Abstract
It has been proposed that JNK-interacting proteins (JIP) facilitate mixed lineage kinase-dependent signal transduction to JNK by aggregating the three components of a JNK module. A new model for the assembly and regulation of these modules is proposed based on several observations. First, artificially induced dimerization of dual leucine zipper-bearing kinase (DLK) confirmed that DLK dimerization is sufficient to induce DLK activation. Secondly, under basal conditions, DLK associated with JIP is held in a monomeric, unphosphorylated and catalytically inactive state. Thirdly, JNK recruitment to JIP coincided with significantly decreased affinity of JIP and DLK. JNK promoted the dimerization, phosphorylation and activation of JIP-associated DLK. Similarly, treatment of cells with okadaic acid inhibited DLK association with JIP and resulted in DLK dimerization in the presence of JIP. In summary, JIP maintains DLK in a monomeric, unphosphorylated, inactive state. Upon stimulation, JNK–JIP binding affinity increases while JIP–DLK interaction affinity is attenuated. Dissociation of DLK from JIP results in subsequent DLK dimerization, autophosphorylation and module activation. Evidence is provided that this model holds for other MLK-dependent JNK modules.
Keywords: dual leucine zipper-bearing kinase (DLK)/JNK/JNK-interacting protein/MAP kinase module/scaffold
Introduction
Signal transduction via protein kinases generically termed mitogen-activated protein kinases (MAPKs) link a variety of extracellular signals to cellular responses as diverse as proliferation, differentiation and apoptosis (reviewed in Whitmarsh and Davis, 1998; Garrington and Johnson, 1999; Schaffer and Weber, 1999; Widman et al., 1999). Biochemical and genetic evidence has established that activation of a prototypical MAPK occurs through sequential activation of a series of upstream kinases: a serine/threonine MAPK kinase kinase (MAPKKK) phosphorylates a dual specificity protein kinase (MAPKK, MKK or MEK) that subsequently phosphorylates and activates a MAPK. Three groups of mammalian MAPKs and the upstream kinases and stimuli that activate them have been studied most extensively. These include the p42/p44MAPKs (extracellular signal-regulated kinases, ERK1 and 2), that are generally activated by mitogens and differentiation-inducing stimuli, the stress-activated protein kinases (p46/p54SAPK or SAPKα, β and γ, and their splice isoforms) and the p38mapks (Gonzalez et al., 1992; Owaki et al., 1992; Charest et al., 1993; Kyriakis et al., 1994; Lee et al., 1994; Gupta et al., 1996). MAPKs phosphorylate a variety of substrates to effect cellular function. MAPKKKs in each group are activated by upstream signals via a variety of incompletely understood mechanisms.
Work in cell culture systems and investigation of genetic mutants in a number of model organisms has begun to establish a variety of distinct physiological roles for individual stress-activated protein kinases and their associated pathways. The stress-activated protein kinases have also been termed JNK protein kinases because they were identified as the principal c-Jun N-terminal kinases. The JNK family kinases are activated by cell stress-inducing stimuli such as heat shock, UV irradiation, hyperosmolarity and ischemia/reperfusion injury, and by activation of specific cell surface receptors (Whitmarsh and Davis, 1996; Ip and Davis, 1998; Savinainen et al., 2001). Indeed, distinct JNK family kinases have been implicated in multiple specific biological processes that include—but are not limited to—embryonic morphogenesis, regulation of the apoptotic response to cellular injury, regulation of thymocyte development and activation, and regulation of proliferation (Su et al., 1994; Raitano et al., 1995; Xia et al., 1995; Chen et al., 1996; Riesgo-Escovar et al., 1996; Sluss et al., 1996; Verheij et al., 1996; Xu et al., 1996; Zanke et al., 1996; Rincon et al., 1997).
The organization, regulation and function of the JNK protein kinase pathways remain incompletely understood. Recognition that specific JNK kinases play unique functional roles, identification of three mammalian JNK kinases and their splice isoforms, and discovery of two MAPKKs and at least 12 MAPKKKs that appear to lie in pathways proximal to the JNKs has led to work aimed at understanding the mechanisms that determine signaling specificity. It is now evident that distinct MAPK pathways that respond to specific stimuli and effect unique cellular responses exist within single eukaryotic cells. Studies in yeast have provided evidence that MAP kinase pathways are assembled from a unique combination of protein kinases into distinct protein complexes or modules (Herskowitz, 1995; Madhani and Fink, 1998). The minimal MAPK module uniformly contains a MAPKKK, a MAPKK and a MAPK. The components of these modules interact via direct protein–protein interactions and/or are tethered to scaffolding proteins (Choi et al., 1994; Kranz et al., 1994; Marcus et al., 1994; Printen and Sprague, 1994; Posas and Saito, 1997; Xia et al., 1998). Importantly, assembly of MAPK modules appears to allow segregation of MAPK signaling components into units that are responsive to independent stimuli, that obtain appropriate subcellular targeting, that are insulated from similar modules and that can regulate functionally distinct substrates (Elion, 1998; Garrington and Johnson, 1999; Cheng et al., 2000).
Identification of JNK-interacting protein 1 (JIP1) as a scaffold protein that interacts in a specific fashion with members of the mixed lineage kinase (MLK) family of MAPKKKs but not MEKK proteins, with MKK7 but not MKK4, and with JNKs first established that mammalian cells organize JNK pathways into modules in a fashion similar to yeast (Yasuda et al., 1999). Three JIP family genes and several splice isoforms have been identified (Whitmarsh et al., 1998; Davis, 2000; Kelkar et al., 2000). JIP proteins can form homo- and hetero-oligomers and are phosphoproteins. While JIP3 is structurally distant from JIP1 and JIP2, each has been demonstrated to associate directly with an MLK protein, with MKK7 and with a JNK (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). It has been proposed that JIP proteins facilitate MLK-dependent signal transduction to JNK possibly by aggregating the three components of a JNK module (Davis, 2000). While JIP can interact with all three components of the MLK-dependent JNK module independently, direct demonstration of the formation of a multimolecular complex is lacking. That JIP proteins potentiate MLK-induced JNK activation has been based largely on observations in mammalian cells where co-transfection of JIP potentiated overexpressed MLK3-induced activation of co-expressed JNK. Importantly, in these experiments, the activation state of JNK associated with the JIP complex was not examined (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). Therefore, the mechanisms that regulate the assembly or activity of these modules require investigation.
The seven MLKs share two structural features (Dorow et al., 1993; Holzman et al., 1994; Fan et al., 1996; Hirai et al., 1996; Tibbles et al., 1996; Sakuma et al., 1997; Gotoh et al., 2000). Each has a kinase catalytic domain whose primary structure most closely resembles that of MAPKKKs. Secondly, closely juxtaposed C-terminal to the catalytic domain, each MLK protein has two leucine/isoleucine zippers separated by a short spacer region. When overexpressed in cells, MLK proteins form catalytically active homo-oligomers that function as MAPKKKs and activate the JNK and p38mapks. Dual leucine zipper-bearing kinase (DLK) homodimerization is mediated by a direct interaction requiring its leucine zipper domain (LZD). LZD dimerization is necessary for DLK phosphorylation and subsequent activation of JNK, since removal or mutation of this domain prevents these outcomes (Nihalani et al., 2000). These observations support the hypothesis that DLK dimerization results in trans-phosphorylation and subsequent activation (Holzman et al., 1994; Leung and Lassam, 1998; Nihalani et al., 2000). However, the mechanism governing DLK dimerization remains undetermined. DLK dimerization does not require DLK phosphorylation or catalytic activity (Mata et al., 1996). Further, the isolated DLK LZD readily associates with itself and appears to associate with any truncated form of DLK as long as these mutants (functional or not) contain the LZD (Nihalani et al., 2000). Collectively, these observations suggest that the DLK LZD–LZD interaction is of high affinity, and that, unchecked, DLK seeks its most favorable configuration by forming stable homodimers. Presuming that DLK homodimerization precedes DLK activation, these observations suggest that a mechanism must exist that regulates the assembly of DLK homodimers.
The data presented in this report suggest a novel model of DLK-dependent JNK module regulation. In this model, DLK is complexed to JIP in a monomeric, catalytically inactive state under basal conditions. Upon appropriate stimulation, DLK is dissociated from JIP1, dimerizes, autophosphorylates and becomes catalytically active, ultimately resulting in JNK activation. DLK dissociation appears to require prior recruitment of JNK to JIP1. Evidence is provided that this model can be generalized to other MLK-dependent JNK modules.
