Abstract
Bone defect remains an intractable issue for clinical orthopedics owing to their varied sizes and irregular shapes. Poly (lactic acid-co-glycolic acid) (PLGA)-based artificial bone grafts has garnered considerable attention in bone repair owing to their outstanding biocompatibility and tunable biodegradability. 3D printing technology is a feasible means due to its ability to construct scaffolds with defined shapes for restoring bone defects in clinical practice. In this review, the physicochemical properties of PLGA and 3D printing technology are briefly introduced. In addition, diverse strategies to improve the osteogenic performance of 3D printed PLGA scaffolds are elaborated. Finally, current challenges and future perspectives of 3D printed PLGA scaffolds applied in clinical practice are proposed.
Keywords: PLGA, 3D printing, Scaffold, Functionalization, Bone regeneration
Graphical abstract
Graphical abstract: Overview of functionalization of 3D-printed PLGA-based scaffolds for bone regeneration (Figure was created with www.figdraw.com).

Highlights
-
•
This review summarizes the latest advances in 3D printed PLGA-based scaffolds for bone regeneration.
-
•
This review highlights how 3D printing technology creates complex scaffold structures to enhance bone regeneration.
-
•
This review identifies current challenges and future research directions in bone regeneration.
1. Introduction
With the rapid advancement of transportation and demographic aging, bone defects are prevalent across all age groups [1]. In China, approximately seven million patients are affected by bone defects every year [2]. While bone tissue has inherent regenerative potential, supercritical defects are unable to heal spontaneously without any clinical intervention [3,4], resulting in delayed healing and bone nonunion [5,6]. This not only impairs the structural stability and physiological function of bone [1], but more importantly has a detrimental influence on individual psychological health [1], and even aggravates the economic burden on the healthcare system. Accordingly, repairing bone defects is an imperative public health issue.
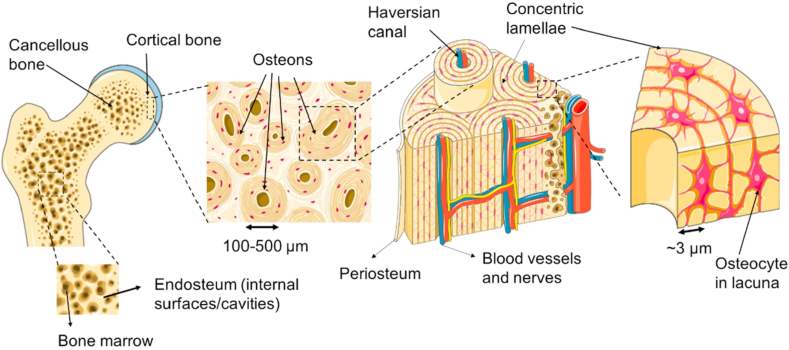
Bone is intricately designed tissues with highly organized hierarchical structures [7,8], which is composed of dense cortical bone and porous cancellous bone [9,10]. Cortical bone is characterized by its arrangement of multiple osteons aligned with the longitudinal axis of the bone [11,12]. Each osteon, functioning as a basic unit of bone tissue, features concentric layers of lamellar bone with blood vessels and nerve fibers traversing through its center [10,11]. On the other hand, cancellous bone exhibits an anisotropic arrangement of trabecular bones, resembling a honeycomb-like structure filled with blood vessels and bone marrow (Fig. 1) [9,11]. This marrow serves as an abundant reservoir of hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) [11], contributing to the regenerative potential of the bone. However, this complex structure presents a significant challenge in orthopedic clinical practice, particularly when it comes to repairing bone defects. Achieving successful repair requires strategies that can replicate the hierarchical organization and anisotropic properties, ensuring optimal functional recovery and structural integrity.
Fig. 1.
A macro-to-micro perspective of cancellous and cortical bone. Reproduced with permission from Ref. [9]. Copyright 2018 Elsevier.
Bone grafting remains a conventional treatment for bone defects [13], with an estimated 2.2 million patients worldwide undergoing this procedure annually [14]. Autografts, known for their non-immunogenicity, osteogenesis, osteoconductivity and osteoinduction, are considered the gold standard [15,16]. However, complex surgical procedures, limited availability of donors, potential complications at donor site, and unknown prognosis hinder their widespread application [17]. Although allografts and xenografts could alleviate donor shortages, they have merely osteoconductivity and lack osteogenesis along with osteoinduction. Additionally, they have the potential for disease transmission and immunological rejection [15,18]. In response to the clinical limitations of conventional grafts, considerable attention has been paid to the advancement of artificial bone grafts.
Artificial bone grafts strive to imitate the natural composition of bone, which comprises an organic phase (collagen, COL) and an inorganic phase (hydroxyapatite, HA) [19]. Polymer materials, with resemblance to COL in structure and mechanical properties, have garnered significant attention [20]. Among these polymers, Poly (lactic acid-co-glycolic acid) (PLGA) stands out owing to its exceptional biocompatibility, tunable biodegradability, and approval by the United States Food and Drug Administration (FDA) and the European Medicine Agency (EMA) [21]. Superior mechanical properties and immune neutrality of PLGA render it an optimal candidate for application in drug delivery [[22], [23], [24]] and tissue engineering [25], particularly bone tissue engineering [26,27]. These attributes position PLGA as a promising polymer for the advancement of artificial bone grafts.
To better simulate natural bone tissue, PLGA is often integrated with inorganic materials to produce hybrid materials that promote bone restoration. Liu et al. combined PLGA with mesoporous bioglass (MBG), enhancing the angiogenic and osteogenic potential of endothelial cells and bone marrow mesenchymal stem cells (BMSCs), respectively [28]. Zhang et al. incorporated HA into PLGA, detecting improved osteogenic differentiation and proliferation compared to pure PLGA scaffolds [29]. Overall, composite scaffolds exhibit superior bone healing potential.
In addition to the regulatory effect of material composition on osteogenesis, the structure of the scaffold is also a decisive factor for bone regeneration. Porous scaffolds not only accommodate for cell adhesion and proliferation [30], but also ensure nutrient transportation, discharge metabolic waste and facilitate angiogenesis [1]. 3D printing technology grants the benefits of low cost, rapid prototyping, and personalized customization, making it a promising technology for preparing porous scaffolds [31]. Moreover, 3D printing technology can adjust the mechanical characteristics of the scaffold to align with those of native bone tissue by coordinating printing settings [20].
However, it is insufficient for an ideal osteogenic scaffold to merely simulate the composition and structure of bone tissue [20]. It should create a favorable microenvironment for bone regeneration, including regulating immune responses during the inflammatory phase, facilitating angiogenesis and osteogenic differentiation of osteoblasts during the bone formation phase, and coordinating the dynamic balance between bone formation and resorption during the bone remodeling phase. Therefore, tremendous efforts have been made to functionalize 3D printed PLGA-based scaffolds to approach these standards.
Over the past decade, a multitude of literature reviews have addressed the application of PLGA-based scaffolds in bone tissue engineering [32,33]. However, the great majority of their focus has either been on optimizing physicochemical properties and degradation behavior [34], or single functional modification and single structural design [35], while this review distinctively underlines the integration of 3D printing technology with multifunctional strategies for bone regeneration. 3D printing technology enables the fabrication of scaffolds tailored to specific bone defects, and allows precise control over their macro- and microstructures. Meanwhile, multifunctional strategies endow scaffolds with a range of biological functions, including osteogenesis, angiogenesis, immunomodulation, and antibacterial properties. The synergistic interaction of 3D printing technology and multifunctional strategies facilitates the development of intelligent, personalized, and multifunctional bone regeneration scaffolds, paving the way for the clinical translation of PLGA-based scaffolds.
Therefore, this review, with a unique focus on the integration of 3D printing technology with multifunctional strategies, offers a comprehensive summary of recent advancements and outlines future challenges and prospects in this emerging field. Firstly, the synthesis methods and physicochemical properties of PLGA are outlined. Subsequently, 3D printing technologies for preparing PLGA-based scaffolds were recapitulated. Afterwards, the functionalization strategies of 3D printed PLGA-based scaffolds to improve bone regeneration are highlighted. Finally, the current challenges and future prospects of functionalized 3D printed PLGA-based scaffolds for bone regeneration are discussed. This review aims to provide valuable insights and sever as a reference for future research on 3D printed PLGA-based scaffolds.
2. Synthesis and physicochemical properties of PLGA
2.1. Synthesis
PLGA is a copolymer of lactic acid and glycolic acid, whose molecular weights range from thousands to hundreds of thousands, depending on their ratios [36,37]. It is synthesized through ring-opening polymerization (ROP) and direct polycondensation [38,39].
ROP is a method for synthesizing PLGA by first dehydrating and cyclizing lactic acid and glycolic acid to obtain lactides and glycolides, respectively [37]. These cyclic intermediates are then subjected to chemical bond cleavage and polymerization to form PLGA [37]. ROP can be further divided into ring opening random polymerization (RORP) [40] and ring opening alternating polymerization (ROAP) [41]. In RORP, lactides and glycolides are synthesized first and polymerized to produce PLGA. However, on account of the higher reactivity of glycolides in contrast to lactides, synthesizing PLGA with random microstructure is challenging [40]. Li et al. developed a novel organophosphazene base as a catalyst to make the reactivity of lactides comparable to that of glycolides, enabling the synthesis of random PLGA copolymers [40]. On the other hand, ROAP aims to synthesize ethylene propylene glycol ester through esterification and cyclization reactions, followed by self-polymerization to obtain PLGA with a regular sequence. Coates et al. developed chirality-directed and pairing-enhanced regioselectivity approaches to synthesize alternating PLGA with controlled sequences [41,42]. Overall, ROP is a mature method for producing with high molecular weight PLGA [43], but its process is cumbersome, laborious and expensive [44,45]. Despite these obstacles, the development of novel catalysts and regioselectivity approaches is still the general trend to synthesize PLGA with tailored microstructures and properties.
Direct polycondensation is a simple method for synthesizing PLGA compared to ROP [44]. It usually involves the dehydration condensation reaction of lactic acid and glycolic acid in the molten state to produce long-chain PLGA copolymers [44]. However, it is difficult to synthesize high molecular weight PLGA. For one thing, condensation water cannot be removed during the synthesis process [45]. For another thing, the thermal stability of the copolymers is poor in the molten state, making them prone to depolymerization or random chain scission at high temperature [45]. Recent research by Kim et al. has shown that it is possible to synthesize high molecular weight PLGA by optimizing reaction conditions, specifically by conducting the esterification reaction of lactic acid and glycolic acid in diphenylether under vacuum [38]. This study highlights that direct polycondensation is able to synthesize high molecular weight PLGA after optimizing reaction condition.
Although ring-opening polymerization and direct polycondensation have been widely used in the synthesis of PLGA, residual catalysts and monomers remain urgent challenges. Scientists are now investigating alternative methods like segmer assembly polymerization [46] and enzyme driven synthesis [47] for creating PLGA with enhanced biosafety, presenting exciting opportunities for the future of polymer synthesis.
2.2. Biocompatibility
PLGA, a widely-used material in the biomedical field, is highly valued for its biocompatibility and low toxicity [48]. This is evident in its non-toxic degradation products, namely lactic acid and glycolic acid [49]. Upon decomposition, these compounds are metabolized by the tricarboxylic acid cycle of body, eventually being eliminated as water and carbon dioxide through the respiration or digestion [50,51]. In the past, concerns were raised about the inflammatory effects of lactic acid, causing hesitation in the use of PLGA [52,53]. However, latest findings have pointed out the beneficial role of lactic acid in biological processes [48,54]. For instance, lactic acid can be converted into pyruvate, which inhibits prolyl hydroxylase 2 (PHD2) [48], stabilize hypoxia inducible factor-1 (HIF-1) [55], and promotes angiogenesis [56]. This new understanding of the positive impact of lactic acid has paved the way for the application of PLGA in various medical treatments and therapies.
Ongoing research continues to explore the biocompatibility of PLGA, with promising findings emerging. For example, Thuaksuban and colleagues utilized 3D printed PLGA membranes to facilitate bone regeneration, assessing their biocompatibility [57]. The results revealed that PLGA (L: G = 90:10) significantly enhanced the proliferation of mouse osteoblasts and fibroblasts for a duration of 14 days [57]. The histological assessment results suggested that the PLGA membrane degraded, disappeared, and formed a cavity during the observation period, without eliciting any adverse immune response [57]. In addition, Gao et al. prepared a non-cellular cylindrical PLGA scaffold with a radially positioned microstructure to repair bone and cartilage defect [54]. After implanting the PLGA scaffold into the defect, it promoted cell migration from surrounding tissues towards the defect region, and induced bone regeneration with the assistance of microstructure [54]. These studies reflect the potential of PLGA in tissue regeneration and lay a solid foundation for its biocompatibility in various medical fields.
PLGA is compatible with cells and tissues, and its degradation products are favorable to vascularized bone regeneration, indicating that PLGA is suitable as a support material for bone tissue engineering.
2.3. Biodegradation
Controllable biodegradability is a key feature of PLGA [58]. The degradation process includes three stages: the quasi-stability stage, the loss of strength stage, and the disruption of scaffold stage [34]. The first stage is characterized by a reduction in scaffold dimensions, an increase in mechanical strength, and a constant weight [34]. The second stage shows a significant decrease in weight and a sharp drop in mechanical strength [34]. The last stage involves the release of acid products, the subsequent pH decrease, increased brittleness and eventual scaffold decomposition [34]. Hast et al. found that the mechanical strength of 3D printed PLGA scaffolds remained unchanged during 0–8 week period but significantly decreased between 8 and 16 weeks, which is consistent with the degradation process of PLGA [59].
The degradation of PLGA can be tailored by multiple factors such as molecular mass, the proportion of lactic acid to glycolic acid, monomer sequences, and end groups [60]. PLGA with high molecular weight can generally maintain the integrity of the polymer structure, meaning it takes more time to degrade [58]. Additionally, the proportion of lactic acid to glycolic acid is also a crucial factor in determining the degradation behavior of PLGA [61]. Typically, as the glycolic acid ratio increases, the rate of degradation becomes faster [62,63]. For instance, PLGA (L: G = 50:50) takes approximately two months for complete degradation [64], while PLGA (L: G = 75:25) takes around five months, and PLGA (L: G = 85:15) takes more than six months [45]. In addition, the monomer sequence has an impact on the degradation of PLGA [65]. A study by Meyer et al. found PLGA with repeated sequences degraded slower than random PLGA [65]. Meanwhile, the end group play an indispensable role in the degradation of PLGA. It has been shown the degradation of PLGA ended with acid groups is 2–3 times faster than PLGA ended with hexyl or ethyl groups [58]. All in all, these factors exert a substantial effect on controlling the degradation rate of PLGA, making it a suitable candidate for different biomedical applications.
