Abstract
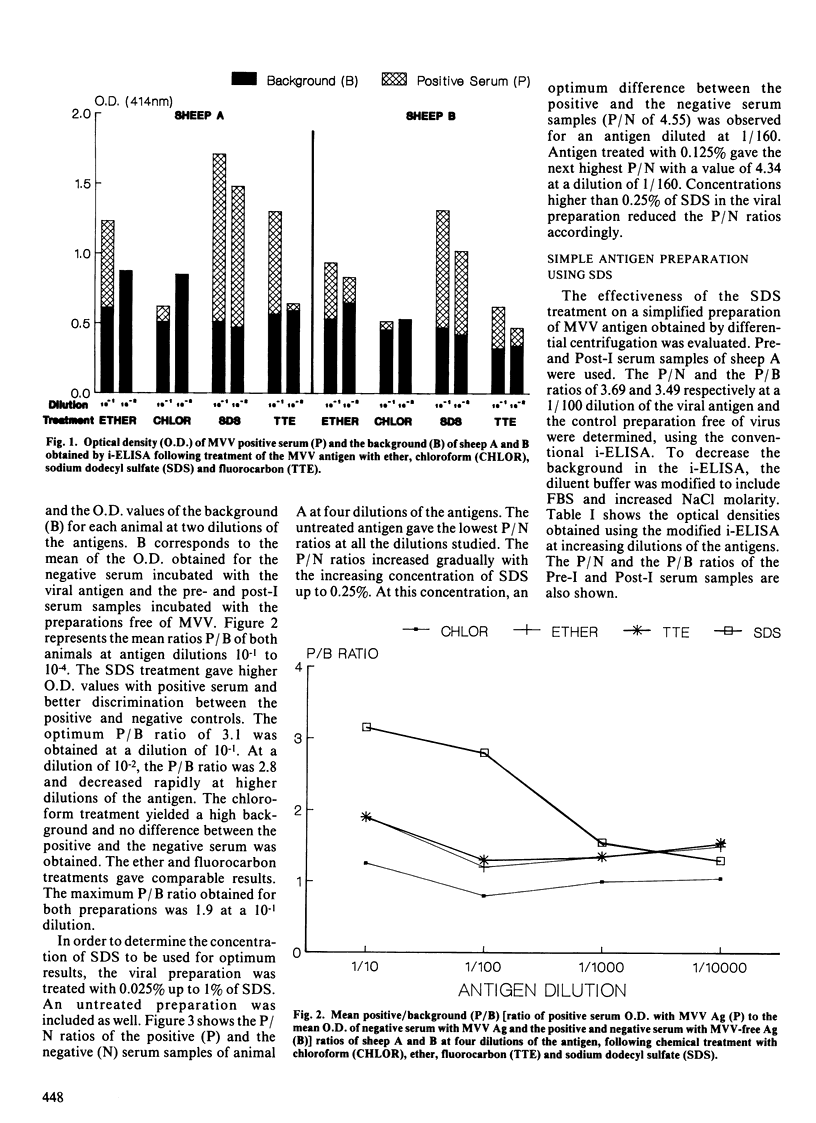
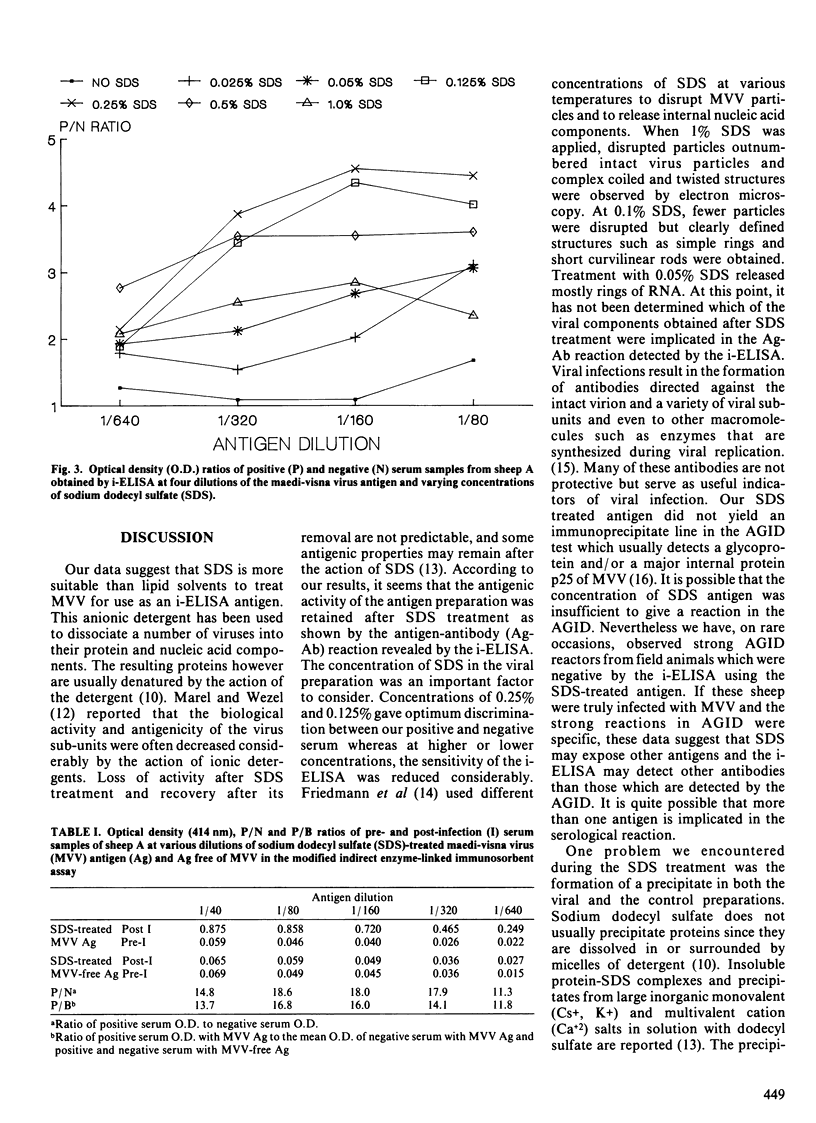
We report the efficacy of an anionic detergent, sodium dodecyl sulfate (SDS) for preparing maedi-visna antigens for an indirect enzyme-linked immunosorbent assay (i-ELISA). Ovine maedi-visna virus (MVV) pelleted by differential centrifugation followed by liquid chromatography was treated with SDS or one of three lipid solvents: ethyl ether, chloroform or fluorocarbon. The SDS-treated antigen resulted in higher optical density values with positive serum and better discrimination between positive and negative serum samples from specific-pathogen-free (SPF) sheep experimentally inoculated with the virus. Optimal results were obtained when MVV was treated with concentrations of 0.25% and 0.125% of SDS. A viral antigen prepared by centrifugation and treatment of a viral pellet with SDS was also suitable for the i-ELISA. This latter technique may facilitate the production of MVV antigens for use in the i-ELISA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. S., Crawford T. B., Banks K. L., McGuire T. C., Perryman L. E. Immune responses of goats persistently infected with caprine arthritis-encephalitis virus. Infect Immun. 1980 May;28(2):421–427. doi: 10.1128/iai.28.2.421-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coackley W., Smith V. W., Houwers D. J. Preparation and evaluation of antigens used in serological tests for caprine syncytial retrovirus antibody in sheep and goat sera. Vet Microbiol. 1984 Oct;9(6):581–586. doi: 10.1016/0378-1135(84)90020-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Friedmann A., Coward J. E., Harter D. H., Lipset J. S., Morgan C. Electron microscopic studies of visna virus ribonucleic acid. J Gen Virol. 1974 Oct;25(1):93–104. doi: 10.1099/0022-1317-25-1-93. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., Gielkens A. L. An ELISA for the detection of maedi/visna antibody. Vet Rec. 1979 Jun 30;104(26):611–611. doi: 10.1136/vr.104.26.611-b. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., Gielkens A. L., Schaake J., Jr An indirect enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to maedi-visna virus. Vet Microbiol. 1982 Jul;7(3):209–219. doi: 10.1016/0378-1135(82)90035-9. [DOI] [PubMed] [Google Scholar]