Results
DLK dimerization precedes DLK phosphorylation and JNK activation
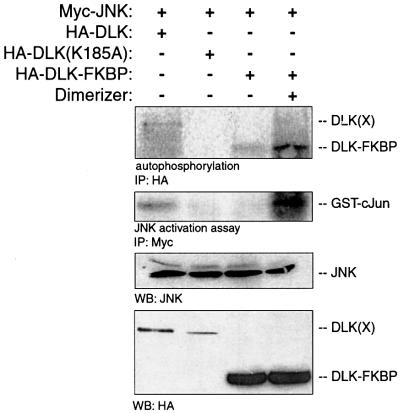
DLK and other MLKs require their LZDs for kinase catalytic activity (Nihalani et al., 2000). We investigated whether DLK dimerization was sufficient to initiate DLK phosphorylation and activation. Chimeric DLK–FK-binding protein (FKBP) constructs were created that could be used to investigate whether artificially induced dimerization of DLK monomers results in DLK phosphorylation and activation. For this construct, the LZDs of DLK and the entire portion of DLK C-terminal to the LZD were replaced with an FKBP domain. When expressed in mammalian cells by transient transfection, DLK–FKBP chimeras were induced to form dimers in the presence of a chemical cross-linker AP20187 (Clackson et al., 1998). Autophosphorylation and activation of DLK–FKBP was evaluated after co-expression by transient transfection of DLK–FKBP and Myc-JNK1. Following immunoprecipitation of DLK–FKBP, autophosphorylation was assessed in vitro after incubation in a kinase buffer containing [γ-32P]ATP. As shown in Figure 1, DLK–FKBP phosphorylation increased significantly in the presence of dimerizer. To examine the capacity of DLK–FKBP dimerization to activate JNK in this system, Myc-JNK was immunoprecipitated from the same cell lysates and was evaluated in a kinase assay using recombinant GST–c-Jun(1–79) as substrate (Figure 1). DLK–FKBP activated JNK only in the presence of dimerizer. Together with previously published observations, these results provide support for the model that in vivo, dimerization initiates autophosphorylation and activation of DLK catalytic activity.
Fig. 1. DLK dimerization results in DLK autophosphorylation and JNK activation. COS 7 cells were transiently transfected with plasmid encoding Myc-JNK (0.5 µg) and with 0.5 µg of either HA-DLK, HA-DLK–FKBP or HA-DLK(K185A). After 24 h, dimerizer (5 nM) was added to the cells as indicated. DLK or its mutants were immunoprecipitated with HA antibodies, incubated in kinase buffer in the presence of [γ-32P]ATP, separated by SDS–PAGE and analyzed by autoradiography. JNK was immunoprecipitated from the same cell lysate and analyzed for catalytic activity. Cell lysates from corresponding experiments were immunoblotted using anti-JNK antibody to demonstrate equivalent expression of JNK. Anti-HA antibody was used to demonstrate the expression of DLK and its mutants.
JIP1 associates with unphosphorylated DLK
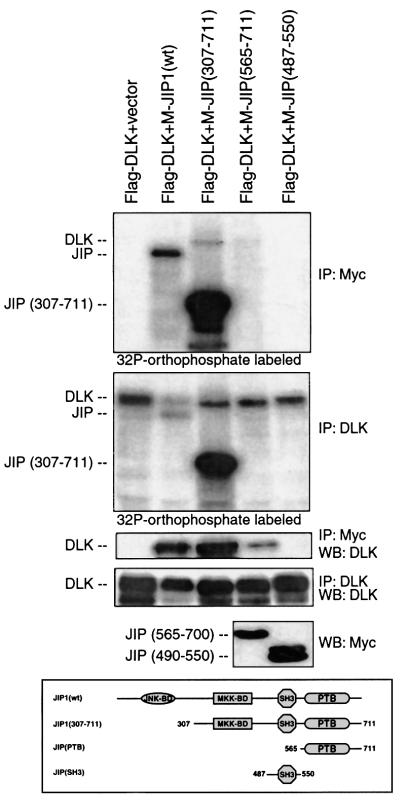
DLK and JIP1 can associate by direct protein–protein interaction involving the N-terminal region of DLK (Whitmarsh et al., 1998; Nihalani et al., 2000). In preliminary work, it was observed that wild-type JIP1 co-immunoprecipitated an unphosphorylated form of DLK based on evaluation of DLK’s electrophoretic mobility by SDS–PAGE (data not shown). To examine this observation in more detail, Flag-DLK and Myc-JIP1 or a variety of JIP1 deletion mutants were co-expressed by transient transfection in COS 7 cells. Following metabolic labeling with [32P]orthophosphate, Flag-DLK or Myc-JIP1 was immunoprecipitated from cell lysates. Immunopre cipitated complexes were analyzed by SDS–PAGE and autoradiography. A reproducible decrease in phosphorylation within the total pool of DLK was noted when DLK was co-expressed with wild-type JIP1 (Figure 2, panel 2). Flag-DLK immunoprecipitated with wild-type Myc-JIP1 was not phosphorylated (Figure 2, compare panels 1 and 3), despite the presence of phosphorylated Flag-DLK in cell lysates (Figure 2, panel 2). Importantly, Myc-JIP1 deletion mutants, Myc-JIP(307–711) and Myc-JIP(565– 700) immunoprecipitated phosphorylated Flag-DLK. Myc-JIP1(490–530) failed to immunoprecipitate Flag- DLK. These results suggested that under basal conditions, wild-type JIP1 interacted only with an unphosphorylated form of DLK. Moreover, overexpression of wild-type JIP1 appeared to inhibit the phosphorylation of a portion of the total pool of DLK. While DLK interacts with at least one region within JIP1 C-terminal to residue 307, the N-terminal region of JIP prevents the association of JIP1 with a phosphorylated form of DLK.
Fig. 2. DLK associated with JIP is not phosphorylated. COS 7 cells were co-transfected as indicated with plasmids (0.2 µg each) encoding Flag-DLK, Myc-JIP1, Myc-JIP(307–711), Myc-JIP(565–700), Myc-JIP(490–550) and vector (to 2 µg), then metabolically labeled with [γ-32P]orthophosphate. DLK or JIP was immunoprecipitated as indicated. Immunoprecipitated complexes were separated by SDS–PAGE and analyzed by autoradiography. Corresponding cell lysates were immunoprecipitated with the indicated antibodies, separated by SDS–PAGE and analyzed by immunoblotting as indicated. Expression of wild-type-JIP1 and JIP(307–711) is seen in the top panel and expression of JIP(565–700) and JIP(490–550) is seen in the bottom panel. Experiments were repeated three times with similar results. M-, Myc.
JIP-1 inhibits DLK dimerization
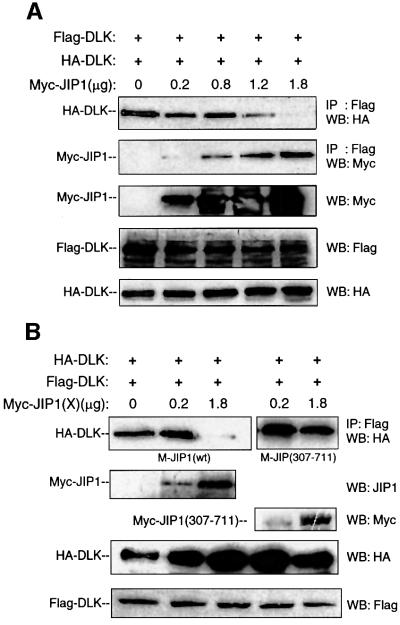
Based on these results and our earlier observations, it was hypothesized that JIP1 maintains DLK in a monomeric form, preventing dimerization-induced phosphorylation and activation. To examine the effect of JIP1 on DLK dimerization, hemagglutinin (HA)-tagged and Flag-tagged DLK were co-expressed with increasing concentrations of Myc-JIP1 in COS 7 cells (Figure 3A). Flag-DLK was immunoprecipitated from cell lysates and immunoprecipitated complexes were analyzed for the presence of HA-DLK. In the absence of Myc-JIP1, HA-DLK was co-immunoprecipitated by Flag-DLK as previously described (Nihalani et al., 2000). However, as the concentration of Myc-JIP1 relative to Flag- and HA-DLK increased, co-immunoprecipitation of Flag-DLK with HA-DLK diminished (Figure 3A). A similar experiment was performed using a mutant of JIP1 lacking its N-terminal region [JIP(307–711)]. In experiments described above, this mutant had been observed to immunoprecipitate phosphorylated DLK (Figure 2). HA-tagged and Flag-tagged DLK were co-expressed with increasing concentrations of either Myc-JIP1 or Myc-JIP1(307–711) in COS 7 cells. Flag-DLK was immunoprecipitated from cell lysates and the immunoprecipitated complexes were analyzed for the presence of HA-DLK (Figure 3B). Wild-type JIP1 again inhibited co-immunoprecipitation of Flag-DLK with HA-DLK. However, Myc-JIP1(307–711) did not inhibit DLK co-immunoprecipitation (Figure 3B). These results provided preliminary evidence that under basal conditions, wild-type JIP1 inhibited DLK oligomerization. This effect appeared to depend on the presence of the N-terminal region (1–306) of JIP1. Taken together with the results presented in Figure 2 above, DLK appeared to bind to wild-type JIP1 in a monomeric, unphosphorylated form.