Customized biodegradable PLGA may coordinate with each stage of bone regeneration, making PLGA stand out among numerous polymers as an artificial bone scaffold material.
2.4. Hydrophobicity
Generally speaking, materials featuring a water contact angle above 90° correspond to hydrophobicity, while materials possessing a water contact angle beyond 90° correspond to hydrophilicity [66]. It has been shown that the water contact angle of pure PLGA is greater than 90° [66], signifying that PLGA is hydrophobic. The intrinsic hydrophobicity of PLGA is determined by the combination of lactic acid and glycolic acid [34]. Polyglycolic acid (PGA) possesses a great deal of hydroxyl and carboxyl groups, which are capable of forming coordination bonds with water molecules, thereby enhancing its interaction with water and making it hydrophilic. However, owing to methyl groups on the side chains of polylactic (PLA), it displays stronger water resistance relative to PGA [27]. Hence, the inherent hydrophobicity of PLGA may be owing to the pendent methyl groups on the PLGA chain [67]. Moreover, the hydrophilicity of PLGA is subject to the proportion of lactic acid to glycolic acid. A greater glycolic acid ratio results in increased hydrophilicity, while elevated lactic acid ratio leads to reduced hydrophilicity [34]. It is critical to take these factors into account with the intention of designing appropriate PLGA-based scaffolds for bone regeneration.
The hydrophilicity of PLGA impacts its biological activity, so many approaches have emerged to improve its hydrophilicity. For example, plasma treatment can reduce the water contact angle of PLGA and enhance its hydrophilicity [68]. Additionally, highly hydrophilic substances such as polyethylene glycol (PEG) and polydopamine (PDA) can be also employed to improve the hydrophilicity of PLGA [35]. Karimipour et al. evaluated the wettability of PLGA and PLGA-PEG composite and found PLGA-PEG composite had better hydrophilicity [69]. Wang et al. constructed a PDA coating on the PLGA scaffold, reducing its water contact angle from 128° to 65° and significantly improving its hydrophilicity [70]. These studies indicate that the hydrophilicity of PLGA can be improved through various ways.
2.5. Mechanical strength
The mechanical strength of PLGA plays a significant role in regulating osteogenesis, and should match with the mechanical strength of cortical and cancellous bone. The compressive modulus, Young's modulus and compressive strength of cancellous bone are 50–500 MPa [71], 100–2000 MPa [72] and 2–12 Mpa [71], respectively, while cortical bone respectively reaches 14–18 GPa [73], 10–25 Pa [72] and 100–230 MPa [74]. However, pure PLGA has drawbacks such as high brittleness and poor toughness, which is not conducive to bone regeneration [75]. Undoubtedly, these factors should be included as evaluation criteria when designing highly biomimetic bone regeneration scaffolds based on PLGA.
Therefore, scientists have been committed to adding inorganic components such as black phosphorus (BP) and silver nanoparticles to PLGA to enhance its mechanical strength. For example, Lai et al. incorporated BP into PLGA to improve its mechanical properties to support new bone formation [76]. Mechanical testing revealed that the compressive strength rose from 1.87 MPa to 2.53 MPa, and the compressive modulus raised from 43.64 MPa to 62.57 MPa. Similarly, Wang et al. added silver nanoparticles to PLGA to prepare a 3D scaffold for infectious bone defects, resulting in an increase in tensile strength from 34.9 MPa to 52.7 MPa and a rise in Young's modulus from 1.96 MPa to 2.46 MPa [77]. In general, it is definitely a feasible approach to incorporate inorganic materials to enhance the mechanical performance of PLGA in an effort to better align with the mechanical attributes of innate bone tissue.
In summary, the intrinsic properties of PLGA exert a profound influence on the design of 3D-printed scaffolds. Firstly, the molecular weight and the lactic-to-glycolic acid ratio of PLGA play a crucial role in the degradation kinetics and mechanical strength of the scaffold, thereby affecting its load-bearing capacity. Secondly, the degradation rate of PLGA scaffolds must be tailored to align with the timeline of bone regeneration. Finally, the rheological behavior and thermal characteristics of PLGA are fundamentally correlated with the printability and structural accuracy of the scaffold. These characteristics not only determine the overall performance of PLGA-based scaffolds, but also highlight the pivotal role of advanced 3D printing technology in achieving architectural precision and functional integration.
3. 3D printing technologies
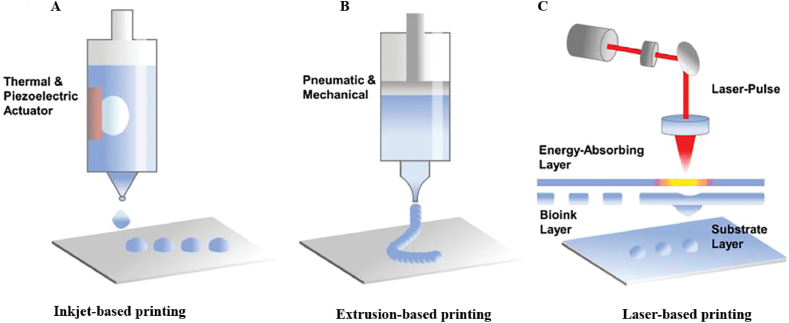
In light of the intricate structure and function of bone tissue, 3D printing technology is employed to fabricate PLGA-based scaffolds for bone regeneration, aiming to harvest bone tissue with homogeneous structures [78]. 3D printing is an advanced manufacturing technique for constructing three-dimensional (3D) scaffolds with complex structures by stacking materials sequentially in layers with the assistance of computer-aided design (CAD) models [79,80]. It also has the ability to create porous structures with specific shapes and orderly interconnected pores [81], which is beneficial for the establishment of vascular networks, nutrients delivery, and the elimination of metabolic excretion. At present, 3D printing technologies include inkjet-based printing, extrusion-based printing and laser-based printing. By leveraging these cutting-edge techniques, researchers are able to produce scaffolds that mimic the native composition and architecture of bone tissue, enhancing the potential for successful bone regeneration therapies.
3.1. Inkjet-based printing
Inkjet-based printing has appeared as a multifunctional and effective method for depositing polymers, biomolecules, and cells onto various substrates at a micro-scale level (Fig. 2A) [82]. This non-contact method is highly cost-effective and offers superior precision and resolution, making it a sought-after technique in various industries [82]. However, one of the limitations of inkjet-based printing is its restriction to bioinks with low viscosity, leading to concerns about the mechanical strength of the printed scaffolds [12]. Additionally, the nozzle of the printer is prone to clogging, which can disrupt the smooth printing process [83]. There are two main types of inkjet-based printing methods, continuous inkjet (CIJ) printing and drop-on-demand (DOD) inkjet printing [84]. CIJ printing involves a continuous flow of liquid through the nozzle, resulting in poor printing quality due to the formation of droplet flow under surface tension forces [84,85]. On the other hand, DOD printing ejects droplets rapidly from a printhead with multiple nozzles in response to a trigger signal [84], allowing for the simultaneous printing of multiple materials [86]. This method is typically driven by heat-related pneumatic pulses, piezoelectric pressure, and electrohydrodynamic forces [12]. In recent years, DOD inkjet printing has gained popularity owing to its ease of operation and versatility in printing various materials [82].
Fig. 2.
(A) Inkjet-based printing, (B) extrusion-based printing, (C) laser-based printing. Reproduced with permission from Ref. [82]. Copyright 2022 Wiley-VCH.
3.2. Extrusion-based printing
Extrusion-based printing is a versatile and easy-to-use 3D printing method that involves using either pneumatic pressure or mechanical forces to extrude and deposit bioink layer by layer via a nozzle onto a build platform (Fig. 2B) [12,87]. Pneumatic-based printing is best for bioink with low viscosity, while mechanical-based printing works well for bioink with high viscosity [82]. The chief merit of extrusion-based printing is that it is able to prepare scaffolds loaded with bioactive substances such as growth factors, drugs, proteins, and even cells [88], which are frequently used in craniomaxillofacial, jaw bone, auditory bone, hip bone, and other sites [[89], [90], [91]]. However, there is a possibility that shear force could cause damage to cells and reduce their viability [12]. This emphasizes the necessity of carefully considering and optimizing printing parameters to ensure successful outcomes.
Fused deposition modeling (FDM) is one of the oldest and most prevalent extrusion-based printing technologies [92], which mainly prints thermoplastic polymer filaments into personalized 3D scaffolds with distinct porosity and pore size, accommodating to the growth and differentiation of stem cells [[93], [94], [95]]. During the printing process, the polymer filaments undergo heating by a nozzle to melted or semi-molten condition, and then extruded and deposited onto a build platform to harden and set [87,88]. Particularly, this technology is advantageous for printing scaffolds with high mechanical performance, making them suitable for simulating the mechanical environment of natural bone tissue [96]. Catros et al. mixed PLGA and HA nanoparticles to fabricate a 3D porous scaffold through FDM, which promoted cell proliferation, and had a positive impact on osteogenic differentiation [97]. FDM has the capacity to prepare scaffolds with exceptional mechanical properties and intricate structures, highlighting its prospect in tissue engineering and regenerative medicine applications.
Low-temperature deposition manufacturing (LDM) is a novel extrusion-based technology, which was first reported for the development of bone tissue engineering scaffolds in 2002 [98]. LDM primarily utilizes viscous polymers to print scaffolds [99], which have lower mechanical strength versus scaffolds constructed by FDM [36]. However, the scaffold made by LDM has a porous structure with variable scales interconnected microchannels [100], which can better simulate the extracellular matrix (ECM) [36]. Xiong et al. introduced Mg and β-tricalcium phosphate (β-TCP) into PLGA and developed a porous hybrid scaffold by LDM, which promoted osteogenic differentiation of mouse calvaria-derived preosteoblastic cell line subclone E1 (MC3T3-E1) cells and enhanced bone regeneration in the rabbit cranial defect area [101].
3.3. Laser-based printing
Lased-based printing, a rapidly advancing 3D printing technology, involves non-contact printing without nozzles. The technique utilizes pulsed laser beams to irradiate the energy-absorbing layer, causing energy evaporation and enabling the bioink to be jetted onto the substrate layer with high precision and resolution(Fig. 2C) [82,92]. It circumvents direct contact between the printhead and bioink, reducing the shear force on cells and avoiding issues such as nozzle clogging and contamination [92]. This printing technology is divided into stereolithography (SLA), selective laser sintering (SLS) and two-photon polymerization (TPP).
SLA represents one of the foundational 3D printing technologies exploited to produce bone tissue engineering scaffolds [83], which operates with ultraviolet laser to print bioinks in a stepwise manner [92]. It has the capability to generate micro or nano scale scaffolds with specific porosity and pore size distribution, as well as interconnected pore structures [102]. In spite of its merits, scaffolds prepared by SLA typically require post-processing before practical application [95].
SLS refers to a rapid prototyping technique that employs near-infrared laser (NIR) to sinter powder materials and fabricates a 3D scaffold via stacking layer by layer [88], which tends to possess a rough and porous surface [102]. Compared with SLA, the scaffolds prepared by SLS have better mechanical strength [103]. However, SLS involves high temperatures, so it cannot integrate cells into the scaffolds [102], and often result in material wastage [96]. Even if there are several drawbacks, SLS remains a promising technology for constructing scaffolds with complicated structures for various applications.
The TPP technology is an cutting-edge 3D printing method that harnesses NIR and double photons absorption to activate polymer materials [87]. This process involves monomers,crosslinking agents, and photoinitiators [102], offering precise control over the polymerization reaction. By carefully choosing the right photoinitiations and NIR wavelengths within 700–800 nm range, micro- or nano 3D scaffolds with tailored shapes can be produced with exceptional accuracy and detail [87].
However, laser-based printing has shortcomings such as low printing speed, high expense, and limited scalability, which limit its widespread application [12].
4. Multifunctional strategies of 3D printed PLGA-based scaffolds for bone regeneration
The 3D printed PLGA-based scaffolds show great promise for bone regeneration due to their biocompatibility, biodegradability, and porous structure. Nevertheless, challenges like hydrophobicity, weak mechanical strength, and limited biological activity have impeded their effectiveness. In order to improve the efficiency and quality of bone regeneration, numerous strategies have been proposed, such as optimizing the scaffold structure, enhancing the physicochemical properties of the scaffold, and endowing the scaffold with the biological activity. Solving these issues intends to improve the efficiency and quality of bone regeneration, thereby expanding the application of bone tissue engineering.
4.1. Strategies to prepare 3D printed PLGA-based scaffolds with personalized shape and hierarchical pore structure
Artificial bone scaffolds are supposed to possess an individualized shape and a multistage microporous structure [104]. The individual shape depends on the bone defect, which could be achieved through 3D printing technology. The hierarchical pore structure means that the scaffold should be provided with both macropores and micropores for bone regeneration simultaneously [105]. Micropores support initial cell adhesion and spreading, while macropores are conducive to cell filtration and migration, nutrient transportation, metabolic waste excretion, angiogenesis and new bone formation [105]. Both are indispensable for osteogenesis. The 3D printed PLGA-based scaffolds only have regular porous structures, which fail to fulfill the requirements of bone regeneration for varying pore sizes. Hence, it is imperative to couple with other methods to construct hierarchical pore structures.
Directional freezing technology is a simple and environment-friendly way to fabricate directional pore structures [106]. Sun et al. utilized a mixed matrix of chitosan and silk fibroin coated with PDA to generate hierarchical pore structure within 3D printed PLGA-HA scaffolds through directional freezing technology (Fig. 3C) [19]. The resulting scaffolds exhibited excellent biocompatibility, promoted the rapid infiltration, adhesion and osteogenic differentiation of BMSCs, and upregulated the expression of osteogenic-related genes. Furthermore, evidence from the rabbit cranial defect experiment suggested that the hierarchical scaffolds not only enhanced angiogenesis but also achieved apparent new bone formation, implying that the scaffold structure exerts a favorable effect on rehabilitating bone defects, and reflecting the potential value of directional freezing technology in bone regeneration.
