Abstract
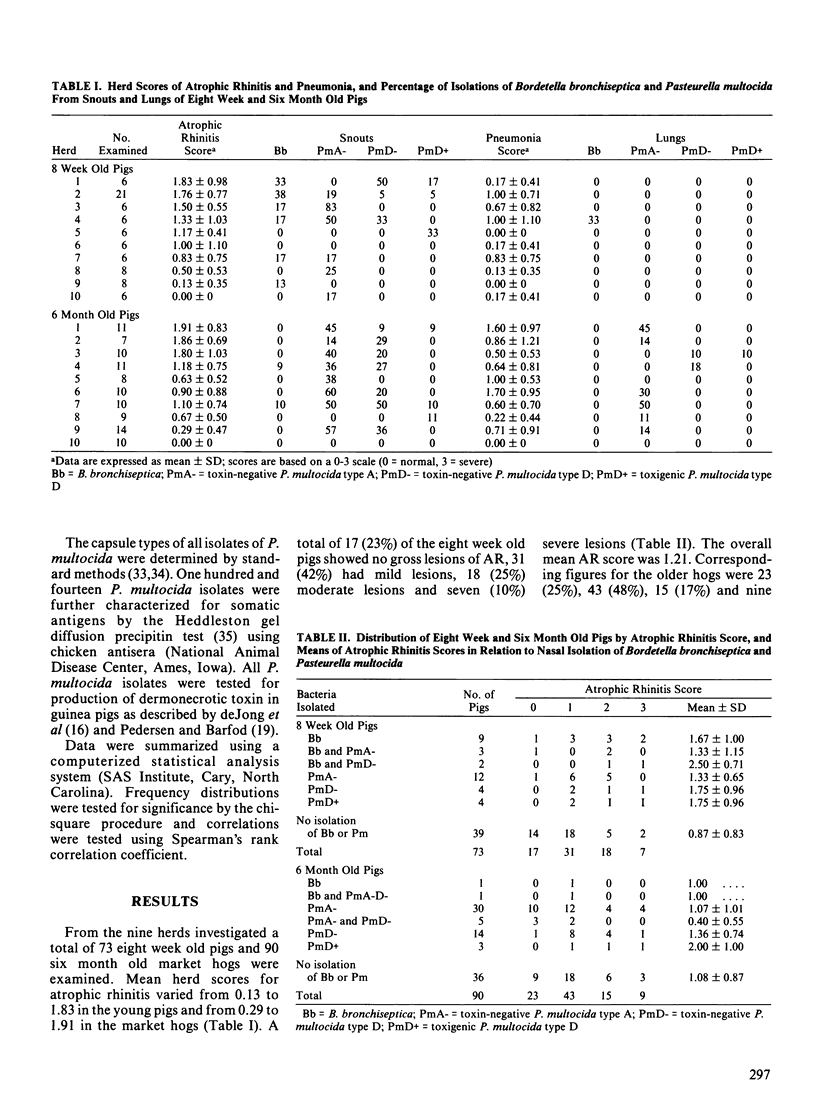
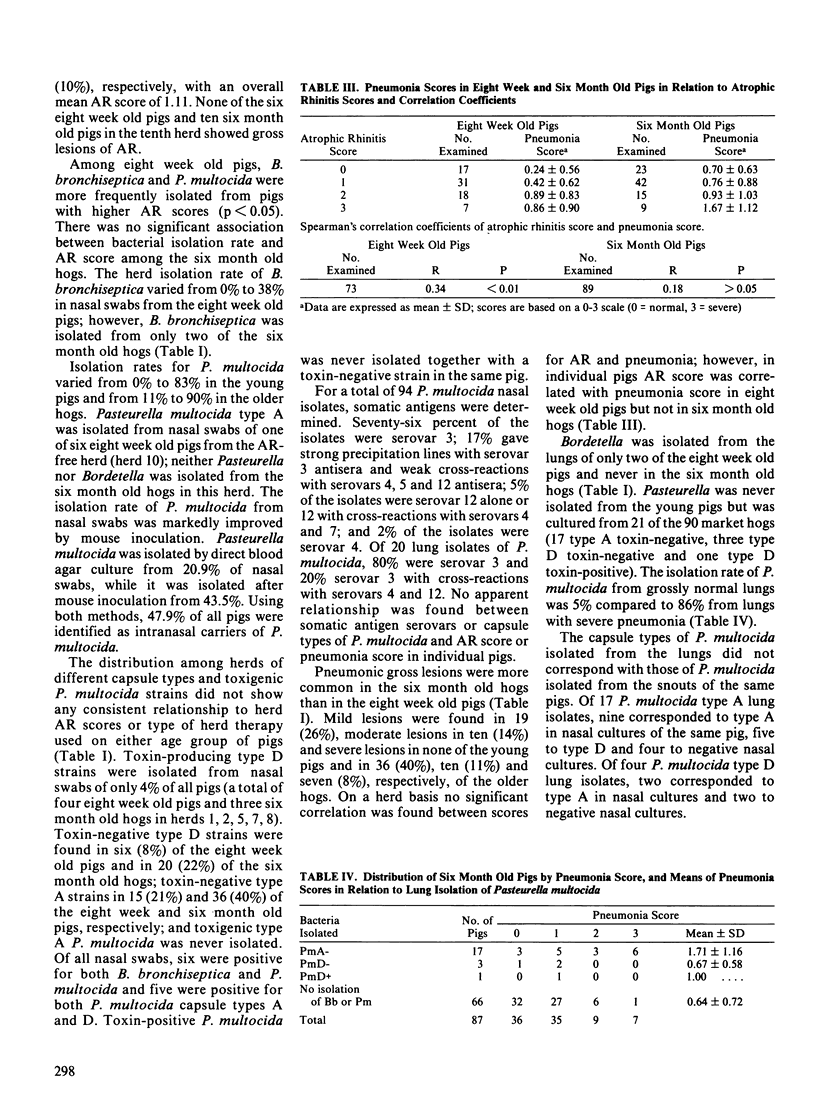
A total of 163 pigs from nine farrow-to-finish herds representing various levels of atrophic rhinitis (AR) were selected for postslaughter examination of AR and pneumonia. Nasal swabs and lungs were cultured for detection of Bordetella bronchiseptica and Pasteurella multocida. Seventy-three pigs were examined at eight weeks of age and 90 contemporaries at six months of age. Mean AR scores were 1.21 and 1.11 for the eight week and six month old pigs, respectively (0 = normal, 3 = severe). In individual pigs increasing AR score was related to increasing pneumonia score in eight week old pigs but not in six month old hogs. In eight week old pigs, B. bronchiseptica and P. multocida were isolated more frequently from pigs with higher AR scores. From nasal swabs of six month old hogs, Bordetella was almost never recovered while Pasteurella was frequently isolated score. Toxigenic type DP. multocida was isolated from nasal cultures of only seven (4%) pigs and from lung cultures of only one pig. Pasteurella was never isolated from lungs of the eight week old pigs and Bordetella never from the six month old hogs. The isolation rate of P. multocida, predominantly type A, from lungs of six month old pigs increased from 11% in grossly normal lungs to 86% in lungs with severe pneumonia. Pigs from one herd free from lesions of AR and pneumonia were also examined; type AP. multocida was isolated from nasal cultures of one of six eight week old pigs. Somatic antigens of P. multocida were determined for 94 nasal and 20 lung isolates. Somatic serovar 3 was found in 93% of the nasal isolates and in all lung isolates.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. R., Krook L., Pond W. G. Atrophic rhinitis in swine. Etiology, pathogenesis, and prophylaxis. Cornell Vet. 1966 Apr;56(Suppl):3–108. [PubMed] [Google Scholar]
- Bäckström L. R., Brim T. A., Collins M. T. Development of turbinate lesions and nasal colonization by Bordetella bronchiseptica and Pasteurella multocida during long-term exposure of healthy pigs to pigs affected by atrophic rhinitis. Can J Vet Res. 1988 Jan;52(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- Bäckström L., Hoefling D. C., Morkoc A. C., Cowart R. P. Effect of atrophic rhinitis on growth rate in Illinois swine herds. J Am Vet Med Assoc. 1985 Oct 1;187(7):712–715. [PubMed] [Google Scholar]
