Abstract
Dose-dependent neuromodulation has been well established; however, the molecular mechanisms underlying astrocytic involvement in this process remain largely unexplored. Using the autoregulation of supraoptic oxytocin (OT) neurons (OTNs) as a model, we investigated the role of distinct astrocytic G proteins and their targets in the dose-dependent effects of OT on OTN activity. The results showed that OT in a low concentration (10 pmol/L, L-OT) excited OTN activity, whereas a high concentration (1 nmol/L, H-OT) inhibited it in brain slices. These effects were abolished upon disruption of astrocytic plasticity using L-aminoadipic acid, a gliotoxin. In primary astrocyte cultures, L-OT slightly reduced the current through astrocyte-specific inwardly rectifying K⁺ channel 4.1 (Kir4.1) while H-OT strongly enhanced it. Selectively blocking Kir4.1 with BaCl₂ (100 µmol/L) did not affect the basal activity but blocked the excitatory effect of L-OT in brain slices. In cultured astrocytes, L-OT mobilized Gαq subunit expression, increased glial fibrillary acidic protein (GFAP) filaments, and quickly expanded astrocytic volume, predominantly visible at the somata. Conversely, H-OT released Gαi subunits and induced progressive volume expansion. Pretreatment of brain slices with U73122 (a Gq inhibitor) or SQ22536 (a Gs inhibitor) suppressed L-OT-induced excitation. Conversely, activation of adenylyl cyclase with forskolin reversed the inhibitory effect of H-OT, and inhibition of Gi with pertussis toxin blocked H-OT-induced inhibition. These findings imply that the dose-dependent effects of OT on OTN activity are mediated, at least partially, by different receptor-coupled G proteins and their subsequent modulation of astrocytic Kir4.1 currents, GFAP expression, and volume dynamics. This mechanism underlying the autoregulation of OTN activity provides an important reference for understanding the concentration-dependent neuromodulation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-025-05193-w.
Keywords: Excitability, Glia, G protein, Hypothalamus, Neurons, Oxytocin
Introduction
Neural function is primarily governed by neuronal firing activity, which is regulated by both extrinsic modulatory factors and intrinsic signals. Among diverse neuronal populations, the firing activity and regulatory mechanism of neuroendocrine cells in the supraoptic nucleus (SON) and paraventricular nucleus are highly representative, particularly for neurons that synthesize and secrete oxytocin (OT). It is well-known that OT neurons (OTNs) play a pivotal role in modulating mental, endocrine, and physical functions in addition to the milk-ejection reflex [1]. These functions are mediated by OT released from their axonal terminals within the brain and posterior pituitary. The amount and pattern of OT release in either tonic or pulsatile pattern [2] are primarily determined by the corresponding firing patterns of OTNs [3]. The activity of OTNs is influenced by a variety of extracellular factors, including synaptic inputs, astrocytic plasticity, interneuron interactions, and the neurochemical environment, alongside intracellular signals and membrane properties [4–7]. A notable feature of OT-mediated neuromodulation is its dose-dependent effect, primarily driven by OT release from the somatodendritic sites of OTNs [5]. While low concentrations of OT (L-OT) enhance the firing rate of OTNs [8], high concentrations (H-OT) suppress their excitability [9]. Clarification of the underlying mechanisms for dose-associated firing activity of OTNs is crucial for understanding the general principles of neural regulation.
Dose-dependent neuronal responses can result from various molecular mechanisms, including opposing effects mediated by distinct receptors, receptor desensitization, negative feedback with increasing dose, or dose-dependent metabolic modulation [10–12] as well as astrocytic plasticity and synaptic development [13]. In OTNs, concentration-dependent effects of OT on OTN activity have been proposed to result from differential activation of Gq, Gi/o, and Gs proteins that are coupled to OT receptor (OTR) [14, 15]. OTR is expressed in both neurons and astrocytes within neural tissues, including OTNs and their surrounding astrocytes [16]. Astrocytes play a critical role in modulating OTN activity through morphological and functional plasticities [17]. However, whether the dose-dependent effects of OT on OTN firing patterns are associated with differential activation of astrocytic OTR-coupled/associated Gq, Gs, and Gi proteins remains unclear.
Previous studies revealed that OT can increase astrocytic Ca2+ release [18] and depolarize astrocytic membrane potential, even in 10 pmol/L [19], a lower physiological level in the cerebrospinal fluid. By contrast, selective OTR knockout in astrocytes expressing glial fibrillary acidic protein (GFAP) decreases OT-evoked astrocytic Ca2+ signaling [16]. Moreover, OT at 10 pmol/L can increase GFAP polymerization in the somatic compartment of astrocytes in the SON by increasing somatic levels of phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2 [19]), an early downstream signal of OTR-Gq signaling [20]. These findings support the activation of OTR-coupled Gq proteins in astrocytes by L-OT. Furthermore, high levels of OT and robust cytosolic Ca2+ increase that could occur following the burst firing in OTNs [21] can reverse L-OT-evoked increases in somatic pERK1/2 and GFAP filaments [19], likely a result of activation of OTR-coupled Gi proteins [15]. It was also reported that the autoregulation of OTNs is related to changes in the activity of OTR-associated Gs protein in astrocytes as indicated by the expression of protein kinase A (PKA, [19]). In addition, astrocytic plasticity in the SON is associated with opposite volemic alterations in the somata versus distal processes, a phenomenon largely determined by the activity of the inwardly rectifying K⁺ channel, Kir4.1 [22] that and GFAP can serve as biomarkers of astrocytic plasticity [17]. Further study is needed to validate the contribution of different OTR-coupled G proteins to the astrocytic plasticity and autoregulation of OTNs.
In this study, we aimed to address the following questions. (1) How do L-OT and H-OT affect the firing activity of OTNs? (2) What roles do astrocytes play in these OT-mediated effects, particularly through the regulation of Kir4.1, GFAP, and astrocytic volume? (3) How do OT-induced changes in astrocytic G protein expression contribute to the autoregulation of OTNs? Our findings demonstrate that the divergent effects of L-OT and H-OT on OTN activity are mediated, at least partially, by the differential activation of astrocytic OTR-coupled/associated Gq, Gs, and Gi proteins.
Materials and Methods
Animals
Male Sprague–Dawley rats (80–120 g), provided by the Animal Center of the Second Affiliated Hospital of Harbin Medical University, were used in this study. All procedures strictly followed the Guideline of National Institutes of Health and were approved by the Institutional Animal Care and Use Committees of Harbin Medical University (HMU2023-03–05: Mechanisms underlying oxytocin modulation of postpartum depression in a dose-dependent manner by acting on different types of G-proteins in astrocytes). Animals were housed in groups of four in polycarbonate cages, with free access to food and water. Room temperature was maintained at 21–23 °C, with a 12 h light–12 h dark cycle.
Preparation of Brain Slices
Acute hypothalamic slices were prepared using the method published previously [23]. Briefly, rats were decapitated, and hypothalamic blocks were dissected in an ice-cold slicing solution that contained the following components (in mmol/L): 83 NaCl, 3 KCl, 5 MgCl2, 3 CaCl2, 1.3 NaH2PO4, 26 NaHCO3, 10 Glucose, 75 Sucrose (pH 7.35 ~ 7.45 after saturation with 95%O2 + 5%CO2 gas mixture; osmolality of 300 ~ 305 mOsm/kg H2O). Coronal hypothalamic slices (300 µm thick) containing the SON were cut using a vibrating microtome (Leica VT1200) and then transferred into normal artificial cerebrospinal fluid (aCSF) that was composed of the following components (in mmol/L): 126 NaCl, 3 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.3 NaH2PO4, 26 NaHCO3, and 10 Glucose; 300 mOsm/kg, pH 7.4, at 35 °C for 30 min and then kept at room temperature for 1 h before further examinations.
