Abstract
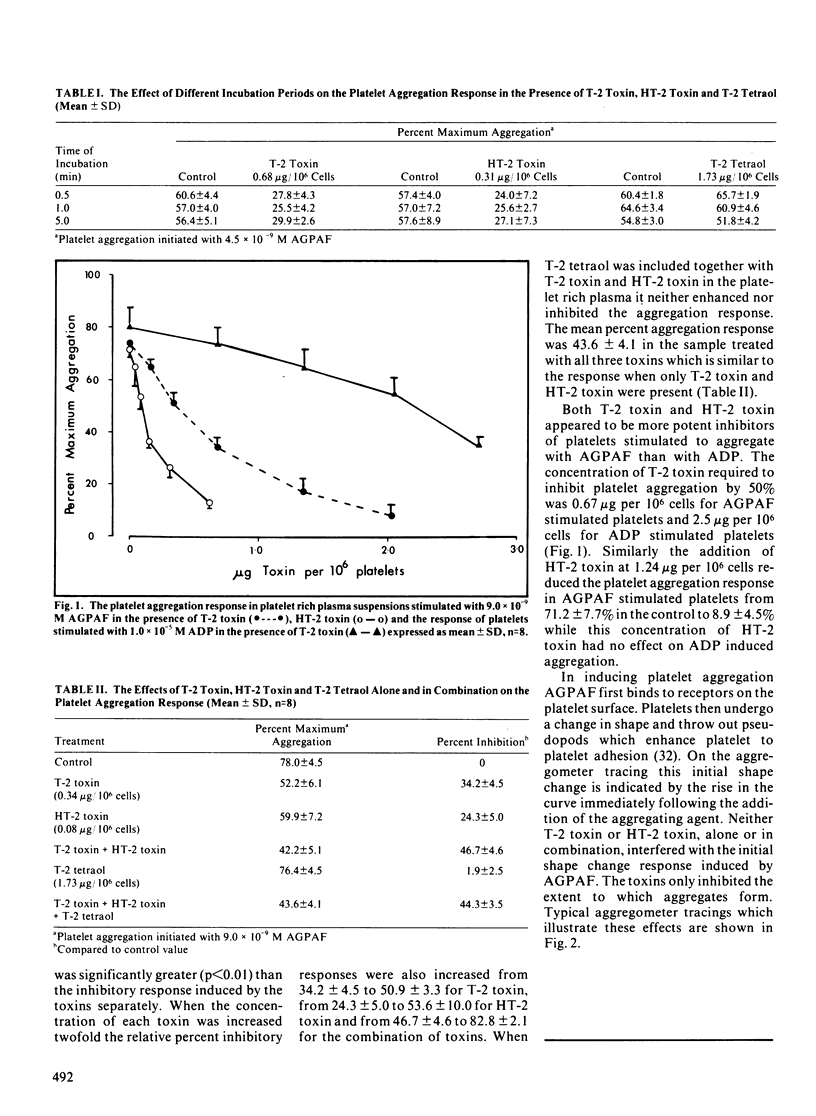
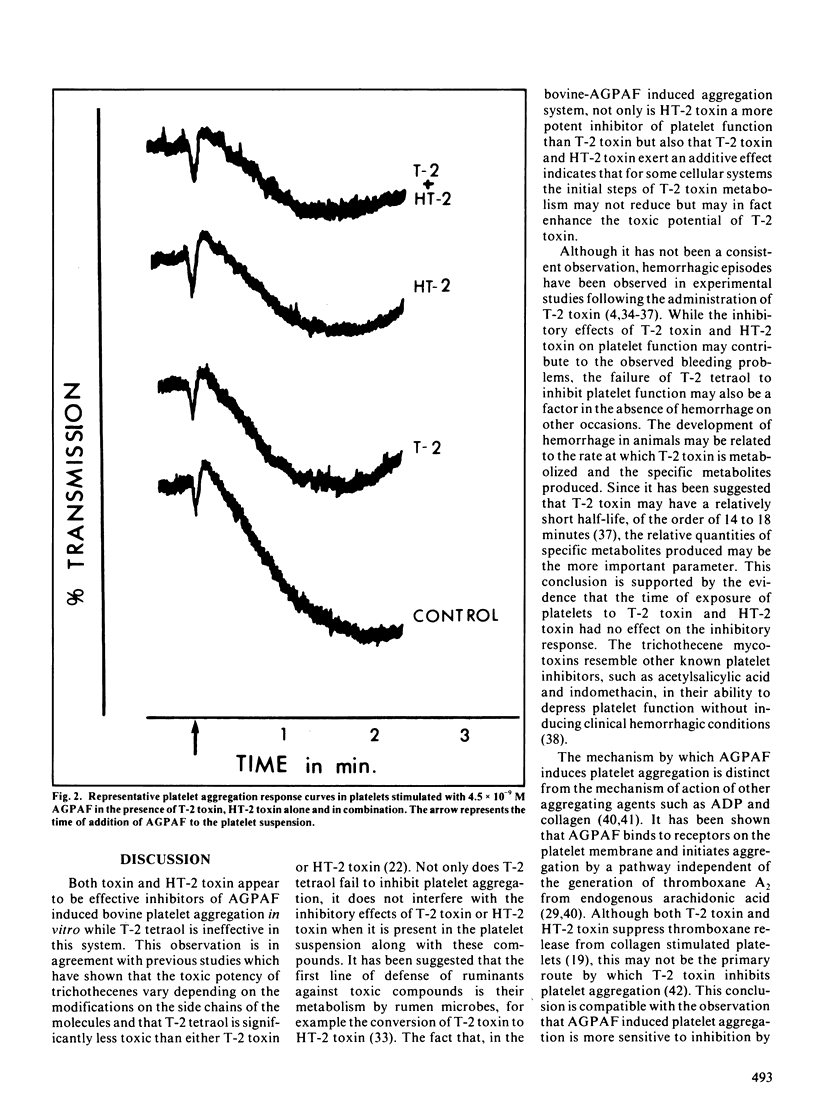
Several species of fungi, which infect cereals and grains, can produce a class of compounds, known as trichothecene mycotoxins, which is characterized by a substituted epoxy-trichothecene ring structure. Cattle are susceptible to intoxication from feeds contaminated with T-2 toxin, one of the more potent trichothecene mycotoxins, while swine refuse to ingest feed contaminated with T-2 toxin. The bovine platelet has been used as a model cell system to evaluate the effects of T-2 toxin and its natural metabolites, HT-2 toxin and T-2 tetraol, on cell function in vitro. Due to the lipophilic nature of these mycotoxins, a biologically active phospholipid was used to stimulate the platelets in the presence and absence of the toxins. The mycotoxin T-2 toxin and its major metabolite HT-2 toxin inhibited platelet activating factor-stimulated bovine platelets, suspended in homologous plasma, in a concentration but not time dependent manner. Significant inhibition of platelet function (p less than 0.01) occurred with 135 ng T-2 toxin per 10(6) platelets and with 77 ng HT-2 toxin per 10(6) platelets. These mycotoxins exerted an additive inhibitory effect on the platelet aggregation response. In contrast, the minor metabolite T-2 tetraol had no inhibitory effect on platelet function and had no influence on the responses of T-2 toxin or HT-2 toxin when the mycotoxins were present together in the platelet suspensions.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley V. R., Swanson S. P., Corley R. A., Buck W. B., Koritz G. D., Burmeister H. R. Pharmacokinetics of the trichothecene mycotoxin, T-2 toxin, in swine and cattle. Toxicon. 1986;24(1):13–23. doi: 10.1016/0041-0101(86)90161-3. [DOI] [PubMed] [Google Scholar]
- Cannon M., Smith K. E., Carter C. J. Prevention, by ribosome-bound nascent polyphenylalanine chains, of the functional interaction of t-2 toxin with its receptor site. Biochem J. 1976 May 15;156(2):289–294. doi: 10.1042/bj1560289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. K., Gentry P. A. Inhibition of bovine platelet function by T-2 toxin, HT-2 toxin, diacetoxyscirpenol and deoxynivalenol. Food Chem Toxicol. 1984 Aug;22(8):643–648. doi: 10.1016/0278-6915(84)90273-4. [DOI] [PubMed] [Google Scholar]