Fig. 3.
(A) Preparation (a) and biological characterization (b) of PLGA/HA scaffolds with multistage porous structure. Reproduced with permission from Ref. [107]. Copyright 2021 The Royal Society of Chemistry. (B) Fabrication and biological characterization of flexible PLGA/HA scaffolds with interconnected pore structure. Reproduced with permission from Ref. [110]. Copyright 2022 American Chemical Society. (C) Schematic illustration of PLGA/HA scaffolds with directional microchannels guiding cranial regeneration. Reproduced with permission from Ref. [19]. Copyright 2024 Wiley-VCH.
Freeze-drying-crosslinking method is also a feasible approach to manufacture multistage porous scaffolds. Li et al. perfused the 3D printed scaffolds containing PLGA and HA with gelatin that has the ECM-like polymer network structure to form multistage porous scaffolds after drying (Fig. 3A) [107]. At 8 weeks of implantation, micro computed tomography (Micro-CT) scans displayed the multistage scaffolds obtained dense trabecular bone and woven bone formation. Compared with the scaffold without a multistage porous structure, the bone volume/total volume (BV/TV) value, the number of bone trabeculae (Tb.N) and the trabecular thickness (Tb.Th) of the multistage scaffold group are higher , while the trabecular separation (Tb.Sp) was the lowest. Hematoxylin and eosin (HE) staining and Masson staining revealed that new bone tissue had penetrated into the porous regions of the scaffold, which was in accordance with the findings from Micro-CT analysis, indicating that the structure of the scaffold might ameliorate its osteogenic performance, which is a positive signal for bone tissue engineering applications.
Supercritical carbon dioxide (SC–CO2) foaming technology is an economical and innocuous pore-making technique, which is widely used to prepare porous polymer scaffolds [108,109]. For example, 3D printed PLGA-HA scaffolds are endowed with interconnected pore structures via SC-CO2 foaming technology (Fig. 3B) [110]. These scaffolds have been found to promote the proliferation and spreading of BMSCs, accelerate biomineralized deposition, and enhance the expression of osteogenic and angiogenic genes, inferring that the pore structure may guide cell adhesion and migration, thereby facilitating osteogenesis.
The hierarchical structure of PLGA-based scaffolds could have a positive effect on the biological behavior of endogenous cells such as adhesion, proliferation, and migration, but it still can not endow these scaffolds with intrinsic osteoinductive properties, limiting their efficiency in bone regeneration.
4.2. Strategies to enhance the physicochemical properties of 3D printed PLGA-based scaffolds
An effective bone repair scaffold must meet several essential criteria: high biocompatibility, suitable mechanical resilience, surface hydrophilicity, controlled biodegradability. Current 3D printed PLGA-based scaffolds fall short in meeting these criteria. To improve their properties, methods like physical blending and surface modification are employed. These techniques enhance the overall performance of scaffolds for successful bone regeneration.
4.2.1. Physical blending
Physical blending is a method that combines materials with different properties to enhance their characteristics and achieve specific objectives. The technique proves to be both convenient and effective method in improving the physicochemical properties of 3D printed scaffolds from PLGA. Incorporating inorganic substances such as HA, β-TCP, carbon nanomaterials and others into PLGA bioinks has shown promising results. These additives can improve hydrophilicity, increase mechanical strength, modulate the degradation kinetics, and support bone growth of the scaffolds. For instance, Li et al. incorporated HA microspheres to the PLGA matrix, which boosted the compressive load-bearing capacity of the PLGA scaffold and improved the adhesion, proliferation, and osteogenic differentiation of BMSCs (Fig. 4A) [111]. HE staining revealed significant bone tissue formation in the HA-containing groups and that the new bone area in the high HA content group was the highest (Fig. 4B). Similarly, the work of Ruan et al. combined HA and β-TCP with PLGA to enhance the mechanical strength of the PLGA scaffold, including Young's modulus and compressive strength, aligning it with the bone defect area, which facilitated new bone formation [112]. Additionally, Calcium sulfate (CaSO4), similar to HA and β-TCP, provides a calcium-rich osteogenic microenvironment [113], and enhance the mechanical strength of polymeric scaffolds owing to their high compressive strength [114]. Zhang et al. blended CaSO4 with PLGA, significantly enhancing the mechanical properties of the PLGA scaffold and ameliorating the migration and osteogenic differentiation of MC3T3-E1 cells [113]. All in all, integrating inorganic materials with PLGA through physical blending provides a promising avenue to improve their performance and efficacy in bone tissue engineering applications.
Fig. 4.
(A) Schematic illustration of the fabrication of BMP-2/PLGA microspheres modified PLGA/CaSO4 scaffolds and osteogenesis in vitro. Reproduced with permission from Ref. [131]. Copyright 2024 Frontiers. (B) Schematic illustration of sustained release of antimicrobial peptide and BMP-2 from 3D-printed PLGA scaffolds for osteogenesis differentiation. Reproduced with permission from Ref. [136]. Copyright 2019 The Royal Society of Chemistry. (C) Schematic diagram of the preparation and biological characterization of PLGA/MBG scaffolds doped with ZIF-8/BMP-2. Reproduced with permission from Ref. [132]. Copyright 2023 Dove Medical Press.
In recent years, carbon nanomaterials, a novel inorganic non-metallic material, have gained popularity in the materials community on account of their remarkable mechanical properties, superior biological activity, and chemical stability [115]. Especially, their role in bone regeneration has also attracted considerable attention. Gomes et al. incorporated carbon quantum dots to the PLGA scaffold to enhance its hydrophilicity, which improved cell adhesion and enhanced bone mineralization of human adipose derived stem cells (ADSCs) [116]. Likewise, Ege et al. produced a 3D printed scaffold based on a mixed bioink of carbon nanotubes and PLGA, which improved the hydrophilicity of the PLGA scaffold, significantly increased the compression modulus, deferred the decomposition of PLGA, and boosted the biomineralization of MC3T3-E1 cells [117]. Carbon nanomaterials hold broad application in bone tissue engineering, and ongoing research will demonstrate their effectiveness and multifunctionality in various biomedical applications.
In general, physical blending is a straightforward process, but its influence on the attributes of the target material is unpredictable. Additionally, exploring optimal mixing ratios for different materials is often time-consuming and laborious.
4.2.2. Surface modification
PLGA lacks natural cell recognition sites and its intrinsic hydrophobicity hinders cell adhesion, migration, proliferation, and even osteogenic differentiation, which is detrimental to bone formation [118]. Surface modification is a conventional and effective approach to impart multiple functions to PLGA scaffolds, including plasma treatment [119], coating [120], grafting [121], and crosslinking [122]. In particular, constructing hydrophilic coatings on the PLGA scaffold surface has shown promise in improving hydrophilicity and promoting osteogenesis.
PDA is a mussel-inspired biomimetic material that can adhere to the surfaces of various substrates [123]. It contains abundant hydroxyl and amino groups, resulting in excellent hydrophilicity [123]. In addition, it can provide recognition sites for cell adhesion, thereby promoting cell adhesion and proliferation [35]. In the light of its outstanding properties, PDA is frequently applied to the PLGA-based scaffolds to enhance their hydrophilicity. This biomimetic material exhibits great promise in biomedical applications owing to its ability to improve the performance of various substrates and support cell growth.
Studies have been conducted where the scaffold containing PLGA and β-TCP were fabricated via 3D printing technology [124]. Subsequently, dopamine self-polymerizes into a PDA coating on the surface of the β-TCP/PLGA scaffold (Fig. 4C) [124]. In vitro experiments suggested that the PDA coated composite scaffold promoted the adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 and this effect became more pronounced with the increase in PDA concentrations. HE and Masson staining revealed that the PDA coated composite scaffold exhibited a more compact and substantial layer of fibrous connective tissues for 2-week implantation and a large amount of new tissue formation for 6-week implantation (Fig. 4D), indicating that the PDA coating enhanced the osteogenic performance of the PLGA-based scaffold.
In recent years, PDA hybrid coatings have emerged to improve the availability of PDA coatings [125]. Priddy and colleagues designed a PDA and HA hybrid coating on the 3D printed PLGA scaffold, achieving higher surface roughness [125]. Compared to PDA and HA coatings, this hybrid coating endowed the PLGA scaffold with the best hydrophilic effect and demonstrated a better ability to promote the adhesion and proliferation of MC3T3-E1 cells. These findings reflect the potential of PDA hybrid coatings in improving the performance of scaffolds in tissue engineering.
To achieve better osteogenic effects, surface modified PLGA-based scaffolds are suggested to be combined with additional bioactive agents such as growth factors, metal ions, and drugs to promote the cell-material interaction, thereby improving osteogenic efficiency. Combining these elements is fundamental to achieve optimal bone regeneration and tissue engineering outcomes.
4.3. Strategies to improve the bioactivity of 3D printed PLGA-based scaffolds
It is challenging to rely solely on scaffold materials to restore bone defects attributing to scarce osteoinductivity. Effectively promoting the osteogenic effect of 3D printed PLGA based scaffolds remains a key focus of current research. The routine approach is to integrate osteoinductive growth factors, peptides, drugs, metal ions into the scaffold. This strategy aims to optimize the capacity of scaffolds to promote bone growth and repair in clinical application.
4.3.1. Growth factor
Bone regeneration is a multifaceted biological process that engages diverse cells, signaling molecules, and numerous growth factor [104]. Growth factors play a role in every stage of bone repair, including inflammation regulation, neovascularization, bone formation, and maintenance of bone homeostasis. Key regulatory growth factors like bone morphogenetic protein (BMP), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-β1 (TGF-β1) are crucial players in the complex mechanism of bone regeneration [102]. Their coordinated efforts ensure the successful restoration and maintenance of healthy bone tissue.
BMPs, members of the TGF-β1 superfamily [126], play a crucial role in inducing bone regeneration [127]. Bone morphogenetic protein-2 (BMP-2) and bone morphogenetic protein-9 (BMP-9) are specially promising in this regard [128]. BMP-2, an FDA-approved osteoinductive growth factor [129], enhances osteogenesis by regulating the recruitment and differentiation of osteoblasts and mesenchymal stromal cells [130]. In one study, BMP-2 was encapsulated in PLGA microspheres and incorporated into 3D printed PLGA and CaSO4 scaffolds to enable sustained release for over 30 days, promoting osteogenic differentiation effectively (Fig. 4A) [131]. Moreover, researchers have developed innovative approaches to further enhance the bioactivity of BMPs. For instance, a bioink composed of PLGA, MBG, and zeolitic imidazolate framework-8 (ZIF-8) embedded with BMP-2 was used to develop a 3D-printed scaffold for continuous BMP-2 release (Fig. 4C) [132]. This strategy not only supported the growth and differentiation of osteoblasts in vitro but also accelerated bone formation in vivo. On the other hand, BMP-9 exhibits the ability to counteract the inhibition of Smad cellular signaling pathway caused by Noggin, thereby promoting osteogenic differentiation in various stem cells [128]. Researchers have successfully immobilized BMP-9 on the surface of 3D printed PLGA scaffolds using the Gly-Phe-Hyp-Gly-Arg (GFOGER) peptide [133]. This approach promoted uniform trabecular bone formation and new bone regeneration, which confirms the prospective role of BMP-9 in bone tissue engineering. Overall, the integration of BMPs, particularly BMP-2 and BMP-9, into 3D printed scaffold has yielded hopeful results in promoting bone regeneration through enhanced osteoblast differentiation and bone tissue formation. These innovative technologies hold enormous potential for advancing bone tissue engineering in the future.
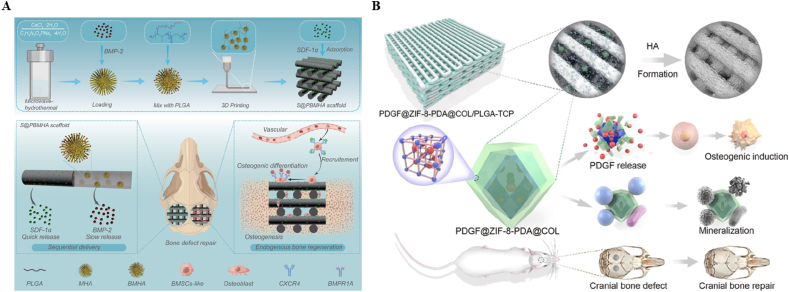
In addition, BMPs can also combine with other bioactive factors to exert synergistic effects, thereby promoting bone regeneration in a sequential and effective manner. Stromal cell-derived growth factor 1 α (SDF-1α) is a chemokine that promotes the recruitment, migration, and homing of MSCs [134]. Researchers have made significant progress in incorporating BMP-2 into engineered porous scaffolds with well-defined pore structures via 3D printing technology [135]. By immobilizing SDF-1α onto the scaffold surface, a controlled and time-dependent release of both BMP-2 and SDF-1α can be achieved [135]. The strategy of releasing SDF-1α early to recruit a large number of MSCs to the defect site, followed by the release of BMP-2 to promote osteogenic differentiation, has proven effective in enhancing bone regeneration (Fig. 5A) [135]. Additionally, the incorporation of antimicrobial peptides like ponectin G1 into scaffolds can provide long-term antibacterial activity without leading to drug-resistant bacteria [102]. Studies have shown that immobilizing antimicrobial peptides, along with BMP-2, on scaffold surfaces through PDA coating can significantly improve cell adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 cells (Fig. 4B) [136]. This innovative approach not only enhances bone regeneration but also ensures sustained antimicrobial activity, contributing to the overall success of bone tissue engineering strategies.
Fig. 5.
(A) Schematic diagram of the fabrication of a functional scaffold with sequential SDF-1α and BMP-2 release and its endogenous bone regeneration experiment. Reproduced with permission from Ref. [135]. Copyright 2023 Wiley-VCH. (B) Schematic illustration of biological characterization of PDGF@ZIF-8-PDA@COL/PLGA-β-TCP composite scaffolds. Reproduced with permission from Ref. [139]. Copyright 2022 Elsevier.