Preparation of Primary Cultures of Hypothalamic Astrocytes and Volume Assay
The primary cultures of astrocytes were prepared from the hypothalamus of newborn pups as previously described [23]. The hypothalamic tissues, mainly including the SON and paraventricular nucleus, were dissected in ice-cold aCSF and then digested in 0.25% trypsin at 37 °C for 20 min. Cells were dissociated by triturating tissue through a glass pipette for 8 ~ 10 times in DMEM/F12 medium (SH30023.01, Hyclone, Utah) supplemented with 10% fetal bovine serum (HLJ-TF041, MRC, Changzhou), 100 units/mL of penicillin, 100 µg/mL of streptomycin, and epidermal growth factor (10 ng/mL). The dissociated cells were plated into culture flasks for 12 ~ 23 days for the maturity of astrocytes, i.e., full expressions of GFAP and aquaporin 4. After purification through the shaking method, and the cultures contained > 99% of astrocytes, as confirmed by GFAP immunostaining [22].
In volume assay, cultured astrocytes were loaded with sulforhodamine dye (SR101, 10 μmol/l, S7635, Sigma-Aldrich, St. Louis, MO), an astrocyte-specific dye [24], for 20 min at 37 °C. Changes in cytosolic fluorescence intensity were used as an indicator of alterations in the cell volume. That is, decrease in the fluorescence intensity alongside a line scan reflects increase in cell volume and cellular swelling, and vice versa.
Electrophysiological Recordings of Brain Slices and Cultured Astrocytes
Whole-cell current-clamp recording was used to record the firing activity of OTNs in the SON of brain slices as previously described [8]. Patch pipettes were filled with a solution containing (in mmol/L) 145 K-gluconate, 10 KCI, 1 MgCl2, 0.01 CaCl2, 10 HEPES, 1 EGTA, 2 Mg3-ATP, and 0.5 Na3-GTP (pH 7.25; 290–300 mOsm/kg). To identify the type of recorded neurons, 0.05% biocytin (B4261, MilliporeSigma, St. Louis, MO) was added to the pipette solution. When filled with the internal solution, pipettes had a resistance of 5–8 MΩ before seal formation. Firing rates were recorded with a Multiclamp 700B amplifier, filtered at 3 kHz, and digitized at 5 kHz. The data were stored and analyzed with a personal computer using pCLAMP 10 software (Molecular Devices, San Jose, CA).
The identification of OTNs was mainly based on the patterns of firing activity as previously reported [25]. In brief, OTNs did not show phasic firing patterns at rest condition or after depolarizing the membrane potential of 5–10 mV in a ramp current over 2 min, which were present in ~ 50% recorded cells. Representative non-phasic firing cells were further confirmed to be oxytocinergic with post hoc immunostaining. In the identification, biocytin was incubated with Alexa Fluor® 488-conjugated streptavidin that exhibits green light under fluorescent illumination, and cells having the co-localization of OT neurophysin (OT-NP) with biocytin were identified as OTNs (inset in Fig. 1).
Fig. 1.
Effect of different concentrations of oxytocin (OT) on the firing activity of OT neurons (OTNs) in the supraoptic nucleus (SON) in brain slices. A Representative recordings showing changes in the firing rates in OTNs after the treatments of low (L-OT, 10 pmol/L, a) and high (H-OT, 1 nmol/L, b) concentrations of OT. The inset shows an image of post hoc identified OTNs, having co-localization of OT neurophysin (OT-NP) with biocytin. B Dotted line graphs summarizing statistical analyses of the firing rate at L-OT (a) and H-OT (b) with bin width of 1 min. *P < 0.05, **P < 0.01 compared to the control (CTR, 0–1 min before OT) by paired t-test
Purified astrocytes in flasks were dissociated and plated onto poly-D-lysine-coated coverslips at 13 days in vitro (DIV 13) for ~ 2 h before recordings as previously reported [22]. Only one astrocyte was recorded per coverslip. Astrocytes were patched in the whole-cell configuration in the aCSF. Recording electrodes with a tip resistance of 5–8 MΩ were filled with a pipette solution containing (in mmol/L) 140 KCI, 10 HEPES, 1 EGTA, 0.5 CaCl2, 1.5 MgCl2, 2 Mg-ATP, and 0.5 Na-GTP (pH 7.25; 290–300 mOsm/kg). The membrane potential of single astrocytes was held at − 70 mV with voltage steps applied in 20 mV increments in the range of − 160 to + 80 mV. The relative current was calculated by dividing current values at − 160 mV of the control (CTR).
Immunohistochemistry
The method was basically the same as previously described [20] with minor modification. In brief, hypothalamic blocks of the brain with intact optic chiasm were cut into 80 μm-thick coronal sections. In immunostaining, the plasma membrane was permeabilized with 0.3% Triton X-100 for 1 h, and then with 5% bovine serum albumin for 2 h to block non-specific binding sites of antibodies. The sections were then incubated with primary antibodies against GFAP (ab4674, 1:500, antichicken antibody, Abcam, RRID: AB_304558), or OT-NP (MABN844, antimouse antibody, 1:3000, Merck Millipore) at 4 ℃ overnight. Species-matched secondary antibodies (ab150171, Donkey Anti-Chicken IgG, Alexa Fluor 647; ab150074, Donkey Anti-Rabbit IgG, Alexa Fluor 555, RRID: AB_2636997; ab150109, Donkey Anti-Mouse IgG, AlexaFluor 488, RRID: AB_2571721; Abcam, Cambridge, UK) were applied for 2 h at room temperature. Lastly, Hoechst (bisbenzimide, B2261, Sigma, 0.5 μg/mL, 30 min, RRID: AB_1589326) was used to label nuclei. Sections were examined with a confocal microscope (Eclipse Ti, Nikon).
In cultured astrocytes, the identification of α-subunits of Gq, Gs, and Gi proteins and GFAP used the same method described previously [23]. In brief, cultured astrocytes were fixed in 4% paraformaldehyde (pH 7.4 in phosphate-buffered solution or PBS) for 30 min and permeated with 0.3% (w/v) Triton X-100 in PBS. Non-specific antibody binding sites were blocked with 5% (w/v) gelatin in PBS. Each coverslip containing astrocytes was covered with a drop of solution that contained primary antibodies against Gαq/11/14 (G-7, sc-365906), Gαs (sc-135914), and Gαi (sc-515658) from Santa Cruz Biotechnology, Inc., and GFAP (ab4674, 1:500) from Abcam. After incubating overnight at 4 ℃ and washing, astrocytes were incubated for 1.5 h at room temperature with species-matched secondary antibodies conjugated with AlexaFluor 488, 555, or 647 (Invitrogen, Waltham, MA). Hoechst was used to label cell nuclei. The fluorescence of different labels was visualized with the laser scanning confocal microscope.
To ensure the comparability of fluorescence measurements between different groups, slices or cell cultures from different treatments were matched in the size/location of imaging fields, and the scanning/imaging conditions. No primary antibody staining was used as a CTR to exclude nonspecific staining.
Experimental Design and Statistical Analyses
Data analyses were performed using the same methods as previously described [23]. The firing rate was calculated every 1 min and expressed as a relative value of CTR (= 1) or actual average frequency (Hz). Each value in the slice was from one SON section of one rat.
Data were expressed as mean ± SEM. Full details of the experimental design of each individual experiment were described in each results. All analyses were performed using SigmaStat program (SPSS 19, Chicago, IL) software. Student’s t-test was used for comparison between two groups. ANOVA was used for comparison among groups of three or more and followed by Bonferroni post-hoc test or other appropriate methods as recommended by the program. P < 0.05 was considered statistically significant.
Results
Effects of OT on the Firing Activity of OTNs
While general excitatory effect of OT in physiological range (10 pmol/L ~ 1 nmol/L) has been considered [26, 27], higher doses of OT may play a post-excitation inhibition or an direct inhibitory role as identified in in vitro studies [9, 28]. To confirm the differential effects of L-OT and H-OT on OTN activity, we performed whole-cell patch-clamp recordings in the SON of brain slices. As shown in Fig. 1, application of L-OT significantly increased the firing rate of OTNs compared to CTR, starting at the third min (1 in CTR versus 1.15 ± 0.05 in L-OT 3 min, t = 3.458, P = 0.0047, n = 13 by paired t-test) and remaining significant at 5 min (1.20 ± 0.06, n = 13, t = 3.651, P = 0.0033 by paired t-test). In contrast, H-OT significantly reduced OTN firing rate at 5 min post-application (0.61 ± 0.14, n = 8, t = 2.691, P = 0.031 compared to the CTR by paired t-test) with or without an initial transient excitation. This result confirms not only the “spike frequency reduction” or post-excitation inhibition of L-OT action [9] but also a direct inhibitory effect of H-OT on the firing activity of OTNs.
