Abstract
Background
Allergic asthma (AA) is a chronic inflammatory disease with limited effective treatments, often co-occurring with atopic dermatitis (AD). Epidemiologic studies have shown a strong association between AD and AA, but their causal relationship remains unclear.
Objectives
This study aimed to identify novel therapeutic targets and candidate drugs for AA.
Methods
We performed Mendelian (MR) analysis to evaluate the causal relationship between AD and AA. To identify potential therapeutic targets, we integrated drug-target MR, colocalization, functional enrichment, protein–protein interaction, and gene expression analyses. Drug candidates were prioritized using the Drug–Gene Interaction Database (DGIdb; dgidb.org) and the Comparative Toxicogenomics Database (CTD; ctdbase.org).
Results
MR analysis confirmed that AD significantly increases the risk of AA (odds ratio = 1.64, P < .001). Integrating drug-target MR and colocalization analysis, we identified Janus kinase 2 (JAK2) as a potential causal gene (posterior probability [PP.H4] = 0.95), supported by functional enrichment, protein–protein interaction, and gene expression analyses. Drug screening via DGIdb and CTD prioritized hydroxyurea as the top candidate (composite score = 0.85), although its efficacy in AA remains to be validated.
Conclusions
We provide the first genomic evidence for a causal link between AD and AA. These findings highlight JAK2 as a potential therapeutic target and hydroxyurea as a candidate for clinical validation, paving the way for future research into shared immunogenetic mechanisms in allergic diseases.
Key words: Atopic dermatitis, allergic asthma, Mendelian randomization, genetic association
Allergic asthma (AA) is a chronic inflammatory disease that frequently co-occurs with atopic dermatitis (AD).1 Despite available treatments, disease control remains suboptimal, underscoring the need for novel therapeutic strategies. A deeper understanding of AA’s molecular mechanisms is crucial for identifying effective drug targets. Mendelian randomization (MR) and colocalization analyses are powerful tools for prioritizing disease-associated genes and identifying potential drug targets.2,3 Applying these methods to AD-related genes could provide insights into shared pathogenic mechanisms with AA and aid in the discovery of novel therapeutic strategies.
Recent studies have utilized MR and colocalization analyses to identify genetic determinants and potential drug targets for allergic diseases.4 One multiomics MR study integrated colocalization and pathway analysis to prioritize asthma-associated genes, such as Janus kinase 2 (JAK2) and ETS1, along with DNA methylation sites linked to asthma risk.5 Another MR analysis highlighted plasma proteins, such as signal transducer and activator of transcription (STAT) 6 and TNFRSF6B, as candidate drug targets for AA, further supporting the role of immune signaling in disease pathogenesis.6
Despite advances in MR-based drug discovery for allergic diseases, the causal relationship linking AD to AA is still poorly understood. Elucidating shared immunologic pathways could reveal novel therapeutic targets for both conditions. AD frequently precedes the onset of other allergic conditions, a progression known as the atopic march.7 AA, characterized by airway inflammation and hyperresponsiveness, shares key immunopathogenic features with AD, particularly activation of TH2-driven cytokine signaling.8, 9, 10 Observational studies consistently report an association between AD and AA, but the extent to which this reflects causality remains unclear because of potential confounding and reverse causation.7,11 MR mitigates these limitations by using genetic variants as instrumental variables (IVs) to infer causality, offering an approach analogous to a randomized controlled trial.12, 13, 14 Unlike traditional epidemiologic approaches, MR minimizes confounding and reverse causation biases because genetic variants are randomly allocated at conception, mimicking the principles of a randomized controlled trial.15 Recent MR studies suggest a potential causal link between AD and AA,16,17 yet the potential causal genes and shared therapeutic targets underlying this association remain largely unexplored.
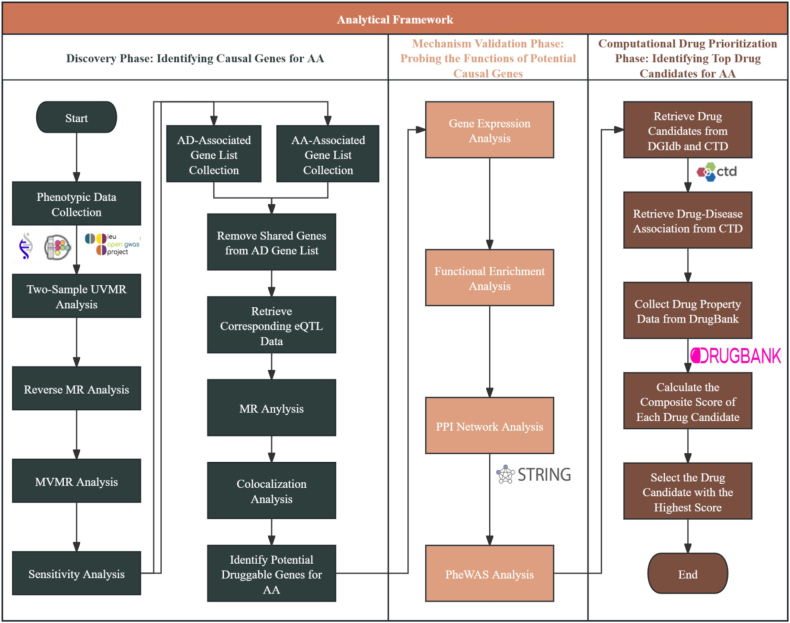
This study was conducted in 3 sequential phases. First, we used large-scale genome-wide association study (GWAS) data to conduct univariable MR (UVMR), reverse MR to examine bidirectional effects, and multivariable MR (MVMR) to account for immune-related and metabolic confounders. Next, expression quantitative trait loci (eQTL) and colocalization analyses were performed to pinpoint genetic variants that influence AA risk via AD-associated gene expression regulation. Functional characterization, including pathway enrichment, protein–protein interaction (PPI) analysis, and gene expression profiling, further delineated the biological relevance of these genes. Finally, a computational drug prioritization framework systematically evaluated therapeutic candidates by integrating genetic evidence with pharmacokinetic and bioavailability parameters.
This study aimed to determine the causal relationship between AD and AA and to identify AD-derived therapeutic targets with translational potential. Our 3-phase integrative approach first established robust bidirectional causality between AD and AA, with AD significantly increasing the risk of AA (odds ratio [OR] = 1.642), independent of immune and metabolic confounders. We next prioritized JAK2 as a potential causal hub gene by integrating eQTL-based MR with Bayesian colocalization, indicating that shared genetic variants may simultaneously influence JAK2 expression and AA susceptibility. Functional analyses further supported JAK2 as a central regulator of TH2-polarized inflammation through dysregulation of the JAK-STAT signaling pathway, with enrichment in cytokine-mediated signaling and immune cell differentiation processes, including TH17 and TH1/TH2 pathways. Finally, hydroxyurea emerged as the top-ranked candidate in our composite prioritization framework on the bases of drug likeness, pharmacokinetics, and association in the literature, although its clinical relevance to AA remains to be explored.
Methods
Study design and data sources
The study was conducted in 3 sequential phases: the discovery, mechanism validation, and computational drug prioritization phases. Fig 1 provides the whole research framework. The first phase leveraged MR to investigate the potential causal association between AD and AA and identified druggable gene targets. We followed the STROBE-MR reporting guidelines to ensure robust genetic instrument selection and to minimize bias.18 The second phase comprised further evaluation of candidate genes for AA using multiomics and functional characterization. The third and final phase screened potential therapies from drug databases using computational methods to prioritize candidates on the bases of pharmacokinetics, regulatory approval, and evidence in the literature.
Fig 1.
Three-phase analytical framework workflow. (1) For discovery phase, GWAS data for AD and AA underwent bidirectional MR (univariable, reverse, and multivariable) to test causality and to shortlist potential druggable genes for AA supported by eQTLs derived from AD after removing genes overlapping with AA. (2) For mechanism validation phase, prioritized genes were functionally profiled by tissue expression (Human Protein Atlas), GO biological process, and KEGG enrichment, high-confidence STRING PPI, and mapping and PheWAS study. (3) For computational drug prioritization phase, gene–drug interactions were retrieved from DGIdb and CTD, filtered for AA relevance, and ranked by DrugBank physicochemical and regulatory attributes using entropy-weighted composite score.
