90 level may affect intracellular signaling pathways, although Hsp90 is among the most abundant cellular proteins. Thus, strict control of Hsp90 expression by HSFs may be required for maintenance of integrity of a cell. The level of Hsp90 expression is also important to suppress morphological phenotypes caused by genetic mutations during evolution (Rutherford and Lindquist, 1998). When Hsp90 function was reduced in Drosophila by thermal stress or mutation of the Hsp90 gene, cryptic mutants were expressed and, surprisingly, these phenotypes continued to be expressed even after Hsp90 function was restored (Rutherford and Lindquist, 1998). HSFs could be a key factor that controls the tendency for evolutionary changes to occur through regulating Hsp90 expression under normal growth conditions in a cell- or tissue-specific manner.
HSF is reported to be required for normal development. Drosophila HSF is necessary for oogenesis and early larval development (Jedlicka et al., 1997). Mouse HSF1 is indispensable for development of placenta and postnatal growth (Xiao et al., 1999). Furthermore, maternal HSF1 is necessary for the development of pre-implantation mouse embryos (Christians et al., 2000). These data suggest that HSFs regulate unknown target genes necessary for development. We identified for the first time a target gene regulated by vertebrate HSFs in normally growing cells. This observation supports the notion that abnormal development in mice lacking HSF1 may be due to lack of correct regulation of target genes. Because changes in gene expression regulated by HSF1 might be small, like that of Hsp90α expression in DT40 cells, detailed analysis may be necessary to identify the genes regulated by HSF, which is necessary for development.
The Hsp90α gene has a heat shock element to which HSFs bind and was induced by HSF activation (Figure 2). However, the existence of a heat shock element is not sufficient for regulation by HSF in the absence of stress because other Hsps are not under the control of HSF. Additional factors might be required for HSF to activate the Hsp90α gene, or HSF might act on other transcription factors that regulate basal expression of Hsp90α. To clarify the molecular mechanism of Hsp90α regulation by HSF, it should be determined whether or not interaction of the DNA-binding domain of HSF with the heat shock element is necessary for correct regulation of Hsp90α expression.
Transcriptional activity of vertebrate HSF1 is regulated by protein kinases such as mitogen-activated protein kinases (MAPKs), glycogen synthase kinase (GSK3) and protein kinase C without inducing trimer formation (Chu et al., 1996; Knauf et al., 1996; Kline et al., 1997). However, it was not clear whether Hsp expression is regulated through HSFs in the absence of stress in vivo because there is no direct evidence that HSF can control constitutive expression of Hsps. In addition to showing that Hsp90α expression is under the control of HSFs, we also found that Hsp90α expression is up-regulated by HSF at early S phase during cell cycle transition (Figure 4). We did not observe any change in the level of Hsp70, Hsc70, Hsp40 or Hsp110 mRNA during cell cycle transition of DT40 cells (data not shown). These observations suggest that intracellular signaling mechanisms may regulate at least Hsp90α expression through HSF regulation.
To our knowledge, this is the first demonstration of a molecular target affected by a high temperature stress that causes cell cycle arrest in vertebrates. Hsp90 may stabilize Cdc2 protein directly or indirectly in normally growing cells. Instability becomes evident due to the reduction in Hsp90α expression even on mild heat stress. Involvement of Hsp90 in Cdc2 stability is supported by the report showing genetic interaction between Hsp90 and Cdc2 in yeast (Munoz and Jimenez, 1999). The cells deficient in HSFs are also highly sensitive to other stresses such as exposure to ionizing radiation and sodium arsenite (Figure 6). Hypersensitivity to each reagent may be caused by a different molecular target. These molecules may be stabilized normally by Hsp90 and other molecular chaperones. Our mutant cells would be useful tools for the identification of molecular targets of various stresses such as hyperthermia and drugs for cancer treatments.