PDGF is a cytokine with dual functions, facilitating the development of new blood vessels and promoting new bone formation [137,138]. This makes PDGF a promising candidate for vascularized bone regeneration [137]. A study conducted by Chang et al. involves infiltrating a COL hydrogel containing PDGF into a 3D printed scaffold (Fig. 5B) [139]. This composite scaffold continuously released PDGF, demonstrating exceptional osteoinductive and osteoconductive properties as well as effective antibacterial activity. Micro CT scans revealed significant progress in bone tissue regeneration within two months of implantation, with the bone defect area being nearly completely filled with new bone tissue. This positive outcome was further supported by histological analyses, including HE staining, Masson staining, and immunohistochemistry, confirming the accelerated bone regeneration potential of the composite scaffold. Overall, the study highlights the promising role of PDGF in enhancing bone regeneration through composite scaffolds technologies.
Enhancing bone regeneration is critical, but the instability of current growth factors limits their effectiveness. Finding bioactive substances with stable performance is crucial to improve osteogenic effects. Stable performance is essential for sustained bone growth, as the short half-life of growth factors can lead to inactivation and uncertain outcomes. Researching more reliable alternatives is necessary.
4.3.2. Peptide
Peptides, derived from growth factor domains, are stable and specific amino acid sequence that are less immunogenic than growth factors [140,141]. They offer a cost-effective and scalable solution as an alternative to growth factors [142,143]. These peptides can be tailored for diverse functions like antibacterial, osteogenic and angiogenic properties along with multifunctional roles [144]. Among them, osteogenic peptide (OP) stands out as a commonly used peptide in bone regeneration. Its customizable nature and ability to simulate bone growth make it a promising tool in the field of regenerative medicine. With their versatility and efficacy, peptides are revolutionizing the way we approach tissue regeneration and healing processes.
Recent studies have demonstrated that OP possesses osteoinductive properties that play a crucial role in guiding MSCs toward bone-forming pathways both in vitro and in vivo. By incorporating graphene oxide (GO) nanosheets adsorbed with the OP into a bioink comprised of PLGA and β-TCP, researchers have been able to create a specialized hierarchical porous scaffold that closely resembles cancellous bone using innovative low-temperature 3D printing technology (Fig. 6A) [145]. The unique scaffold not only facilitates the sustained delivery of OP, attracting BMSCs for growth and promoting their differentiation into bone-forming cells, but also significantly enhances bone regeneration in vivo. Another pioneering approach involved the creation of a composite scaffold composed of PLGA/β-TCP nanocomposite struts loaded with OP and BMSCs encapsulated within gelatin/gelatin methacryloyl (GelMA) hydrogel rods using a dual-nozzle low-temperature hybrid 3D printing technique (Fig. 6C) [146]. The incorporation of OP within this composite scaffold was found to effectively simulate the osteogenic differentiation of BMSCs, leading to increased expression of alkaline phosphatase (ALP) expression and enhanced biomineralization, ultimately contributing to improved bone formation.
Fig. 6.
(A) Schematic illustration for the fabrication and function of OP/GO/β-TCP/PLGA scaffolds. Reproduced with permission from Ref. [145]. Copyright 2019 MDPI. (B) Schematic illustration of the preparation and biological characterization of OP@COL/CHX@GO/β-TCP/PLGA scaffolds. Reproduced with permission from Ref. [150]. Copyright 2022 Elsevier. (C) Schematic illustration of BMSCs-gelatin-GelMA/OP-β-TCP-PLGA scaffolds via low temperature hybrid 3D printing. Reproduced with permission from Ref. [146]. Copyright 2022 IOP Publishing.
Bone defects are vulnerable to bacterial infections, leading to long-term inflammation that hinders bone formation [[147], [148], [149]]. To combat this, antibiotics are often used [102]. However, to enhance bone regeneration while preventing infections, it is beneficial to combine antibiotics with OP. Researchers like Zhou et al. have developed a dual-release scaffold using GO nanosheets loaded with chlorhexidine and OP encapsulated in a COL hydrogel, incorporated into a 3D-printed scaffold made from biocompatible materials (Fig. 6B) [150]. This scaffold delivers chlorhexidine rapidly for immediate antibacterial effects, while the slow-release chlorhexidine helps prevent future infections. Additionally, sustained release of OP aids in promoting bone regeneration. In vivo experiments, this dual-release scaffold demonstrated significant efficacy in treating infectious bone defects, offering a promising new approach for addressing such challenging conditions. By combining antibacterial agents with OP in a controlled-release scaffold, researchers are pioneering novel approaches for managing infected bone lesions.
Repairing bone defects is sophisticated process that not only entails combating the risk of bacterial infection but also necessitates the restoration of blood supply to facilitate successful bone regeneration. In light of these challenges, researchers have turned to fusion peptides that embody multiple functionalities to address these critical aspects. Deng et al. manufactured a PLGA and HA composite scaffold via 3D printing technology and immobilized fusion peptides with osteogenic, angiogenic, and antibacterial properties on the composite scaffold surface [144]. The composite scaffold modified with this fusion peptide exhibited good antibacterial effects and upregulated the expression of osteogenic factors such as Runt-related transcription factor 2 (Runx2), ALP, BMP-2, osteocalcin (OCN) in BMSCs, as well as the expression of angiogenic factors such as VEGF and HIF-1α in Ea.hy 926 cells. After 3 months of implantation, newly formed bone was observed growing from the edge of the defect to the center in the composite scaffold modified with fusion peptide group, and the BV/TV value was the highest. HE and Masson staining showed that the most mature bone tissue filled the bone defect treated with the composite scaffold modified with fusion peptides. Immunohistochemistry detected more pronounced expression of OCN and Collagen Type I (COL I) in the composite scaffold modified with fusion peptide group, implying that fusion peptides have more advantages in promoting bone regeneration. This innovative approach brings great hope for promoting effective bone defect repair and denotes a new moment of bone regeneration treatment strategies.
Although peptides have lower costs and better stability compared to growth factors, they are customized, time-consuming, and have a short plasma half-life. Compared to drugs, their prices are relatively expensive, which limits their clinical popularization. Advances in technology such as biosynthesis are poised to drive down production costs and enhance efficiency, paving the way for wider clinical usage. As these innovations continue to evolve, the potential for peptides to become more accessible and cost-effective in healthcare settings appears increasingly probable.
4.3.3. Drug
The utilization of 3D printed PLGA scaffolds for drug delivery offers a promising solution to reduce systemic adverse effects and improve the biological activity of medications. By enabling local sustained release, these scaffolds can minimize the potential toxic effects of drugs while increasing their stability and availability in the body. As a result, there is a rising interest in the advancement of biomaterial-based drug delivery method.
Puerarin, known for its ability to promote bone formation and inhibit bone resorption [151,152], was successfully incorporated into a 3D printed PLGA/β-TCP scaffold by Wang and colleagues [153]. The integration of puerarin did not compromise the structure, mechanical properties, or biodegradability of the scaffold. After 8 weeks of implantation, the puerarin-enriched scaffold demonstrated significant advancement in new bone tissue regeneration and blood vessels formation at the calvarial bone defect site. Additionally, it shows higher BV/TV, bone density and increased blood vessels compared to the control group. Immunohistochemistry analysis revealed an elevated expression of crucial factors like hypoxia inducible factor-1α (HIF-1α), VEGF, and BMP-2, indicating that the puerarin-enriched scaffold effectively promotes bone repair by enhancing osteogenesis and angiogenesis.
Biotin is a water-soluble B complex vitamin consisting of a ureido ring and a tetrahydrothiophene ring, which is involved in bone development [154]. Studies have shown that biotin upregulated the expression of COL I, Runx2, and BMP-2, promoting the repair of the rabbit femoral head defects [155]. Liu et al. doped biotin into the bioink of PLGA and β-TCP, and fabricated the bone repair scaffold via low-temperature 3D printing technology (Fig. 7A) [156]. The biotin doped bone repair scaffold enhanced calcium deposition and upregulated the expression of BMP-2 and Runx2. HE staining revealed dense interconnected bone structures after a 4-week implantation period, and clear new bone structures similar to natural bone tissue at 8 weeks. On the whole, these findings suggest that biotin can be developed as a promising component in bone repair therapy.
Fig. 7.
(A) Schematic illustration of the preparation of biotin-doped β-TCP/PLGA scaffolds and bone regeneration in vivo. Reproduced with permission from Ref. [156]. Copyright 2023 AccScience Publishing. (B) Preparation of HA-15-loaded β-TCP/PLGA bone tissue scaffolds along with in vitro MSCs culture and in vivo scaffold implantation in rabbit. Reproduced with permission from Ref. [157]. Copyright 2021 AccScience Publishing.
H15 is a osteogenesis-promoting drug, which could reduce the endoplasmic reticulum stress and establish a favorable microenvironment for osteogenesis [157]. Tang et al. noticed the function of H15 and loaded it into a 3D printed PLGA/β-TCP scaffold for bone regeneration (Fig. 7B) [157]. The scaffold loaded with H15 upregulated the expression of ALP and OCN genes and enhanced calcium deposition. Micro CT scans and microfilm angiography revealed a notable rise in bone formation and new blood vessel growth in the group treated with the H15-loaded scaffold. This cutting-edge strategy demonstrates considerable promise in optimizing the outcomes of bone regeneration therapies.
Drug delivery systems are instrumental in improving the effectiveness of the bone repair and regeneration treatments for patients with various bone disorders. The precise administration of therapeutic agents enhances the process, offering hope to those in need. The research on drug delivery systems is highly promising and is expected to revolutionize the intervention of bone defects. This advancement in medical technology has the power to profoundly influence the lives of individuals suffering from bone-related issues.
4.3.4. Metal ion
The human body is home to a variety of vital metal ions like calcium ion (Ca2+), magnesium ion (Mg2+), zinc ion (Zn2+), strontium ion (Sr2+), copper ion (Cu2+), which are essential for numerous physiological reactions [158]. Notably, these metal ions significantly contribute to the formation and maintenance of healthy bones. For example, Mg2+ holds a pivotal role in promoting bone and vascular formation, as well as the production of HA through stabilizing amorphous calcium phosphate [20]. Similarly, Sr2+ aids in inhibiting bone resorption and stimulating new bone growth [159]. Additionally, Zn2+ supports the growth and differentiation of osteoblasts, as well as the synthesis of COL, leading to improved bone mineralization and formation [160]. Incorporating these essential metal ions into 3D printed PLGA-based scaffolds has proven to enhance their biological activity, particularly in the realm of bone regeneration.
Magnesium (Mg) is a critical element for maintaining bone health and metabolism [158,161], with Mg compounds such as magnesium oxide (MgO), magnesium phosphate (MgP) and magnesium hydroxide (Mg (OH)2) holding significant research value. Researchers have utilized low-temperature 3D printing technology to create innovative scaffolds that incorporate Mg, leading to enhanced osteogenesis and angiogenesis, as well as increased new bone formation and improved bone quality [162]. Evidence suggests that Mg composites can match or even outperform pure Mg in promoting bone growth, while also enhancing the physicochemical characteristics of scaffolds made from PLGA [163]. In one study, MgO and HA were blended with a PLGA matrix to form a porous scaffold through 3D printing [163]. MgO influenced the degradation of PLGA, while Mg2+ facilitated the attachment, growth, and osteogenic differentiation of BMSCs, resulting in increased bone formation. Another study combined PLGA with MgO, and then applied a PDA coating to enhance surface hydrophilicity, further promoting the differentiation of BMSCs toward bone-forming cells [164]. MgP has shown particular promise for osteogenesis, as it can provide both Mg2+ and phosphate ion (PO43−) necessary for bone formation and mineralization. Researchers have successfully incorporated MgP into a bioink formulated from PLGA and nano-hydroxyapatite (n-HA) to create a scaffold that accelerates degradation, buffers acidic byproducts, and enhances both osteogenic and angiogenic properties [165]. Furthermore, the role of Mg2+ in bone regeneration extends beyond just bone formation and can influence the entire process. By developing a stage-regulative scaffold containing decellularized bone matrix microparticles, Mg (OH)2 nanoparticles, and PLGA using low-temperature 3D printing technology, researchers effectively modulated the release of Mg2+ to coordinate early inflammation, intermediate neovascularization, and later bone formation, facilitating sequential bone regeneration (Fig. 8A) [166]. In general, integrating magnesium and its compounds into 3D printed scaffolds is likely to enhance the quantity and quality of bone regeneration, and is a hopeful avenue to propel bone tissue engineering.
Fig. 8.
(A) Schematic illustration of a novel 3D-printed PLGA/DBM-MPs/MH-NPs scaffold for the enhancement of endogenous bone regeneration. Reproduced with permission from Ref. [166]. Copyright 2023 Wiley-VCH. (B) Schematic illustration for synthesis of the LPS/Sr-doped micro/nano HA/PCL/PLGA composite scaffold and evaluation of its immune-mediated angiogenesis and osteogenesis in vitro and in vivo. Reproduced with permission from Ref. [167]. Copyright 2024 Elsevier. (C) Schematic demonstration of the GO/Cu/β-TCP/PLGA scaffold preparation, in vitro and in vivo experimental procedures. Reproduced with permission from Ref. [173]. Copyright 2022 Wiley-VCH.
In addition, Sr2+, Zn2+, and lithium ion (Li+) also have superior osteoinductivity. For example, Li et al. designed a Sr-containing 3D printed composite scaffold consisting of PLGA, polycaprolactone (PCL), and Sr-doped micro/nano HA, whose surface was further modified with lipopolysaccharide (LPS), which triggered an inflammatory response by inducing macrophage homing and M1 polarization [167]. The release of Sr2+ subsequently inhibited the inflammatory response by promoting M2 macrophage polarization, indirectly fostering osteogenesis, and directly facilitating osteogenesis by mediating the differentiation of osteogenic precursor cells (Fig. 8B). Furthermore, both Zn and Sr are trace elements naturally found in the human body and have shown potential for enhancing osteogenesis [158]. Zhao et al. developed a 3D printed scaffold capable of continuously releasing Zn2+ by incorporating Zn submicron particles with PLGA and β-TCP, leading to anti-inflammatory and osteogenic effects [168]. Additionally, Li+ has been found to inhibit the activity of GSK-3β, increase the accumulation of β-catenin, and activate the classical Wnt signaling pathway to promote osteogenesis [169]. Researchers such as Liu et al. have successfully integrated Li-containing MBG into PLGA for a 3D printed composite scaffold [170]. This innovation effectively counteracted the suppressive effects of high glucose on BMSCs proliferation, migration, and osteogenic differentiation, showcasing remarkable osteogenic potential [170].