Genetic summary statistics for AD were obtained from the National Human Genome Research Institute–European Bioinformatics Institute GWAS catalog (www.ebi.ac.uk/gwas)19 with study ID GCST90244787,20 and data for AA were sourced from the FinnGen consortium (www.finngen.fi/en).21 The AD dataset included 60,653 cases and 804,329 controls, while the AA dataset comprised 13,450 cases and 270,290 controls, with all individuals of European ancestry. In addition to the data from these mentioned sources, information on 6 traits was obtained from the IEU Open GWAS Project website (gwas.mricieu.ac.uk).22 The immune-related traits analyzed included eosinophil, lymphocyte, and basophil cell counts. The data for these traits were sourced from the Blood Cell Consortium. Specifically, the dataset IDs ieu-b-33, ieu-b-32, and ieu-b-29 were utilized for this analysis. The data for other traits, such as triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol, were derived from the UK Biobank GWAS repository, with dataset IDs ieu-b-111, ieu-b-110, and ieu-b-109, respectively. Expression quantitative trait loci data were obtained from eQTLGen Consortium (www.eqtlgen.org).23 Table E1 in the in the Online Repository at www.jaci-global.org provides more details. For MR analyses investigating AD- and AA-associated genes, gene lists were curated from the DISEASES database (diseases.jensenlab.org).24 In the third phase, candidate drug names were identified through the Drug–Gene Interaction Database (DGIdb; dgidb.org)25 and the Comparative Toxicogenomics Database (CTD; ctdbase.org),26 with detailed drug information retrieved from DrugBank (go.drugbank.com).27
Discovery phase: Identifying causal genes for AA
Causal relationship between AD and AA
To identify potential causal genes associated with AA derived from AD, we conducted a multistep MR analysis, leveraging genetic summary statistics and gene expression datasets following standard MR methodologies.13 We initially used a 2-sample UVMR to assess the causal relationship between AD and AA. The exposure data comprised phenotypic data for AD, while the outcome data consisted of phenotypic data for AA.
MR relies on 3 key assumptions: (1) relevance, where genetic variants used as IVs are strongly associated with the exposure; (2) independence, meaning that these IVs are not related to confounders that influence both the exposure and the outcome; and (3) exclusion restriction, indicating that IVs affect the outcome solely through the exposure, with no direct effects or horizontal pleiotropy.13 These assumptions enable MR to estimate causal effects while minimizing bias from confounding and reverse causation.
To satisfy the core assumptions of MR, the genetic instrument variable selection and harmonization workflow was implemented as follows. First, single nucleotide polymorphisms (SNPs) reaching genome-wide significance were extracted from the exposure GWAS summary statistics, with a significance threshold set to 5 × 10−8. Linkage disequilibrium clumping was performed by PLINK v1.9,28 with a clumping window of 10,000 kbp and an r2 threshold of less than 0.001 to retain independent SNPs, prioritizing the most significant SNP per locus. Allele directions were harmonized by excluding palindromic SNPs with minor allele frequencies between 0.4 and 0.6 and SNPs missing beta or standard error estimates in the outcome dataset. Horizontal pleiotropic outliers were detected by MR-PRESSO with a global test significance threshold of .05,29 with iterative outlier removal until heterogeneity was reduced until heterogeneity assessed by the Cochran Q statistic yielded P > .05. Radial MR was applied to quantify individual SNP contributions to heterogeneity through the residual sum of squares,30 and directional pleiotropy was evaluated via MR-Egger regression, where an intercept P > .05 confirmed the exclusion restriction assumption. Finally, instrument strength was validated by retaining only SNPs with an F statistic greater than 10 to mitigate weak instrument bias.
Dissecting AD-AA association
After completing the study of the causality of AD on AA, we performed reverse MR, treating AA as the exposure and AD as the outcome. This analysis allowed us to confirm the directionality of the causal link. We conducted MVMR to account for potential confounders to further investigate this association.31 On the basis of prior literature, 6 traits with potential relevance to both AD and AA were included as covariates in the analysis. These included triglycerides,32 LDL cholesterol,33 HDL cholesterol,34 eosinophil count,35 lymphocyte count,36 and basophil count.37 While previous studies have reported associations between these traits and allergic diseases, their specific role in influencing the AD-AA relationship remains uncertain. Incorporating these variables aimed to refine causal estimates and mitigate potential confounding effects.
MR for causal gene identification
We first obtained gene lists associated with AD and AA from the DISEASES database to identify specific genes driving the causal relationship between these conditions. Genes shared between the two diseases were excluded from the AD gene list to ensure specificity, and the remaining AD-specific genes were mapped to their expression quantitative trait loci using data from the eQTLGen Consortium. These eQTL data were used as exposure in a subsequent MR analysis, with AA as the outcome. The same quality control procedures were applied for IV selection and clumping to maintain analytical rigor.
Positive control for methodologic validation
To ensure the robustness of our MR framework, we conducted two positive control analyses using eQTL data for IL4R and IL4 as exposures and AA GWAS summary statistics as the outcome. IL4R was included as a positive control because of its well-documented genetic association with asthma and its role as a therapeutic target.38 As a key regulator of TH2-driven immune responses, IL4 is directly implicated in asthma pathogenesis.39 Reproducing the expected causal associations in both analyses would provide strong methodologic validation for our analytic pipeline in the context of hypothesis testing.
Colocalization analysis for candidate gene prioritization
To further refine the list of candidate causal genes, we integrated eQTL data for genes associated with AA in the prior MR step and performed colocalization analysis with AA GWAS data. This analysis was used to assess whether the observed associations between gene expression and AA were consistent with shared genetic variants or arose from distinct genetic signals.40 Genes were considered to have high-confidence colocalization evidence if the posterior probability (PP.H4) exceeded 0.8, supporting the possibility that the observed eQTL and GWAS signals share a common causal variant. Genes meeting this criterion were prioritized as candidate genes for AA.
Mechanism validation phase: Probing the functions of potential causal genes
Gene expression analysis
To investigate the potential of the potential causal genes identified in the discovery phase to contribute to the etiologic processes of AD and AA, their expression profiles across human tissues were analyzed using data from the Human Protein Atlas (www.proteinatlas.org).41 This platform integrates transcriptomic and proteomic data, enabling a comprehensive assessment of gene expression in different tissues. This approach aimed to determine whether the potential causal genes exhibit tissue-specific expression patterns associated with AA pathophysiology, providing insights into their biological relevance and potential mechanisms of action.
Functional enrichment analysis
Functional enrichment analyses were performed using the STRING database (string-db.org) to further characterize the biological roles of the potential causal genes.42 Gene Ontology (geneontology.org) biological process enrichment analysis was conducted to identify biological processes associated with these genes, focusing on immune regulation, inflammatory responses, and other AA-relevant pathways. Kyoto Encyclopedia of Genes and Genomes (KEGG, genome.jp/kegg) pathway analysis was performed to identify signaling pathways through which the potential causal genes may regulate disease processes. In addition, Reactome (reactome.org) pathway analysis was conducted to provide complementary mechanistic insights that were based on a more detailed molecular annotation framework.
STRING was used to construct a PPI network from the candidate genes identified in upstream analyses. First-shell interacting proteins were automatically added to the input to provide a broader biological context for the enrichment analyses. The statistical background was set to the whole genome, and multiple comparison corrections were applied using the false discovery rate (FDR) method, with a significance threshold of FDR ≤ 0.05. This approach enabled the identification of biological processes and pathways potentially associated with the network context of the candidate genes in AA.
PPI network analysis
PPI networks were constructed by STRING to contextualize the potential causal genes within broader biological networks. Highest-confidence interactions (confidence score > 0.9) were included to ensure the reliability of the networks. These analyses helped identify key hub proteins and interacting partners, providing insights into how the potential causal genes may cooperate with other molecules to influence disease-related biological processes. The inclusion of additional proteins in the network was considered during interpretation.
Pleiotropic disease association analysis via disease ontology enrichment and phenome-wide association
To comprehensively evaluate the pleiotropic disease associations and potential adverse consequences of targeting the identified candidate gene, we performed both disease ontology (DO) enrichment and phenome-wide association (PheWAS) analyses. DO enrichment was conducted by the DOSE R v4.0.0 package,43 which performs DO semantic and enrichment analysis. The input gene set included JAK2 and its first-shell PPI partners, derived from the STRING platform. Gene symbols were converted to Entrez IDs using the ‘org.Hs.eg.db’ v3.20.0 annotation package, and enrichment was performed using the ‘enrichDO(e)’ function in the DOSE package. Results were visualized by bar plots showing the top 10 enriched disease terms.