Effects of l-Aminoadipic Acid (L-AAA) on OT-Modulated Firing Activity
Astrocytes in the SON are essential modulators of OTN activity [16, 20, 29]. However, it remains to examine whether astrocytes are involved in the actions of L-OT and H-OT differentially. The presence of OTRs on astrocytes [16, 30] including those that surround OTNs in the SON [31] provides a mechanistic basis for L-OT and H-OT to induce distinct astrocytic plasticity via different OTR signaling. This plasticity may subsequently modulate OTN activity in a concentration-dependent manner [15]. In this study, we tested effects of L-OT and H-OT on OTN activity after disabling astrocytic plasticity with 0.25 mmol/L L-AAA for 30 min [32, 33], a specific gliotoxin that has been used extensively to disable astrocytic plasticity [20, 34]. As shown in Fig. 2, L-AAA significantly reduced basal firing rate of OTNs (4.19 ± 0.42 Hz in CTR versus 0.34 ± 0.10 Hz in L-AAA, n = 7, t = 11.38, P < 0.0001 by paired t-test), a result similar to a previous report [20]. In the presence of L-AAA, both L-OT (0.35 ± 0.11 Hz, n = 7, t = 0.271, P = 0.7953 by paired t-test) and H-OT (0.38 ± 0.10 Hz, n = 7, t = 1.442, P = 0.1995 by paired t-test) lost their significant effects on OTN firing activity. This finding supports the proposal that the effects of both L-OT and H-OT on OTNs are involved in OTR-mediated OT modulation of astrocytic plasticity [19, 20].
Fig. 2.
Effects of l-aminoadipic acid (L-AAA) on OT-modulated electrical activity of OTNs in the SON of brain slices. A and B Representative recordings showing changes in the firing rates in OTNs (a) and line graphs showing statistical analyses (b) of L-AAA effect on L-OT (A) and H-OT (B) actions, respectively. Other annotations refer to Fig. 1
Effects of OT on Kir4.1 Currents of Astrocytes
The effects of disabling astrocytic plasticity on OT-modulated OTN activity indicate that astrocytes play a pivotal role in the effects of L-OT and H-OT on OTNs. Astrocytic plasticity is largely dependent on astrocyte-specific Kir4.1 current that influences multiple functions of astrocytes [35] and is a key machinery of astrocytic GFAP expression and volume regulation in the SON [22]. To clarify the mechanisms of OT effect on astrocytic plasticity, we recorded Kir4.1 currents in primarily cultured astrocytes in whole-cell patch-clamp configuration. Kir4.1 was identified by application of BaCl2 (100 μmol/L, a specific blocker of Kir4.1 [36]), the specificity of which on astrocytic Kir4.1 has been verified with the use of VU0134992, another specific blocker of Kir4.1 [22]. As shown in Fig. 3, treatment of the astrocytes with L-OT for 2 min, the amplitude of Kir4.1 current was significantly reduced at − 160 mV compared with that of the CTR (− 1 in CTR vs. − 0.77 ± 0.04 in L-OT, n = 5, t = 4.426, P = 0.012, paired t-test). H-OT inversely increased the Kir4.1 current (− 0.84 ± 0.02 in CTR vs. − 1.31 ± 0.21 in H-OT at − 140 mV, n = 10, t = 2.273, P = 0.046, paired t-test). This result supports the possibility that both L-OT and H-OT modulate astrocytic plasticity by differently modulating astrocytic Kir4.1 currents, a finding similar to the effect of hypotonic solution on astrocytic plasticity [22].
Fig. 3.
Effect of L-OT and H-OT on Ba2+- sensitive inwardly rectifying K+ current Kir4.1 of the primary cultures of hypothalamic astrocytes. A and B Kir4.1 currents in representative waveforms (a) and in the current–voltage curves (b) in response to L-OT (A) or H-OT (B) application for 2 min, respectively. Note that, the membrane potential of single astrocytes was held at − 70 mV with voltage steps of 20 mV increments from − 160 to + 80 mV; *P < 0.05 compared to the CTR by paired t-test. Other annotations refer to Fig. 1
Firing Activity of OTNs After Blocking Kir4.1 in Brain Slices
The minor effect of L-OT on Kir4.1 at − 160 mV raises a question whether changes in Kir4.1 currents are involved in L-OT modulation of OTN activity via altering astrocytic plasticity. To answer this question, SON slices were incubated with BaCl2 at 100 µmol/L before L-OT application. As shown in Fig. 4, BaCl2 itself did not significantly change the basal firing rate of OTNs, suggesting that BaCl2 at 10 µmol/L does not significantly influence the basal activity of both astrocytes and OTNs although Ba2+ is known to block the TWIK-related potassium channel-1, large conductance, Ca2+- activated K+ channel, and Kir2.1 at higher concentrations [37–39]. Moreover, the excitatory effect of L-OT on OTNs became insignificant with BaCl2 application (CTR: 6.07 ± 1.1 Hz; BaCl2: 4.5 ± 1.1 Hz; BaCl2 + L-OT: 4.77 ± 1.3 Hz, n = 8, F = 0.5504, P = 0.587 by ANOVA). This finding, similar to the effect of hypotonic solution on astrocytic plasticity [22], indicates that astrocytic Kir4.1 may not influence the basal activity of astrocytes significantly, but is essential for L-OT-evoked astrocytic plasticity and its subsequent actions on OTNs.
Fig. 4.
Effects of blocking Kir4.1 on L-OT-modulated OTN firing activity in the brain slices. A Representative full recording (top panel) and expanded episodes (bottom panel) showing changes in OTN firing rate in response to L-OT in the presence of BaCl2. B Line (a) and dotted (b) graphs showing the results of statistical analysis of L-OT effects in the presence of BaCl2. Other annotations refer to Fig. 1
Effects of OT on Astrocyte Volume
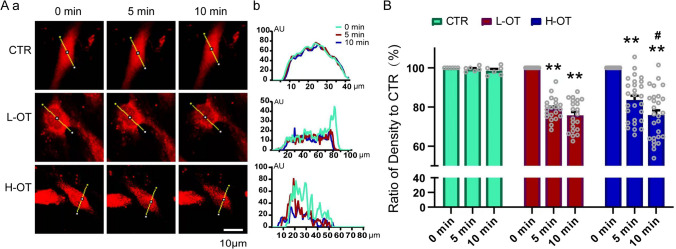
Kir4.1 expressed exclusively in glial cells, is implicated in extracellular K+ homeostasis, maintenance of astrocyte resting membrane potential, regulation of cell volume, and facilitation of glutamate uptake [35]. While all these functions can influence astrocytic plasticity, Kir4.1 involvement in astrocytic volume regulation in the SON was confirmed in our recent study [22] and thus, we tested effects of L-OT and H-OT on astrocytic volumes in SR101-loaded astrocytes (10 μmol/L, 37 °C, 20 min), a method that has been used to evaluate astrocytic volume previously [22, 23]. Since reduction in the high-contrast fluorescence intensity of SR101 in astrocytic somata and their processes reflects increase in astrocytic volume [40], we assayed the changes in the fluorescence intensity as an index of volemic alteration. As shown in Fig. 5, there was no obvious change in the fluorescence intensity in the CTR group over 10 min. Both L-OT and H-OT significantly increased the volume of astrocytes at 5 min as indicated by the decrease in the fluorescence intensity (L-OT: 79.0 ± 1.24% of the CTR, n = 21, t = 5.122, P < 0.0001; H-OT: 83.6 ± 2.10%, n = 27, t = 4.067, P < 0.0001 by Bonferroni test after ANOVA). At 10 min, the decrease in the fluorescence intensity did not change significantly compared to that at 5 min in response to L-OT (L-OT: 75.8 ± 1.71%, n = 21, t = 5.122, P < 0.0001 vs. CTR; P > 0.05 vs. 5 min, by Bonferroni test after ANOVA); however, H-OT for 10 min caused further decrease in the fluorescence intensity compared to that at 5 min (75.9 ± 2.44%, n = 27, t = 4.067, P < 0.0001 vs. CTR and P < 0.05 vs. 5 min by Bonferroni test after ANOVA). This result indicates that L-OT and H-OT can differently increase astrocytic volume, at least at the somata, a finding in agreement with that occurs at different stages of suckling [19, 20].