- Cosgriff T. M., Bunner D. L., Wannemacher R. W., Jr, Hodgson L. A., Dinterman R. E. The hemostatic derangement produced by T-2 toxin in cynomolgus monkeys. Toxicol Appl Pharmacol. 1986 Mar 15;82(3):532–539. doi: 10.1016/0041-008x(86)90288-7. [DOI] [PubMed] [Google Scholar]
- Cosgriff T. M., Bunner D. P., Wannemacher R. W., Jr, Hodgson L. A., Dinterman R. E. The hemostatic derangement produced by T-2 toxin in guinea pigs. Toxicol Appl Pharmacol. 1984 Dec;76(3):454–463. doi: 10.1016/0041-008x(84)90349-1. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., Cannon M., Davies J. Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Proc Natl Acad Sci U S A. 1974 Jan;71(1):30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E., Davies J. E. Inhibition of initiation, elongation, and termination of eukaryotic protein synthesis by trichothecene fungal toxins. Antimicrob Agents Chemother. 1977 Mar;11(3):491–499. doi: 10.1128/aac.11.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. C., Hancock D. S., Schiefer H. B., Babiuk L. A. Experimental T-2 toxicosis in sheep. Can J Comp Med. 1983 Jul;47(3):291–297. [PMC free article] [PubMed] [Google Scholar]
- Gareis M., Hashem A., Bauer J., Gedek B. Identification of glucuronide metabolites of T-2 toxin and diacetoxyscirpenol in the bile of isolated perfused rat liver. Toxicol Appl Pharmacol. 1986 Jun 15;84(1):168–172. doi: 10.1016/0041-008x(86)90424-2. [DOI] [PubMed] [Google Scholar]
- Gyongyossy-Issa M. I., Khachatourians G. G. Interaction of T-2 toxin with murine lymphocytes. Biochim Biophys Acta. 1984 Mar 23;803(3):197–202. doi: 10.1016/0167-4889(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Gyongyossy-Issa M. I., Khanna V., Khachatourians G. G. Characterisation of hemolysis induced by T-2 toxin. Biochim Biophys Acta. 1985 Feb 15;838(2):252–256. doi: 10.1016/0304-4165(85)90086-8. [DOI] [PubMed] [Google Scholar]
- Hayes M. A., Bellamy J. E., Schiefer H. B. Subacute toxicity of dietary T-2 toxin in mice: morphological and hematological effects. Can J Comp Med. 1980 Apr;44(2):203–218. [PMC free article] [PubMed] [Google Scholar]
- Kiessling K. H., Pettersson H., Sandholm K., Olsen M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl Environ Microbiol. 1984 May;47(5):1070–1073. doi: 10.1128/aem.47.5.1070-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuri N. R., Smalley E. B., Nichols R. E. Toxicologic studies of Fusarium tricinctum (Corda) Snyder et Hansen from moldy corn. Am J Vet Res. 1971 Nov;32(11):1843–1850. [PubMed] [Google Scholar]
