Abstract
Two feline coronaviruses were characterized to determine their biological properties in vitro and their antigenic relatedness to a previously recognized feline infectious peritonitis virus and canine coronavirus. The viruses, designated WSU 79-1146 and WSU 79-1683, were shown to have comparable growth curves with the prototype feline infectious peritonitis virus. Treatment of the feline infectious peritonitis virus strains with 0.25% trypsin indicated that they were relatively resistant to proteolytic inactivation when compared with the feline enteric coronavirus strain. This observation may serve as a useful in vitro marker to distinguish closely related members of the feline coronavirus group. Plaque assay results indicated that the feline infectious peritonitis virus strains produced large homogeneous plaques in comparison to the feline enteric coronavirus strain and canine coronavirus, which showed a heterogenous plaque size distribution. No naturally temperature sensitive mutants were detected in either of the feline coronavirus populations. Both of the viruses were antigenically related to feline infectious peritonitis virus and to a lesser extent to canine coronavirus by virus neutralization.
Full text
PDF

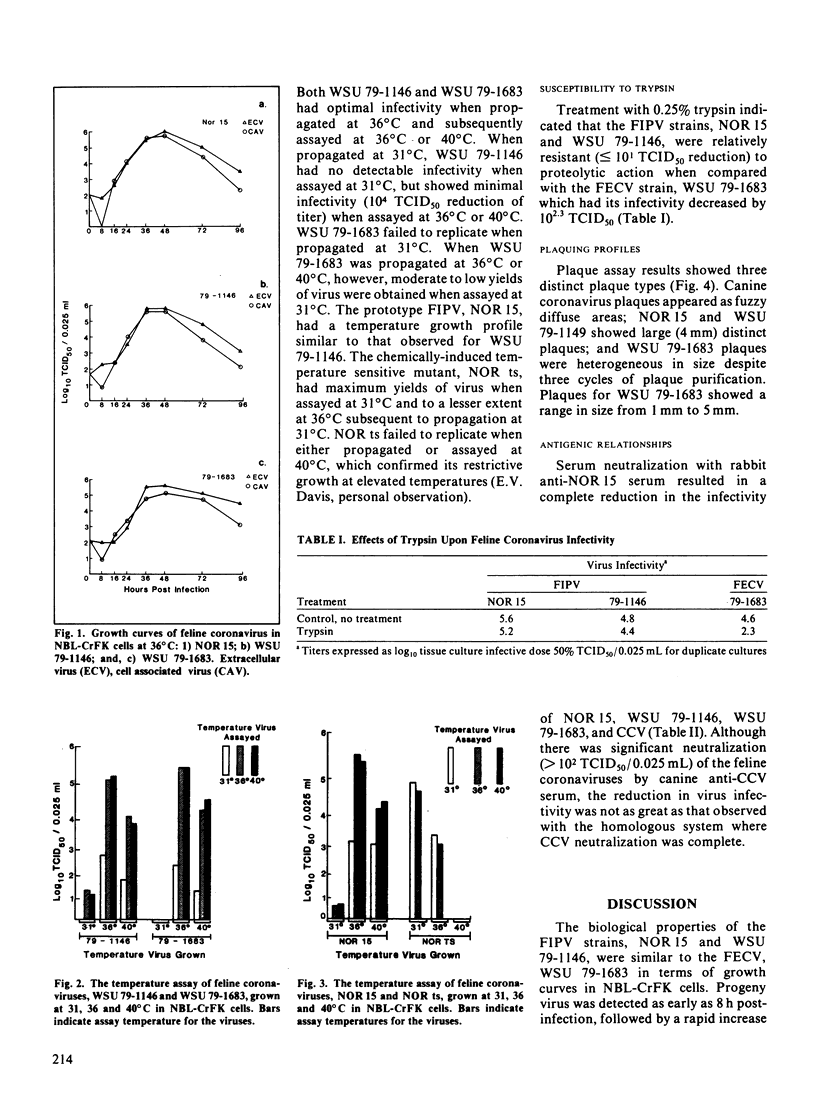
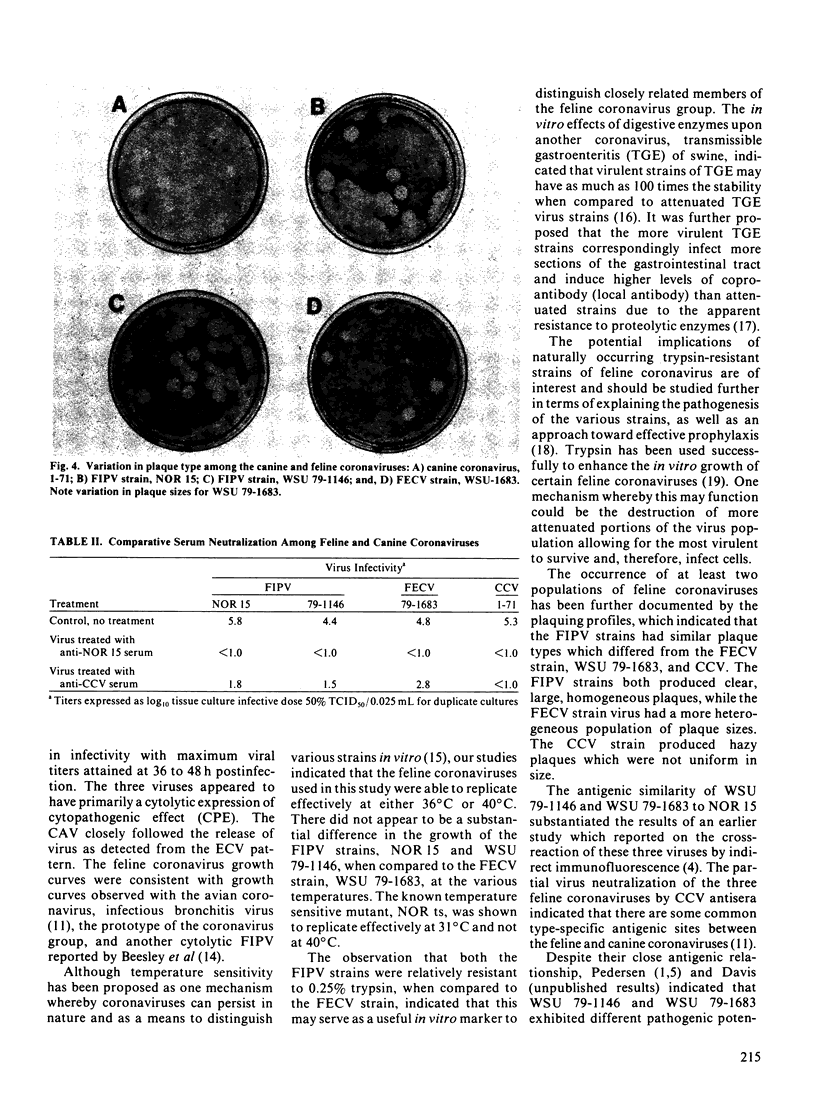

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. R. Feline infectious peritonitis. An immune-mediated coronaviral vasculitis. Vet Clin North Am Small Anim Pract. 1984 Sep;14(5):971–984. doi: 10.1016/S0195-5616(84)50102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aynaud J. M., Nguyen T. D., Bottreau E., Brun A., Vannier P. Transmissible gastroenteritis (TGE) of swine: survivor selection of TGE virus mutants in stomach juice of adult pigs. J Gen Virol. 1985 Sep;66(Pt 9):1911–1917. doi: 10.1099/0022-1317-66-9-1911. [DOI] [PubMed] [Google Scholar]
- Beesley J. E., Hitchcock L. M. The ultrastructure of feline infectious peritonitis virus in feline embryonic lung cells. J Gen Virol. 1982 Mar;59(Pt 1):23–28. doi: 10.1099/0022-1317-59-1-23. [DOI] [PubMed] [Google Scholar]
- Chen K. S. Enzymatic and acidic sensitivity profiles of selected virulent and attenuated transmissible gastroenteritis viruses of swine. Am J Vet Res. 1985 Mar;46(3):632–636. [PubMed] [Google Scholar]
- Chen K. S., Kahn D. E. A double-protease-resistant variant of transmissible gastroenteritis virus and its ability to induce lactogenic immunity. Am J Vet Res. 1985 Aug;46(8):1632–1636. [PubMed] [Google Scholar]