Fig. 5.
Effect of L-OT and H-OT on astrocytic volume in the primary cultures. A Representative images (a) and time-associated curves of fluorescence intensity (b) of SR101-loaded hypothalamic astrocytes, wherein the line in b represents the fluorescence density at the yellow straight line in Aa. B Bar-graphs summarizing time-dependent changes in total fluorescence intensity, and dotted graphs showing individual intensity of the cells tested. **, P < 0.01 compared to 0 min; #, P < 0.05 compared to 5 min. AU, arbitrary units
Effects of OT on the Expression of GFAP Filaments in Cultured Astrocytes
GFAP is the major cytoskeletal component of mature astrocytes and is essential for the maintenance of astrocytic structure and functions [41]. Assaying GFAP expression is not only crucial for understanding astrocytic reactivity, neuroinflammation, and neural injury responses [42, 43], but also helpful for determining astrocytic volume dynamics [20]. Here, we observed OT’s effects on the expression of GFAP in cultured astrocytes wherein GFAP filaments are primarily detectable at the somata. The results showed that L-OT significantly increased the intensity of GFAP filaments (CTR: 49.0 ± 5.4 A.U., n = 14, L-OT: 63.4 ± 3.2 A.U., n = 15, H-OT: 50.2 ± 4.7 A.U., n = 12, F = 3.361, P = 0.045 by least significant difference test after ANOVA, Fig. 6A) but decreased the expression of 50 KDa GFAP monomers in these cells (L-OT: 0.71 ± 0.10, n = 9, t = 2.983, P = 0.018 by Welch’s t-test; H-OT: 1.04 ± 0.30, n = 8, P > 0.05, Fig. 6B). The opposite change in GFAP filament and GFAP monomer in response to L-OT is in agreement with a previous report that mild increase in OT levels at earlier stage of suckling causes somatic swelling and retraction of the distal processes while high OT levels following the milk-ejection reflex exerts an opposite effect [19, 20].
Fig. 6.
Effect of L-OT and H-OT on GFAP expressions in the primary cultures of hypothalamic astrocytes. A Representative imaging showing the fluorescence intensity of GFAP filaments (a) and dotted graphs summarizing the statistical analysis of OT effects (b). B Representative Western blotting bands (a) and dotted graphs showing statistical analysis of OT effects (b). *, P < 0.05 compared to the CTR; #, P < 0.05 compared to L-OT
Effects of OT on the Expression of Astrocytic G Proteins
OTR-positive astrocytes were found in multiple brain regions including the SON [31], and selective OTR deletion in GFAP+ astrocytes reduces OT-evoked Ca2+ signaling [16, 30], indicating that OT’s effects on astrocytic plasticity (e.g., Kir4.1 currents, volume changes, and GFAP expression) are mediated by astrocytic OTR. As OTR is a single gene product [26], the differential effects of L-OT and H-OT on astrocytes are more likely associated with OTR-coupled G proteins [15]. To test this hypothesis, we examined the effects of L-OT and H-OT on the expression of α subunits of Gq, Gs and Gi proteins in cultured astrocytes using immunocytochemistry (Fig. 7). Relative to the CTR (47.24 ± 4.08 A.U., n = 26), L-OT significantly increased expressions of the α subunits of Gq proteins while H-OT did not significantly influence it (L-OT: 69.85 ± 5.36 A.U., n = 15, t = 3.606, P = 0.0019 compared to CTR; H-OT: 45.38 ± 3.48 A.U., n = 22, t = 3.779, P = 0.0011 compared to L-OT, by Bonferroni’s multiple comparisons test after ANOVA). In response to L-OT and H-OT for 10 min, Gs intensity reduced significantly, particularly following H-OT (CTR: 83.55 ± 0.50 A.U., n = 13; L-OT: 63.81 ± 4.50 A.U., n = 15, t = 3.391, P = 0.0036 compared to CTR; H-OT: 51.8 ± 7.21 A.U., n = 9, t = 4.765, P = 0.0006 compared to CTR, by Dunn’s multiple comparisons test after Kruskal–Wallis test). In the expression of Gi proteins, L-OT had no significant effect (59.38 ± 4.47 A.U., n = 23) compared to the CTR (45.14 ± 3.49 A.U., n = 26, P = 0.0623) while H-OT significantly increased Gi proteins compared to the CTR (76.12 ± 3.84 A.U., n = 7, P = 0.0004 compared to CTR, by Dunn’s multiple comparisons test after Kruskal–Wallis test). Notably, L-OT also decreased the ratio of GFAP colocalized with Gi protein (CTR, 99.11 ± 0.37% vs. L-OT, 94.49 ± 1.17%, P = 0.0009 by Dunn’s multiple comparisons test after Kruskal–Wallis test). This finding suggests that L-OT and H-OT can increase the release of Gαq subunits and Gαi subunits, respectively, since isolated Gα subunits have greater accessibility and flexibility to the antibody than the Gα subunits when it is within the Gαβγ heterotrimer [44]. This result supports that L-OT increases GFAP polymerization in the somatic compartment of astrocytes in the SON by enhanced OTR-Gq signaling, while H-OT likely activates Gi protein to reduce somatic GFAP filaments [20, 45]. Moreover, Gs proteins are suppressed by both L-OT and H-OT at 10 min, particularly the effect of H-OT, and the latter finding is consistent with the suppression of adenylyl cyclase (AC) by Gi proteins. Conversely, reduced Gi-GFAP colocalization under L-OT suggests Gi inhibition in astrocytes, consistent with a prior proposal [15].
Fig. 7.
Effects of L-OT and H-OT on G protein expressions and the relative spatial distribution of GFAP filaments with Gq, Gs, and Gi in the primary cultures of hypothalamic astrocytes. A–C Representative fluorescence images showing the distribution of GFAP (green), Gq (A, red)/Gs(B, red)/Gi(C, red) and nuclei (blue) (a), respectively, and dotted graphs showing statistical analyses of the average intensity of Gq/Gs/Gi (left panel in b), and the ratio of GFAP co-localized with Gq/Gs/Gi proteins (right panel in b), respectively. Note that, yellow bar = 40 μm; **, P < 0.01, ***, P < 0.001 compared with CTR; ##, P < 0.01 compared with L-OT. Arrows represent the sites where the two proteins are differentially expressed
G Protein Signaling Pathway Underlying the Effect of L-OT and H-OT
While L-OT and H-OT differently modulate astrocyte G protein signaling, the functional implications of distinct OTR-G protein signals remain to be examined. These G protein actions are mediated through their downstream signals, e.g., phospholipase C (PLC)-β for Gαq subunit, AC and PKA for Gαs subunit, and AC/PKA for Gαi subunit [15]. To explore the signaling pathway mediating L-OT actions, we pre-treated the slices with U73122 (10 μmol/L, a specific blocker of PLC [46]) and then recorded the firing rate of OTNs in brain slices. As shown in Fig. 8, compared with the CTR (4.89 ± 1.39 Hz, n = 8), U73122 application for 10 min did not significantly affect the basal firing rate of OTNs (4.56 ± 1.78 Hz, n = 8, t = 0.462, P = 0.658 by Paired t-test). However, in the presence of U73122, the excitatory effect of L-OT on the firing rate disappeared (3.33 ± 1.83 Hz, n = 8, F = 1.217, P = 0.320, by repeated measurement ANOVA) (see Fig. 8A). This result indicates that the excitatory effect of L-OT on OTNs is mediated by OTR-Gαq subunit signaling in astrocytes and/or OTNs.