Cu2+ is renowned for its antibacterial effects, but current investigations have shown that they also contribute to promoting bone regeneration by stimulating osteogenesis and angiogenesis [171]. This makes them a prospective option for combating infectious bone defects. In a study by Ma et al., a scaffold based on PLGA and Cu-loaded ZIF-8 nanoparticles was developed using 3D printing technology [172]. This scaffold demonstrated exceptional antibacterial properties and high bioactivity, enhancing the adhesion, spreading, proliferation, and osteogenic differentiation of MSCs [172]. Additionally, Lei et al. successfully created a porous scaffold by integrating the GO/Cu nanocomposite into the bioink of PLGA and β-TCP for infected bone defects [173]. The resulting scaffold showed remarkable antibacterial effects as well as superior osteogenic and angiogenic abilities, leading to improved cancellous bone formation in models of bacterial-contaminated bone defect (Fig. 8C).
Metal ions incorporated into scaffolds offer a more stable option compared to growth factors, peptides, and drugs. However, Potential biological toxicity should be taken into account, with metal ion concentrations controlled within safe limits. It is crucial to control this balance to assure the effectiveness and safety of the scaffold.
5. Conclusion and future perspectives
Considerable advancements have been witnessed in recent years within artificial bone graft engineering, with some products even progressing to clinical trial stages. Notably, artificial bone grafts on PLGA have emerged as an encouraging option for bone repair owing to their impressive biocompatibility, biodegradability, osteoconductivity, and ease of customizable processing. Biocompatibility has a crucial influence on enabling cell function and bone regeneration. Additionally, the adjustable biodegradability of PLGA enables the synchronization of scaffold degradation with new bone formation, promoting effective bone regeneration. The osteoconductive properties of PLGA support the growth of new blood vessels and bone tissue. Furthermore, the flexibility of PLGA permits the development of intricate 3D printed scaffolds tailored to the unique needs of each patient, facilitating angiogenesis, osteogenesis, cell proliferation, and migration, ultimately enhancing the process of bone regeneration.
So far, various strategies have been developed to improve the performance of 3D printed PLGA-based scaffolds for bone regeneration. First, 3D printing technology can produce scaffolds with customized shapes, but cannot manufacture scaffolds with sophisticated hierarchical structures like native bone tissue. 3D printing technology could be combined with other hole-making approaches to construct PLGA-based scaffolds with complex hierarchical architectures. Second, pure PLGA scaffolds exhibit high brittleness and poor toughness, and are prone to collapse, making them unable to support new bone formation. Inorganic materials can be incorporated to improve their mechanical properties to better match those of natural bone tissue. Third, pure PLGA scaffolds have poor hydrophilicity, which is not conducive to the initial attachment, proliferation, and growth, and even osteogenic differentiation of cells. It is feasible to construct hydrophilic coatings on the surface of PLGA scaffold to enhance hydrophilicity and bone regeneration. Finally, PLGA-based scaffolds lack osteoinductivity, which may lead to delayed bone healing. Bioactive factors can be integrated into PLGA-based scaffolds to mobilize endogenous or exogenous cells and growth factors to facilitate bone regeneration. On the whole, these innovative strategies manage to improve the osteogenic performance of 3D printed PLGA-based scaffolds for bone tissue engineering.
Although functionalized 3D printed PLGA-based scaffolds can achieve effective bone regeneration, there is still a long way to go to translate 3D printed PLGA-based scaffolds from the laboratory to clinical practice. To the best of knowledge, further research is expected to address the following issues. First, the research subjects in the experimental stage are healthy young animals, whose metabolism is vigorous and favorable for bone regeneration. However, the patients encountered in clinical practice are not all young people, but mostly elderly people, and even those with prior health conditions like diabetes. The osteogenic effect of 3D printed PLGA-based scaffolds may be compromised in clinical settings. Second, natural bone tissue has the perceptual function and can avoid harmful stimulus. Bone regeneration not only focuses on new bone formation and the reconstruction of vascular networks, but also pays attention to the role of sensory and sympathetic nerves in bone development and regeneration. Third, 3D printed PLGA-based scaffolds are supposed to be sensitive and able to respond promptly to internal and external stimuli to release bioactive factors on-demand. Last but not least, the role of immunoregulation in bone regeneration has attracted widespread attention in recent years. The connection between 3D printed PLGA-based scaffolds and immunoregulation is still unknown. Deciphering their interactions is of great significance for the development of more effective bone graft substitutes.
The clinical implementation of 3D-printed PLGA scaffolds still faces numerous challenges, among which sterilization methods are particularly crucial. The currently prevalent sterilization methods, including ethylene oxide gas sterilization, gamma irradiation, and electron beam irradiation, all have their own advantages and disadvantages. Ethylene oxide gas sterilization primarily relies on low temperatures, which can maintain the morphology and mechanical properties of PLGA scaffolds. Prolonged ventilation after sterilization is necessary to remove residues, otherwise there are potential biological safety concerns. Gamma irradiation possesses a high penetration depth and can sterilize complex porous structures, but high doses can lead to the breakage of PLGA molecular chains and a decrease in molecular weight, thereby weakening the mechanical properties and altering the degradation rate. Electron beam irradiation has a moderate penetration ability, making it suitable only for small and medium-sized scaffolds, but high doses can also damage their structure. Therefore, an optimal sterilization method has not yet been universally established. In terms of scale-up manufacturing, the high cost of medical-grade PLGA, low 3D printing efficiency, and high quality-control costs all restrict the industrialization process. Meanwhile, long-term safety still requires further evaluation. The acidic by-products generated by PLGA degradation may trigger chronic inflammation and immune reactions locally, and may also affect systemic organs through blood circulation, posing potential systemic risks. Moreover, the sterilization process may introduce new potential risks. More importantly, prior to regulatory approval by the FDA, EMA or the National Medical Products Administration (NMPA), 3D-printed PLGA scaffolds not only need to be systematically validated for biocompatibility, degradation behavior, mechanical properties, and bone regeneration effects in medium to large animal models, but also complete Phase I small-scale safety assessments, Phase II medium-scale efficacy verification, and Phase III large-scale multicenter clinical trials in sequence.
In summary, despite the current challenges faced by 3D printed PLGA-based scaffolds in clinical practice, the rapid advancements in technology hold great promise for overcoming these hurdles. It is anticipated that these scaffolds will thoroughly comply with the requirements of clinical application and grant notable benefits to patients. Therefore, it is plausible to expect that 3D printed PLGA-based scaffolds will meet the rigorous standards of clinical practice in the near future. They have the potential to revolutionize tissue engineering and regenerative medicine, providing patients with new and effective treatment options.
Author contributions
Conceptualization, H.L. and Z.L.; writing-original draft preparation, X.Y.; writing-review and editing, X.Y., Y.W., Y.L., Z.W. and H.L.; visualization, X.Y.; supervision, Z.L.; project administration, Z.L and H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Data availability statement
The data presented in this study can be requested from the corresponding author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by the institution of National Natural Science Foundations of China (No. 82160192) and Guangxi Science and Technology Program (2023AB23037).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Zhe Liu, Email: ndfskqyy175@ncu.edu.cn.
Hongbing Liao, Email: hongbing_liao@gxmu.edu.cn.
References
- 1.Chen J., Luo J., Feng J., Wang Y., Lv H., Zhou Y. Spatiotemporal controlled released hydrogels for multi-system regulated bone regeneration. J Contr Release. 2024;372:846–861. doi: 10.1016/j.jconrel.2024.06.065. [DOI] [PubMed] [Google Scholar]
- 2.Han Z., Li A., Yu Y., Dai K., Yin W., Li X., Wang J., Yu M., Qi X., Li Q. Synergistic osteogenesis and angiogenesis in promoting bone repair by levistolide A-induced smad pathway activation. Compos B Eng. 2024;275 [Google Scholar]
- 3.Zhao X., Zhuang Y., Cao Y., Cai F., Lv Y., Zheng Y., Yang J., Shi X. Electrospun biomimetic periosteum capable of controlled release of multiple agents for programmed promoting bone regeneration. Adv Healthcare Mater. 2024 doi: 10.1002/adhm.202303134. [DOI] [PubMed] [Google Scholar]
- 4.Kim J., Park S., Park J.-Y., Jung U.-W., Jung S., Oh Y., Lee M., Heo S.-e., Choi B., Cha J.-K., Hong J. Dual-phase blocks for regeneration of critical-sized bone defects. Nano Today. 2024;54 [Google Scholar]
- 5.Wang J., Wu Y., Li G., Zhou F., Wu X., Wang M., Liu X., Tang H., Bai L., Geng Z., Song P., Shi Z., Ren X., Su J. Engineering large‐scale self‐mineralizing bone organoids with bone matrix‐inspired hydroxyapatite hybrid bioinks. Adv Mater. 2024;36 doi: 10.1002/adma.202309875. [DOI] [PubMed] [Google Scholar]
- 6.He Y., Luo Z., Nie X., Du Y., Sun R., Sun J., Lin Z., Wan R., Chen W., Feng X., Li F., Liu X., Chen S., Qiu J., Li J., Zhao Z. An injectable multi-functional composite bioactive hydrogel for bone regeneration via immunoregulatory and osteogenesis effects. Adv Compos Hybrid Mater. 2025;8 [Google Scholar]
- 7.Yang Z., Yu X., Chen J., Ma W., Hao J., Wu C. Bioactive scaffolds with ordered Micro/Nano‐Scale topological surface for vascularized bone regeneration. Small. 2025 doi: 10.1002/smll.202500975. [DOI] [PubMed] [Google Scholar]
- 8.Shan J., Cheng L., Li X., Liu W., Liu Z., Chai Y., Yu Y., Wang X., Wen G. End-tail soaking strategy toward robust and biomimetic sandwich-layered hydrogels for full-thickness bone regeneration. Bioact Mater. 2025;49:486–501. doi: 10.1016/j.bioactmat.2025.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes D., Martins-Cruz C., Oliveira M.B., Mano J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240–275. doi: 10.1016/j.biomaterials.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M., Lin R., Wang X., Xue J., Deng C., Feng C., Zhuang H., Ma J., Qin C., Wan L., Chang J., Wu C. 3D printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu G., Zhang T., Chen M., Yao K., Huang X., Zhang B., Li Y., Liu J., Wang Y., Zhao Z. Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact Mater. 2021;6:4110–4140. doi: 10.1016/j.bioactmat.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan X., Zhu W., Yang Z., He N., Chen F., Han X., Zhou K. Recent advances in 3D printing of smart scaffolds for bone tissue engineering and regeneration. Adv Mater. 2024 doi: 10.1002/adma.202403641. [DOI] [PubMed] [Google Scholar]
- 13.Liu X., Lu S., Wang T., Wang X., Yang K., Yang H. Advances and prospects of 3D printed antibacterial bone implants: a systematic review. J Mater Sci Technol. 2024;200:227–242. [Google Scholar]
- 14.Li W., Shen Q., Tong T., Tian H., Lian X., Wang H., Yang K., Dai Z., Li Y., Chen X., Wang Q., Yang D., Wang F., Hao F., Wang L. Sequential simulation of regeneration-specific microenvironments using scaffolds loaded with nanoplatelet vesicles enhances bone regeneration. Bioact Mater. 2025;50:475–493. doi: 10.1016/j.bioactmat.2025.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B., Huang Y., Cai Q., Du Z., Li X. Biomaterials for diabetic bone repair: influencing mechanisms, multi-aspect progress and future prospects. Compos B Eng. 2024:274. [Google Scholar]
- 16.Cao Y., Liu C., Ye W., Zhao T., Fu F. Functional hydrogel interfaces for cartilage and bone regeneration. Adv Healthcare Mater. 2025;14 doi: 10.1002/adhm.202403079. [DOI] [PubMed] [Google Scholar]
- 17.Xu R., You Y., Zheng W., Ma L., Chang Y., Pan S., He Y., Zhou M., Xu Z., Chen T., Liu H. Selenoprotein‐regulated hydrogel for ultrasound‐controlled microenvironment remodeling to promote bone defect repair. Adv Funct Mater. 2024;34 [Google Scholar]
- 18.Fan Y., Ran H., Wang Z., Ning C., Zhai J., Yu P. Semiconductive biomaterials for pathological bone repair and regeneration. Adv Funct Mater. 2024;34 [Google Scholar]
- 19.Cheng Y., Li X., Gu P., Mao R., Zou Y., Tong L., Li Z., Fan Y., Zhang X., Liang J., Sun Y. Hierarchical scaffold with directional microchannels promotes cell ingrowth for bone regeneration. Adv Healthcare Mater. 2024;13 doi: 10.1002/adhm.202303600. [DOI] [PubMed] [Google Scholar]