To assess the pleiotropic effects of potential causal genes and evaluate their possible adverse effects, PheWAS analysis was conducted by AZ-PheWAS (azphewas.com).44 This online tool enables the systematic examination of gene-level associations across a wide range of clinical traits and diseases. Analyses were restricted to individuals of European ancestry. For each gene–phenotype pair, the most statistically significant collapsing model was selected according to platform-defined criteria. As part of the therapeutic target evaluation, the analysis considered potential adverse effects that might arise if these genes were targeted for treatment.
Computational drug prioritization phase: Identifying top drug candidates for AA
Identification of candidate drugs
To identify potential therapeutic drugs for AA, we performed a systematic integration of publicly available databases. Drug candidates were first retrieved by querying genes identified in earlier phases by the DGIdb and CTD. In the DGIdb, we extracted a drug list of gene–drug interaction results after inputting the gene name. Simultaneously, CTD’s Chemical–Gene Interaction Query was also used to obtain compounds known to interact with the same genes. The drug lists from both databases were merged to generate a comprehensive set of candidate drugs.
To focus on AA, we queried the CTD for disease data related to the list of drugs and screened the results to include only drugs linked to respiratory tract diseases, immune system diseases, and pathologic processes, ensuring relevance to asthma pathophysiology. Additionally, only drugs with direct therapeutic evidence in CTD were retained. This filtering process resulted in a set of prioritized candidate drugs, which required further validation to evaluate their potential in AA.
Prioritization of drug candidates
We used a multicriteria decision analysis to rank the candidate drugs systematically and identify the most promising therapeutic drug. These criteria used for ranking include regulatory approval status because it reflects prior safety and efficacy evaluations, thus providing an indicator of clinical readiness. Physicochemical properties, including water solubility, lipophilicity (logP), and polar surface area (Å2), were incorporated because of their influence on drug absorption, distribution, and permeability. Bioavailability was considered to assess systemic drug exposure after administration, while compliance with the Lipinski rule of 5 provided an overall measure of oral drug likeness. To assess the scientific and clinical relevance, we extracted the number of PubMed-indexed studies referencing each drug from CTD as a measure of research interest and prior investigations. These parameters were retrieved from DrugBank and CTD.
All collected data were first standardized to ensure comparability across different scales. The entropy weight method was used to assign weights to each criterion, giving higher importance to attributes with greater variability among candidate drugs.45 A composite score was then calculated for each drug, integrating its pharmacologic properties, regulatory status, bioavailability, and support in the literature. The drug with the highest composite score was prioritized as the most promising therapeutic candidate for AA. Importantly, the prioritization framework does not reflect predicted therapeutic efficacy alone but rather a weighted composite of pharmacokinetic properties, bioavailability, regulatory history, and literature attention.
Statistical and sensitivity analyses
Causal effects were estimated by the inverse variance weighted (IVW) method. Sensitivity analyses included MR-Egger regression to detect pleiotropy, MR-PRESSO to correct for outliers, Cochran Q test to assess heterogeneity, and leave-one-out analysis to evaluate reliance on individual SNPs. Bayesian colocalization was performed to identify shared genetic signals between eQTL and GWAS data, with PP.H4 > 0.8 indicating strong colocalization.
The entropy weight method was applied in Excel to determine criterion weights according to variability. All other statistical analyses were conducted in R using the TwoSampleMR, MR-PRESSO, and ‘coloc’ packages, with statistical significance set at P < .05.46
Results
Causal relationship between AD and AA
MR reveals a causal relationship between AD and AA
After selecting genome-wide significant SNPs, applying linkage disequilibrium clumping, and harmonizing exposure–outcome allele directions, 45 SNPs were retained for preliminary analysis. Outliers exhibiting horizontal pleiotropy were iteratively excluded using multiple runs of MR-PRESSO, and radial MR further removed SNPs that contributed disproportionately to heterogeneity. The final set of 24 SNPs demonstrated strong instrument strength, with all F statistics exceeding 10. The beta, standard error, and P value of the IVs’ associations with exposure and outcome are listed in Table E2 in the Online Repository at www.jaci-global.org.
Table I presents the potential causal effect of AD on AA. The IVW method provided the most precise estimate, indicating a significant association between AD and an increased risk of AA, with an OR of 1.64, a 95% confidence interval (CI) of 1.49 to 1.81, and P = 1.59 × 10−22. The weighted median approach yielded a similar estimate, with an OR of 1.59, a 95% CI of 1.38 to 1.83, and P = 1.51 × 10−10. The weighted mode and simple mode methods produced ORs of 1.55 and 1.53, with P = 1.51 × 10−3 and 5.08 × 10−3, respectively, indicating consistency across methods. The MR-Egger method produced a slightly lower estimate, with an OR of 1.45, a 95% CI of 1.12 to 1.88, and P = .01.
Table I.
MR estimates for AD and AA
| Analysis type | Exposure | Outcome | Method | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Univariable | AD | AA | MR-Egger | 1.45 (1.12-1.88) | .01 |
| — | — | — | Weighted median | 1.59 (1.38-1.83) | <.001 |
| — | — | — | IVW | 1.64 (1.49-1.81) | <.001 |
| — | — | — | Simple mode | 1.53 (1.17-2.00) | .005 |
| — | — | — | Weighted mode | 1.55 (1.22-1.96) | .002 |
| Reverse MR | AA | AD | MR-Egger | 1.17 (0.99-1.37) | .08 |
| — | — | — | Weighted median | 1.25 (1.19-1.33) | <.001 |
| — | — | — | IVW | 1.23 (1.18-1.28) | <.001 |
| — | — | — | Simple mode | 1.28 (1.16-1.41) | <.001 |
| — | — | — | Weighted mode | 1.27 (1.15-1.40) | <.001 |
| Multivariable | AD | AA | Multivariable IVW | 1.38 (1.27-1.49) | <.001 |
| — | HDL | — | — | 0.99 (0.90-1.09) | .83 |
| — | LDL | — | — | 1.04 (0.94-1.16) | .43 |
| — | Triglycerides | — | — | 1.06 (0.95-1.17) | .29 |
| — | Basophils | — | — | 0.93 (0.81-1.08) | .37 |
| — | Lymphocytes | — | — | 0.90 (0.82-1.00) | .04 |
| — | Eosinophils | — | — | 1.40 (1.26-1.54) | <.001 |
Sensitivity analyses provided evidence for the robustness of these findings. The MR-Egger intercept test did not provide evidence for substantial horizontal pleiotropy, with an intercept estimate of 0.01, a standard error of 0.01, and P = .32, suggesting that directional pleiotropy is unlikely to bias the causal estimate (see Table E3 in the Online Repository at www.jaci-global.org). The Steiger directionality test provided evidence for the correct causal direction from AD to AA, but P = .17 did not reach statistical significance (see Table E4 in the Online Repository). The forest plot (Fig 2, A) illustrates the individual and overall MR estimates, providing evidence of AD’s potential causal effect on AA. The scatter plot (Fig 2, B) shows the alignment of SNP-specific causal estimates across different MR methods, supporting the consistency of causal inference. Heterogeneity tests did not provide evidence for substantial heterogeneity. The Cochran Q statistic for the IVW method was 16.32 with 23 degrees of freedom and P = .84, while for the MR-Egger method, the Q statistic was 15.28 with 22 degrees of freedom and P = .85 (see Table E5 in the Online Repository). The funnel plot (Fig 2, C) appears symmetrical, suggesting no strong evidence of horizontal pleiotropy or publication bias. Fig 2, D, shows that leave-one-out analysis suggested that no single SNP disproportionately influenced the results.
Fig 2.
UVMR results and sensitivity diagnostics for causal effect of AD on AA. (A) Forest plot of SNPs with their causal estimates (black dots, 95% CIs). Red horizontal bars represent overall IVW estimate (top) and MR-Egger slope estimate (bottom).(B) Scatter plot of SNP effects on AD (x-axis) vs AA (y-axis). Regression lines from 5 MR estimators (IVW, MR-Egger, simple mode, weighted median, and weighted mode) all display positive slope, indicating consistent causal direction across methods. (C) Funnel plot. Approximate symmetry around vertical IVW line suggests limited directional pleiotropy. (D) Leave-one-out analysis. IVW estimate recalculated after sequential removal of each SNP; no reestimate departs materially from overall IVW line (red), confirming robustness.