Fig. 8.
Effect of L-OT and H-OT on OTN firing activity after pharmacological modulation of different G protein signaling pathways in the brain slices. A–D Exemplary firing activity of OTNs in patch-clamp recordings (a) and statistical analyses in line graphs (b) following the pre-incubation of U73122 (A), SQ22536 (B), forskolin (C), and pertussis toxin (PTX, D), respectively. *, P < 0.05 by paired t-test
In OTR-G protein signaling, PKA, downstream of cyclooxygenase 2-prostaglandin-Gs protein-AC pathway [15], is an essential mediator of OT/prostaglandin-evoked activation of OTNs [47, 48]. Thus, we also pre-treated the slices with SQ22536 (50 μmol/L, an AC inhibitor of the Gαs pathway [49] and then observed L-OT effects. The presence of SQ22536 inhibited the firing activity of OTNs (CTR: 5.43 ± 1.00 Hz vs. SQ22536: 3.23 ± 0.84 Hz, n = 9, t = 3.156, P = 0.0135 by Paired t-test). Further administration of L-OT failed to induce the increase of OTN firing rate (2.53 ± 0.64 Hz, t = 0.761, P > 0.999 by Bonferroni’s multiple comparisons test after repeated measurement ANOVA; Fig. 8B). This finding is consistent with the reports of Li and colleagues [47, 48], supporting the key roles of activation of OTR-associated Gs proteins in the basal excitability and excitation of OTNs evoked by L-OT.
Relative to the effect of L-OT, effects of H-OT are likely mediated by the inhibition of AC/PKA signaling through activating Gi proteins [50]. To test this possibility, we first confirmed the excitatory effect of activation of AC with 10 μmol/L forskolin, an activator of AC [51] (CTR: 3.88 ± 0.45 Hz; forskolin: 4.61 ± 0.50 Hz, n = 4, t = 3.377, P = 0.043 by Paired t-test). Following the inhibition of H-OT on the basal firing rate of OTNs (2.86 ± 0.78 Hz in CTR vs. 1.67 ± 0.52 Hz in H-OT for 5 min, n = 9, t = 3.273, P = 0.0113 by paired t-test), administration of forskolin for 10 min reversed the inhibitory effect of H-OT (2.85 ± 0.96 Hz, F = 1.949, P = 0.195 by repeated measures ANOVA summary, Fig. 8C). Moreover, after blocking Gi/o-proteins with pertussis toxin (PTX, 1 µg/ml; > 6 h), both L-OT and H-OT further increased the firing rate of OTNs (2.76 ± 0.94 Hz in CTR vs. 3.64 ± 0.86 Hz in L-OT for 5 min, n = 5, P < 0.05 by paired t-test; 3.24 ± 0.50 Hz in CTR vs. 3.92 ± 0.50 Hz in H-OT for 5 min, n = 5 P < 0.05 by paired t-test; Fig. 8D). These results support the mediation of OTR-coupled Gi/o proteins in H-OT inhibition of OTN activity as proposed [15].
Discussion
Dose-Dependent Effects of OT on OTNs and Astrocytic Plasticity
The present study demonstrated that L-OT exerts excitatory effects on OTNs whereas H-OT inhibits their activity. This finding supports the previous report about the dose- and time-dependent OT effects on OTNs [9]. Moreover, disruption of astrocytic plasticity using the gliotoxin L-AAA abolished both the excitatory effect of L-OT and the inhibitory effect of H-OT on OTN activity in hypothalamic tissues. This finding together with our previous reports [19, 20] confirms the essential role of astrocytic plasticity in the autoregulation of OTN activity [52]. Certainly, OT can modulate OTN activity through direct actions on OTNs or indirect effects on presynaptic neurons; however, astrocytic plasticity is clearly a dominant mediator of OT effects on OTNs. This proposal is supported by the fact that OTR is present in astrocytes [16, 30] including those in the SON [31] and that L-OT induces depolarization of astrocytic membrane potential in the SON [19]. Thus, it is possible for L-OT to function through inhibiting Kir4.1 activity and redistributing astrocytic volume from the distal processes to the somata. By contrast, H-OT, via activating Kir4.1 currents and causing expansion of astrocytic processes, decreases OTN activity.
It is well-established that volume transfer or redistribution from the distal processes to the somata of astrocytes and the resultant retraction of astrocytic processes can enhance synaptic innervation of neurons, the coupling of nearby neurons through gap junctions, and apposition of adjacent neurons [20]. These structural changes simultaneously reduce the absorption of extracellular K⁺ and glutamate accumulating during neural activation, thereby elevating neuronal excitability and firing rates. Conversely, expansion of astrocytic processes decreases synaptic innervation, neuronal coupling, and apposition density while enhancing the absorption of ionic/neurotransmitter released during neural activation [20], ultimately suppressing neuronal excitability [17, 53]. For instance, an increase in perisynaptic astrocytic processes enhances astrocytic coverage of synapses, which increases glutamate uptake by glutamate transporters and decreases glutamate releasing into the extracellular space and extrasynaptic activation [54]. Resultantly, their adjacent neurons receive diminished excitatory synaptic input and neurochemical activation, thereby exhibiting reduced excitability. Conversely, a decrease in synaptic coverage by the perisynaptic astrocyte processes increases neuronal excitability. The question is how L-OT and H-OT differentially modulate OTN activities through modulating astrocytic plasticity in both cultures and slice preparations.
Molecular Mechanisms for L-OT and H-OT Modulation of Astrocytic Plasticity
The present study revealed that L-OT and H-OT can differentially modulate astrocyte-specific Kir4.1 activity, GFAP expression, and astrocytic volume. These OT concentration-dependent effects on Kir4.1 are consistent with the dose-dependent effects of OT on OTN activity. L-OT-induced inhibition of Kir4.1 currents could reduce astrocytic absorption of extracellular K+ and glutamate [35], thereby increasing excitatory neurochemical environment and OTN firing rate. A major question is about the extent of Kir4.1 contribution to L-OT actions. As shown in Fig. 3, L-OT had only a significant effect on BaCl2-sensitive Kir4.1 at − 160 mV (Fig. 3), which cannot fully explain L-OT effect on the spontaneous firing of SON neurons around the resting membrane potential. However, we could not exclude the possibility that BaCl2-sensitive Kir4.1 functions across a broad scope of membrane potential, since Ba2+ itself had no significant effect on the firing rate of OTNs but did block L-OT action around the resting membrane potential in brain slices. It is also noticeable that the inhibitory trend of L-OT on Kir4.1 current existed over a broad scope from − 160 mV to − 20 mV, which possibly turns to a statistically significant level in a larger sample test. Consistently, our recent study showed that the blocking effect of Ba2+ at 100 µmol/L on Kir4.1 is the same as the action of a specific Kir4.1 blocker, VU0134992. Similar to the L-OT effects, the effect of hypotonic challenge on Kir4.1 was significant only at − 160 mV in cultured astrocytes; however, both Ba2+ at 100 µmol/L and VU0134992 blocked post-inhibition excitation of vasopressin neurons in the SON in brain slices [22]. It was also reported that reduced Kir4.1 activity can lead to astrocyte swelling, prominently at astrocyte processes where changes in Kir4.1 activity are stronger than that in astrocytic somata [55]. Thus, it is reasonable to propose that the action of Ba2+ on astrocytic process Kir4.1 in slices may be stronger and broader than that observed in the somata of cultured astrocytes and that the effect of Ba2+ blockade of L-OT-evoked excitation is, at least partially, attributable to the blockade of Kir4.1, although the involvement of other mechanisms [56] in astrocytes and OTNs remains to be identified.