Fig. 3. Wild-type JIP1 but not JIP(307–711) inhibits DLK dimerization. COS 7 cells were co-transfected with HA-DLK (0.1 µg), Flag-DLK (0.1 µg) and increasing quantities of Myc-JIP1 plasmids as indicated. (A) Flag-DLK complexes were immunoprecipitated from cell lysates using anti-Flag antibody, separated by SDS–PAGE and immunoblotted with either anti-HA or anti-Myc antibody. Correspond ing cell lysates were immunoblotted to evaluate the relative expression of Myc-JIP, HA-DLK and Flag-DLK proteins. (B) Cells were co-transfected with HA-DLK (0.1 µg), Flag-DLK (0.1 µg) and the indicated quantity of Myc-JIP or Myc-JIP(307–711) plasmids; samples were analyzed as above.
JIP-associated DLK does not phosphorylate its specific substrate
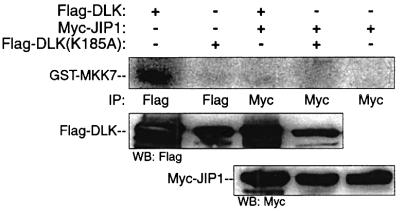
Since JIP1 had been demonstrated to associate with an unphosphorylated and monomeric form of DLK, it was anticipated that JIP1-associated DLK would be catalytically inactive. For this reason, the ability of JIP1-associated DLK to phosphorylate its specific substrate MKK7 in vitro was investigated (Merritt et al., 1999). Immunopre cipitated overexpressed DLK alone or DLK associated with JIP1 were incubated with recombinant GST–MKK7 in a buffer containing radiolabeled ATP and magnesium (Figure 4). Unlike DLK alone, JIP1-associated DLK did not phosphorylate recombinant MKK7 in vitro.
Fig. 4. JIP-associated DLK does not phosphorylate its specific substrate. COS 7 cells were co-transfected with plasmids encoding Flag-DLK (0.5 µg) or Flag-DLK(K185A) (0.5 µg) and Myc-JIP1 (0.2 µg) as indicated. Cell lysates were immunoprecipitated using the indicated antibodies. Immunoprecipitates were analyzed for DLK catalytic activity in vitro using GST–MKK7 as substrate. Immunoprecipitated complexes from corresponding experiments were separated by SDS–PAGE and were immunoblotted as indicated.
Evaluation of the activation state of JIP-associated JNK expressed in cells
It had been proposed that JIP proteins serve as scaffolds that facilitate MLK-dependent signal transduction to JNK. This proposal had been based largely on observations in mammalian cells where co-transfection of JIP potentiated overexpressed MLK3-induced activation of co-expressed JNK. In these experiments, JNK catalytic activity was assessed in vitro after immunoprecipitation from the total pool of epitope-tagged JNK rather than by specifically examining JNK activity associated with the putative JIP scaffold protein (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). In experiments performed in a similar manner, COS 7 cells were transfected with plasmid encoding Flag-JNK and HA-DLK. Additional co-transfection of JIP1 augmented DLK-induced JNK activation to a modest degree (Figure 5, lanes 7, 1 and 2). This experiment was repeated multiple times with similar results.
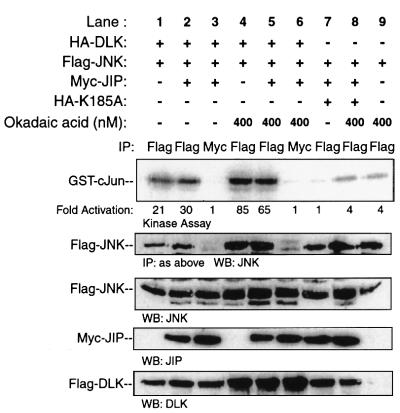
Fig. 5. Evaluation of the activation state of JIP-associated JNK expressed in cells. COS 7 cells were co-transfected as indicated with plasmids encoding Flag-JNK (0.5 µg), HA-DLK (0.2 µg), HA-DLK(K185A) (0.2 µg) and Myc-JIP1 (0.2 µg). At 24 h post-transfection, the indicated samples were treated for 3 h with 400 nM okadaic acid. Immunoprecipitation was performed with the indicated antibodies and immune complex-associated JNK was analyzed for catalytic activity using GST–c-Jun as substrate. Corresponding immunoprecipitated complexes were immunoblotted with anti-JNK antibody. Cell lysates from corresponding experiments were immunoblotted with the indicated antibodies to evaluate the expression of JNK, JIP and DLK. Fold activation is indicated relative to the control experiment shown in lane 7 for Flag immunoprecipitation experiments, and to the control experiment shown in lane 3 for Myc immunoprecipitation experiments.
MLK3 or DLK, MKK7 and JNK individually associate with JIP1 via a direct protein–protein interaction (Whitmarsh et al., 1998; Nihalani et al., 2000). There fore, we initially assumed that JIP forms a simultaneous complex with each of these proteins. Based on published models of scaffold function, it was also assumed that JNK module signal transduction should occur within the intact protein complex (Whitmarsh et al., 1998; Yasuda et al., 1999). For these reasons, COS 7 cells were co-transfected with plasmids encoding HA-DLK, Flag-JNK and Myc- JIP1. By immunoprecipitating Myc-JIP1, protein complexes containing JIP were isolated. Complex-associated JNK activity was assessed in an in vitro kinase assay using GST–c-Jun(1–79) as substrate. JIP1-associated JNK was catalytically inactive (Figure 5, lane 3). However, only a small amount of JNK appeared to associate with JIP under these experimental conditions. Treatment of mammalian cells with okadaic acid results in DLK phosphorylation (Mata et al., 1996; and see below) and JNK activation (Barancik et al., 1999; and Figure 5, lanes 4 and 5). Moreover, the affinity of JNK for JIP3 increases following appropriate cellular stimulation (Kelkar et al., 2000). Indeed, in these experiments, increased co-immunoprecipitation of JNK with JIP1 was detected when COS 7 cells were treated with okadaic acid (Figure 5, compare lanes 3 and 6 in the second panel). Therefore, even under conditions that facilitated JNK recruitment to JIP1, activation of JNK associated with JIP1 could not be demonstrated. In summary, in this artificial experimental system, the presence of JIP appeared to augment JNK activation modestly within the total pool of cellular JNK. However, activation of the fraction of JNK associated with JIP was not detected even when assessed under experimental conditions that resulted in increased recruitment of JNK to JIP.
The affinity of JIP for DLK is dependent on the presence of JNK
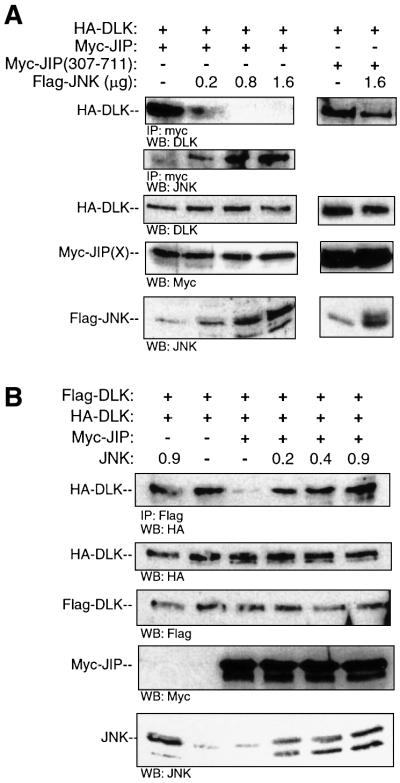
In order to resolve the paradox presented by the results above, we tested the possibility that JIP1 does not bind DLK and JNK simultaneously. COS 7 cells were co-transfected with plasmid encoding HA-DLK and Myc- JIP1 and with increasing quantities of plasmid encoding Flag-JNK. JIP was immunoprecipitated from cell lysates and immunoprecipitated complexes were analyzed for HA-DLK or for Flag-JNK (Figure 6A). Without exogenous JNK present, DLK readily immunoprecipitated with JIP1. However, expression of increased exogenous JNK in cells resulted in increased association of JIP and JNK and simultaneously resulted in markedly attenuated affinity of JIP1 for DLK (Figure 6A). This result suggested that DLK and JNK, that have distinct binding domains on JIP, do not bind JIP simultaneously. Importantly, overexpression of JNK in COS 7 cells did not alter the affinity between DLK and JIP(307–711) (Figure 6A). Therefore, the N-terminal region of JIP1, containing the JNK-binding domain, appeared to be required to alter the affinity of JIP1 for DLK in the presence of JNK.