- Küster L. J., Filep J., Frölich J. C. Mechanism of PAF-induced platelet aggregation in man. Thromb Res. 1986 Aug 15;43(4):425–433. doi: 10.1016/0049-3848(86)90087-3. [DOI] [PubMed] [Google Scholar]
- Liggitt H. D., Leid R. W., Huston L. Aggregation of bovine platelets by acetyl glyceryl ether phosphorylcholine (platelet activating factor). Vet Immunol Immunopathol. 1984 Aug;7(1):81–87. doi: 10.1016/0165-2427(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Lorenzana R. M., Beasley V. R., Buck W. B., Ghent A. W., Lundeen G. R., Poppenga R. H. Experimental T-2 toxicosis in swine. I. Changes in cardiac output, aortic mean pressure, catecholamines, 6-keto-PGF1 alpha, thromboxane B2, and acid-base parameters. Fundam Appl Toxicol. 1985 Oct;5(5):879–892. doi: 10.1016/0272-0590(85)90170-8. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Doebler J. A., Anthony A. Scanning cytophotometric analysis of brain neuronal nuclear chromatin changes in acute T-2 toxin-treated rats. Toxicol Appl Pharmacol. 1986 Sep 15;85(2):207–214. doi: 10.1016/0041-008x(86)90114-6. [DOI] [PubMed] [Google Scholar]
- Oldham J. W., Allred L. E., Milo G. E., Kindig O., Capen C. C. The toxicological evaluation of the mycotoxins T-2 and T-2 tetraol using normal human fibroblasts in vitro. Toxicol Appl Pharmacol. 1980 Jan;52(1):159–168. doi: 10.1016/0041-008x(80)90255-0. [DOI] [PubMed] [Google Scholar]
- Patterson D. S., Matthews J. G., Shreeve B. J., Roberts B. A., McDonald S. M., Hayes A. W. The failure of trichothecene mycotoxins and whole cultures of Fusarium tricinctum to cause experimental haemorrhagic syndromes in calves and pigs. Vet Rec. 1979 Sep 15;105(11):252–255. doi: 10.1136/vr.105.11.252. [DOI] [PubMed] [Google Scholar]
- Petrie L., Robb J., Stewart A. F. The identification of T-2 toxin and its association with a haemorrhagic syndrome in cattle. Vet Rec. 1977 Oct 15;101(16):326–326. doi: 10.1136/vr.101.16.326. [DOI] [PubMed] [Google Scholar]
- Rosenstein Y., Lafarge-Frayssinet C. Inhibitory effect of Fusarium T2-toxin on lymphoid DNA and protein synthesis. Toxicol Appl Pharmacol. 1983 Sep 15;70(2):283–288. doi: 10.1016/0041-008x(83)90104-7. [DOI] [PubMed] [Google Scholar]