- Evermann J. F., Baumgartener L., Ott R. L., Davis E. V., McKeirnan A. J. Characterization of a feline infectious peritonitis virus isolate. Vet Pathol. 1981 Mar;18(2):256–265. doi: 10.1177/030098588101800214. [DOI] [PubMed] [Google Scholar]
- Horzinek M. C., Ederveen J., Egberink H., Jacobse-Geels H. E., Niewold T., Prins J. Virion polypeptide specificity of immune complexes and antibodies in cats inoculated with feline infectious peritonitis virus. Am J Vet Res. 1986 Apr;47(4):754–761. [PubMed] [Google Scholar]
- Horzinek M. C., Lutz H., Pedersen N. C. Antigenic relationships among homologous structural polypeptides of porcine, feline, and canine coronaviruses. Infect Immun. 1982 Sep;37(3):1148–1155. doi: 10.1128/iai.37.3.1148-1155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobse-Geels H. E., Horzinek M. C. Expression of feline infectious peritonitis coronavirus antigens on the surface of feline macrophage-like cells. J Gen Virol. 1983 Sep;64(Pt 9):1859–1866. doi: 10.1099/0022-1317-64-9-1859. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Fleming J. O., Stohlman S. A., Fujiwara K. Genetic heterogeneity of murine coronaviruses. Arch Virol. 1983;78(3-4):167–175. doi: 10.1007/BF01311312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki K., Noro K., Yamamoto H., Tsubokura M. Studies on avian infectious bronchitis virus (IBV). II. Propagation of IBV in several cultured cells. Arch Virol. 1979;60(2):115–122. doi: 10.1007/BF01348027. [DOI] [PubMed] [Google Scholar]
- Otsuki K., Yamamoto H., Tsubokura M. Studies on avian infectious bronchitis virus (IBV). I. Resistance of IBV to chemical and physical treatments. Arch Virol. 1979;60(1):25–32. doi: 10.1007/BF01318094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C., Evermann J. F., McKeirnan A. J., Ott R. L. Pathogenicity studies of feline coronavirus isolates 79-1146 and 79-1683. Am J Vet Res. 1984 Dec;45(12):2580–2585. [PubMed] [Google Scholar]
- Siddell S., Wege H., Ter Meulen V. The biology of coronaviruses. J Gen Virol. 1983 Apr;64(Pt 4):761–776. doi: 10.1099/0022-1317-64-4-761. [DOI] [PubMed] [Google Scholar]
- Talbot P. J., Salmi A. A., Knobler R. L., Buchmeier M. J. Topographical mapping of epitopes on the glycoproteins of murine hepatitis virus-4 (strain JHM): correlation with biological activities. Virology. 1984 Jan 30;132(2):250–260. doi: 10.1016/0042-6822(84)90032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]