- Klein J. R., Martin J., Griffing S., Nathanson N., Gorham J., Shen D. T., Petursson G., Georgsson G., Palsson P. A., Lutley R. Precipitating antibodies in experimental visna and natural progressive pneumonia of sheep. Res Vet Sci. 1985 Mar;38(2):129–133. [PubMed] [Google Scholar]
- McGrath M., Witte O., Pincus T., Weissman I. L. Retrovirus purification: method that conserves envelope glycoprotein and maximizes infectivity. J Virol. 1978 Mar;25(3):923–927. doi: 10.1128/jvi.25.3.923-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J., Macmillan J. The validity of addict notifications. Br J Psychiatry. 1979 Mar;134:264–268. doi: 10.1192/bjp.134.3.264. [DOI] [PubMed] [Google Scholar]
- Ressang A. A., Gielkens A. L., Quak S., Mastenbroek N., Tuppert C., De Castro A. Studies on bovine leukosis. VI. Enzyme linked immunosorbent assay for the detection of antibodies to bovine leukosis virus. Ann Rech Vet. 1978;9(4):663–666. [PubMed] [Google Scholar]
- Schroeder B. A., Oliver R. E., Cathcart A. The development and evaluation of an ELISA for the detection of antibodies to caprine arthritis-encephalitis virus in goat sera. N Z Vet J. 1985 Dec;33(12):213–215. doi: 10.1080/00480169.1985.35240. [DOI] [PubMed] [Google Scholar]
- Simard C. L., Briscoe M. R. An enzyme-linked immunosorbent assay for detection of antibodies to maedi-visna virus in sheep. II. Comparison to conventional agar gel immunodiffusion test. Can J Vet Res. 1990 Oct;54(4):451–456. [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Ueda S., Samejima T. Enzyme-linked immunosorbent assay for diagnosis of equine infectious anemia. Vet Microbiol. 1982 Sep;7(4):307–315. doi: 10.1016/0378-1135(82)90010-4. [DOI] [PubMed] [Google Scholar]
- Verwoerd D. W., Payne A. L., York D. F., Myer M. S. Isolation and preliminary characterization of the jaagsiekte retrovirus (JSRV). Onderstepoort J Vet Res. 1983 Dec;50(4):309–316. [PubMed] [Google Scholar]
- Vitu C., Russo P., Filippi P., Vigne R., Querat G., Giauffret A. Une technique ELISA pour la détection des anticorps anti-virus maedi-visna. Etude comparative avec l'immunodiffusion en gelose et la fixation du complement. Comp Immunol Microbiol Infect Dis. 1982;5(4):469–481. doi: 10.1016/0147-9571(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. ELISA techniques in virology. Lab Res Methods Biol Med. 1982;5:59–81. [PubMed] [Google Scholar]