Fig. 6. The presence of JNK results in decreased affinity of JIP1 for DLK and promotes DLK dimerization. (A) COS 7 cells were co-transfected with plasmids encoding HA-DLK (0.5 µg) and either Myc-JIP1 (0.5 µg) or Myc-JIP1(307–711) (0.5 µg) and increasing quantities of plasmid encoding Flag-JNK as indicated. Cell lysates were immunoprecipitated with anti-Myc antibody, separated on SDS–PAGE and immunoblotted with anti-DLK antibody. Cell lysates from corresponding experiments were immunoblotted with the indicated antibodies to evaluate the expression of JNK, JIP and DLK. (B) COS 7 cells were co-transfected as indicated with HA-DLK (0.05 µg), Flag-DLK (0.05 µg), Myc-JIP1 (0.9 µg), the indicated quantity of JNK and vector (to 2 µg). Cell lysates were immuno precipitated with anti-Flag antibody and immunoblotted with HA antibody. Corresponding cell lysates were immunoblotted with the indicated antibodies to evaluate the expression of HA-DLK, Flag-DLK, Myc-JIP1 and JNK.
JNK promotes DLK dimerization, phosphorylation and activation in the presence of JIP1
Since JNK attenuated the interaction affinity of JIP1 and DLK and following from our observations above, we hypothesized that JNK would also promote DLK dimerization in the presence of JIP1. COS 7 cells were transfected simultaneously with plasmid encoding HA-DLK, Flag-DLK, Myc-JIP1 (in a quantity shown above to be sufficient to attenuate DLK oligomerization) and an increasing quantity of JNK. Flag-DLK was immunoprecipitated from cell lysates and immuno precipitated complexes were analyzed for HA-DLK. As observed above, DLK oligomerization was attenuated in the presence of JIP1. However, JNK promoted the oligomerization of DLK even in the presence of JIP1 (Figure 6B). Together with results obtained above, these results suggest that in the presence of excess JNK, DLK dissociates from JIP1 and oligomerizes.
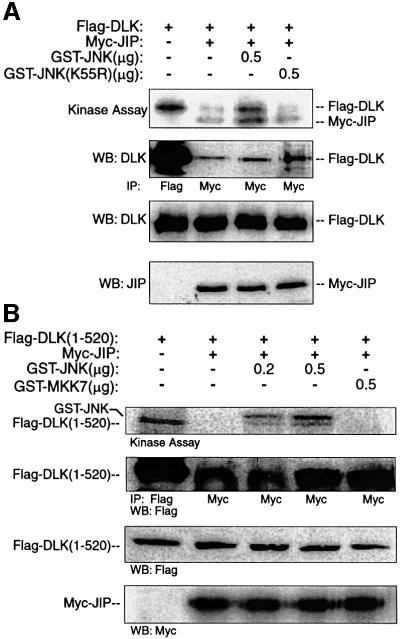
Since DLK oligomerization results in DLK autophosphorylation, it was hypothesized that DLK would become phosphorylated in the presence of excess JNK. Because it had been demonstrated above that simultaneously transfecting JIP, DLK and JNK precluded co-immunoprecipitation of DLK with JIP, an alternative experimental approach was employed. The induction of DLK phosphorylation in pre-formed DLK–JIP complexes was tested in an in vitro model system. COS 7 cells were transiently transfected with plasmid encoding Flag-DLK and Myc- JIP1. JIP1–DLK complexes were obtained after immunoprecipitation of Myc-JIP1 (Figure 7A, panel 2). Phosphorylation of DLK and JIP was assessed in vitro by incubating JIP1–DLK complexes in a kinase buffer containing radiolabeled ATP before and after addition of excess recombinant GST–JNK protein. In the absence of GST–JNK, JIP1 and JIP1-associated DLK remained unphosphorylated (Figure 7A, top panel, lane 2) consistent with observations above that JIP-associated DLK was catalytically inactive. Following addition of GST–JNK, both JIP1 and DLK became phosphorylated (Figure 7A, top panel, lane 3). In contrast, addition of recombinant kinase-dead JNK did not induce DLK or JIP1 phosphorylation in this system. Analogous results were obtained when these experiments were repeated in a similar manner by substituting DLK(1–520) for wild-type DLK (Figure 7B). In summary, in the presence of excess JNK, DLK associated with JIP can become phosphorylated. Since JIP is a known substrate of JNK (Kelkar et al., 2000), it is plausible that JNK phosphorylation of JIP results in DLK dissociation and subsequent phosphorylation. However, the possibility that DLK is phosphorylated directly by JNK cannot be excluded and will require further investigation.
Fig. 7. JNK promotes JIP-associated DLK phosphorylation in vitro. COS 7 cells were co-transfected as indicated with plasmids encoding the Flag-DLK (A) or Flag-DLK(1–520) (0.5 µg) (B) and Myc-JIP1 (0.5 µg). Cell lysates were immunoprecipitated using either anti-Flag or anti-Myc antibody as indicated. Immunoprecipitated complexes were analyzed in vitro for DLK phosphorylation by incubating them in kinase buffer containing [γ-32P]ATP and the indicated quantity of GST–JNK, kinase-dead GST–JNK(K55R) or GST–MKK7. Immunoprecipitated complexes were separated by SDS–PAGE and analyzed by autoradiography. Alternatively, immunoprecipitated complexes were immunoblotted with anti-Flag antibody. Expression of proteins was assessed by immunoblotting corresponding cell lysates.
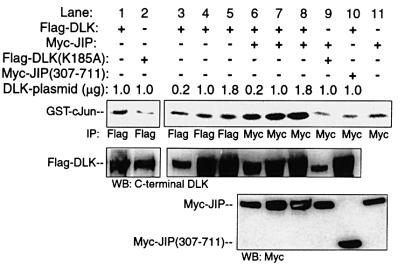
In the absence of JIP, overexpressed DLK activated JNK in an in vitro reconstitution assay (Figure 8, lanes 1–5; and previously published in Merritt et al., 1999). As established above, JIP1-associated DLK is unphosphorylated and catalytically inactive. Therefore, without an activating stimulus, JIP–DLK complexes might not be anticipated to activate JNK in an in vitro reconstitution assay in which recombinant MKK7 and JNK are added to pre-formed complexes of JIP–DLK. However, excess JNK resulted in DLK dissociation from JIP and subsequent DLK oligomerization and phosphorylation. For this reason, it was hypothesized that in the presence of excess catalytically competent JNK, DLK would become activated, resulting in subsequent JNK activation. COS 7 cells were transiently transfected with plasmid encoding Myc-JIP1 and either Flag-DLK or catalytically inactive Flag-DLK(K185A). JIP1–DLK complexes or JIP1–DLK (K185A) complexes were obtained after immunoprecipitation of Myc-JIP1 from cell lysates. GST–JNK activation was determined by incubating the immunoprecipitated complexes with a mixture of bacterially expressed and purified recombinant GST–MKK7, GST–JNK and GST– c-Jun in a kinase buffer containing radiolabeled ATP. In this system, GST–JNK activity was elevated when incubated in the presence of DLK–JIP complexes relative to controls in which DLK was absent or in which catalytically inactive DLK(K185A) was substituted for wild-type DLK (Figure 8, compare lanes 7 and 9 with lanes 4–6). Therefore, JNK activation was dependent on DLK activity in this system. To test the relevance of wild-type JIP in this system, pre-formed complexes of DLK and JIP[307–711] (lacking JIP’s JNK-binding domain) were employed. Pre-formed DLK–Myc-JIP(307–711) complexes did not activate GST–JNK above background in this system (Figure 8, lane 8). These results suggested that unless JNK can interact with JIP, JIP-bound DLK is restricted from activating JNK.
Fig. 8. JIP N-terminus is required for JNK activation by JIP-associated DLK in vitro. Myc-JIP1 (0.2 µg), Myc-JIP1(307–711) (0.2 µg) or vector control were co-transfected with either inactive Flag-DLK(K185A) or various quantities of Flag-DLK plasmid. DLK, DLK(K185A) or JIP1 was immunoprecipitated from the cell lysates using the indicated antibodies. Immunoprecipitates were then assayed in vitro for their ability to activate GST–JNK. Kinase buffer containing recombinant GST–MKK7, GST–JNK, GST–c-Jun and [γ-32P]ATP was added to immunoprecipitated complexes and incubated at 30°C for 15 min. Immunoprecipitates were separated on SDS–PAGE, transferred to nitrocellulose and autoradiographed. Immunoblots from corresponding cell lysates were used to evaluate the relative expression of DLK and JIP in each reaction. Similar results were obtained in three independent experiments.