Reverse and MVMR analyses confirm the independence of AD’s effect on AA
To assess the possibility of reverse causation, we evaluated whether AA has a causal effect on AD (Table I). The IVW method yielded an OR of 1.23 with a 95% CI of 1.18 to 1.28 and P = 3.61 × 10−25, suggesting a significant association between AA and an increased risk of AD. The weighted median method yielded a similar result, with an OR of 1.25, a 95% CI of 1.19 to 1.33, and P = 6.99 × 10−16, further supporting the robustness of the findings. The weighted mode and simple mode methods provided consistent estimates, reinforcing causal inference. Sensitivity analyses indicated that the results were robust. The Cochran Q test did not suggest substantial heterogeneity, with P = .34 for the IVW method and .30 for the MR-Egger method (Table E5). The MR-Egger intercept test did not provide strong evidence for horizontal pleiotropy, with an intercept estimate of 0.01 and P = .54 (Table E3). The Steiger directionality test supported the causal direction from AA to AD; however, P = .167 did not reach statistical significance (Table E4).
Additionally, as Table I shows, lipid and immune cell traits were potential confounders in estimating the independent causal effect of AD on AA after adjusting for lipid traits, including HDL, LDL, and triglycerides, and immune cell counts, including basophils, lymphocytes, and eosinophils. After accounting for these potential confounders, AD remained significantly associated with an increased risk of AA, with an MVMR estimated OR of 1.38, a 95% CI of 1.27 to 1.49, and P = 2.71 × 10−13, indicating that the causal relationship remained robust even after adjusting for these covariates. Among the included covariates, lymphocyte count showed a statistically significant association with AA, with an OR of 0.90, a 95% CI of 0.82 to 1.00, and P = .04, suggesting a potential role of lymphocytes in asthma pathogenesis. Additionally, eosinophil count was strongly associated with AA, with an OR of 1.38, a 95% CI of 1.27 to 1.49, and P = 3.57 × 10−10, indicating its likely involvement in asthma development. In contrast, HDL, LDL, triglycerides, and basophils did not reach statistical significance, suggesting that they may not act as major confounders in the causal association between AD and AA. We found no evidence of horizontal pleiotropy or heterogeneity in the MVMR analysis (Tables E3 and E5).
JAK2 identified as causal hub gene linking AD and AA through shared genetic variants
Gene sets associated with AD and AA were curated from the DISEASES database to identify potential causal drivers of AD-AA comorbidity. After excluding 520 overlapping genes, 1,106 AD-specific candidates were retained for MR and colocalization analyses. eQTL data from the eQTLGen Consortium were available for 325 of these genes, which were subsequently used as exposures in a 2-sample MR analysis with AA as the outcome. MR analysis identified 50 genes with significant associations (see Table E6 in the Online Repository at www.jaci-global.org).
Positive control MR analysis supported the validity of our causal inference framework. For IL4R, all 5 MR methods yielded consistent and highly significant inverse associations with AA. The IVW method showed an OR of 0.74 (95% CI, 0.73-0.76; P = 1 × 10−251). We found no evidence of heterogeneity or directional horizontal pleiotropy. Steiger filtering confirmed the correct causal direction (see Table E7 in the Online Repository at www.jaci-global.org). For IL4, the IVW analysis was highly significant, with an OR of 0.50 (95% CI, 0.48-0.52; P = 1 × 10−232), and results were consistent across all MR methods except MR-Egger. While the possibility of horizontal pleiotropy warrants cautious interpretation, the consistency and strength of the association across multiple methods provide support for a genuine causal effect (see Table E8 in the Online Repository).
Colocalization analysis was performed to assess whether shared genetic variants were involved in these associations. Among the 50 MR-identified genes, only JAK2 exhibited strong colocalization evidence, with PP.H4 = 0.95, supporting the possibility that its eQTL signal and the AA GWAS signal originate from the same causal variant.
Functional and molecular characterization of potential causal genes
JAK2 exhibits broad expression with notable levels in immune and respiratory tissues
RNA expression analysis showed that the gene is broadly expressed across multiple human tissues and exhibits low tissue specificity. The highest expression levels were observed in heart muscle, bone marrow, lymph nodes, spleen, and lungs, with relatively high expression in immune-related tissues (lymph nodes, spleen) and the respiratory system (lungs). The gene also exhibited moderate expression in the skin (Fig 3).
Fig 3.
Tissue-wide RNA expression profile of JAK2. Bar height represents normalized transcripts per million (nTPM) obtained from consensus transcriptomics dataset, which merges Human Protein Atlas and GTEx RNA sequencing data after internal normalization. Bars are color coded by tissue group. JAK2 transcripts are detected in every tissue examined, with relatively higher expression in heart muscle, bone marrow, lymph node, spleen, and lung, and lower but still measurable levels in most other tissues, indicative of broad expression and low tissue specificity.
JAK2 as a central node in immune-cytokine and hormone signaling network
As shown in Fig 4, A, Gene Ontology (geneontology.org) biological process enrichment analysis revealed significant involvement of JAK2 in multiple immune-related and hormone-responsive signaling pathways. The most enriched terms included the cytokine-mediated signaling pathway (FDR = 1.04 × 10−12), cellular response to cytokine stimulus (FDR = 1.16 × 10−12), and receptor signaling pathway via JAK-STAT (FDR = 2.24 × 10−12). Additional enrichment was observed in the growth hormone receptor signaling pathway (FDR = 3.19 × 10−9) and the IFN-γ–mediated signaling pathway (FDR = 1.24 × 10−7), and in the cellular response to peptide hormone stimulus (FDR = 1.63× 10−6), suggesting JAK2’s involvement in coordinating immune and hormone signaling processes.
Fig 4.
Functional enrichment of JAK2-centered gene set. Seed set comprising JAK2 and its first-shell STRING interactors was analyzed by STRING’s enrichment module to identify functional overrepresentation. Bubble plots show top 10 enriched terms ranked by signal score. Circle size is proportional to number of genes annotated to each term; color encodes FDR, where smaller FDR values are shown in lighter colors to indicate higher statistical significance. Functional terms were grouped at semantic similarity of ≥0.8. (A) GO-BP enrichment. “Receptor signaling pathway via JAK-STAT” ranks highest. (B) KEGG enrichment. “JAK-STAT signaling pathway” ranks highest by signal score. (C) Reactome pathway enrichment. Top-ranked terms include “growth hormone receptor signaling,” “inactivation of CSF3 signaling,” and “signaling by KIT mutants.” GO-BP, Gene Ontology (geneontology.org) biological process.
KEGG pathway enrichment analysis further supported these findings, reinforcing the centrality of JAK-STAT signaling. The JAK-STAT signaling pathway was the most significantly enriched (FDR = 5.66 × 10−18), followed by TH17 cell differentiation (FDR = 7.51 × 10−10) and TH1 and TH2 cell differentiation (FDR = 4.49 × 10−8). Additional significant enrichment was observed in necroptosis (FDR = 5.96 × 10−9), AGE-RAGE signaling pathway in diabetic complications (FDR = 6.92 × 10−8), prolactin signaling pathway (FDR = 1.16 × 10−10), and growth hormone synthesis, secretion, and action (FDR = 1.78 × 10−11), indicating enrichment across immune and hormone signaling networks (Fig 4, B).
Reactome pathway enrichment analysis provided high-resolution insights into the downstream molecular mechanisms associated with JAK2. The most significantly enriched pathways included the IL-6 signaling pathway (FDR = 1.09 × 10−8), IL-9 signaling pathway (FDR = 6.69 × 10−9), IL-21 signaling pathway (FDR = 8.54 × 10−9), and IL-20 family signaling (FDR = 7.54 × 10−10), suggesting the central positioning of JAK2 within cytokine-driven immune networks. Additional enrichment was observed in leptin signaling (FDR = 8.00 × 10−14), erythropoietin-activated STAT5 (FDR = 3.95 × 10−9), and inactivation of CSF3 signaling (FDR = 2.55 × 10−12), indicating potential cross talk between JAK2-associated immune and endocrine signaling processes (Fig 4, C).