By contrast, H-OT enhances Kir4.1 activity and causes delayed astrocytic swelling relative to L-OT in cultured astrocytes. The increased Kir4.1 currents can facilitate inward K⁺ flow and glutamate uptake, thereby reducing extracellular K⁺ and glutamate levels and then OTN firing rate [35]. Such modulations may occur following burst firing in OTNs in the milk-ejection reflex, during which high-frequency firing induces Ca2⁺ influx that could trigger a bolus release of OT from the somatodendritic sites [21, 57], creating a condition like H-OT. Thus, H-OT may reduce extracellular K+ and glutamate levels by strengthening astrocytic Kir4.1 activity.
Consistent with functions of Kir4.1 in OT actions, L-OT and H-OT may also modulate OTN activity by regulating astrocytic volume, particularly at the distal processes around OTNs. In cultured astrocytes, L-OT increased GFAP filaments, which is in agreement with the quick increase in the somatic volume; the slow increases in astrocytic volume in response to H-OT were associated with insignificant change in GFAP filaments, mainly visible at the somata, suggesting less expansion of astrocytic somata and more expansion of the distal processes. If this H-OT effect occurs in brain slices, it should inhibit the activity of their adjacent neurons in general view [17]. Although these alterations in cultured astrocytes cannot be linked to the effects of L-OT and H-OT in brain slices directly, comparative analysis of OT actions on cultured astrocytes versus slice astrocytes provides valuable clues supporting the differential effects of OT-evoked astrocytic plasticity on OTN activity.
In cultured astrocytes, OT acts on the entire cell evenly, which is mainly detected at the somata; however, in the SON tissues, OT released from OTNs primarily targets the distal processes of astrocytes surrounding OTNs’ somata, thereby eliciting different responses from the cultures. Our previous studies have revealed that L-OT causes volume transfer from distal processes to the somata, as indicated by changes in GFAP filament expression and volumetric measurements using Bauer-peptide illustration. This redistribution results from increased PKA expression in the processes and enhanced pERK1/2 expression in the soma. By contrast, following bust firing in OTNs, decreased somatic pERK1/2 expression and separation of PKA from GFAP in the process drive volume transfer from astrocytic soma to the distal processes [19, 20]. It means that the increases in somatic GFAP filaments and volume evoked by L-OT in cultured astrocytes reflect reduced GFAP filaments and volume in the processes of astrocytes in brain slices, while the gradual increase in somatic volume during H-OT corresponds to the expansion of astrocytic processes in the slices. Therefore, excitation of OTNs under the influence of L-OT possibly results from the regulatory volume reduction of astrocytic processes alongside inhibition of Kir4.1 activity around OTNs, whereas the inhibition effect of H-OT is likely due to (re)expansion of astrocytic processes alongside activation of Kir4.1 activity.
Differential OT Effects Mediated by Different G Proteins-Coupled/Associated with OTR
The present study further revealed that astrocytic OTR-coupled Gq proteins and associated Gs-proteins likely mediate L-OT effects, whereas OTR-coupled Gi proteins mediate H-OT effects on OTNs. As reported, astrocytes express OTR [16, 30] [31] that is encoded by a single gene and thus, the differential actions of L-OT and H-OT on astrocytic plasticity are more likely due to dose-associated activation of different OTR-coupled G proteins as proposed [15]. This proposal is supported by present findings that L-OT increases Gαq subunit mobilization and inhibits Gαi subunit release, while H-OT promotes Gαi subunit release (Fig. 7).
Further functional study clearly supports this proposal. As shown in Fig. 8, blocking PLC, the downstream signals of Gq proteins, also blocked the excitatory effect of L-OT on OTNs in the SON. This aligns with the report that Kir inhibition by OT was mediated by the Gq-PLC activation in the immortalized gonadotropin-releasing hormone-positive GN11 cell line [50]. The present results support the involvement of astrocytic OTR-Gαq-PLC pathway in L-OT-induced OTN excitation although L-OT can activate OTNs directly through many other approaches [15]. Between this pathway and Kir4.1, Ca2⁺ mobilization may serve as a mediator since calcineurin inhibitors can stimulate Kir4.1 activity [58]. Thus, L-OT-induced Kir4.1 suppression likely results from mildly elevated Ca2⁺ levels downstream to Gαq subunit.
Furthermore, inhibition of Gs signaling with the AC blocker reduced both basal firing rate and the excitatory effect of L-OT, suggesting that the AC-PKA pathway not only maintains basal OTN activity but also facilitates L-OT responses. Although that L-OT slightly reduced the expression of Gαs subunits could argue against L-OT activation of Gs proteins, it can be explained by a post-activation inhibition, receptor internalization, or other mechanisms remaining to be identified. Importantly, the reduction of GFAP colocalization with Gαi subunits under the influence of L-OT indicates the dominance of AC/PKA signaling. Consistent with this fact, that activation of AC with forskolin increased OTN firing activity, even after H-OT treatment, further supports the involvement of the Gs-AC-PKA pathway in L-OT-evoked OTN excitability as reported [47, 48], which could occur in astrocytes in the SON [59].
The functional study also supports that the inhibitory effect of H-OT on OTN activity is associated with enhanced Gi protein activation. Clearly, application of the AC activator forskolin effectively abolished the inhibitory effect of H-OT on OTNs, while blocking Gi proteins with PTX reversed the inhibitory effect of H-OT. It was consistent with the report that OT activation of Kir currents is through Gi/o proteins [50]. These findings indicate that OTR-coupled Gi protein activation is a key mechanism underlying H-OT-mediated inhibition, potentially involving direct OTR-Gi coupling as well as suppression of OTR-Gq and Gs signaling [15].
Strengths and Limitations
The biodiversity and limited number of sampling in a study restrain the power of a single type of sampling and bio-technique to reveal the natural mechanisms underlying a bio-activity. Correspondingly, combining cell cultures and tissue sections becomes a common technique in biological research. Using cell cultures helps explore the inherent property of cellular events under clear environmental factors while the use of tissue sections allows identification of cellular interactions under more natural conditions. Moreover, combination of different methods in one study can better reflect the underlying mechanisms than any single method. Based on this idea, in combination with previous findings, our results provide this set updated information about astrocytic G proteins-associated dose-dependent effects of the autoregulation of OTN activity.
The limitations of the current study is also clear, such as the lack of a direct mechanistic link between the observed phenomena, the temporal mismatching between astrocytes in cultures and in slices, weaker data showing dynamic changes in OTR and G protein signaling in different compartments of the astrocytes, the relatively non-specificity of pharmacological agents and poor localization of G protein signals from astrocytes versus OTNs in OT actions when it is applied in slices, etc. Thus, further studies should consider overcoming these limitations using different models and methods, such as optogenetics and a combination of patch-clamping recording with real-time confocal microscopy to show spatiotemporal features of these molecular and functional activities. Hence, astrocytic-neuronal signaling pathway involving astrocytic OTR-G protein-astrocytic plasticity-OTN activity would be better clarified.
Conclusion
As a whole, the mechanisms underlying astrocytic OTR-coupled G protein mediation of L-OT and H-OT effects on OTNs can be proposed as the followings. L-OT primarily activates astrocytic OTR-Gq proteins and the associated Gs proteins in certain spatiotemporal order. This cascade triggers a redistribution or transfer of GFAP filaments and cytosolic volume from the distal processes to astrocytic somata, which increases interneuronal interactions. Concurrent inhibition of Kir4.1 reduces K⁺/glutamate clearance, thereby elevating their extracellular concentrations. They together enhance OTN excitability. By contrast, H-OT activates Gi proteins directly or following a transient activation of OTR-Gq proteins, leading to the suppression of AC-PKA signaling, which allows GFAP repolymerization and volemic expansion in the distal processes while potentiating Kir4.1 activity. Resultantly increased extracellular K+ and glutamate uptake and decreased in interneuronal interactions reduce the excitatory environment around OTNs, and ultimately inhibit OTN activity (Supplemental Fig. 1). Since glial neuronal interactions in the SON is a model system in neuroscience research [60] while dose-dependent responses of neurons are a common phenomenon of the neural activity, clarification how distinct G proteins mediate OT’s dose-dependent effects on astrocytic plasticity and neuronal activity in the SON will greatly facilitate our understandings of the cellular and molecular mechanisms underlying neural activity.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Drs. Ping Wang, Hui Zhu, Dan Cui, Donyang Li and Tong Li for advices, and critical reading and/or technical assistance of Hongyang Wang, Jiawei Yu and Guichuan Chen.