Okadaic acid induces dissociation of DLK from JIP and DLK oligomerization in the presence of JIP1
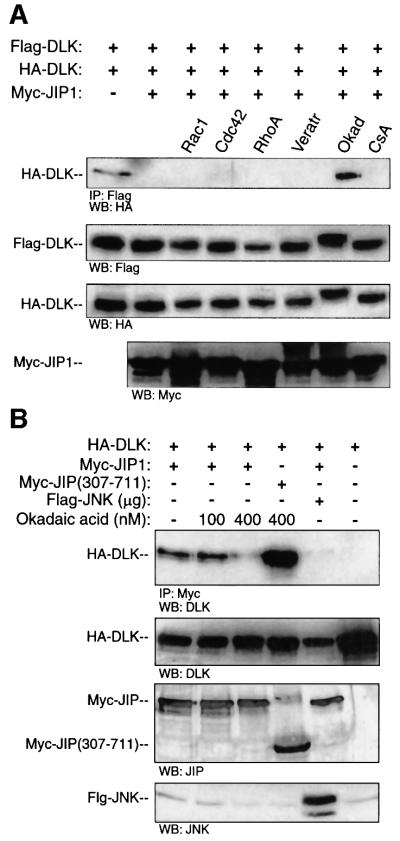
The data presented above suggests a model of DLK-dependent JNK module regulation. In this model, DLK is complexed to JIP in a monomeric, catalytically inactive state under basal conditions. Upon appropriate stimulation, DLK is dissociated from JIP1, dimerizes, autophosphorylates and becomes catalytically active. DLK dissociation may require prior recruitment of JNK to JIP1. We sought to test this model in experiments in which the molar concentration of JNK was not being varied relative to that of JIP and DLK by applying an exogenous stimulus known to result in the phosphorylation of endogenous DLK in cells. Presently, okadaic acid is the only known stimulus that results both in increased phosphorylation of endogenous DLK present in a variety of cells and in JNK activation (Mata et al., 1996; Barancik et al., 1999; and data not shown). Unlike MLK3, DLK does not bind Rho-family small GTPases (Teramoto et al., 1996; and data not shown). Initially, we tested the effect of okadaic acid on DLK oligomerization in the presence of JIP1. COS 7 cells were transiently transfected with plasmid encoding HA-tagged and Flag-tagged DLK and with Myc-JIP1 in a quantity previously shown to inhibit DLK dimerization. As noted previously, HA-DLK was not co-immunoprecipitated with Flag-DLK. However, after treatment of cell culture with okadaic acid, HA-DLK was co-immunoprecipitated readily with Flag-DLK (Figure 9A, panel 1). This correlated with a decrease in the mobility of DLK on SDS–PAGE (Figure 9A, panel 2), an observation previously demonstrated to be due to a change in the phosphorylation state of DLK (Mata et al., 1996). Treatment of cells with veratridine or cyclosporin A did not allow DLK oligomerization in the presence of JIP1. Further, co-transfection of constitutively activated mutants of Rac1, Cdc42Hs or RhoA did not relieve JIP1’s ability to prevent DLK dimerization (Figure 9A). Since the small GTPases do not bind DLK and are not believed to play a role in activating this MLK, these results provide evidence of the specificity of the effect of okadaic acid on the JIP1–DLK complex.
Fig. 9. Okadaic acid treatment results in decreased affinity of JIP for DLK and DLK dimerization in the presence of JIP. (A) COS 7 cells were transfected with plasmids encoding HA-DLK (0.1 µg), Flag-DLK (0.1 µg) and Myc-JIP1 (1.0 µg) as indicated. The indicated cells were transfected simultaneously with activated forms of either Myc-V12Cdc42Hs, Myc-V12Rac1 or Myc-L63RhoA. Where indicated, cells were treated for 3 h with either veratridine (20 µg), okadaic acid (400 nM) or cyclosporin A (30 µM). Flag-DLK was immuno precipitated from cell lysates; immunoprecipitated complexes were separated by SDS–PAGE and were immunoblotted with anti-HA antibody. Immunoblots from the corresponding cell lysates were used to evaluate the relative expression of Flag-DLK, HA-DLK and Myc-JIP. (B) COS 7 cells were co-transfected with plasmids encoding HA-DLK (0.5 µg) and Myc-JIP1 (0.5 µg). Cells were treated for 3 h with the indicated concentrations of okadaic acid. Cell lysates were immunoprecipitated with anti-Myc antibody, separated by SDS–PAGE and immunoblotted with anti-DLK antibody. Cell lysates from the corresponding experiments were immunoblotted with the indicated antibodies to evaluate the expression of JIP and DLK. These experiments were repeated three times with similar results.
To be consistent with the proposed model, treatment of COS 7 cells with okadaic acid should also result in decreased affinity of JIP1 for DLK. COS 7 cells were co-transfected with plasmid encoding HA-DLK and Myc-JIP1 or Myc-JIP(307–711). At 24 h post-transfection, a subset of cells were treated with okadaic acid. JIP1 was immunoprecipitated from cell lysates and immunoprecipitated complexes were analyzed for HA-DLK. In untreated cells or cells treated with 100 nM okadaic acid, JIP1 co-immunoprecipitated DLK from cell lysates. However, treatment of cells with 400 nM okadaic acid resulted in attenuated affinity of JIP1 and DLK (Figure 9B). In contrast, the affinity of JIP(307–711) for DLK was not affected by treatment of cells with the same concentration of okadaic acid.
MLK3 oligomerization is inhibited by JIP1, and constitutively active Cdc42Hs promotes MLK3 oligomerization in the presence of JIP1
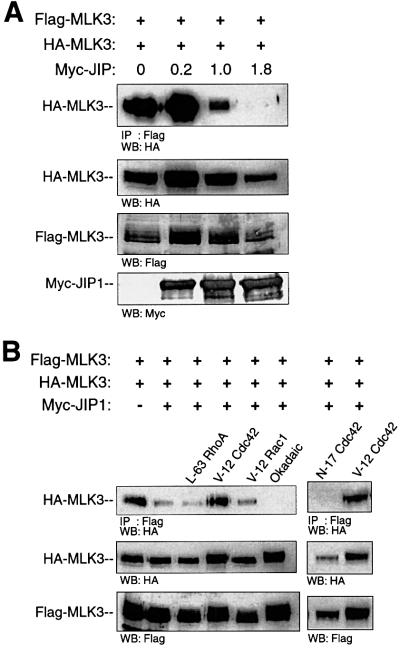
We sought to determine whether the proposed model of JIP1-regulated DLK dimerization could be generalized to include other MLKs. Like DLK, MLK3 can bind JIP1 and requires oligomerization for catalytic activity (Leung and Lassam, 1998; Whitmarsh et al., 1998). To examine the effect of JIP-1 on MLK3 dimerization, HA- and Flag-tagged MLK3 were co-expressed with increasing concentrations of Myc-JIP1 in COS 7 cells (Figure 10A). Flag-MLK3 was immunoprecipitated from cell lysates and immunoprecipitated complexes were analyzed for the presence of HA-MLK3. In the absence of Myc-JIP1, HA-MLK3 was co-immunoprecipitated by Flag-MLK3 as previously described (Leung and Lassam, 1998). How ever, as the concentration of Myc-JIP1 relative to Flag- and HA-MLK3 increased, co-immunoprecipitation of Flag-MLK3 with HA-MLK3 diminished (Figure 10A). These results suggest that, like DLK, MLK3 oligomerization is inhibited in the presence of JIP1.
Fig. 10. JIP inhibits dimerization of MLK3 and V12Cdc42Hs promotes MLK3 dimerization in the presence of JIP. (A) COS 7 cells were co-transfected with Flag-MLK3 (0.1 µg), HA-MLK3 (0.1 µg) and increasing quantities of Myc-JIP plasmids as indicated. Flag-MLK3 complexes were immunoprecipitated from cell lysates using anti-Flag antibody, separated by SDS–PAGE and immunoblotted with anti-HA antibody. Corresponding cell lysates were immunoblotted to evaluate the relative expression of Myc-JIP, HA-MLK3 and Flag-MLK3 proteins. (B) COS 7 cells were transfected with Flag-MLK3 (0.05 µg), HA-MLK3 (0.05 µg), Myc-JIP (1.0 µg) and either V12Rac1, V12Cdc42Hs or L63RhoA plasmids as indicated. Where indicated, 24 h after transfection, cells were treated for 3 h with okadaic acid (400 nM). As above, immunoprecipitation and immunoblotting were used to assess the association of HA-MLK3 and Flag-MLK3 in the presence of JIP. Experiments were repeated three times with similar results.