- Carter G. R., Rundell S. W. Identification of type A strains of P multocida using staphylococcal hyaluronidase. Vet Rec. 1975 Apr 12;96(15):343–343. doi: 10.1136/vr.96.15.343. [DOI] [PubMed] [Google Scholar]
- Carter G. R., Subronto P. Identification of type D strains of Pasteurella multocida with acriflavine. Am J Vet Res. 1973 Feb;34(2):293–294. [PubMed] [Google Scholar]
- Chanter N., Rutter J. M., Mackenzie A. Partial purification of an osteolytic toxin from Pasteurella multocida. J Gen Microbiol. 1986 Apr;132(4):1089–1097. doi: 10.1099/00221287-132-4-1089. [DOI] [PubMed] [Google Scholar]
- Eliás B., Hámori D. Data on the aetiology of swine atrophic rhinitis. V. The role of genetic factors. Acta Vet Acad Sci Hung. 1976;26(1):13–19. [PubMed] [Google Scholar]
- Flesjå K. I., Ulvesaeter H. O. Pathological lesions in swine at slaughter. III. Inter-relationship between pathological lesions, and between pathological lesions and 1) carcass quality and 2) carcass weight. Acta Vet Scand Suppl. 1980;(74):1–22. [PubMed] [Google Scholar]
- Giles C. J., Smith I. M., Baskerville A. J., Brothwell E. Clinical bacteriological and epidemiological observations on infectious atrophic rhinitis of pigs in southern England. Vet Rec. 1980 Jan 12;106(2):25–28. doi: 10.1136/vr.106.2.25. [DOI] [PubMed] [Google Scholar]
- Heddleston K. L., Gallagher J. E., Rebers P. A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972 Jul-Sep;16(4):925–936. [PubMed] [Google Scholar]
- Kielstein P., Eliás B. Zur Bedeutung von Bordetella bronchiseptica und Pasteurella multocida bei der Rhinitis atrophicans suum. Zentralbl Veterinarmed B. 1985 Oct;32(9):694–705. [PubMed] [Google Scholar]
- Nakai T., Kume K., Yoshikawa H., Oyamada T., Yoshikawa T. Changes in the nasal mucosa of specific-pathogen-free neonatal pigs infected with Pasteurella multocida or Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1986 Aug;48(4):693–701. doi: 10.1292/jvms1939.48.693. [DOI] [PubMed] [Google Scholar]
- Nakai T., Sawata A., Tsuji M., Samejima Y., Kume K. Purification of dermonecrotic toxin from a sonic extract of Pasteurella multocida SP-72 serotype D. Infect Immun. 1984 Nov;46(2):429–434. doi: 10.1128/iai.46.2.429-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada T., Yoshikawa T., Yoshikawa H., Shimizu M., Nakai T., Kume K. Lesions induced in the nasal turbinates of neonatal pigs inoculated with Pasteurella multocia and/or Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1986 Apr;48(2):377–387. doi: 10.1292/jvms1939.48.377. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Barfod K. Effect on the incidence of atrophic rhinitis of vaccination of sows with a vaccine containing Pasteurella multocida toxin. Nord Vet Med. 1982 Jul-Sep;34(7-9):293–302. [PubMed] [Google Scholar]