Materials and methods
Construction of targeting vectors
Chicken HSF1 genomic clones were isolated by screening an EMBL3 library of genomic DNA from adult chicken liver (Clontech) using a 1.8 kb EcoRI fragment of pCHSF1 (Nakai and Morimoto, 1993) as a probe. We also isolated a 5.5 kb PCR fragment (which corresponds to the 5′ arm of the HSF1-zeo construct in Figure 1A) of the HSF1 gene using genomic DNA from DT40 cells. To generate targeting constructs HSF1-bsr and HSF1-puro, a 9.0 kb HindIII–SalI fragment was first inserted into the pGEM1 vector (Promega) in which the BamHI site was mutated [pGEM1B(–)]. A 3.0 kb BamHI–ApaI fragment was then removed and self-ligated by creating a BamHI site. A cassette containing a blastcydin or puromycin resistance gene flanked by a BamHI site was inserted into the vector. As the third allele of the HSF1 gene was not mutated by the construct with these arms, we further generated an HSF1-zeo targeting construct. A 5.0 kb PCR fragment and a 1.9 kb SphI fragment were inserted into the pZeoSV2 vector (Invitrogen) (detailed information available on request).
Cell culture, transfection and screening
We previously generated HSF3-deficient DT40 chicken B lymphocytes by transfection with HSF3 gene-targeting constructs containing the neomycin- or hygromycin-resistant gene by electroporation (Tanabe et al., 1998). Cells deficient in HSF1 were generated by sequential transfection into wild-type cells of a circular HSF1 gene-targeting construct containing blastcydin or puromycin genes, or a zeocine resistance gene linearized by NotI. The HSF1 gene is located on the second chromosome, which is present in three copies. Cells deficient in both HSF1 and HSF3 were generated similarly by transfection into HSF3-null cells.
Generation of Hsp90α-restored cells
To generate an expression vector of full-length chicken Hsp90α, expression vector K390S (a kind gift form Dr M.-G.Catelli), which expresses Hsp90α lacking the four N-terminal amino acids, was modified to express full-length cHsp90α by site-directed mutagenesis. The Hsp90α cDNA insert was then subcloned into expression vector pCMV/Blue (BD PharMingen, San Diego, CA). The resulting plasmid pCMV/Hsp90α was co-transfected into cells deficient in HSF1 and HSF3 with a plasmid containing the hisD gene. These cells were maintained in the presence of 750 µg/ml histidinol. The level of Hsp90α in each clone isolated was determined by western blot analysis using antiserum αcHsp90α (Kawazoe et al., 1999). HSF1-restored cells were generated similarly by transfection of cHSF1 expression vector pHβ-cHSF1 (Nakai et al., 1995).
Western blot analysis and gel shift assay
To examine the protein level of each HSF, we used specific antisera, αHSF1β, αHSF2α and αHSF3γ, which were generated previously (Nakai et al., 1995). A gel shift assay and a supershift experiment using αHSF1γ, αHSF2δ and αHSF3γ antisera was performed essentially as described previously (Nakai et al., 1995, 1997). To examine Cdc2 protein, rabbit polyclonal IgG specific for Cdc2 (Santa Cruz, sc-53) was used.
Northern blot analysis
Northern blot analysis was performed using specific probes essentially as described previously (Tanabe et al., 1998). A chicken Hsc70 cDNA fragment (615 bp) (de la Rosa et al., 1998) was amplified by RT–PCR using total RNA from DT40 cells, and subcloned into the pCR-XL-TOPO vector (Invitrogen). The plasmid pcHsc70 was digested with EcoRI and a 0.6 kb fragment was used as a probe after 32P labeling. Primers used for PCR were: cHsc70-1, 5′-ACCTTCGATATTGATGCC-3′; and cHsc70-2, 5′-GCAATATACCCAGATGTGCC-3′.
Cell cycle analysis
The cell cycle was analyzed essentially as described previously (Sonoda et al., 1998). Cells were labeled with 20 µM bromodeoxyuridine (BrdU; Amersham Pharmacia) for 10 min, washed with phosphate-buffered saline (PBS), and fixed in 70% ethanol at 4°C for 30 min. Cells were then soaked in 2 M HCl containing 0.5% Triton X-100 at room temperature for 30 min. After washing, cells were incubated with mouse anti-BrdU antibody (1:100 dilution, PharMingen, CA) in PBS/1% bovine serum albumin (BSA) at room temperature for 30 min. After washing with PBS/1% BSA, cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (1:200 dilution, Cappel) in PBS/1% BSA at room temperature for 30 min. After washing, cells were suspended in PBS containing 25 µg/ml propidium iodide and analyzed using a FACScan flow cytometer (Becton Dickinson, CA). Fluorescence data were displayed as dot plots using the Cell Quest program (Becton Dickinson, CA).