- Rousseaux C. G., Nicholson S., Schiefer H. B. Fatal placental hemorrhage in pregnant CD-1 mice following one oral dose of T-2 toxin. Can J Comp Med. 1985 Jan;49(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- Schappert K. T., Khachatourians G. G. Influence of the membrane on T-2 toxin toxicity in Saccharomyces spp. Appl Environ Microbiol. 1984 Apr;47(4):681–684. doi: 10.1128/aem.47.4.681-684.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley E. B. T-2 toxin. J Am Vet Med Assoc. 1973 Dec 1;163(11):1278–1281. [PubMed] [Google Scholar]
- Smith K. E., Cannon M., Cundliffe E. Inhibition at the initiation level of eukaryotic protein synthesis by T-2 toxin. FEBS Lett. 1975 Jan 15;50(1):8–12. doi: 10.1016/0014-5793(75)81028-3. [DOI] [PubMed] [Google Scholar]
- Trusal L. R. Metabolism of T-2 mycotoxin by cultured cells. Toxicon. 1986;24(6):597–603. doi: 10.1016/0041-0101(86)90180-7. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J. Present concepts on the mechanisms of platelet aggregation. Biochem Pharmacol. 1981 Feb 15;30(4):263–271. doi: 10.1016/0006-2952(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Visconti A., Mirocha C. J. Identification of various T-2 toxin metabolites in chicken excreta and tissues. Appl Environ Microbiol. 1985 May;49(5):1246–1250. doi: 10.1128/aem.49.5.1246-1250.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver G. A., Kurtz H. J., Mirocha C. J., Bates F. Y., Behrens J. C., Robison T. S., Swanson S. P. The failure of purified T-2 mycotoxin to produce hemorrhaging in dairy cattle. Can Vet J. 1980 Jul;21(7):210–213. [PMC free article] [PubMed] [Google Scholar]
- Yarom R., More R., Eldor A., Yagen B. The effect of T-2 toxin on human platelets. Toxicol Appl Pharmacol. 1984 Apr;73(2):210–217. doi: 10.1016/0041-008x(84)90326-0. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Sakamoto T., Okamoto K. In vitro formation of 3'-hydroxy T-2 and 3'-hydroxy HT-2 toxins from T-2 toxin by liver homogenates from mice and monkeys. Appl Environ Microbiol. 1984 Jan;47(1):130–134. doi: 10.1128/aem.47.1.130-134.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T., Swanson S. P., Mirocha C. J. In vitro metabolism of T-2 toxin in rats. Appl Environ Microbiol. 1980 Nov;40(5):901–906. doi: 10.1128/aem.40.5.901-906.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