Author Contributions
XYL and SL wrote the first draft, SL, XYL, YJ, YL,CH, YPL collected and analyzed data; XW, SJ, and DQ provided critical ideas; XYL supervised the study; XYL and YFW designed the study, and YFW edited the final draft.
Funding
The research was supported by the Fund of the Ministry of Science and Technology of China (grant No. G2021011014L); Excellent Young Teachers Basic Research Support Program of Heilongjiang Provincial (grant No.YQJH2023031); the Fundamental Research Funds for the Provincial Universities (XYL, 2023); and Core Teacher Program of Harbin Medical University (grant No.JJ2023LH1248). The sponsors are not involved in study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the article for publication.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval
This study was performed in accordance with the Guideline of National Institutes of Health and approved by the Institutional Animal Care and Use Committees of Harbin Medical University (HMU2023-03–05).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiao-Yu Liu and Shuo Ling contributed equally to this work.
Contributor Information
Xiao-Yu Liu, Email: liuxiaoyuhayida@163.com.
Yu-Feng Wang, Email: 20231059@zcmu.edu.cn.
References
- 1.Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, Ferris CF, Nazarloo HP et al (2020) Is oxytocin “nature’s medicine”? Pharmacol Rev 72(4):829–861. 10.1124/pr.120.019398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higuchi T, Tadokoro Y, Honda K, Negoro H (1986) Detailed analysis of blood oxytocin levels during suckling and parturition in the rat. J Endocrinol 110(2):251–256 [DOI] [PubMed] [Google Scholar]
- 3.Lincoln DW, Wakerley JB (1975) Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol 250(2):443–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine RA, Seymour AJ, Campbell RE, Grattan DR, Brown CH (2018) Integrative neuro-humoral regulation of oxytocin neuron activity in pregnancy and lactation. J Neuroendocrinol. 10.1111/jne.12569 [DOI] [PubMed] [Google Scholar]
- 5.Leng G, MacGregor DJ (2018) Models in neuroendocrinology. Math Biosci 305:29–41. 10.1016/j.mbs.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 6.Armstrong WE, Foehring RC, Kirchner MK, Sladek CD (2019) Electrophysiological properties of identified oxytocin and vasopressin neurones. J Neuroendocrinol 31(3):e12666. 10.1111/jne.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leithead AB, Tasker JG, Harony-Nicolas H (2021) The interplay between glutamatergic circuits and oxytocin neurons in the hypothalamus and its relevance to neurodevelopmental disorders. J Neuroendocrinol 33(12):e13061. 10.1111/jne.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Jiang Y, Wang X, Liu H, Li D, Ling S, Jia S, Liu X et al (2023) Excitatory effects of astrocytic hydrogen sulfide on the electrical activity of oxytocin neurons in the supraoptic nucleus. Neuroendocrinology 113(3):343–360. 10.1159/000526812 [DOI] [PubMed] [Google Scholar]
- 9.Wang YF, Ponzio TA, Hatton GI (2006) Autofeedback effects of progressively rising oxytocin concentrations on supraoptic oxytocin neuronal activity in slices from lactating rats. Am J Physiol Regul Integr Comp Physiol 290(5):R1191-1198. 10.1152/ajpregu.00725.2005 [DOI] [PubMed] [Google Scholar]
- 10.Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C (2015) Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health 14:13. 10.1186/1476-069x-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Kocher SD, Lewis MM, Mailman RB (2022) Dose-dependent regulation on prefrontal neuronal working memory by dopamine D(1) agonists: evidence of receptor functional selectivity-related mechanisms. Front Neurosci 16:898051. 10.3389/fnins.2022.898051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos D, Gonzalez-Perez F, Navarro X, Del Valle J (2016) Dose-dependent differential effect of neurotrophic factors on in vitro and in vivo regeneration of motor and sensory neurons. Neural Plast 2016:4969523. 10.1155/2016/4969523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawal O, Ulloa Severino FP, Eroglu C (2022) The role of astrocyte structural plasticity in regulating neural circuit function and behavior. Glia 70(8):1467–1483. 10.1002/glia.24191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busnelli M, Chini B (2018) Molecular basis of oxytocin receptor signalling in the brain: what we know and what we need to know. Curr Top Behav Neurosci 35:3–29. 10.1007/7854_2017_6 [DOI] [PubMed] [Google Scholar]
- 15.Liu XY, Ling S, Liu Y, Jia S, Wang X, Li T, Li D, Hou C et al (2024) Dual oxytocin receptor-G protein signaling in the autoregulation of activities of oxytocin neurons. Neuroendocrinology 114(2):134–157. 10.1159/000534490 [DOI] [PubMed] [Google Scholar]
- 16.Wahis J, Baudon A, Althammer F, Kerspern D, Goyon S, Hagiwara D, Lefevre A, Barteczko L et al (2021) Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat Neurosci 24(4):529–541. 10.1038/s41593-021-00800-0 [DOI] [PubMed] [Google Scholar]
- 17.Wang SC, Parpura V, Wang YF (2021) Astroglial regulation of magnocellular neuroendocrine cell activities in the supraoptic nucleus. Neurochem Res 46(10):2586–2600. 10.1007/s11064-020-03172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo J, Hariri OR, Micevych P (2009) An interaction of oxytocin receptors with metabotropic glutamate receptors in hypothalamic astrocytes. J Neuroendocrinol 21(12):1001–1006. 10.1111/j.1365-2826.2009.01922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Qin D, Wang YF (2017) Oxytocin rapidly changes astrocytic GFAP plasticity by differentially modulating the expressions of pERK 1/2 and protein kinase A. Front Mol Neurosci 10:262. 10.3389/fnmol.2017.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YF, Hatton GI (2009) Astrocytic plasticity and patterned oxytocin neuronal activity: dynamic interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience 29(6):1743–1754. 10.1523/jneurosci.4669-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkinson MR, Kim JS, Iremonger KJ, Brown CH (2021) Visualising oxytocin neurone activity in vivo: the key to unlocking central regulation of parturition and lactation. J Neuroendocrinol 33(11):e13012. 10.1111/jne.13012 [DOI] [PubMed] [Google Scholar]
- 22.Jiang YH, Li T, Liu Y, Liu X, Jia S, Hou C, Chen G, Wang H et al (2023) Contribution of inwardly rectifying K(+) channel 4.1 of supraoptic astrocytes to the regulation of vasopressin neuronal activity by hypotonicity. Glia 71(3):704–719. 10.1002/glia.24306 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wang XR, Jiang YH, Li T, Ling S, Wang HY, Yu JW, Jia SW et al. (2023) Interactions between the astrocytic volume-regulated anion channel and aquaporin 4 in hyposmotic regulation of vasopressin neuronal activity in the supraoptic nucleus. Cells 12(13). 10.3390/cells12131723 [DOI] [PMC free article] [PubMed]
- 24.Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F (2004) Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1(1):31–37. 10.1038/nmeth706 [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Li T, Liu Y, Jia S, Liu X, Jiang Y, Wang P, Parpura V et al (2021) Aquaporin 4 differentially modulates osmotic effects on vasopressin neurons in rat supraoptic nucleus. Acta Physiol (Oxf) 232(3). 10.1111/apha.13672 [DOI] [PMC free article] [PubMed]
- 26.Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81(2):629–683. 10.1152/physrev.2001.81.2.629 [DOI] [PubMed] [Google Scholar]