Staining of DNA and α-tubulin
Cells were washed with PBS and adhered to glass slides by using a centrifugal cell collector (Tomy, Tokyo, Japan) according to the manufacturer’s instructions. Cells adhering to the slides were fixed with 4% paraformaldehyde at room temperature for 15 min, and were permeabilized in 0.5% NP-40 in PBS for 15 min. After washing three times with 0.5% BSA/PBS, cells were incubated with FITC- conjugated mouse anti-α-tubulin antibody (Sigma) at 37°C for 1 h. After washing, cells were mounted in Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories CA) and visualized using fluorescence microscopy (Axiophot, Zeiss). The numbers of mitotic cells were counted.
Acknowledgments
Acknowledgements
We thank S.Ishii and S.Takeda for critically reading the manuscript, R.I.Morimoto, D.J.Thiele, S.Yonehara and E.Nishida for discussion, M.G.Catelli, T.Kina, E.Sonoda, Y.Yamashita, K.Sakamaki, Y.Miyata and N.Tokuda for reagents and advice, and M.Tanabe and S.Inoue for technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, the Core Research for Evolutional Science and Technology, Japan Science and Technology Corporation, the Uehara Memorial Foundation and the Inamori Foundation.
References
- Aligue R., Akhavan-Niak,H. and Russell,P. (1994) A role for Hsp90 in cell cycle control: Weel1 tyrosine kinase activity requires interaction with Hsp90. EMBO J., 13, 6099–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. and von Saint Paul,U. (1973) Brain temperature as related to gross motor activity in the unanesthetized chicken. Physiol. Behav., 10, 529–533. [DOI] [PubMed] [Google Scholar]
- Baler R., Dahl,G. and Voellmy,R. (1993) Activation of human heat shock genes is accompanied by oligomerization, modification and rapid translocation of heat shock transcription factor HSF1. Mol. Cell. Biol., 13, 2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K.A., Farrelly,F.W., Finkelstein,D.B., Taulien,J. and Lindquist,S. (1989) Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol., 9, 3919–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians E., Davis,A.A., Thomas,S.D. and Benjamin,I.J. (2000) Maternal effect of Hsf1 on reproductive success. Nature, 407, 693–694. [DOI] [PubMed] [Google Scholar]
- Chu B., Soncin,F., Price,B.D., Stevenson,A. and Caldwood,S.K. (1996) Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J. Biol. Chem., 271, 30847–30857. [DOI] [PubMed] [Google Scholar]
- Cutforth T. and Rubin,G.M. (1994) Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell, 77, 1027–1036. [DOI] [PubMed] [Google Scholar]
- de la Rosa E.J., Vega-Nunez,E., Morales,A.V., Serna,J., Rubio,E. and de Pablo,F. (1998) Modulation of the chaperone heat shock cognate 70 by embryonic (pro)insulin correlates with prevention of apoptosis. Proc. Natl Acad. Sci. USA, 95, 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S.J. (1996) Cell cycle checkpoints: preventing an identity crisis. Science, 274, 1664–1672. [DOI] [PubMed] [Google Scholar]
- Fröhlich K.-U. and Madeo,F. (2000) Apoptosis in yeast—a monocellular orgamism exhibits altruistic behaviour. FEBS Lett., 473, 6–9. [DOI] [PubMed] [Google Scholar]
- Galea-Lauri J., Richardson,A.J., Latchman,D.S. and Katz,D.R. (1996) Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-1 and cycloheximide. J. Immunol., 157, 4109–4118. [PubMed] [Google Scholar]
- Jedlicka P., Mortin,M.A. and Wu,C. (1997) Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J., 16, 2452–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome V., Vourc’h,C., Baulieu,E.E. and Catelli,M.G. (1993) Cell cycle regulation of the chicken hsp90α expression. Exp. Cell Res., 205, 44–51. [DOI] [PubMed] [Google Scholar]
- Kawazoe Y., Tanabe,T., Sasai,N., Nagata,K. and Nakai,A. (1999) HSF3 is a major heat shock responsive factor during chicken embryonic development. Eur. J. Biochem., 265, 688–697. [DOI] [PubMed] [Google Scholar]
- Kline M.P. and Morimoto,R.I. (1997) Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol. Cell. Biol., 17, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf U., Newton,E.M., Kyriakis,J. and Kingston,R.E. (1996) Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev., 10, 2782–2793. [DOI] [PubMed] [Google Scholar]
- Lindquist S. (1986) The heat-shock response. Annu. Rev. Biochem., 55, 1151–1191. [DOI] [PubMed] [Google Scholar]