MLK3 can be activated in vivo by activated Cdc42Hs (Bock et al., 2000). Therefore, given the observation that JIP1 inhibited the dimerization of MLK3, we tested whether activated Cdc42Hs or other activated Rho-family small GTPases could promote MLK3 dimerization in the presence of JIP1. COS 7 cells were transfected with plasmid encoding HA-MLK3, Flag-MLK3, Myc-JIP1 (in a quantity expected to be sufficient to attenuate MLK3 oligomerization) and with either constitutively active or inactive mutants of RhoA, Cdc42Hs or Rac1. Flag-MLK3 was immunoprecipitated from cell lysates and immunoprecipitated complexes were analyzed for HA-MLK3. As observed above, MLK3 oligomerization was inhibited in the presence of JIP1. However, activated Cdc42Hs, but not activated RhoA or Rac1, promoted the oligomerization of MLK3 even in the presence of JIP1 (Figure 10B). Moreover, expression of inactive Cdc42Hs did not relieve JIP1’s ability to inhibit MLK3 oligomerization. Since okadaic acid promoted the dimerization of DLK in the presence of JIP1, we examined whether treatment of cells with this phosphatase inhibitor would promote the dimerization of MLK3 in the presence of JIP1. Unlike DLK, okadaic acid had no effect on JIP1’s ability to inhibit MLK3 dimerization (Figure 10B). Taken together, these results provide evidence that the proposed model of JIP1-regulated DLK dimerization and activation can be applied generally to the interaction of JIP with other MLKs. Moreover, upstream stimuli that allow MLK dimerization and presumably activation in the presence of JIP1 appear to be specified by the type of MLK involved.
Discussion
The minimal unit of a MAPK pathway is comprised of a MAPKKK, a MAPKK and a MAPK that are assembled into a distinct protein complex referred to as a module (Herskowitz, 1995; Madhani and Fink, 1998). The three components of these modules appear to interact via direct protein–protein interactions and in some instances are tethered to scaffolding proteins (Choi et al., 1994; Kranz et al., 1994; Marcus et al., 1994; Printen and Sprague, 1994; Posas and Saito, 1997; Xia et al., 1998). The assembly of MAPK modules creates units that are responsive to independent stimuli, are targeted appropriately to a subcellular compartment, are insulated from similar modules and can regulate functionally distinct substrates. Two important related roles have been proposed for scaffold proteins in this setting. First, by assembling specific components into a complex, scaffold proteins are thought to contribute to a mechanism that determines signaling specificity. Secondly, by stabilizing complex formation, the scaffold might facilitate or catalyze signal transduction from the MAPKKK to the MAPK. It has been proposed that JIP proteins behave as JNK module scaffolds that possess these attributes (Whitmarsh et al., 1998). Consistent with this hypothesis, JIP proteins interact independently with a specific MAPKKK (MLK but not MEKK protein kinases), MAPKK (MKK7 but not MKK4) and JNK. Moreover, preliminary experimental evidence suggests that JIP may facilitate MLK-dependent JNK activation (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). Despite these initial observations, experimental evidence that JIP simultaneously binds all three module components is lacking and experimental evidence for signal transduction facilitation within the module remains difficult to interpret.
It is commonly assumed that MAPK modules are composed of three kinase components in order to achieve signal amplification via a signaling ‘cascade’ mechanism. This model requires free diffusion of component kinases. Burack and Shaw, and others have pointed out that a protein scaffold prevents component diffusion, imposes a fixed stoichiometric relationship between components of a given module and should prevent signal amplification (Burack and Shaw, 2000). For these theoretical reasons, the expectation that JIP should amplify MLK-induced JNK activation by aggregating three JNK module components is problematic. This hypothesis was based on transient co-transfection experiments in mammalian cells. In these experiments, co-expression of JIP appeared to augment overexpressed MLK3-induced JNK activation as assessed at a single experimental time point. JNK catalytic activity was assessed in vitro after immunoprecipitation from the total pool of epitope-tagged overexpressed JNK (Whitmarsh et al., 1998; Yasuda et al., 1999; Kelkar et al., 2000). In experiments carried out in a similar manner here, co-expression of JIP modestly, but reproducibly, augmented DLK-induced JNK activation. Importantly, these experiments assay the catalytic activity of the total pool of overexpressed JNK rather than that of isolated JNK associated with the putative JIP module.
Experiments presented herein begin to clarify the function of JIP and may reconcile theory with experimental observation. First, the assumption that JIP binds an MLK protein kinase, MKK7, and a JNK protein kinase simultaneously appears to be incorrect. Our results demonstrate that DLK and JNK do not appear to co-exist simultaneously on JIP, particularly following module stimulation. In data provided previously by Davis et al. and confirmed above, appropriate cellular stimulation results in recruitment of JNK to the JIP scaffold (Kelkar et al., 2000). Importantly, we have observed that JNK recruitment coincides with significantly decreased affinity of JIP and DLK. Secondly, under basal conditions, DLK associated with JIP is held in a monomeric, unphosphorylated and catalytically inactive state; DLK does not become activated until it is dissociated from JIP. This indicates that sequential signaling from MLK to MKK7 to JNK does not occur while DLK is associated with JIP.
These observations suggest a mechanism that is consistent with theoretical considerations regarding the limitations imposed by scaffold proteins on signal amplification. As noted above, MLK-induced catalytic activation of the JNK pool that is largely not associated with JIP does appear to be increased modestly in the presence of JIP. Whether this occurs as a result of signal amplification within a pool of freely diffusing components or by signal facilitation within scaffolded complexes remains to be clarified. However, the data presented suggest that classical cascade-like signal amplification within this module can occur after DLK dissociation from JIP when the 1:1 molar relationship between JIP-bound DLK and MKK7 is relieved and activated DLK oligomers gain ready access to multiple nearby MKK7 molecules. Indeed, previously published observations suggest that the affinity of DLK for MKK7 is sufficient for this interaction to occur without stabilization by a scaffold (Mata et al., 1996). Whether sequential phosphorylation and activation of MKK7 and JNK occur while these kinases are associated with JIP requires additional investigation. If MKK7 and JNK remain simultaneously associated with JIP following DLK dissociation, this complex would retain many of the special attributes of a two-component scaffolded system including the potential for signal facilitation (Levchenko et al., 2000).
Ferrel and co-workers have suggested that the three-component MAPK module serves to provide for a switch-like response to appropriately graded stimuli (Huang and Ferell, 1996; Ferrell and Machleder, 1998). Their model was based on assumptions that the module has freely diffusing multiple components and that the dual phosphorylation events required for activation of both MAPKK and MAPK occur in a non-processive fashion. Evidence has been provided that MAPK phosphorylation does occur in a non-processive fashion (Huang and Ferrell, 1996). However, the model employed by Ferrell and co-workers did not include a scaffold protein that, if present, would restrict diffusion and would favor processive rather than non-processive phosphorylation reactions. Our results suggest an alternative mechanism by which an all-or-nothing switch-like response might occur in the presence of a scaffold protein. As noted in the Introduction, DLK LZD–LZD interaction is of high affinity and, unchecked, DLK appears to seek its most favorable configuration by forming stable homo-oligomers. LZD-dependent DLK oligomerization is both necessary and sufficient for DLK phosphorylation and subsequent activation (Nihalani et al., 2000; and results herein). JIP serves to regulate activation of DLK by preventing DLK’s oligomerization in the basal state. Upon appropriate stimulation, JIP appears to allow DLK activation by releasing DLK to seek its favored conformation.
Since their initial identification, JIP proteins have been ascribed the role of either inhibitor or facilitator of JNK activation (Dickens et al., 1997; Davis, 2000). Based on mathematical modeling of a two-component scaffold, Levchenko et al. (2000) have argued that JIP may inhibit or facilitate JNK module signal transduction depending on the relative concentration of scaffold and component kinases. Their model assumed that kinase components bind to scaffold independently of each other, exhibiting no cooperativity in binding. Without additional data, this assumption appeared plausible for JIP, since component kinases bind to independent JIP-binding domains. At a high concentration of scaffold relative to component kinases, scaffold will serve to sequester components from each other, preventing functional complex formation. Levchenko terms this effect ‘combinatorial inhibition’. At an optimal relative concentration of component kinases to scaffold, the Levchenko model predicts that signal transduction will be facilitated relative to signal transduction that might occur between components that are freely diffusing. Importantly, the assumptions employed by the Levchenko model are not entirely consistent with our experimental observations. Combinatorial inhibition theory predicts that increasing JNK concentration relative to a fixed concentration of DLK and JIP should have no effect on the binding affinity of DLK and JIP. Instead, our results suggest that JNK–JIP interactions negatively influence DLK and JIP binding affinity (negative cooperativity). Decreased JIP–DLK binding affinity was observed either in the presence of molar excess JNK or under experimental conditions that resulted in increased interaction affinity of JIP and JNK. The observation that JNK excess did not influence DLK–JIP(307–711) interaction suggests that the N-terminal region of JIP is functionally relevant. While requiring additional investigation, this conclusion is not surprising since the JIP N-terminal region contains the JNK-binding domain and potential JNK phosphorylation sites (Whitmarsh et al., 1998; Kelkar et al., 2000).
A detailed structural analysis of the DLK and JIP complex would help clarify the mechanism by which JIP inhibits DLK dimerization. The N-terminal region of JIP is clearly a determinant of this behavior. Since the regulation of both DLK dimerization and DLK–JIP binding affinity are determined by the N-terminus of JIP, it is likely that the mechanisms governing DLK dissociation and dimerization are linked.