- 20.Wang W., Wang L., Zhang B., Shang S., Zhao C., Zhang W., Chen J., Zhou C., Zhou H., Feng S. 3D printing of personalized magnesium composite bone tissue engineering scaffold for bone and angiogenesis regeneration. Chem Eng J. 2024;484 [Google Scholar]
- 21.Jing C., Chen S., Bhatia S.S., Li B., Liang H., Liu C., Liang Z., Liu J., Li H., Liu Z., Tan H., Zhao L. Bone-targeted polymeric nanoparticles as alendronate carriers for potential osteoporosis treatment. Polym Test. 2022;110 [Google Scholar]
- 22.Guan H., Wang W., Jiang Z., Zhang B., Ye Z., Zheng J., Chen W., Liao Y., Zhang Y. Magnetic aggregation‐induced bone‐targeting nanocarrier with effects of Piezo1 activation and osteogenic–angiogenic coupling for osteoporotic bone repair. Adv Mater. 2023;36 doi: 10.1002/adma.202312081. [DOI] [PubMed] [Google Scholar]
- 23.Jin S., Gao J., Yang R., Yuan C., Wang R., Zou Q., Zuo Y., Zhu M., Li Y., Man Y., Li J. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact Mater. 2022;8:559–572. doi: 10.1016/j.bioactmat.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahar R., Chakraborty A., Nainwal N., Bahuguna R., Sajwan M., Jakhmola V. Application of PLGA as a biodegradable and biocompatible polymer for pulmonary delivery of drugs. AAPS PharmSciTech. 2023;24 doi: 10.1208/s12249-023-02502-1. [DOI] [PubMed] [Google Scholar]
- 25.Sutrisno L., Chen H., Yoshitomi T., Kawazoe N., Yang Y., Chen G. PLGA–collagen–BPNS bifunctional composite mesh for photothermal therapy of melanoma and skin tissue engineering. J Mater Chem B. 2022;10:204–213. doi: 10.1039/d1tb02366g. [DOI] [PubMed] [Google Scholar]
- 26.Sun F., Sun X., Wang H., Li C., Zhao Y., Tian J., Lin Y. Application of 3D-Printed, PLGA-based scaffolds in bone tissue engineering. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S., Xia X., Huang J., Yuan C., Zuo Y., Li Y., Li J. Recent advances in PLGA-Based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127:56–79. doi: 10.1016/j.actbio.2021.03.067. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Zhang S., Zhang X., Ji L., Yu H., Wang J., Liu C. Porous PLGA/MBG scaffold enhanced bone regeneration through osteoimmunomodulation. Compos B Eng. 2024:272. [Google Scholar]
- 29.Wenzhi S., Dezhou W., Min G., Chunyu H., Lanlan Z., Peibiao Z. Assessment of nano-hydroxyapatite and poly (lactide-co-glycolide) nanocomposite microspheres fabricated by novel airflow shearing technique for in vivo bone repair. Mater Sci Eng C. 2021:128. doi: 10.1016/j.msec.2021.112299. [DOI] [PubMed] [Google Scholar]
- 30.Lei Z., Li C., Yuan Z., Wang X., Cai G., Shang P., Zhao Y., Rafique M., El-Newehy M., Adulhameed M.M., Shafiq M., Song L., Zheng H., Mo X. Boron-doped silica/chitosan-based elastic three-dimensional sponge scaffold for bone regeneration. Carbohydr Polym. 2025;358 doi: 10.1016/j.carbpol.2025.123491. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M., Huang Z., Wang X., Liu X., He W., Li Y., Wu D., Wu S. Personalized PLGA/BCL scaffold with hierarchical porous structure resembling periosteum‐bone complex enables efficient repair of bone defect. Adv Sci. 2024 doi: 10.1002/advs.202401589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S., Xia X., Huang J., Yuan C., Zuo Y., Li Y., Li J. Recent advances in PLGA-Based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127:56–79. doi: 10.1016/j.actbio.2021.03.067. [DOI] [PubMed] [Google Scholar]
- 33.Zhao D., Zhu T., Li J., Cui L., Zhang Z., Zhuang X., Ding J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact Mater. 2021;6:346–360. doi: 10.1016/j.bioactmat.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan M., Abdelnabi H.A., Mohsin S. Harnessing the potential of PLGA nanoparticles for enhanced bone regeneration. Pharmaceutics. 2024;16 doi: 10.3390/pharmaceutics16020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan B., Hua Y., Wang J., Shao T., Wang S., Gao X., Gao J. Surface modification progress for PLGA-based cell scaffolds. Polymers (Basel) 2024;16 doi: 10.3390/polym16010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun F., Sun X., Wang H., Li C., Zhao Y., Tian J., Lin Y. Application of 3D-Printed, PLGA-based scaffolds in bone tissue engineering. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayyoob M., Kim Y.J. Effect of chemical composition variant and oxygen plasma treatments on the wettability of PLGA thin films, synthesized by direct copolycondensation. Polymers (Basel) 2018;10 doi: 10.3390/polym10101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayyoob M., Yang X., Park H.-J., Park S.-y., Kim J.H., Nam S.W., Kim Y.J. Synthesis of bioresorbable poly(Lactic-co-glycolic acid)s through direct polycondensation: an economical substitute for the synthesis of polyglactin via ROP of lactide and glycolide. Fibers Polym. 2019;20:887–895. [Google Scholar]
- 39.Little A., Wemyss A.M., Haddleton D.M., Tan B., Sun Z., Ji Y., Wan C. Synthesis of Poly(Lactic acid-co-glycolic acid) copolymers with high glycolide ratio by ring-opening polymerisation. Polymers (Basel) 2021;13 doi: 10.3390/polym13152458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y., Li D., Kou X., Wang R., Liu F., Li Z. Ultrafast ring-opening copolymerization of lactide with glycolide toward random poly(lactic-co-glycolic acid) copolymers by an organophosphazene base and urea binary catalysts. Polym Chem. 2022;13:1861–1868. [Google Scholar]
- 41.Lu Y., Swisher J.H., Meyer T.Y., Coates G.W. Chirality-directed regioselectivity: an approach for the synthesis of alternating poly(Lactic-co-glycolic acid) J Am Chem Soc. 2021;143:4119–4124. doi: 10.1021/jacs.1c00248. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y., Coates G.W. Pairing-enhanced regioselectivity: synthesis of alternating poly(lactic-co-glycolic acid) from racemic methyl-glycolide. J Am Chem Soc. 2023;145:22425–22432. doi: 10.1021/jacs.3c05941. [DOI] [PubMed] [Google Scholar]
- 43.Little A., Wemyss A.M., Haddleton D.M., Tan B., Sun Z., Ji Y., Wan C. Synthesis of Poly(Lactic acid-co-glycolic acid) copolymers with high glycolide ratio by ring-opening polymerisation. Polymers. 2021;13 doi: 10.3390/polym13152458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin H., Wang R., Ge H., Zhang X., Zhu Z. Synthesis and structure control of l‐lactic acid–glycolic acid copolymer by homo‐copolymerization. J Appl Polym Sci. 2014;132 [Google Scholar]
- 45.Ayyoob M., Kim Y. Effect of chemical composition variant and oxygen plasma treatments on the wettability of PLGA thin films, synthesized by direct copolycondensation. Polymers. 2018;10 doi: 10.3390/polym10101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nifant’ev I.E., Tavtorkin A.N., Shlyakhtin A.V., Ivchenko P.V. Chemical features of the synthesis, degradation, molding and performance of poly(lactic-co-glycolic) acid (PLGA) and PLGA-based articles. Eur Polym J. 2024;215 [Google Scholar]
- 47.Nicolás P., Lassalle V.L., Ferreira M.L. Evaluation of biocatalytic pathways in the synthesis of polyesters: towards a greener production of surgical sutures. Polym Adv Technol. 2022;34:64–78. [Google Scholar]
- 48.Chereddy K.K., Payen V.L., Préat V. PLGA: from a classic drug carrier to a novel therapeutic activity contributor. J Contr Release. 2018;289:10–13. doi: 10.1016/j.jconrel.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Krok-Borkowicz M., Reczyńska K., Rumian Ł., Menaszek E., Orzelski M., Malisz P., Silmanowicz P., Dobrzyński P., Pamuła E. Surface-modified poly(l-lactide-co-glycolide) scaffolds for the treatment of osteochondral critical size defects—in vivo studies on rabbits. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21207541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmowafy E.M., Tiboni M., Soliman M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. Journal of Pharmaceutical Investigation. 2019;49:347–380. [Google Scholar]
- 51.Su Y., Zhang B., Sun R., Liu W., Zhu Q., Zhang X., Wang R., Chen C. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28:1397–1418. doi: 10.1080/10717544.2021.1938756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Go E.J., Kang E.Y., Lee S.K., Park S., Kim J.H., Park W., Kim I.H., Choi B., Han D.K. An osteoconductive PLGA scaffold with bioactive β-TCP and anti-inflammatory Mg(OH)2 to improve in vivo bone regeneration. Biomater Sci. 2020;8:937–948. doi: 10.1039/c9bm01864f. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y., Zhang T., Li M., Ouyang Z., Gao F., Liu C., Li C., Liu D., Zhou Z. PLGA hybrid porous microspheres as human periodontal ligament stem cell delivery carriers for periodontal regeneration. Chem Eng J. 2021;420 [Google Scholar]
- 54.Dai Y., Shen T., Ma L., Wang D., Gao C. Regeneration of osteochondral defects in vivo by a cell‐free cylindrical poly(lactide‐co‐glycolide) scaffold with a radially oriented microstructure. J Tissue Eng Regen Med. 2017;12 doi: 10.1002/term.2592. [DOI] [PubMed] [Google Scholar]
- 55.Sun L., Niu H., Wu Y., Dong S., Li X., Kim B.Y.S., Liu C., Ma Y., Jiang W., Yuan Y. Bio-integrated scaffold facilitates large bone regeneration dominated by endochondral ossification. Bioact Mater. 2024;35:208–227. doi: 10.1016/j.bioactmat.2024.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang L., Song Z., Wang J., Bian M., Zou J., Zou Y., Ge J., Lu S. Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration. Open Life Sci. 2024;19 doi: 10.1515/biol-2022-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petposri S., Thuaksuban N., Buranadham S., Suwanrat T., Punyodom W., Supphaprasitt W. Physical characteristics and biocompatibility of 3D-Printed polylactic-co-glycolic acid membranes used for guided bone regeneration. J Funct Biomater. 2023;14 doi: 10.3390/jfb14050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun X., Xu C., Wu G., Ye Q., Wang C. Poly(Lactic-co-glycolic acid): applications and future prospects for periodontal tissue regeneration. Polymers. 2017;9 doi: 10.3390/polym9060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith A.N., Ulsh J.B., Gupta R., Tang M.M., Peredo A.P., Teinturier T.D., Mauck R.L., Gullbrand S., Hast M.W. Characterization of degradation kinetics of additively manufactured PLGA under variable mechanical loading paradigms. J Mech Behav Biomed Mater. 2024;153 doi: 10.1016/j.jmbbm.2024.106457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Netti P.A., Biondi M., Frigione M. Experimental studies and modeling of the degradation process of poly(Lactic-co-glycolic acid) microspheres for sustained protein release. Polymers. 2020;12 doi: 10.3390/polym12092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y., Kim C.S., Saylor D.M., Koo D. Polymer degradation and drug delivery in PLGA‐based drug–polymer applications: a review of experiments and theories. J Biomed Mater Res B Appl Biomater. 2016;105:1692–1716. doi: 10.1002/jbm.b.33648. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z., Zhang X., Fu Y., Jin Y., Weng Y., Bian X., Chen X. Degradation behaviors of polylactic acid, polyglycolic acid, and their copolymer films in simulated marine environments. Polymers. 2024;16 doi: 10.3390/polym16131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y., Cheng D., Niu B., Wang X., Wu X., Wang A. Properties of poly (lactic-co-glycolic acid) and progress of poly (lactic-co-glycolic Acid)-Based biodegradable materials in biomedical research. Pharmaceuticals. 2023;16 doi: 10.3390/ph16030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swider E., Koshkina O., Tel J., Cruz L.J., de Vries I.J.M., Srinivas M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38–51. doi: 10.1016/j.actbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Washington M.A., Swiner D.J., Bell K.R., Fedorchak M.V., Little S.R., Meyer T.Y. The impact of monomer sequence and stereochemistry on the swelling and erosion of biodegradable poly(lactic-co-glycolic acid) matrices. Biomaterials. 2017;117:66–76. doi: 10.1016/j.biomaterials.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 66.Bazgir M., Zhang W., Zhang X., Elies J., Saeinasab M., Coates P., Youseffi M., Sefat F. Degradation and characterisation of electrospun polycaprolactone (PCL) and poly(lactic-co-glycolic acid) (PLGA) scaffolds for vascular tissue engineering. Materials. 2021;14 doi: 10.3390/ma14174773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bharadwaz A., Jayasuriya A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C. 2020;110 doi: 10.1016/j.msec.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu P., Sun L., Liu P., Yu W., Zhang Q., Zhang W., Ma J., Liu P., Shen J. Surface modification of porous PLGA scaffolds with plasma for preventing dimensional shrinkage and promoting scaffold-cell/tissue interactions. J Mater Chem B. 2018;6:7605–7613. doi: 10.1039/c8tb02374c. [DOI] [PubMed] [Google Scholar]
- 69.Asghari Niari S., Rahbarghazi R., Salehi R., Kazemi L., Fathi Karkan S., Karimipour M. Fabrication, characterization and evaluation of the effect of PLGA and PLGA-PEG biomaterials on the proliferation and neurogenesis potential of human neural SH-SY5Y cells. Microsc Res Tech. 2022;85:1433–1443. doi: 10.1002/jemt.24006. [DOI] [PubMed] [Google Scholar]
- 70.Li H., Zheng L., Wang M. Biofunctionalized nanofibrous bilayer scaffolds for enhancing cell adhesion, proliferation and osteogenesis. ACS Appl Bio Mater. 2021;4:5276–5294. doi: 10.1021/acsabm.1c00414. [DOI] [PubMed] [Google Scholar]