- McMillan D.R., Xiao,X., Shao,L., Graves,K. and Benjamin,I.J. (1998) Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem., 273, 7523–7528. [DOI] [PubMed] [Google Scholar]
- Morano K.A., Santoro,N., Koch,K.A. and Thiele,D.J. (1999) A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol. Cell. Biol., 19, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R.I. (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Genes Dev., 12, 3788–3796. [DOI] [PubMed] [Google Scholar]
- Munoz M.J. and Jimenez,J. (1999) Genetic interactions between Hsp90 and the Cdc2 mitotic machinery in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet., 261, 242–250. [DOI] [PubMed] [Google Scholar]
- Nakai A. (1999) New aspect in the vertebrate heat shock factor system: HSF3 and HSF4. Cell Stress Chaperone, 4, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A. and Morimoto,R.I. (1993) Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol. Cell. Biol., 13, 1983–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A., Kawazoe,Y., Tanabe,M., Nagata,K. and Morimoto,R.I. (1995) The DNA-binding properties of two heat shock factors, HSF1 and HSF3 are induced in the avian erythroblast cell line HD6. Mol. Cell. Biol., 15, 5268–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A., Tanabe,M., Kawazoe,Y., Inazawa,J., Morimoto,R.I. and Nagata,K. (1997) HSF4, a new member of human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol., 17, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A., Suzuki,M. and Tanabe,M. (2000) Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1. EMBO J., 19, 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S.L. and Lindquist,S. (1998) Hsp90 as a capacitor for morphological evolution. Nature, 396, 336–342. [DOI] [PubMed] [Google Scholar]
- Sarge K.D., Murphy,S.P. and Morimoto,R.I. (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol., 13, 1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Sasaki,M.S., Buerstedde,J.M., Bezzubova,O., Shinohara,A., Ogawa,H., Takata,M., Yamaguchi-Iwai,Y. and Takeda,S. (1998) Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J., 17, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P.K. (1991) Heat shock factor and the heat shock response. Cell, 65, 363–366. [DOI] [PubMed] [Google Scholar]
- Sorger P.K. and Pelham,H.R. (1988) Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell, 54, 855–864. [DOI] [PubMed] [Google Scholar]
- Stepanova L., Leng,X., Parker,S.B. and Harper,J.W. (1996) Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev., 10, 1491–1502. [DOI] [PubMed] [Google Scholar]
- Tanabe M., Nakai,A., Kawazoe,Y. and Nagata,K. (1997) Different thresholds in the responses of two heat shock transcription factors, HSF1 and HSF3. J. Biol. Chem., 272, 15389–15395. [DOI] [PubMed] [Google Scholar]
- Tanabe M., Kawazoe,Y., Takeda,S., Morimoto,R.I., Nagata,K. and Nakai,A. (1998) Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO J., 17, 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe M., Sasai,N., Nagata,K., Liu,X.-D., Liu,P.C.C., Thiele,D.J. and Nakai,A. (1999) The mammalian HSF4 gene generates both an activator and a repressor of heat shock genes by alternative splicing. J. Biol. Chem., 274, 27845–27856. [DOI] [PubMed] [Google Scholar]
- Townsley F.M. and Ruderman,J.V. (1998) Proteolytic ratchets that control progression through mitosis. Trends Cell Biol., 8, 238–244. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos N.O. (1993) Tissue-specific expression of heat shock proteins 70 and 90: potential implication for differential sensitivity of tissues to glucocorticoids. Mol. Cell. Endocrinol., 98, 49–54. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Seto,D. and Parker,C.S. (1988) Isolation of the gene encoding the S.cerevisiae heat shock transcription factor. Cell, 54, 841–853. [DOI] [PubMed] [Google Scholar]
- Wu C. (1995) Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol., 11, 441–469. [DOI] [PubMed] [Google Scholar]
- Xiao X., Zuo,X., Davis,A.A., McMillan,D.R., Curry,B.B., Richardson,J.A. and Benjamin,I.J. (1999) HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J., 18, 5943–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov P., Boucherie,H. and Mann,C. (1997) A yeast heat shock transcription factor (Hsf1) mutant is defective in both Hsc82/Hsp82 synthesis and spindle pole body duplication. J. Cell Sci., 110, 1879–1891. [DOI] [PubMed] [Google Scholar]