PPI network of JAK2 reveals key signaling partners
PPI network analysis was performed with JAK2 as the input (Fig 5), which identified its direct interaction partners. The highest confidence interactions (confidence score > 0.9) were observed with STAT1, STAT3, STAT5A, and STAT5B as well as with key receptors including IFNGR1 and IFNGR2, GHR, and LEPR. SOCS3, a known negative regulator of JAK-STAT pathway, was also among the interacting proteins.
Fig 5.
Degree-coded PPI network centered on JAK2. Interactions (STRING combined score of >0.9) were imported into Cytoscape. Node color saturation and diameter are scaled in parallel with interaction degree, with darker and larger nodes denoting higher degrees and paler, smaller nodes denoting lower degrees. Dark red, JAK2; orange-red, degree 6-7 partners; and light salmon, degree 3-4 partners.
JAK2 is associated with neoplastic and immune-related conditions across DO and phenotypic landscapes
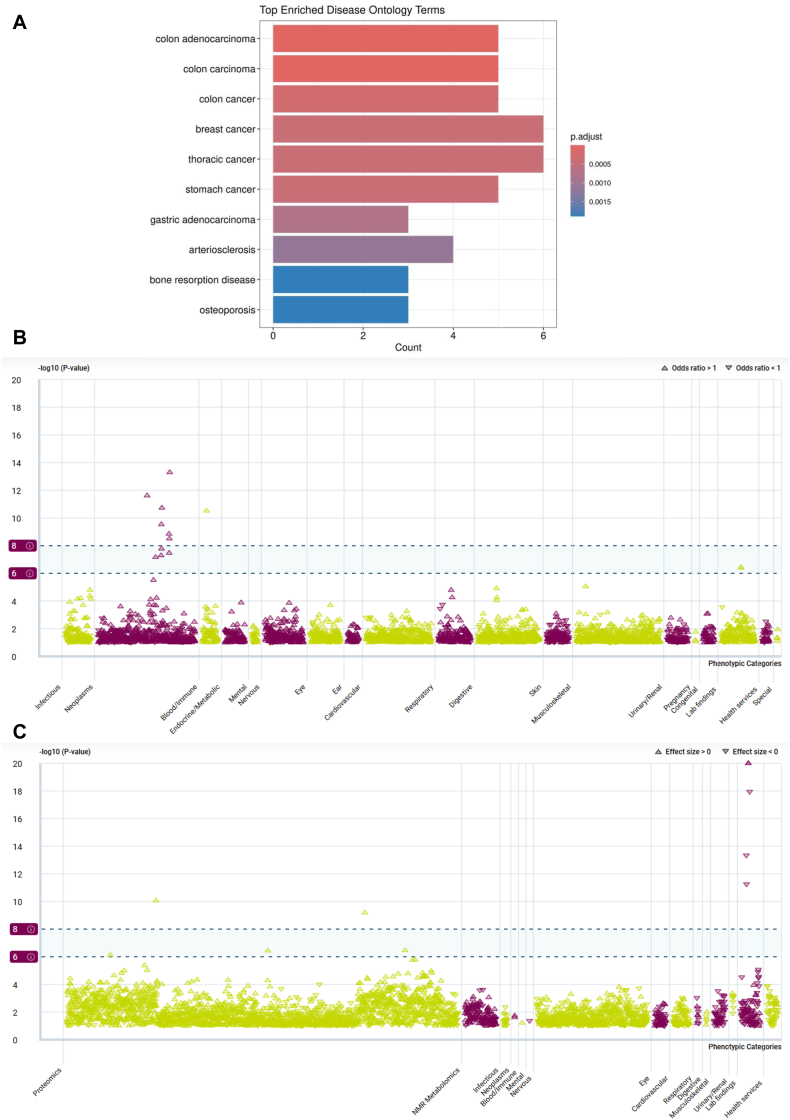
Fig 6, A, shows the top 10 results of DO enrichment analysis. The most significantly enriched terms included colon adenocarcinoma (adjusted P [Padj] = 1.14 × 10−6), colon carcinoma (Padj = 3.39 × 10−6), colon cancer (Padj = 2.71 × 10−4), breast cancer (Padj = 4.15 × 10−4), thoracic cancer (Padj = 4.15 × 10−4), stomach cancer (Padj = 4.15 × 10−4), and gastric adenocarcinoma (Padj = 7.76 × 10−4). Additional enrichment was observed in arteriosclerosis (Padj = 1.14 × 10−3), bone resorption disease (Padj = 1.89 × 10−3), and osteoporosis (Padj = 1.89 × 10−3).
Fig 6.
Pleiotropic disease associations of JAK2 across ontology and phenotypic landscapes. (A) DO enrichment analysis of JAK2 and its first-shell protein interactors, performed by DOSE R package. Top 10 significantly enriched disease terms are shown. Bar length indicates gene count; color intensity, adjusted P values. Neoplastic and immune-related conditions are prominently enriched. (B) PheWAS results for binary traits. Manhattan-style plots display gene-level associations obtained from AZ-PheWAS using collapsing burden models for JAK2. Phenotypes are grouped by clinical category on x-axis; significance (−log10P value) is shown on y-axis. Upward-pointing triangles indicate increased odds (OR > 1); downward-pointing triangles, decreased odds (OR < 1); and dashed lines, significance thresholds: suggestive (−log10P = 6, ∼1 × 10−6) and significant (−log10P = 8, ∼1 × 10−8). Strong signals were observed for hematologic malignancies, especially myeloproliferative neoplasms. (C) PheWAS results for quantitative traits. Similar plotting conventions as in (B) apply. Top associations were detected for eosinophil count, eosinophil percentage, platelet count, and plateletcrit, indicating JAK2’s involvement in hematologic and immune-related quantitative traits.
Fig 6, B, presents PheWAS analysis of JAK2 in binary traits, revealing significant associations with various hematologic neoplasms. All significant associations demonstrated OR > 1, including osteomyelofibrosis (OR = 104.02), polycythemia vera (OR = 99.27), and chronic myeloproliferative diseases (OR = 34.82), indicating a strong phenotypic enrichment pattern in myeloid malignancy domains (see Table E9 in the Online Repository at www.jaci-global.org). Fig 6, C, displays associations with continuous traits, where JAK2 was significantly associated with blood cell parameters. Eosinophil count (β = −0.16) and eosinophil percentage (β = −0.15) showed negative associations, while platelet count (β = 0.27) and plateletcrit (β = 0.25) showed positive associations. Additionally, increased levels of inflammation-related proteins were also associated. These findings suggest a broad immunohematologic association profile for JAK2 across both neoplastic and immune-related traits (see Table E10 in the Online Repository).
Prioritization of therapeutic drug candidates for AA
Screening and selection of drugs associated with AA
After removing duplicates, 102 unique compounds were identified in the DGIdb and CTD. To refine the list of drugs relevant to AA, disease association data from CTD were used, reducing the number of candidates to 22.
Computational prioritization and top-ranked drug evaluation
The remaining 22 drugs were evaluated according to pharmacokinetic properties, bioavailability, and drug likeness, incorporating parameters such as water solubility, logP, polar surface area, and compliance with the Lipinski rule of 5. After removing outliers, 18 drugs were retained for subsequent calculations (see Table E11 in the Online Repository at www.jaci-global.org). Table II presents the normalized results.
Table II.