- Pedersen K. B., Barfod K. The aetiological significance of Bordetella bronchiseptica and Pasteurella multocida in atrophic rhinitis of swine. Nord Vet Med. 1981 Dec;33(12):513–522. [PubMed] [Google Scholar]
- Pijoan C., Morrison R. B., Hilley H. D. Serotyping of Pasteurella multocida isolated from swine lungs collected at slaughter. J Clin Microbiol. 1983 Jun;17(6):1074–1076. doi: 10.1128/jcm.17.6.1074-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler R. B., Brogden K. A. Pasteurella multocida isolated from rabbits and swine: serologic types and toxin production. Am J Vet Res. 1986 Apr;47(4):730–737. [PubMed] [Google Scholar]
- Runnels L. J. Infectious atrophic rhinitis of swine. Vet Clin North Am Large Anim Pract. 1982 Nov;4(2):301–319. doi: 10.1016/s0196-9846(17)30107-6. [DOI] [PubMed] [Google Scholar]
- Rutter J. M. Atrophic rhinitis in swine. Adv Vet Sci Comp Med. 1985;29:239–279. [PubMed] [Google Scholar]
- Rutter J. M., Mackenzie A. Pathogenesis of atrophic rhinitis in pigs: a new perspective. Vet Rec. 1984 Jan 28;114(4):89–90. doi: 10.1136/vr.114.4.89. [DOI] [PubMed] [Google Scholar]
- Rutter J. M. Quantitative observations on Bordetella bronchiseptica infection in atrophic rhinitis of pigs. Vet Rec. 1981 May 23;108(21):451–454. doi: 10.1136/vr.108.21.451. [DOI] [PubMed] [Google Scholar]
- SWITZER W. P. Studies on infectious atrophic rhinitis. V. Concept that several agents may cause turbinate atrophy. Am J Vet Res. 1956 Jul;17(64):478–484. [PubMed] [Google Scholar]
- Sawata A., Nakai T., Tuji M., Kume K. Dermonecrotic activity of Pasteurella multocida strains isolated from pigs in Japanese field. Nihon Juigaku Zasshi. 1984 Apr;46(2):141–148. doi: 10.1292/jvms1939.46.141. [DOI] [PubMed] [Google Scholar]
- Straw B. E., Bürgi E. J., Hilley H. D., Leman A. D. Pneumonia and atrophic rhinitis in pigs from a test station. J Am Vet Med Assoc. 1983 Mar 15;182(6):607–611. [PubMed] [Google Scholar]
- Straw B. E., Leman A. D., Robinson R. A. Pneumonia and atrophic rhinitis in pigs from a test station--a follow-up study. J Am Vet Med Assoc. 1984 Dec 15;185(12):1544–1546. [PubMed] [Google Scholar]