- 71.Rasoulianboroujeni M., Fahimipour F., Shah P., Khoshroo K., Tahriri M., Eslami H., Yadegari A., Dashtimoghadam E., Tayebi L. Development of 3D-printed PLGA/TiO(2) nanocomposite scaffolds for bone tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019;96:105–113. doi: 10.1016/j.msec.2018.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X.L., Zhao Y.Q., Miao L., An Y.X., Wu F., Han J.Y., Han J.Y., Tay F.R., Mu Z., Jiao Y., Wang J. Strategies for promoting neurovascularization in bone regeneration. Mil Med Res. 2025;12:9. doi: 10.1186/s40779-025-00596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins M.N., Ren G., Young K., Pina S., Reis R.L., Oliveira J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv Funct Mater. 2021;31 [Google Scholar]
- 74.Chang B., Liu X. Osteon: structure, turnover, and regeneration. Tissue Engineering Part B: Reviews. 2022;28:261–278. doi: 10.1089/ten.teb.2020.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qi J., Feng S., Liu X., Xing L., Chen D., Xiong C. Morphology, thermal properties, mechanical property and degradation of PLGA/PTMC composites. J Polym Res. 2020;27 [Google Scholar]
- 76.Long J., Yao Z., Zhang W., Liu B., Chen K., Li L., Teng B., Du X.F., Li C., Yu X.F., Qin L., Lai Y. Regulation of osteoimmune microenvironment and osteogenesis by 3D‐Printed PLAG/black phosphorus scaffolds for bone regeneration. Adv Sci. 2023;10 doi: 10.1002/advs.202302539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen F., Han J., Guo Z., Mu C., Yu C., Ji Z., Sun L., Wang Y., Wang J. Antibacterial 3D-Printed silver nanoparticle/poly lactic-co-glycolic acid (PLGA) scaffolds for bone tissue engineering. Materials. 2023;16 doi: 10.3390/ma16113895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou S., Liu S., Wang Y., Li W., Wang J., Wang X., Wang S., Chen W., Lv H. Advances in the study of bionic mineralized collagen, PLGA, magnesium ionomer materials, and their composite scaffolds for bone defect treatment. J Funct Biomater. 2023;14 doi: 10.3390/jfb14080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu G., Zhang X., Chen X., He Y., Cheng L., Huo M., Yin J., Hao F., Chen S., Wang P., Yi S., Wan L., Mao Z., Chen Z., Wang X., Cao Z., Lu J. Additive manufacturing of structural materials. Mater Sci Eng R Rep. 2021;145 [Google Scholar]
- 80.Zhao Y.-Q., Yang J.-H., Ding X., Ding X., Duan S., Xu F.-J. Polycaprolactone/polysaccharide functional composites for low-temperature fused deposition modelling. Bioact Mater. 2020;5:185–191. doi: 10.1016/j.bioactmat.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu J., Wei J., Liu J., Yuan L., Li Y., Luo X., Li Y., Li J. A novel strategy for fabrication of polyamide 66/Nanohydroxyapatite composite bone repair scaffolds by low-temperature three-dimensional printing. ACS Biomater Sci Eng. 2024;10:4073–4084. doi: 10.1021/acsbiomaterials.4c00457. [DOI] [PubMed] [Google Scholar]
- 82.Wang S., Zhao S., Yu J., Gu Z., Zhang Y. Advances in translational 3D printing for cartilage, bone, and osteochondral tissue engineering. Small. 2022;18 doi: 10.1002/smll.202201869. [DOI] [PubMed] [Google Scholar]
- 83.Xu Y., Zhang F., Zhai W., Cheng S., Li J., Wang Y. Unraveling of advances in 3D-Printed polymer-based bone scaffolds. Polymers. 2022;14 doi: 10.3390/polym14030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daly R., Harrington T.S., Martin G.D., Hutchings I.M. Inkjet printing for pharmaceutics – a review of research and manufacturing. Int J Pharm. 2015;494:554–567. doi: 10.1016/j.ijpharm.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 85.Scoutaris N., Ross S., Douroumis D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm Res. 2016;33:1799–1816. doi: 10.1007/s11095-016-1931-3. [DOI] [PubMed] [Google Scholar]
- 86.Cheng J., Yu S., Wang R., Ge Q. Digital light processing based multimaterial 3D printing: challenges, solutions and perspectives. Int J Extrem Manuf. 2024;6 [Google Scholar]
- 87.Wu N., Li J., Li X., Wang R., Zhang L., Liu Z., Jiao T. 3D printed biopolymer/black phosphorus nanoscaffolds for bone implants: a review. Int J Biol Macromol. 2024;279 doi: 10.1016/j.ijbiomac.2024.135227. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y., Li W., Zhang C., Wu Z., Liu J. Recent developments of biomaterials for additive manufacturing of bone scaffolds. Adv Healthcare Mater. 2020;9 doi: 10.1002/adhm.202000724. [DOI] [PubMed] [Google Scholar]
- 89.Slots C., Jensen M.B., Ditzel N., Hedegaard M.A.B., Borg S.W., Albrektsen O., Thygesen T., Kassem M., Andersen M.Ø. Simple additive manufacturing of an osteoconductive ceramic using suspension melt extrusion. Dent Mater. 2017;33:198–208. doi: 10.1016/j.dental.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 90.Golafshan N., Willemsen K., Kadumudi F.B., Vorndran E., Dolatshahi‐Pirouz A., Weinans H., van der Wal B.C.H., Malda J., Castilho M. 3D‐Printed regenerative magnesium phosphate implant ensures stability and restoration of hip dysplasia. Adv Healthcare Mater. 2021;10 doi: 10.1002/adhm.202101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun H., Hu C., Zhou C., Wu L., Sun J., Zhou X., Xing F., Long C., Kong Q., Liang J., Fan Y., Zhang X. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater Des. 2020;189 [Google Scholar]
- 92.Xu H., Zhang Y., Zhang Y., Zhao Z., Xue T., Wang J., Li M., Zhao S., Zhang H., Ding Y. 2024. 3D bioprinting advanced biomaterials for craniofacial and dental tissue engineering – a review, materials & design; p. 241. [Google Scholar]
- 93.Chen Y., Li W., Zhang C., Wu Z., Liu J. Recent developments of biomaterials for additive manufacturing of bone scaffolds. Adv Healthcare Mater. 2020;9 doi: 10.1002/adhm.202000724. [DOI] [PubMed] [Google Scholar]
- 94.Su X., Wang T., Guo S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther. 2021;16:63–72. doi: 10.1016/j.reth.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hamza M., Kanwal Q., Hussain M.I., Khan K., Asghar A., Liu Z., Liu C., Chen Z. Recent progress in 3D printed piezoelectric materials for biomedical applications. Mater Sci Eng R Rep. 2025;164 [Google Scholar]
- 96.Fan J., Ding Z., Cai Y., Lai Y., Huang C., Jiang B., Zhou Z., Luo Z. Revolutionizing bone regeneration: vascularized bone tissue engineering with advanced 3D printing technology. Aggregate. 2025 [Google Scholar]
- 97.Babilotte J., Martin B., Guduric V., Bareille R., Agniel R., Roques S., Héroguez V., Dussauze M., Gaudon M., Le Nihouannen D., Catros S. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater Sci Eng C. 2021:118. doi: 10.1016/j.msec.2020.111334. [DOI] [PubMed] [Google Scholar]
- 98.Xiong Z., Yan Y., Wang S., Zhang R., Zhang C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr Mater. 2002;46:771–776. [Google Scholar]
- 99.Su X., Wang T., Guo S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regenerative Therapy. 2021;16:63–72. doi: 10.1016/j.reth.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun T., Wang J., Huang H., Liu X., Zhang J., Zhang W., Wang H., Li Z. Low-temperature deposition manufacturing technology: a novel 3D printing method for bone scaffolds. Front Bioeng Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1222102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Hu J., Li B., Xia J., Zhang T., Xiong Z. Towards the clinical translation of 3D PLGA/β-TCP/Mg composite scaffold for cranial bone regeneration. Materials. 2024;17 doi: 10.3390/ma17020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J., Zhou H., Fan Y., Gao G., Ying Y., Li J. 3D printing for bone repair: coupling infection therapy and defect regeneration. Chem Eng J. 2023;471 [Google Scholar]
- 103.Slots C., Jensen M.B., Ditzel N., Hedegaard M.A., Borg S.W., Albrektsen O., Thygesen T., Kassem M., Andersen M.O. Simple additive manufacturing of an osteoconductive ceramic using suspension melt extrusion. Dent Mater. 2017;33:198–208. doi: 10.1016/j.dental.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 104.Feng P., Zhao R., Tang W., Yang F., Tian H., Peng S., Pan H., Shuai C. Structural and functional adaptive artificial bone: materials, fabrications, and properties. Adv Funct Mater. 2023;33 [Google Scholar]
- 105.Wang C., Huang W., Zhou Y., He L., He Z., Chen Z., He X., Tian S., Liao J., Lu B., Wei Y., Wang M. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020;5:82–91. doi: 10.1016/j.bioactmat.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du G., Zhou Y., Tian X., Wu G., Xi Y., Zhao S. High-performance 3D directional porous LiFePO4/C materials synthesized by freeze casting. Appl Surf Sci. 2018;453:493–501. [Google Scholar]
- 107.Dou Y., Huang J., Xia X., Wei J., Zou Q., Zuo Y., Li J., Li Y. A hierarchical scaffold with a highly pore-interconnective 3D printed PLGA/n-HA framework and an extracellular matrix like gelatin network filler for bone regeneration. J Mater Chem B. 2021;9:4488–4501. doi: 10.1039/d1tb00662b. [DOI] [PubMed] [Google Scholar]
- 108.Yang C., Song S., Chen F., Chen N. Fabrication of PVDF/BaTiO3/CNT piezoelectric energy harvesters with bionic balsa wood structures through 3D printing and supercritical carbon dioxide foaming. ACS Appl Mater Interfaces. 2021;13:41723–41734. doi: 10.1021/acsami.1c11843. [DOI] [PubMed] [Google Scholar]
- 109.Furtado A.I., Bonifácio V.D.B., Viveiros R., Casimiro T. Design of molecularly imprinted polymers using supercritical carbon dioxide technology. Molecules. 2024;29 doi: 10.3390/molecules29050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gu P., Xu Y., Liu Q., Wang Y., Li Z., Chen M., Mao R., Liang J., Zhang X., Fan Y., Sun Y. Tailorable 3DP flexible scaffolds with porosification of filaments facilitate cell ingrowth and biomineralized deposition. ACS Appl Mater Interfaces. 2022 doi: 10.1021/acsami.2c07649. [DOI] [PubMed] [Google Scholar]
- 111.Wei J., Yan Y., Gao J., Li Y., Wang R., Wang J., Zou Q., Zuo Y., Zhu M., Li J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Biomater Adv. 2022;133 doi: 10.1016/j.msec.2021.112618. [DOI] [PubMed] [Google Scholar]
- 112.Zhang C., Chen Z., Liu J., Wu M., Yang J., Zhu Y., Lu W.W., Ruan C. 3D-printed pre-tapped-hole scaffolds facilitate one-step surgery of predictable alveolar bone augmentation and simultaneous dental implantation. Compos B Eng. 2022;229 [Google Scholar]
- 113.Liu T., Li Z., Zhao L., Chen Z., Lin Z., Li B., Feng Z., Jin P., Zhang J., Wu Z., Wu H., Xu X., Ye X., Zhang Y. Customized design 3D printed PLGA/calcium sulfate scaffold enhances mechanical and biological properties for bone regeneration. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.874931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu Y., Li Y., Zhou X., Li H., Guo M., Zhang P. Glucose microenvironment sensitive degradation of arginine modified calcium sulfate reinforced poly(lactide-co-glycolide) composite scaffolds. J Mater Chem B. 2024;12:508–524. doi: 10.1039/d3tb01595e. [DOI] [PubMed] [Google Scholar]
- 115.Liu H., Chen J., Qiao S., Zhang W. Carbon-based nanomaterials for bone and cartilage regeneration: a review. ACS Biomater Sci Eng. 2021;7:4718–4735. doi: 10.1021/acsbiomaterials.1c00759. [DOI] [PubMed] [Google Scholar]
- 116.Nasrin A., Hassan M., Mirabet M.M., Windhab N., Gomes V.G. 3D‐printed bioresorbable poly(lactic‐co‐glycolic acid) and quantum‐dot nanocomposites: scaffolds for enhanced bone mineralization and inbuilt co‐monitoring. J Biomed Mater Res. 2021;110:916–927. doi: 10.1002/jbm.a.37340. [DOI] [PubMed] [Google Scholar]
- 117.Kaya H., Arıcı Ş., Bulut O., Bilgili F., Ege D. CNT incorporation improves the resolution and stability of porous 3D printed PLGA/HA/CNT scaffolds for bone regeneration. Biomedical Materials. 2023;18 doi: 10.1088/1748-605X/acf25d. [DOI] [PubMed] [Google Scholar]
- 118.Yan B., Hua Y., Wang J., Shao T., Wang S., Gao X., Gao J. Surface modification progress for PLGA-based cell scaffolds. Polymers. 2024;16 doi: 10.3390/polym16010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vu P.T., Conroy J.P., Yousefi A.M. The effect of argon plasma surface treatment on poly(lactic-co-glycolic acid)/collagen-based biomaterials for bone tissue engineering. Biomimetics. 2022;7 doi: 10.3390/biomimetics7040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fu C., Jiang Y., Yang X., Wang Y., Ji W., Jia G. Mussel-inspired gold nanoparticle and PLGA/L-Lysine-g-Graphene oxide composite scaffolds for bone defect repair. Int J Nanomed. 2021;16:6693–6718. doi: 10.2147/IJN.S328390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Y., Chu L., Yang S., Zhang H., Qin L., Guillaume O., Eglin D., Richards R.G., Tang T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018;79:265–275. doi: 10.1016/j.actbio.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 122.Amirian J., Tripathi G., Kang H.-J., Lee B.-T. Porous BMP-2 immobilized PLGA/glycol chitosan scaffold with enhanced hydrophilicity, mineralization and osteogenesis. Mater Lett. 2022;308 [Google Scholar]
- 123.Fu Y., Yang L., Zhang J., Hu J., Duan G., Liu X., Li Y., Gu Z. Polydopamine antibacterial materials. Mater Horiz. 2021;8:1618–1633. doi: 10.1039/d0mh01985b. [DOI] [PubMed] [Google Scholar]
- 124.Xu Z., Wang N., Liu P., Sun Y., Wang Y., Fei F., Zhang S., Zheng J., Han B. Poly(Dopamine) coating on 3D-Printed poly-lactic-co-glycolic Acid/β-Tricalcium phosphate scaffolds for bone tissue engineering. Molecules. 2019;24 doi: 10.3390/molecules24234397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen W., Nichols L., Teer L., Clinton K., Priddy L.B. A hybrid coating of polydopamine and nano-hydroxyapatite enhances surface properties of 3D printed poly(lactic-co-glycolic acid) scaffolds. J Mater Sci. 2022;57:13011–13026. [Google Scholar]