List of drug candidates after data normalization
| MeSH ID | Drug | Regulatory approval | No. PubMed IDs | Water solubility (mg/mL) | logP | Polar surface area (Å2) | Bioavailability | Rule of 5 | Weighted composite score |
|---|---|---|---|---|---|---|---|---|---|
| D006918 | Hydroxyurea | 1 | 0.31 | 1.00 | 0.59 | 1.00 | 1 | 1 | 0.85 |
| D014635 | Valproic acid | 1 | 0.82 | 0.01 | 1.00 | 0.86 | 1 | 1 | 0.68 |
| D000068877 | Imatinib | 1 | 0.49 | 0 | 0.93 | 1.00 | 1 | 1 | 0.64 |
| D000077547 | Crizotinib | 1 | 0.43 | 0 | 0.88 | 1.00 | 1 | 1 | 0.62 |
| C479163 | Tofacitinib | 1 | 0.18 | 0 | 1.00 | 1.00 | 1 | 1 | 0.60 |
| D000069347 | Erlotinib | 1 | 0.18 | 0 | 0.98 | 1.00 | 1 | 1 | 0.59 |
| D015760 | Alfentanil hydrochloride | 1 | 0.04 | 0 | 1.00 | 1.00 | 1 | 1 | 0.57 |
| C000596027 | Baricitinib | 1 | 0.03 | 0 | 1.00 | 1.00 | 1 | 1 | 0.57 |
| D014223 | Triamterene | 1 | 0.01 | 0 | 1.00 | 1.00 | 1 | 1 | 0.57 |
| C531550 | Olaparib | 1 | 0 | 0 | 1.00 | 1.00 | 1 | 1 | 0.57 |
| D000077209 | Decitabine | 1 | 0.16 | 0.02 | 0.56 | 1.00 | 1 | 1 | 0.55 |
| C561234 | Pacritinib | 1 | 0 | 0 | 0.74 | 1.00 | 1 | 1 | 0.54 |
| D017239 | Paclitaxel | 1 | 1.00 | 0 | 0.97 | 0.63 | 0 | 0 | 0.46 |
| C532162 | Pictilisib | 0 | 0.08 | 0 | 1.00 | 1.00 | 1 | 0 | 0.35 |
| D020123 | Sirolimus | 1 | 0.35 | 0 | 0.73 | 0.66 | 0 | 0 | 0.33 |
| C001277 | Geldanamycin | 0 | 0 | 0 | 1.00 | 0.86 | 1 | 0 | 0.32 |
| C531198 | Dactolisib | 0 | 0 | 0 | 0.76 | 1.00 | 1 | 0 | 0.31 |
| C112765 | Tanespimycin | 0 | 0 | 0 | 1.00 | 0.81 | 0 | 0 | 0.20 |
To ensure an unbiased ranking framework, weights were assigned to each criterion on the basis of its variability among candidate drugs. Water solubility had the highest weight of 0.28, followed by the number of PubMed-indexed studies at 0.16 and regulatory approval status at 0.12. Other parameters, including logP at 0.11, polar surface area at 0.11, bioavailability at 0.11, and compliance with the Lipinski rule of 5 at 0.11, contributed more evenly to the final composite score. Hydroxyurea emerged as the highest-ranked candidate, with a composite score of 0.85. Although primarily approved for hematologic conditions, hydroxyurea also shows therapeutic associations with respiratory and immune-related diseases, such as bronchogenic carcinoma and pulmonary hypertension, according to the CTD. These associations are presented in Table E12 in the Online Repository at www.jaci-global.org and provide a supportive context for its computational prioritization.
Discussion
This study, structured across 3 sequential analytical phases, provides the first genetic evidence supporting a bidirectional causal relationship between AD and AA, prioritizes JAK2 as a potential causal gene through integrated MR and colocalization analyses, and validates its mechanistic relevance through expression profiling and enrichment analyses, which reveal its central positioning in immune and hormone signaling networks. Finally, hydroxyurea is computationally nominated as a top-ranked therapeutic candidate using a pharmacologically informed prioritization framework. AD significantly increases the risk of AA, while AA also exerts a weaker but significant causal effect on AD. MVMR confirms that the association from AD to AA remained independent of immune and metabolic confounders. Integrating eQTL-MR and Bayesian colocalization prioritized JAK2 as a candidate gene linking AD to AA, with additional functional analyses revealing its central role in cytokine-mediated signaling, particularly the JAK-STAT pathway. Our positive control results demonstrate that our MR approach successfully recapitulates known causal relationships and reinforces the credibility of our findings. Among the 50 MR-prioritized genes, the colocalization result (PP.H4 = 0.95) for JAK2 strengthens its causal relevance, although the remaining genes warrant further multiomics validation.
To validate the mechanistic relevance of JAK2 in AA pathophysiology, we conducted a series of post-MR functional characterizations. Gene expression profiling revealed that JAK2 is highly expressed in immune-relevant tissues. Functional enrichment analysis of JAK2 and its first-shell interacting proteins identified significant overrepresentation in cytokine receptor binding, JAK-STAT signaling, and interleukin-mediated pathways, reinforcing its central role in immune signaling cascades. PPI network analysis highlighted JAK2 as a central hub node within this signaling architecture, linking key transcription factors and cytokine receptors implicated in asthma-related immune dysregulation. Further, DO enrichment confirmed that this JAK2-centered network is significantly enriched for neoplastic and immune-associated disorders, such as colon adenocarcinoma, breast cancer, and arteriosclerosis. Complementary PheWAS analysis at the gene level for JAK2 revealed strong phenotypic associations with hematologic traits and immune-mediated diseases, such as eosinophil count and myeloproliferative neoplasms. Together, these multidimensional validations support the pleiotropic and immunopathologic relevance of JAK2, underscoring its functional plausibility as a shared causal factor linking AD and AA. When we applied a pharmacologically informed prioritization framework, hydroxyurea emerged as the top-ranked candidate compound for AA. It is important to note that this ranking reflects a composite score integrating pharmacokinetics, regulatory approval, and literature-based evidence and is not a prediction of therapeutic efficacy. Although hydroxyurea has therapeutic associations with other immune and respiratory conditions as per CTD, its efficacy in AA has not been validated.
Our findings align with previous studies that have identified associations between AD and various comorbidities, including asthma. A systematic review of MR studies highlighted genetic associations between AD and these comorbidities, suggesting a shared genetic basis and the presence of potential causal links between these conditions.15 The identification of JAK2 as a key gene linking the AD-AA relationship is consistent with existing literature emphasizing the role of the JAK-STAT pathway in the pathogenesis of AD. This pathway is known to contribute to immune dysregulation and inflammatory responses characteristic of AD.47 In the mechanism validation phase, we further investigated JAK2’s role as a candidate therapeutic target for AA. Expression analysis revealed that JAK2 is broadly expressed, with notable enrichment in immune-relevant tissues, consistent with its established role in immune regulation and inflammation.8 Previous studies have highlighted the role of JAK2 in TH2-driven inflammatory responses, particularly in allergic diseases like asthma and AD.48 Our findings align with these studies by demonstrating JAK2’s involvement in cytokine signaling pathways, including the JAK-STAT cascade and cytokine receptor interactions. These pathways are central to the shared immune dysregulation underlying AD and AA, supporting the biological rationale for targeting JAK2 in this comorbid framework.47 Mechanistically, IL-6 activates the JAK2/STAT3 pathway to drive airway inflammation, particularly in non-TH2 asthma phenotypes.48 IL-10 signals through the same axis to promote macrophage polarization and suppress excessive inflammation.49 In AD, acute lesions are dominated by TH2 cytokines such as IL-4 and IL-13, while chronic stages involve JAK2-mediated IL-6 and IFN-γ signaling, contributing to a TH1/TH17 shift.11 Although anti-inflammatory cytokines like IL-10 and TGF-β are present, they often fail to fully counteract chronic inflammation, especially in lichenified lesions.47 In AA, the classical eosinophilic response is driven by IL-4, IL-5, and IL-13, whereas JAK2-dependent IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) pathways are linked to steroid-resistant, neutrophilic subtypes.48 Similar to AD, IL-10–mediated regulation may be insufficient in certain AA endotypes, particularly in TH2-low or persistent inflammation contexts.8 Unlike prior studies that primarily focused on JAK2’s role in individual diseases, such as myeloproliferative disorders,50 our work integrates DO and PheWAS analyses to provide a more comprehensive assessment of JAK2’s therapeutic potential and associated risks. DO enrichment linked JAK2-associated networks to immune-related conditions and neoplastic diseases.51 PheWAS analysis further identified significant gene-level associations at the JAK2 locus with hematologic malignancies, as well as immune-related traits like eosinophil count and platelet crit. These findings underscore the therapeutic potential of JAK2 but also highlight its broad phenotypic effects, including risks of hematologic complications.52 Furthermore, the efficacy of JAK inhibitors in treating AD and their potential application in asthma management underscores the relevance of JAK2 in these diseases.48 Our computational prioritization framework screened drug candidates that also identified tofacitinib, imatinib, and sirolimus/rapamycin as in previous studies,53 although they were not ranked first. Hydroxyurea emerged as the top candidate in our multicriteria prioritization approach, which integrated drug-likeness, regulatory status, and literature-based support. Although not traditionally used in asthma, hydroxyurea has known associations with pulmonary and immune-related conditions such as bronchogenic carcinoma and pulmonary, as reported in CTD (Table E12). While hydroxyurea is known to modulate immune responses and has been linked to JAK2 signaling in hematologic settings,54,55 its potential impact on inflammatory mediators such as IL-6 and GM-CSF in allergic conditions is not yet fully understood. However, its direct effects on IL-6 and GM-CSF expression in allergic contexts remain unclear and require further mechanistic investigation. Given that these cytokines are implicated in steroid-resistant and TH2-low asthma subtypes,8 hydroxyurea may exert context-specific anti-inflammatory effects relevant to these endotypes. While earlier studies have suggested a progression from AD to other atopic disorders—that is, the atopic march56—our analysis provides robust genetic evidence supporting this bidirectional causality.