- 27.Fischer S, Duffield C, Swaney WT, Bolton RL, Davidson AJ, Hurst JL, Stockley P (2024) Egalitarian cooperation linked to central oxytocin levels in communal breeding house mice. Communications biology 7(1):1193. 10.1038/s42003-024-06922-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terenzi MG, Ingram CD (2005) Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience 134(1):345–354. 10.1016/j.neuroscience.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 29.Bakos J, Srancikova A, Havranek T, Bacova Z (2018) Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast 2018:4864107. 10.1155/2018/4864107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinung CP, Boi L, Pandamooz S, Mazaud D, Ghezali G, Rouach N, Neumann ID (2024) OXTR-mediated signaling in astrocytes contributes to anxiolysis. Mol Psychiatry. 10.1038/s41380-024-02870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YF, Hatton GI (2006) Mechanisms underlying oxytocin-induced excitation of supraoptic neurons: prostaglandin mediation of actin polymerization. J Neurophysiol 95(6):3933–3947. 10.1152/jn.01267.2005 [DOI] [PubMed] [Google Scholar]
- 32.Takanashi K, Shibata K, Mizuno K, Komatsu R, Koizumi S (2021) Goshajinkigan attenuates paclitaxel-induced neuropathic pain via cortical astrocytes. Pharmacol Res Perspect 9(6):e00850. 10.1002/prp2.850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neill E, Chiara Goisis R, Haverty R, Harkin A (2019) L-alpha-aminoadipic acid restricts dopaminergic neurodegeneration and motor deficits in an inflammatory model of Parkinson’s disease in male rats. J Neurosci Res 97(7):804–816. 10.1002/jnr.24420 [DOI] [PubMed] [Google Scholar]
- 34.Hong S, Kim Y, Kwon Y, Cho SH (2024) Antidepressant effect of heracleum moellendorffii extract on behavioral changes in astrocyte ablation mouse model of depression by modulating neuroinflammation through the inhibition of lipocalin-2. Nutrients 16(13):2049. 10.3390/nu16132049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML (2016) The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta neuropathologica 132(1):1–21. 10.1007/s00401-016-1553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y et al (2018) Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554(7692):323–327. 10.1038/nature25752 [DOI] [PubMed] [Google Scholar]
- 37.Bello RA, Magleby KL (1998) Time-irreversible subconductance gating associated with Ba2+ block of large conductance Ca2+-activated K+ channels. J Gen Physiol 111(2):343–362. 10.1085/jgp.111.2.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma XY, Yu JM, Zhang SZ, Liu XY, Wu BH, Wei XL, Yan JQ, Sun HL et al (2011) External Ba2+ block of the two-pore domain potassium channel TREK-1 defines conformational transition in its selectivity filter. J Biol Chem 286(46):39813–39822. 10.1074/jbc.M111.264788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilles O (2022) K(+) -independent Kir blockade by external Cs(+) and Ba(2). Physiol Rep 10(5):e15200. 10.14814/phy2.15200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimmerjahn A, Helmchen F (2012) In vivo labeling of cortical astrocytes with sulforhodamine 101 (SR101). Cold Spring Harb Protoc 3:326–334. 10.1101/pdb.prot068155 [DOI] [PubMed] [Google Scholar]
- 41.Li D, Liu X, Liu T, Liu H, Tong L, Jia S, Wang YF (2020) Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia 68(5):878–897. 10.1002/glia.23734 [DOI] [PubMed] [Google Scholar]
- 42.Jurga AM, Paleczna M, Kadluczka J, Kuter KZ (2021) Beyond the GFAP-astrocyte protein markers in the brain. Biomolecules 11(9):1361. 10.3390/biom11091361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang IH, Sternweis PC (1990) Purification of unique alpha subunits of GTP-binding regulatory proteins (G proteins) by affinity chromatography with immobilized beta gamma subunits. J Biol Chem 265(30):18707–18712 [PubMed] [Google Scholar]
- 45.Kim JH, Rahman MH, Lee WH, Suk K (2021) Chemogenetic stimulation of the G(i) pathway in astrocytes suppresses neuroinflammation. Pharmacol Res Perspect 9(6):e00822. 10.1002/prp2.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alqudah M, Razzaq RA, Alfaqih MA, Al-Shboul O, Al-Dwairi A, Taha S (2022) Mechanism of oxytocin-induced contraction in rat gastric circular smooth muscle. International journal of molecular sciences 24(1):441. 10.3390/ijms24010441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D, Liu X, Liu H, Li T, Jia S, Wang X, Wang P, Qin D et al (2021) Key roles of cyclooxygenase 2-protein kinase a-hyperpolarization-activated cyclic nucleotide-gated channel 3 pathway in the regulation of oxytocin neuronal activity in lactating rats with intermittent pup-deprivation. Neuroscience 452:13–25. 10.1016/j.neuroscience.2020.10.016 [DOI] [PubMed] [Google Scholar]
- 48.Li D, Liu X, Li T, Wang X, Jia S, Wang P, Wang YF (2021) Involvement of protein kinase A in oxytocin neuronal activity in rat dams with pup deprivation. Neurochem Res 46(4):980–991. 10.1007/s11064-020-03218-5 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Huang Q, Xiong X, Yin T, Chen S, Yuan W, Zeng G, Huang Q (2023) Acacetin alleviates energy metabolism disorder through promoting white fat browning mediated by AC-cAMP pathway. J Physiol Biochem. 10.1007/s13105-023-00947-3 [DOI] [PubMed]
- 50.Gravati M, Busnelli M, Bulgheroni E, Reversi A, Spaiardi P, Parenti M, Toselli M, Chini B (2010) Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J Neurochem 114(5):1424–1435. 10.1111/j.1471-4159.2010.06861.x [DOI] [PubMed] [Google Scholar]
- 51.Tesic B, Samardzija Nenadov D, Tomanic T, Fa Nedeljkovic S, Milatovic S, Stanic B, Pogrmic-Majkic K, Andric N (2023) DEHP decreases steroidogenesis through the cAMP and ERK1/2 signaling pathways in FSH-stimulated human granulosa cells. Cells 12(3):398. 10.3390/cells12030398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YF, Hamilton K (2009) Chronic vs. acute interactions between supraoptic oxytocin neurons and astrocytes during lactation: role of glial fibrillary acidic protein plasticity. Scientific World Journal 9:1308–1320. 10.1100/tsw.2009.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theodosis DT, Poulain DA, Oliet SH (2008) Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88(3):983–1008. 10.1152/physrev.00036.2007 [DOI] [PubMed] [Google Scholar]
- 54.Zhou B, Zuo YX, Jiang RT (2019) Astrocyte morphology: diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther 25(6):665–673. 10.1111/cns.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dibaj P, Kaiser M, Hirrlinger J, Kirchhoff F, Neusch C (2007) Kir4.1 channels regulate swelling of astroglial processes in experimental spinal cord edema. J Neurochem 103(6):2620–2628. 10.1111/j.1471-4159.2007.04979.x [DOI] [PubMed] [Google Scholar]
- 56.Reed MM, Blazer-Yost B (2022) Channels and transporters in astrocyte volume regulation in health and disease. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 56(S2):12–30. 10.33594/000000495 [DOI] [PubMed] [Google Scholar]
- 57.Tobin VA, Bull PM, Arunachalam S, O’Carroll AM, Ueta Y, Ludwig M (2008) The effects of apelin on the electrical activity of hypothalamic magnocellular vasopressin and oxytocin neurons and somatodendritic peptide release. Endocrinology 149(12):6136–6145. 10.1210/en.2008-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang DD, Duan XP, Mutig K, Rausch F, Xiao Y, Zheng JY, Lin DH, Wang WH (2023) Calcineurin inhibitors stimulate Kir4.1/Kir5.1 of the distal convoluted tubule to increase NaCl cotransporter. JCI insight 8(7):e165987. 10.1172/jci.insight.165987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li D, Li T, Yu J, Liu X, Jia S, Wang X, Wang P, Wang YF (2021) Astrocytic modulation of supraoptic oxytocin neuronal activity in rat dams with pup-deprivation at different stages of lactation. Neurochem Res 46(10):2601–2611. 10.1007/s11064-020-03129-5 [DOI] [PubMed] [Google Scholar]
- 60.Perkinson MR, Augustine RA, Bouwer GT, Brown EF, Cheong I, Seymour AJ, Fronius M, Brown CH (2021) Plasticity in intrinsic excitability of hypothalamic magnocellular neurosecretory neurons in late-pregnant and lactating rats. International journal of molecular sciences 22(13):7140. 10.3390/ijms22137140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.