- 126.Zhu L., Liu Y., Wang A., Zhu Z., Li Y., Zhu C., Che Z., Liu T., Liu H., Huang L. Application of BMP in bone tissue engineering. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.810880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bi Z., Shi X., Liao S., Li X., Sun C., Liu J. Strategies of immobilizing BMP-2 with 3D-printed scaffolds to improve osteogenesis. Regen Med. 2023;18:425–441. doi: 10.2217/rme-2022-0222. [DOI] [PubMed] [Google Scholar]
- 128.Bharadwaz A., Jayasuriya A.C. Osteogenic differentiation cues of the bone morphogenetic protein-9 (BMP-9) and its recent advances in bone tissue regeneration. Mater Sci Eng C. 2021:120. doi: 10.1016/j.msec.2020.111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jiang F., Qi X., Wu X., Lin S., Shi J., Zhang W., Jiang X. Regulating macrophage-MSC interaction to optimize BMP-2-induced osteogenesis in the local microenvironment. Bioact Mater. 2023;25:307–318. doi: 10.1016/j.bioactmat.2023.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dorogin J., Hochstatter H.B., Shepherd S.O., Svendsen J.E., Benz M.A., Powers A.C., Fear K.M., Townsend J.M., Prell J.S., Hosseinzadeh P., Hettiaratchi M.H. Moderate‐affinity affibodies modulate the delivery and bioactivity of bone morphogenetic Protein‐2. Adv Healthcare Mater. 2023;12 doi: 10.1002/adhm.202300793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao L., Zhao X., Deng F., Ye X., Shen Z., Xia Y., Zhang Y. Integration of BMP-2/PLGA microspheres with the 3D printed PLGA/CaSO4 scaffold enhances bone regeneration. Frontiers in Materials. 2024;11 [Google Scholar]
- 132.Ma R., Su Y., Cao R., Wang K., Yang P. Enhanced osteogenic activity and bone repair ability of PLGA/MBG scaffolds doped with ZIF-8 nanoparticles loaded with BMP-2. Int J Nanomed. 2023;18:5055–5072. doi: 10.2147/IJN.S423985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song X., Li X., Wang F., Wang L., Lv L., Xie Q., Zhang X., Shao X. Bioinspired protein/peptide loaded 3D printed PLGA scaffold promotes bone regeneration. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.832727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mao Y., Chen Y., Li W., Wang Y., Qiu J., Fu Y., Guan J., Zhou P. Physiology‐inspired multilayer nanofibrous membranes modulating endogenous stem cell recruitment and osteo‐differentiation for staged bone regeneration. Adv Healthcare Mater. 2022;11 doi: 10.1002/adhm.202201457. [DOI] [PubMed] [Google Scholar]
- 135.Wei J., Xia X., Xiao S., Jin S., Zou Q., Zuo Y., Li Y., Li J. Sequential dual‐biofactor release from the scaffold of mesoporous HA microspheres and PLGA matrix for boosting endogenous bone regeneration. Adv Healthcare Mater. 2023;12 doi: 10.1002/adhm.202300624. [DOI] [PubMed] [Google Scholar]
- 136.Chen L., Shao L., Wang F., Huang Y., Gao F. Enhancement in sustained release of antimicrobial peptide and BMP-2 from degradable three dimensional-printed PLGA scaffold for bone regeneration. RSC Adv. 2019;9:10494–10507. doi: 10.1039/c8ra08788a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee J., Lee S., Ahmad T., Madhurakkat Perikamana S.K., Lee J., Kim E.M., Shin H. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120192. [DOI] [PubMed] [Google Scholar]
- 138.Liu W., Wang Q., Luo H., Luo B., Zhao F., Kang Y., Zhang Y., Shao L. Nanographene oxide promotes angiogenesis by regulating osteoclast differentiation and platelet-derived growth factor secretion. ACS Nano. 2024;18:22390–22403. doi: 10.1021/acsnano.4c06979. [DOI] [PubMed] [Google Scholar]
- 139.Ni T., Zhu Y., Hao L., Chen Y., Cheng T. Preparation of photothermal-sensitive PDGF@ZIF-8-PDA@COL/PLGA-TCP composite scaffolds for bone defect repair. Mater Des. 2022:217. [Google Scholar]
- 140.Cui Y., Wang J., Tian Y., Fan Y., Li S., Wang G., Peng C., Liu H., Wu D. Functionalized decellularized bone matrix promotes bone regeneration by releasing osteogenic peptides. ACS Biomater Sci Eng. 2023;9:4953–4968. doi: 10.1021/acsbiomaterials.3c00413. [DOI] [PubMed] [Google Scholar]
- 141.Cheng C.T., Vyas P.S., McClain E.J., Hoelen T.-C.A., Arts J.J.C., McLaughlin C., Altman D.T., Yu A.K., Cheng B.C. The osteogenic peptide P-15 for bone regeneration: a narrative review of the evidence for a mechanism of action. Bioengineering. 2024;11 doi: 10.3390/bioengineering11060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang C., Lai J., Li K., Zhu S., Lu B., Liu J., Tang Y., Wei Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact Mater. 2021;6:137–145. doi: 10.1016/j.bioactmat.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li R., Zhou C., Chen J., Luo H., Li R., Chen D., Zou X., Wang W. Synergistic osteogenic and angiogenic effects of KP and QK peptides incorporated with an injectable and self-healing hydrogel for efficient bone regeneration. Bioact Mater. 2022;18:267–283. doi: 10.1016/j.bioactmat.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Z., Tian G., Liu L., Li Y., Xu S., Du Y., Li M., Jing W., Wei P., Zhao B., Ma S., Deng J. A 3D-printed PLGA/HA composite scaffold modified with fusion peptides to enhance its antibacterial, osteogenic and angiogenic properties in bone defect repair. J Mater Res Technol. 2024;30:5804–5819. [Google Scholar]
- 145.Zhang Y., Wang C., Fu L., Ye S., Wang M., Zhou Y. Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3D printing for repairing critical-sized bone defect. Molecules. 2019;24 doi: 10.3390/molecules24091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lai J., Wang C., Liu J., Chen S., Liu C., Huang X., Wu J., Pan Y., Xie Y., Wang M. Low temperature hybrid 3D printing of hierarchically porous bone tissue engineering scaffolds with in situ delivery of osteogenic peptide and mesenchymal stem cells. Biofabrication. 2022;14 doi: 10.1088/1758-5090/ac84b0. [DOI] [PubMed] [Google Scholar]
- 147.Zhang H., Sun H., Zhang L., Zhang B., Zhang M., Luo Z., Tan Y., Tang R., Sun J., Zhou X., Fan Y., Zhang X., Zhao Z., Zhou C. Coaxial 3D printing scaffolds with sequential antibacterial and osteogenic functions to effectively repair infected mandibular defects. Adv Funct Mater. 2024 [Google Scholar]
- 148.Wang M., Li H., Yang Y., Yuan K., Zhou F., Liu H., Zhou Q., Yang S., Tang T. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact Mater. 2021;6:1318–1329. doi: 10.1016/j.bioactmat.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Han Z., Xiong J., Jin X., Dai Q., Han M., Wu H., Yang J., Tang H., He L. Advances in reparative materials for infectious bone defects and their applications in maxillofacial regions. J Mater Chem B. 2024;12:842–871. doi: 10.1039/d3tb02069j. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y., Wang H., Huangfu H., Zhang X., Zhang H., Qin Q., Fu L., Wang D., Wang C., Wang L., Zhou Y. 3D printing of bone scaffolds for treating infected mandible bone defects through adjustable dual-release of chlorhexidine and osteogenic peptide. Mater Des. 2022:224. [Google Scholar]
- 151.Yang Y., Chen D., Li Y., Zou J., Han R., Li H., Zhang J. Effect of puerarin on osteogenic differentiation in vitro and on new bone formation in vivo. Drug Des Dev Ther. 2022;16:2885–2900. doi: 10.2147/DDDT.S379794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin S., Ke D., Lin Y., Fu X., Yu Y. Puerarin inhibits the migration of osteoclast precursors and osteoclastogenesis by inhibiting MCP-1 production. Biosci Biotechnol Biochem. 2020;84:1455–1459. doi: 10.1080/09168451.2020.1738912. [DOI] [PubMed] [Google Scholar]
- 153.Cao H., Li L., Li L., Meng X., Liu Y., Cheng W., Zhang P., Gao Y., Qin L., Wang X. New use for old drug: local delivery of puerarin facilitates critical-size defect repair in rats by promoting angiogenesis and osteogenesis. Journal of Orthopaedic Translation. 2022;36:52–63. doi: 10.1016/j.jot.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao J., Yang B., Yarmolenka M.A., Lian R., Jiang X., Zhao J., Rogachev A.V. Osteogenic potential evaluation of biotin combined with magnesium-doped hydroxyapatite sustained-release film. Biomater Adv. 2022;135 doi: 10.1016/j.msec.2022.112679. [DOI] [PubMed] [Google Scholar]
- 155.Cheng T., Cao J., Wu T., Jiang X., Yarmolenko M.A., Rogachev A.A., Rogachev A.V. Study on osteoinductive activity of biotin film by low-energy electron beam deposition. Biomater Adv. 2022;135 doi: 10.1016/j.bioadv.2022.212730. [DOI] [PubMed] [Google Scholar]
- 156.Xue P., Tan X., Xi H., Chen H., He S., Sun G., Gu C., Jiang X., Du B., Liu X. Low-temperature deposition 3D printing biotin-doped PLGA/β-TCP scaffold for repair of bone defects in osteonecrosis of femoral head. International Journal of Bioprinting. 2023;10 [Google Scholar]
- 157.Zheng C., Attarilar S., Li K., Wang C., Liu J., Wang L., Yang J., Tang Y. 3D-printed HA15-loaded β-Tricalcium Phosphate/poly (lactic-co-glycolic acid) bone tissue scaffold promotes bone regeneration in rabbit radial defects. International Journal of Bioprinting. 2021;7 doi: 10.18063/ijb.v7i1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luo Y., Liu H., Zhang Y., Liu Y., Liu S., Liu X., Luo E. Metal ions: the unfading stars of bone regeneration—from bone metabolism regulation to biomaterial applications. Biomater Sci. 2023;11:7268–7295. doi: 10.1039/d3bm01146a. [DOI] [PubMed] [Google Scholar]
- 159.Liu J., Shi Y., Zhao Y., Liu Y., Yang X., Li K., Zhao W., Han J., Li J., Ge S. A multifunctional metal–phenolic nanocoating on bone implants for enhanced osseointegration via early immunomodulation. Adv Sci. 2024;11 doi: 10.1002/advs.202307269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang S., Gu R., Wang F., Zhao X., Yang F., Xu Y., Yan F., Zhu Y., Xia D., Liu Y. 3D-Printed PCL/Zn scaffolds for bone regeneration with a dose-dependent effect on osteogenesis and osteoclastogenesis. Mater Today Bio. 2022;13 doi: 10.1016/j.mtbio.2021.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dai Y., Wu J., Wang J., Wang H., Guo B., Jiang T., Cai Z., Han J., Zhang H., Xu B., Zhou X., Wang C. Small. 2024. Magnesium ions promote the induction of immunosuppressive bone microenvironment and bone repair through HIF‐1α‐TGF‐β axis in dendritic cells. [DOI] [PubMed] [Google Scholar]
- 162.Lai Y., Li Y., Cao H., Long J., Wang X., Li L., Li C., Jia Q., Teng B., Tang T., Peng J., Eglin D., Alini M., Grijpma D.W., Richards G., Qin L. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 163.Xia X., Huang J., Wei J., Jin S., Zou Q., Zuo Y., Li J., Li Y. Magnesium oxide regulates the degradation behaviors and improves the osteogenesis of poly(lactide-co-glycolide) composite scaffolds. Compos Sci Technol. 2022;222 [Google Scholar]
- 164.Tan L., Ye Z., Zhuang W., Mao B., Li H., Li X., Wu J., Sang H. 3D printed PLGA/MgO/PDA composite scaffold by low-temperature deposition manufacturing for bone tissue engineering applications. Regenerative Therapy. 2023;24:617–629. doi: 10.1016/j.reth.2023.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu M., Li L., Zheng C., Wang Y., Zhang B., Wang L., Li Z., Zhang Y., Zhou Y., Zhou C., Min L., Fan Y., Tu C. 3D printed porous PLGA/n-HA/MgP composite scaffolds with improved osteogenic and angiogenic properties. Mater Des. 2023:234. [Google Scholar]
- 166.Yuan Y., Xu Y., Mao Y., Liu H., Ou M., Lin Z., Zhao R., Long H., Cheng L., Sun B., Zhao S., Zeng M., Lu B., Lu H., Zhu Y., Chen C. Three birds, one stone: an osteo‐microenvironment stage‐regulative scaffold for bone defect repair through modulating early osteo‐immunomodulation, middle neovascularization, and later osteogenesis. Adv Sci. 2023 doi: 10.1002/advs.202306428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang J., Wei J., Xia X., Xiao S., Jin S., Zou Q., Zuo Y., Li Y., Li J. A sequential macrophage activation strategy for bone regeneration: a micro/nano strontium-releasing composite scaffold loaded with lipopolysaccharide. Mater Today Bio. 2024;26 doi: 10.1016/j.mtbio.2024.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li C., Sun F., Tian J., Li J., Sun H., Zhang Y., Guo S., Lin Y., Sun X., Zhao Y. Continuously released Zn2+ in 3D-printed PLGA/β-TCP/Zn scaffolds for bone defect repair by improving osteoinductive and anti-inflammatory properties. Bioact Mater. 2023;24:361–375. doi: 10.1016/j.bioactmat.2022.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shang L., Liu Z., Ma B., Shao J., Wang B., Ma C., Ge S. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact Mater. 2021;6:1175–1188. doi: 10.1016/j.bioactmat.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen Y., Chen L., Wang Y., Lin K., Liu J. Lithium-containing bioactive glasses enhanced 3D-printed PLGA scaffolds for bone regeneration in diabetes. Compos B Eng. 2022:230. [Google Scholar]
- 171.Wu Y., Huo S., Liu S., Hong Q., Wang Y., Lyu Z. Cu–Sr bilayer bioactive glass nanoparticles/polydopamine functionalized polyetheretherketone enhances osteogenic activity and prevents implant‐associated infections through spatiotemporal immunomodulation. Adv Healthcare Mater. 2023;12 doi: 10.1002/adhm.202301772. [DOI] [PubMed] [Google Scholar]
- 172.Zou F., Jiang J., Lv F., Xia X., Ma X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J Nanobiotechnology. 2020;18:39. doi: 10.1186/s12951-020-00594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Y., Li M., Zhou B., Jiang X., Zhang D., Luo H., Lei S. Novel therapeutic strategy for bacteria‐contaminated bone defects: reconstruction with multi‐biofunctional GO/Cu‐Incorporated 3D scaffolds. Adv Therapeut. 2022;5 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study can be requested from the corresponding author.