While recent systematic reviews have summarized MR studies that explore potential causal links between AD and its comorbidities, including asthma, most remain unidirectional and do not incorporate post-MR functional validation or colocalization-based gene prioritization.15 Our study builds on this foundation by using a bidirectional MR framework to assess reciprocal causality between AD and AA while adjusting for immune and metabolic confounders through MVMR. Furthermore, we extended beyond causal inference by integrating eQTL-based colocalization to prioritize JAK2 as a potential drug target for AA. Unlike earlier studies that focused on JAK2 in hematologic malignancies and myeloproliferative neoplasms,57, 58, 59 we applied an integrative analytic pipeline that combines bidirectional MR with eQTL colocalization to identify JAK2 as a gene whose genetically regulated expression causally links AD and AA. To our knowledge, this is the first study to implicate JAK2 in allergic comorbidity through genetic expression regulation, extending prior work focused on its downstream inflammatory roles or therapeutic inhibition. Finally, we applied a pharmacologically informed scoring framework to identify hydroxyurea as a top candidate for asthma treatment. This represents a novel therapeutic hypothesis not reported in previous allergy-related MR studies. The combined use of causal inference, gene prioritization, immunologic pathway analysis, and drug prioritization that is based on mechanistic relevance constitutes a comprehensive methodologic and translational advance beyond prior work in the field.
Despite its strengths, this study has several limitations that should be acknowledged. First, the GWAS datasets that we used only comprise European populations, so it remains uncertain whether our findings apply to other populations. Second, the reliance on publicly available GWAS summary statistics introduces potential variability, as differences in cohort characteristics, genotyping platforms, and phenotype definitions may influence the findings. Building on our integrative analyses, JAK2 was identified as a potential target gene. However, it is not yet clear whether the dysregulation involves gain or loss of function. This distinction is crucial for determining whether JAK2 should be inhibited or activated in future therapies, which will require functional studies in allergic models. Additionally, although our computational drug prioritization approach integrates multiple parameters, it is inherently limited by the availability and accuracy of pharmacokinetic and pharmacodynamic data. These constraints may prevent a full assessment of a compound’s therapeutic potential or adverse effects in the context of AA. Notably, hydroxyurea’s clinical role in AA has not been previously reported. Thus, we classify it as a hypothesis-generating candidate rather than a confirmed therapeutic. Its prioritization warrants further experimental validation in disease-relevant models before consideration for clinical translation.
Future research should focus on addressing the limitations of this study while building on its findings. Given the population specificity of the genetic data used, replication in cohorts of diverse ancestries is essential to ensure the generalizability of the observed associations. Functional studies are warranted to elucidate the biological mechanisms linking AD and AA, with particular attention to the role of JAK2 in disease pathogenesis. Experimental validation of JAK-STAT pathway dysregulation using relevant cellular and animal models could further clarify its therapeutic potential. Although hydroxyurea emerged as the top candidate in our computational prioritization, its pharmacologic effects in the context of AA remain untested. Rigorous preclinical and clinical studies are needed to evaluate its efficacy and safety in allergic inflammation. Moreover, integrating multiomics datasets, such as transcriptomic and proteomic profiles, may uncover additional mechanistic insights and facilitate the discovery of novel therapeutic targets. Finally, future MR studies incorporating longitudinal data and polygenic risk scores could enhance causal inference by accounting for disease heterogeneity and temporal dynamics. These efforts will be essential for translating genetic discoveries into effective, personalized therapeutic strategies.
In conclusion, this study provides new insights into the genetic and molecular mechanisms linking AD and AA, establishing a bidirectional causal relationship between the two diseases. The identification of JAK2 as a central regulator of AD-AA comorbidity emphasizes the therapeutic potential of targeting the JAK-STAT pathway in treating TH2-mediated allergic inflammation. Furthermore, our computational drug prioritization identifies hydroxyurea as a candidate for further investigation. These findings contribute to a deeper understanding of disease pathogenesis and offer new directions for targeted therapeutic strategies. Future research should build on these results to refine mechanistic insights and assess the translational potential of identified targets in clinical settings.
Key messages.
-
•
Integrative MR and colocalization analyses establish a bidirectional causal relationship between AD and AA.
-
•
Drug-target MR analysis identifies JAK2 as a key causal gene linking AD and AA through shared genetic variants, implicating dysregulation of the JAK-STAT signaling pathway in TH2-driven allergic inflammation.
-
•
Hydroxyurea is computationally prioritized as a candidate for further investigation in AA, with its therapeutic relevance remaining to be experimentally validated.
Disclosure statement
Supported by the City University of Hong Kong Internal Funds for External Grant Schemes (grant 9678233).
Declaration of generative AI and AI-assisted technologies in the writing process: The authors acknowledge the use of OpenAI’s GPT-4 for grammar correction and improving the report’s clarity. All the authors carefully reviewed and validated all content generated by the AI to ensure accuracy and originality.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Acknowledgments
We thank HE. Qian for valuable suggestions and insightful feedback during the revision of this report. We also thank LEUNG Ka Yu, CAI Shuyi, and ZHU Han for their support during the early stages of this project.