It remains to be determined whether JNK recruitment to JIP initiates DLK dissociation from JIP and subsequent module activation, or merely occurs concomitantly. The factors that regulate JNK–JIP binding affinity are unknown. The importance of JNK catalytic activity and JIP phosphorylation events in this process requires investigation.
In summary, the data presented support the proposal of a modified model of JIP scaffold function. In this model, DLK is complexed to JIP in a monomeric, catalytically inactive state under basal conditions. Upon appropriate stimulation, DLK is dissociated from JIP1, dimerizes, autophosphorylates and becomes catalytically active. DLK dissociation may require prior recruitment of JNK to JIP. The model provides for switch-like regulation of module activation and appears to preserve the theoretical signaling properties of generic MAPK modules.
Materials and methods
Reagents
Polyclonal antibodies to the C-terminal 223 amino acids and to the N-terminal 250 amino acids of DLK were described previously (Mata et al., 1996; Merritt et al., 1999). GST–N-terminal DLK fusion protein, GST–MKK7 and GST–c-Jun(1–79) were prepared as described previously (Mata et al., 1996; Merritt et al., 1999). Anti-Flag epitope monoclonal antibody (M2, Sigma), anti-HA antibody (Sigma), anti-JNK antibody (Santa Cruz Biotechnology, Inc.) and the anti-Myc epitope monoclonal antibody (9E10, Oncogene Science) were obtained commercially. Antibodies to JIP proteins were gifts of Dr Benjamin Margolis. Cell culture grade chemicals, okadaic acid, cyclosporin A and veratridine were purchased from Sigma. GST–SAP kinase1b/JNK3 was obtained from Upstate Biotechnology.
Eukaryotic expression constructs
Construction and characterization of mammalian expression constructs encoding Flag-DLK, Myc-DLK, Flag-DLK(K185A), Flag-MKK7α1 and Flag-DLK(1–520) were described previously (Merritt et al., 1999). pCDNA3-Flag-MLK-3 was provided by Dr James Woodgett (University of Toronto). HA-DLK, HA-DLK(K185A) and HA-MLK3 mammalian constructs were prepared by standard PCR cloning techniques. The DLK–FKBP12 construct was prepared using a dimerizer kit provided by Ariad Pharmaceuticals. Briefly, DLK lacking its C-terminal region and the LZD [DLK(1–404)] was amplified by PCR as an NheI–NheI fragment and cloned into the XbaI site of the pC4Fv1E plasmid that contains FKBP12 harboring a F36V mutation and a C-terminal HA epitope (Clackson et al., 1998). The pCDNA3 expression construct expressing Myc-JIP-1, HA-JIP1(1–307), Myc-Cdc42HsV12, Myc-Cdc42HsN17, Myc-RhoAL63 and Myc-RacV12 were gifts of Dr Benjamin Margolis (Meyer et al., 1999).
Cell culture
Transient transfections were carried out in COS 7 cells. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (Life Technologies Inc.) and 200 U/ml of penicillin and streptomycin (Boehringer Mannheim). Transfections were performed using Fugene-6 (Boehringer Mannheim). For dimerization of FKBP12 chimeras, transfected cells were treated for 3 h with chemical dimerizer AP20187 (Ariad Pharmaceuticals), a derivative of AP1903 (Clackson et al., 1998). It is conceptually related to FK1012 (Morgenstern and Land, 1990; Pomerantz et al., 1995) and has been engineered so as not to bind to endogenous FKBP12. AP20187 was dissolved in ethanol to a stock concentration of 2.5 µM and used at a final concentration of 5 nM. Control experiments demonstrated that exposure of COS 7 cells to 5 nM AP20187 for 3 h did not activate JNK activity above control (data not shown).
Orthophosphate labeling of cells
Twenty four hours after transfection, cells were washed and incubated with phosphate-free DMEM supplemented with 10% dialyzed fetal bovine serum containing 1 mCi/ml [γ-32P]ATP orthophosphate (Amersham) for 5 h at 37°C.
Immunoprecipitation and immunoblotting
Immunoprecipitations were performed using the indicated antibodies as described elsewhere (Nihalani et al., 2000).
JNK activation assays
Cell lysates were prepared 24 h after transfection using 1 ml of lysis buffer [50 mM HEPES pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium vanadate, 50 mM sodium fluoride, 20 mM β-glycerophosphate, 10% glycerol, 1% Triton X-100 and a cocktail of protease inhibitors (Boehringer Mannheim, catalog no. 1836170)]. Kinase assays for JNK or JIP1 immune complexes were performed as described previously (Nihalani et al., 2000). Briefly, the immunoprecipitated mixtures from the lysates were incubated at 30°C for 30 min in 50 µl of kinase buffer (25 mM HEPES pH 7.2, 10% glycerol, 100 mM NaCl, 20 mM MgCl2, 0.1 mM sodium vanadate and a cocktail of protease inhibitors) containing 25 µM ATP, 5 µCi of [γ-32P]ATP (3000 Ci/mmol) and 2 µg of GST–c-Jun (1–79). Samples were processed as previously described.
In vitro JNK activation assays
In vitro JNK activation was determined essentially as described previously (Merritt et al., 1999; Nihalani et al., 2000). In vitro reconstituted coupled kinase assays were performed by combining the indicated JIP immune complexes with GST–MKK7 in kinase buffer containing 50 µm ATP and incubated at 30°C for 60 min. Kinase buffer containing recombinant GST–JNK, GST–c-Jun(1–79) and 5 µCi of [γ-32P]ATP (3000 Ci/mmol, Amersham) were then added to the mixtures and incubated further at 30°C for 15 min. Reactions were terminated by addition of Laemmli buffer, boiled, resolved by SDS–PAGE, transferred to nitrocellulose membranes and autoradiographed.
Acknowledgments
Acknowledgements
We gratefully acknowledge Dr Benjamin Margolis for gifts of JIP1 antibody and JIP1 expression plasmids, for helpful discussions and for sharing data before publication. This work was supported by a grant from the National Institutes of Health (DK52886) to L.B.H. and by a post-doctoral fellowship from the American Heart Association (D.N.).
References
- Barancik M., Htun,P. and Schaper,W. (1999) Okadaic acid and anisomycin are protective and stimulate the SAPK/JNK pathway. J. Cardiovasc. Pharmacol., 34, 182–190. [DOI] [PubMed] [Google Scholar]
- Bock B.C., Vacratsis,P.O., Qamirani,E. and Gallo,K.A. (2000) Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. J. Biol. Chem., 275, 14231–14241. [DOI] [PubMed] [Google Scholar]
- Burack R.W. and Shaw,A.S. (2000) Signal transduction: hanging on scaffold. Curr. Opin. Cell Biol., 12, 211–216. [DOI] [PubMed] [Google Scholar]
- Charest D.L., Mordet,G., Harder,K.W., Jirik,F. and Pelech,S.L. (1993) Molecular cloning, expression and characterization of the human mitogen-activated protein kinase p44erk1. Mol. Cell. Biol., 13, 4679–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.R., Meyer,C.F. and Tan,T.H. (1996) Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in γ radiation-induced apoptosis. J. Biol. Chem., 271, 631–634. [DOI] [PubMed] [Google Scholar]
- Cheng J., Yang,J., Xia,Y., Karin,M. and Su,B. (2000) Synergistic interaction of MEK kinase2, c-Jun N-terminal kinase (JNK) kinase 2 and JNK1 results in efficient and specific JNK1 activation. Mol. Cell. Biol., 20, 2334–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.Y., Satterberg,B., Lyons,D.M. and Elion,E.A. (1994) Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S.cerevisiae. Cell, 78, 499–512. [DOI] [PubMed] [Google Scholar]
- Clackson T. et al. (1998) Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl Acad. Sci. USA, 95, 10437–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Dickens M., Rogers,J.S., Cavanagh,J., Raitano,A., Xia,Z., Halpern,J.R., Greenberg,M.E., Sawyers,C.L. and Davis,R.J. (1997) A cytoplasmic inhibitor of the JNK signal transduction pathway. Science, 277, 693–696. [DOI] [PubMed] [Google Scholar]
- Dorow D.S., Devereux,L., Dietzsch,E. and De Kretser,T. (1993) Identification of a new family of human epithelial protein kinases containing two leucine/isoleucine-zipper domains. Eur. J. Biochem., 213, 701–710. [DOI] [PubMed] [Google Scholar]
- Elion E.A. (1998) Routing MAP kinase cascades. Science, 281, 1625–1626. [DOI] [PubMed] [Google Scholar]
- Fan G., Merritt,S.E., Kortenjann,M., Shaw,P.E. and Holzman,L.B. (1996) Dual leucine zipper-bearing kinase (DLK) activates p46SAPK and p38mapk but not ERK2. J. Biol. Chem., 271, 24788–24793. [DOI] [PubMed] [Google Scholar]
- Ferrell J.E. Jr and Machleder,E.M. (1998) The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science, 280, 895–898. [DOI] [PubMed] [Google Scholar]
- Garrington T.P. and Johnson,G.L. (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol., 11, 211–218. [DOI] [PubMed] [Google Scholar]