Supplementary data
References
- 1.Paller A.S., Spergel J.M., Mina-Osorio P., Irvine A.D. The atopic march and atopic multimorbidity: many trajectories, many pathways. J Allergy Clin Immunol. 2019;143:46–55. doi: 10.1016/j.jaci.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Yang C., Fagan A.M., Perrin R.J., Rhinn H., Harari O., Cruchaga C. Mendelian randomization and genetic colocalization infer the effects of the multi-tissue proteome on 211 complex disease-related phenotypes. Genome Med. 2022;14:140. doi: 10.1186/s13073-022-01140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouyang Y., Chen Y., Chen K., Tang Z., Shi G., Qu C., et al. Mendelian randomization and colocalization analysis reveal novel drug targets for myasthenia gravis. Hum Genomics. 2024;18:43. doi: 10.1186/s40246-024-00607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z., Li S., Cai G., Gao Y., Yang H., Li Y., et al. Mendelian randomization analysis identifies druggable genes and drugs repurposing for chronic obstructive pulmonary disease. Front Cell Infect Microbiol. 2024;14:1386506. doi: 10.3389/fcimb.2024.1386506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Hu J., Qin D., Han D., Hu J. A multi-omics Mendelian randomization identifies putatively causal genes and DNA methylation sites for asthma. World Allergy Organ J. 2024;17 doi: 10.1016/j.waojou.2024.101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z., Li Q., Li Y., Wu J. Identification of plasma protein markers of allergic disease risk: a Mendelian randomization approach to proteomic analysis. BMC Genomics. 2024;25:503. doi: 10.1186/s12864-024-10412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spergel J.M., Paller A.S. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht B.N., Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076–1083. doi: 10.1038/ni.3829. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z., Zhu X., Liu C.L., Shi H., Shen S., Yang Y., et al. Shared genetics of asthma and mental health disorders: a large-scale genome-wide cross-trait analysis. Eur Respir J. 2019;54 doi: 10.1183/13993003.01507-2019. [DOI] [PubMed] [Google Scholar]
- 11.Yaneva M., Darlenski R. The link between atopic dermatitis and asthma-immunological imbalance and beyond. Asthma Res Pract. 2021;7:16. doi: 10.1186/s40733-021-00082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey Smith G., Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 13.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawlor D.A., Harbord R.M., Sterne J.A.C., Timpson N., Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 15.Elhage K.G., Kranyak A., Jin J.Q., Haran K., Spencer R.K., Smith P.L., et al. Mendelian randomization studies in atopic dermatitis: a systematic review. J Invest Dermatol. 2024;144:1022–1037. doi: 10.1016/j.jid.2023.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira M.A., Vonk J.M., Baurecht H., Marenholz I., Tian C., Hoffman J.D., et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49:1752–1757. doi: 10.1038/ng.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demenais F., Margaritte-Jeannin P., Barnes K.C., Cookson W.O.C., Altmüller J., Ang W., et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50:42–53. doi: 10.1038/s41588-017-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skrivankova V.W., Richmond R.C., Woolf B.A.R., Yarmolinsky J., Davies N.M., Swanson S.A., et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326:1614–1621. doi: 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 19.Cerezo M., Sollis E., Ji Y., Lewis E., Abid A., Bircan K.O., et al. The NHGRI-EBI GWAS Catalog: standards for reusability, sustainability and diversity. Nucleic Acids Res. 2025;53(D1):D998–D1005. doi: 10.1093/nar/gkae1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budu-Aggrey A., Kilanowski A., Sobczyk M.K., Shringarpure S.S., Mitchell R., Reis K., et al. European and multi-ancestry genome-wide association meta-analysis of atopic dermatitis highlights importance of systemic immune regulation. Nat Commun. 2023;14:6172. doi: 10.1038/s41467-023-41180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurki M.I., Karjalainen J., Palta P., Sipilä T.P., Kristiansson K., Donner K.M., et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–518. doi: 10.1038/s41586-022-05473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyon M.S., Andrews S.J., Elsworth B., Gaunt T.R., Hemani G., Marcora E. The variant call format provides efficient and robust storage of GWAS summary statistics. Genome Biol. 2021;22:32. doi: 10.1186/s13059-020-02248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Võsa U., Claringbould A., Westra H.J., Bonder M.J., Deelen P., Zeng B., et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021;53:1300–1310. doi: 10.1038/s41588-021-00913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grissa D., Junge A., Oprea T.I., Jensen L.J. Diseases 2.0: a weekly updated database of disease–gene associations from text mining and data integration. Database (Oxford) 2022;2022:baac019. doi: 10.1093/database/baac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannon M., Stevenson J., Stahl K., Basu R., Coffman A., Kiwala S., et al. DGIdb 5.0: rebuilding the drug–gene interaction database for precision medicine and drug discovery platforms. Nucleic Acids Res. 2023;52(D1):D1227–D1235. doi: 10.1093/nar/gkad1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis A.P., Wiegers T.C., Sciaky D., Barkalow F., Strong M., Wyatt B., et al. Comparative Toxicogenomics Database’s 20th anniversary: update 2025. Nucleic Acids Res. 2024;53(D1):D1328–D1334. doi: 10.1093/nar/gkae883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knox C., Wilson M., Klinger C.M., Franklin M., Oler E., Wilson A., et al. DrugBank 6.0: the DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024;52(D1):D1265–D1275. doi: 10.1093/nar/gkad976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden J., Spiller W., Del Greco M.F., Sheehan N., Thompson J., Minelli C., et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47:1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson E., Davey Smith G., Windmeijer F., Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48:713–727. doi: 10.1093/ije/dyy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y., Li Y. Association between lipid-lowering drugs and allergic diseases: a Mendelian randomization study. World Allergy Organ J. 2024;17 doi: 10.1016/j.waojou.2024.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seong M.K., Shin M. Low-density lipoprotein cholesterol is associated with atopic dermatitis in Korean adolescents. Int Arch Allergy Immunol. 2023;184:1230–1236. doi: 10.1159/000533401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trakaki A., Marsche G. High-density lipoprotein (HDL) in allergy and skin diseases: focus on immunomodulating functions. Biomedicines. 2020;8:558. doi: 10.3390/biomedicines8120558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaway Z., Kim C.K. Eosinophil-derived neurotoxin levels can predict allergic disease development and atopic march in children. Clin Exp Pediatr. 2025;68:398–405. doi: 10.3345/cep.2024.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipińska-Opałka A., Leszczyńska-Pilich M., Będzichowska A., Tomaszewska A., Rustecka A., Kalicki B. The role of regulatory B lymphocytes in allergic diseases. Biomedicines. 2024;12:2721. doi: 10.3390/biomedicines12122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obata K., Mukai K., Tsujimura Y., Ishiwata K., Kawano Y., Minegishi Y., et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 38.Valette K., Li Z., Bon-Baret V., Chignon A., Bérubé J.C., Eslami A., et al. Prioritization of candidate causal genes for asthma in susceptibility loci derived from UK Biobank. Commun Biol. 2021;4:700. doi: 10.1038/s42003-021-02227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinke J.W., Borish L. Th2 cytokines and asthma—interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2:66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giambartolomei C., Vukcevic D., Schadt E.E., Franke L., Hingorani A.D., Wallace C., et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., et al. Tissue-based map of the human proteome. Science. 2015;347(6220) doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 42.Szklarczyk D., Kirsch R., Koutrouli M., Nastou K., Mehryary F., Hachilif R., et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2022;51(D1):D638–D646. doi: 10.1093/nar/gkac1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu G., Wang L.G., Yan G.R., He Q.Y. DOSE: an R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics. 2015;31:608–609. doi: 10.1093/bioinformatics/btu684. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q., Dhindsa R.S., Carss K., Harper A.R., Nag A., Tachmazidou I., et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature. 2021;597(7877):527–532. doi: 10.1038/s41586-021-03855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guiaşu S. Weighted entropy. Rep Math Phys. 1971;2:165–179. [Google Scholar]
- 46.Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao L., Zhang H., Chan L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2 doi: 10.4161/jkst.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georas S.N., Donohue P., Connolly M., Wechsler M.E. JAK inhibitors for asthma. J Allergy Clin Immunol. 2021;148:953–963. doi: 10.1016/j.jaci.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan F., Fu X., Shi H., Chen G., Dong P., Zhang W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bader M.S., Meyer S.C. JAK2 in myeloproliferative neoplasms: still a protagonist. Pharmaceuticals. 2022;15:160. doi: 10.3390/ph15020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen L.T., Attfield K.E., Feldmann M., Fugger L. Allosteric TYK2 inhibition: redefining autoimmune disease therapy beyond JAK1-3 inhibitors. eBioMedicine. 2023;97 doi: 10.1016/j.ebiom.2023.104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bose P., Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood. 2017;130:115–125. doi: 10.1182/blood-2017-04-742288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kruse R.L., Vanijcharoenkarn K. Drug repurposing to treat asthma and allergic disorders: progress and prospects. Allergy. 2018;73:313–322. doi: 10.1111/all.13305. [DOI] [PubMed] [Google Scholar]
- 54.Tefferi A., Hutchens C., Saliba A.N., Loscocco G.G., Begna K., Hogan W.J., et al. Molecular and cytokine correlates of anemia response to treatment with DISC-0974 (an anti-hemojuvelin antibody) in non-transfusion–dependent patients with myelofibrosis. Blood. 2024;144(suppl 1):6678. [Google Scholar]
- 55.Rinaldi C.R., Simmonds M., Walker C., Amirian N., Musleh M., Squires A.M. Therapeutically targeting the CALR/CD47 pathway in MPN to reactivate natural immunosurveillance mechanisms within the body to enhance treatment outcomes. J Clin Oncol. 2024;42(23 suppl):170. [Google Scholar]
- 56.Bantz S.K., Zhu Z., Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202. doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison C.N., Barbui T., Bose P., Kiladjian J.J., Mascarenhas J., McMullin M.F., et al. Polycythaemia vera. Nat Rev Dis Primer. 2025;11:26. doi: 10.1038/s41572-025-00608-3. [DOI] [PubMed] [Google Scholar]
- 58.Abu-Zeinah G., Erdos K., Lee N., Lebbe A., Bouhali I., Khalid M., et al. Are thrombosis, progression, and survival in ET predictable? Blood Cancer J. 2024;14:103. doi: 10.1038/s41408-024-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y.H., Wei C.H., Lin C.C., Gurnari C., Awada H., Benajiba L., et al. Synergistic effect of concurrent high molecular risk mutations and lower JAK2 mutant variant allele frequencies on prognosis in patients with myelofibrosis—insights from a multicenter study. Leukemia. 2025;39:144–154. doi: 10.1038/s41375-024-02422-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.