- Gonzalez F.A., Raden.D.L., Rigby,M.R. and Davis,R.J. (1992) Heterogenous expression of four AMP kinase isoforms in human tissues. FEBS Lett., 304, 170–178. [DOI] [PubMed] [Google Scholar]
- Gotoh I., Adachi,M. and Nishida,E. (2000) Identification and characterization of a novel MAP kinase kinase kinase, MLTK. J. Biol. Chem., 276, 4276–4286. [DOI] [PubMed] [Google Scholar]
- Gupta S., Barrett,T., Whitmarsh, A,J., Cavanagh,J., Sluss,H.K., Derijard,B. and Davis,R.J. (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J., 15, 2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. (1995) MAP kinase pathways in yeast: for mating and more. Cell, 80, 187–197. [DOI] [PubMed] [Google Scholar]
- Hirai S., Izawa,M., Osada,S., Spyrou,G. and Ohno,S. (1996) Activation of the JNK pathway by distantly related protein kinases, MEKK and MUK. Oncogene, 12, 641–650. [PubMed] [Google Scholar]
- Holzman L.B., Merritt,S.E. and Fan,G. (1994) Identification, molecular cloning and characterization of dual leucine zipper bearing kinase. A novel serine/threonine protein kinase that defines a second subfamily of mixed lineage kinases. J. Biol. Chem., 269, 30808–30817. [PubMed] [Google Scholar]
- Huang C.Y. and Ferrell,J.E.,Jr (1996) Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA, 93, 10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip Y.T. and Davis,R.J. (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol., 10, 205–219. [DOI] [PubMed] [Google Scholar]
- Kelkar N., Gupta,S., Dickens,M. and Davis,R.J. (2000) Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol. Cell. Biol., 20, 1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz J.E., Satterberg,B. and Elion,E.A. (1994) The MAP kinase Fus3 associates with and phosphorylates the upstream signaling component Ste5. Genes Dev., 8, 313–327. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M., App,H., Banerjee,P., Nikolakaki,E., Dai,T., Rubie,E.A., Ahmad,M.F.A., Avruch,J. and Woodgett,J.R. (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature, 369, 156–160. [DOI] [PubMed] [Google Scholar]
- Lee J.C. et al. (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature, 372, 739–746. [DOI] [PubMed] [Google Scholar]
- Leung I.W.-L. and Lassam,N. (1998) Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. J. Biol. Chem., 273, 32408–32415. [DOI] [PubMed] [Google Scholar]
- Levchenko A., Bruck,J. and Sternberg,P.W. (2000) Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl Acad. Sci. USA, 97, 5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani H.D. and Fink,G.R. (1998) The riddle of MAP kinase signaling specificity. Trends Genet., 14, 151–155. [DOI] [PubMed] [Google Scholar]
- Marcus S., Polverino,A., Barr,M. and Wigler,M. (1994) Complexes between STE5 and components of the pheromone-responsive mitogen-activated protein kinase module. Proc. Natl Acad. Sci. USA, 91, 7762–7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Merritt,S.E., Fan,G., Yu,G.G. and Holzman,L.B. (1996) Characterization of dual leucine zipper-bearing kinase, a mixed lineage kinase present in synaptic terminals whose phosphorylation state is regulated by membrane depolarization via calcineurin. J. Biol. Chem., 271, 16888–16896. [DOI] [PubMed] [Google Scholar]
- Merritt S.E., Mata,M., Nihalani,D., Zhu,C., Hu,X. and Holzman,L.B. (1999) The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate. J. Biol. Chem., 274, 10195–10202. [DOI] [PubMed] [Google Scholar]
- Meyer D., Liu,A. and Margolis,B. (1999) Interaction of c-Jun amino-terminal kinase interacting protein-1 with p190 rhoGEF and its localization in differentiated neurons. J. Biol. Chem., 274, 35113–35118. [DOI] [PubMed] [Google Scholar]
- Morgenstern J.P. and Land,H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res., 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihalani D., Merritt,S. and Holzman,L.B. (2000) Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J. Biol. Chem., 275, 7273–7279. [DOI] [PubMed] [Google Scholar]
- Owaki H., Makar,R., Boulton,T.G., Cobb,M.H. and Geppert.T.D. (1992) Extracellular signal-regulated kinases in T cells: characterization of human ERK1 and ERK2 cDNAs. Biochem. Biophys. Res. Commun., 182, 1416–1422. [DOI] [PubMed] [Google Scholar]
- Pomerantz J.L., Sharp,P.A. and Pabo,C.O. (1995) Structure-based design of transcription factors. Science, 267, 93–96. [DOI] [PubMed] [Google Scholar]
- Posas F. and Saito,H. (1997) Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science, 276, 1702–1705. [DOI] [PubMed] [Google Scholar]
- Printen J.A. and Sprague,G.F.,Jr (1994) Protein–protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics, 138, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitano A.B., Halpern,J.R., Hambuch,T.M. and Sawyers,C.L. (1995) The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc. Natl Acad. Sci. USA, 92, 11746–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesgo-Escovar J.R., Jenni,M., Fritz,A. and Hafen,E. (1996) The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev., 10, 2759–2768. [DOI] [PubMed] [Google Scholar]
- Rincon M., Derijard,B., Chow,C.W., Davis,R.J. and Flavell,R.A. (1997) Reprogramming the signalling requirement for AP-1 (activator protein-1) activation during differentiation of precursor CD4+ T-cells into effector Th1 and Th2 cells. Genes Funct., 1, 51–68. [DOI] [PubMed] [Google Scholar]
- Sakuma H., Ikeda,A., Oka,S., Kozutsumi,Y., Zanetta,J.P. and Kawasaki,T. (1997) Molecular cloning and functional expression of a cDNA encoding a new member of mixed lineage protein kinase from human brain. J. Biol. Chem., 272, 28622–28629. [DOI] [PubMed] [Google Scholar]
- Savinainen A., Garcia,P.E., Dorow,D., Marshall,J. and Liu,F.Y. (2001) Kainate receptor activation induces mixed lineage kinase-mediated cellular signaling cascades via post-synaptic density protein 95. J. Biol. Chem., 276, 11382–11386. [DOI] [PubMed] [Google Scholar]
- Schaffer H.J. and Weber,M.J. (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol., 19, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss H.K., Han,Z., Barrett,T., Davis,R.J. and Ip,T. (1996) A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev., 10, 2745–2758. [DOI] [PubMed] [Google Scholar]
- Su B., Jacinto,E., Hibi,M., Kallunki,T., Karin,M. and Ben-Neriah,Y. (1994) JNK is involved in signal integration during costimulation of T lymphocytes. Cell, 77, 727. [DOI] [PubMed] [Google Scholar]
- Teramoto H., Coso,O.A., Miyata,H., Igishi,T., Miki,T. and Gutkind,J.S. (1996) Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J. Biol. Chem., 271, 27225–27228. [DOI] [PubMed] [Google Scholar]
- Tibbles L.A.,Y.L., Ing,F., Kiefer,J., Chan,N., Iscove,J.R., Woodgett and Lassam,N.J. (1996) MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J., 15, 7026–7035. [PMC free article] [PubMed] [Google Scholar]
- Verheij M. et al. (1996) Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature, 380, 75–79. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A.J. and Davis,R.J. (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med., 74, 589–607. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A.J. and Davis,R.J. (1998) Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem. Sci., 23, 481–485. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A.J., Cavanagh,J., Tournier,C., Yasuda,J. and Davis,R.J. (1998) A mammalian scaffold complex that selectively mediates MAP kinase activation. Science, 281, 1671–1674. [DOI] [PubMed] [Google Scholar]
- Widman C., Gibson,S., Jarpe,M.B. and Johnson,G.L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev., 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Xia Y., Wu,Z., Su,B., Murray,B. and Karin,M. (1998) JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev., 12, 3369–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Dickens,M., Raingeaud,J., Davis,R.J. and Greenberg,M.E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Xu X., Heidenreich,O., Kitajima,I., McGuire,K., Li,Q., Su,B. and Nerenberg,M. (1996) Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene, 13, 135–142. [PubMed] [Google Scholar]
- Yasuda J., Whitmarsh,A.J., Cavanagh,J., Sharma,M. and Davis,R.J. (1999) The JIP group of mitogen-activated protein kinase scaffolding proteins. Mol. Cell. Biol., 19, 7245–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke B.W., Boudreau,K., Rubie,E., Winnett,E., Tibbles,L.A., Zon,L., Kyriakis,J., Liu,F.F. and Woodgett,J.R. (1996) The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol., 6, 606–613. [DOI] [PubMed] [Google Scholar]