Abstract
Inositol acylation is an obligatory step in glycosylphosphatidylinositol (GPI) biosynthesis whereas mature GPI anchors often lack this modification. The GPI anchors of Trypanosoma brucei variant surface glycoproteins (VSGs) undergo rounds of inositol acylation and deacylation during GPI biosynthesis and the deacylation reactions are inhibited by diisopropylfluorophosphate (DFP). Inositol deacylase was affinity labelled with [3H]DFP and purified. Peptide sequencing was used to clone GPIdeAc, which encodes a protein with significant sequence and hydropathy similarity to mammalian acyloxyacyl hydrolase, an enzyme that removes fatty acids from bacterial lipopolysaccharide. Both contain a signal sequence followed by a saposin domain and a GDSL-lipase domain. GPIdeAc–/– trypanosomes were viable in vitro and in animals. Affinity-purified HA-tagged GPIdeAc was shown to have inositol deacylase activity. However, total inositol deacylase activity was only reduced in GPIdeAc–/– trypanosomes and the VSG GPI anchor was indistinguishable from wild type. These results suggest that there is redundancy in T.brucei inositol deacylase activity and that there is another enzyme whose sequence is not recognizably related to GPIdeAc.
Keywords: acyloxyacyl hydrolase/glycosylphosphatidyl inositol/inositol deacylase/saposin/Trypanosoma brucei
Introduction
Glycosylphosphatidylinositol (GPI) membrane anchors are widely distributed among the eukaryotes. They anchor proteins to the outer leaflet of the plasma membrane and are thought to be associated with other functions, such as signal transduction and protein targeting, via association with membrane microdomains or ‘lipid rafts’ (Simons and Ikonen, 1997).
Protein-linked GPI anchors and GPI-related glycolipids are particularly abundant in protozoa (Ferguson, 1999). The tsetse-transmitted African trypanosomes that cause human sleeping sickness are protected in the mammalian bloodstream by a surface coat of GPI-anchored variant surface glycoprotein (VSG) (Cross, 1996) and GPI biosynthesis has been validated as a therapeutic target (Nagamune et al., 2000).
GPI biosynthesis has been studied in several organisms (reviewed recently by Ferguson et al., 1999; Kinoshita and Inoue, 2000; McConville and Menon, 2000; Morita et al., 2000). In all cases, GPI biosynthesis involves the addition of GlcNAc to phosphatidylinositol (PI) to give GlcNAc-PI, which is then de-N-acetylated to form GlcN-PI. Notable differences between the Trypanosoma brucei and mammalian GPI biosynthetic pathways occur from GlcN-PI onwards (Figure 1), including the addition of extra ethanolamine phosphate groups to mammalian anchors (Puoti and Conzelmann, 1993), fatty-acid remodelling of T.brucei anchors (Masterson et al., 1990) and the timing of inositol acylation and deacylation (Güther and Ferguson, 1995; Doerrler et al., 1996). In T.brucei, inositol acylation (i.e. the addition of a fatty acid to the 2-OH of the myo-inositol residue) occurs only after the addition of the first Man residue to GlcN-PI and intermediates from Man1GlcN-(acyl)PI onwards are in equilibrium between their inositol acylated and non-acylated forms through the action of inositol acyltransferase(s) and inositol deacylase(s). In mammalian cells, inositol acylation occurs at the level of GlcN-PI and strictly precedes mannosylation to Man1GlcN-(acyl)PI. Furthermore, once added, the acyl chain remains attached until the mature GPI anchor is added to protein in the endoplasmic reticulum (ER). Thereafter, it may remain or be removed by an inositol deacylase (Chen et al., 1998), depending on the cell type (Wong and Low, 1992).
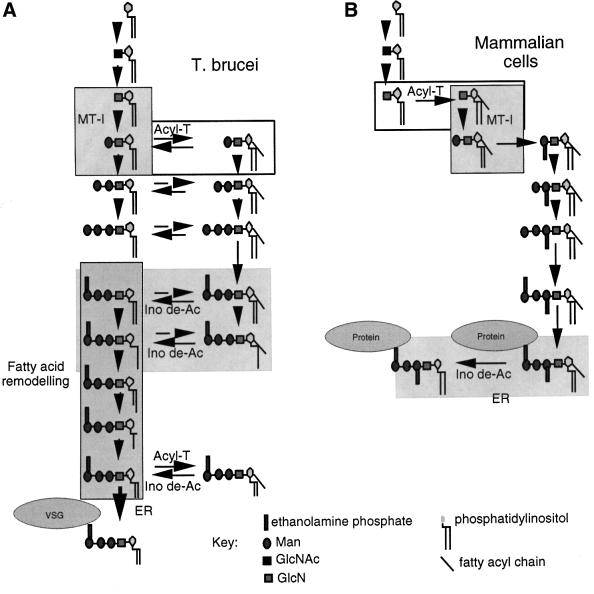
Fig. 1. Biosynthetic schemes for GPI assembly and transfer in (A) bloodstream-form T.brucei and (B) human mammalian cells. MT-1, GPI mannosytransferase I; Acyl-T, inositol acyltransferase; Ino de-Ac, inositol deacylase.
Several genes associated with GPI biosynthesis have been cloned by complementation of GPI– mammalian and yeast mutants (Miyata et al., 1993; Hamburger et al., 1995; Leidich et al., 1995; Benghezal et al., 1996; Nakamura et al., 1997; Maeda et al., 2001) and by identifying proteins that associate physically with known components of the pathway (Watanabe et al., 2000). However, thus far, no inositol deacylase mutants have been identified.
Here, we affinity label and purify a T.brucei inositol deacylase, clone the corresponding gene, confirm the activity of the recombinant enzyme and analyse the biochemical phenotype of the gene knockout mutant.
Results
Affinity labelling and extraction of the putative trypanosome inositol deacylase
We reported previously the inhibition of T.brucei inositol deacylase with diisopropylfluorophosphate (DFP) and its insensitivity to a range of serine esterase/protease inhibitors (Güther and Ferguson, 1995). Aliquots of washed trypanosome membranes (1010 cell equivalents) were pre-incubated with a cocktail of esterase and protease inhibitors and subsequently labelled with [3H]DFP. The membranes were washed until there was no radioactivity in the supernatant and then stripped of peripheral membrane proteins and soluble ER lumen proteins with 1 M KCl and sodium carbonate buffer, respectively. The latter treatments released very little radioactivity from the membranes. Various concentrations of the detergents CHAPS, Zwittergent 3-16 and nOG were used to extract the membranes. The best yield of solubilized radioactivity was achieved with 8% (w/v) nOG. Following initial solibilization, the nOG concentration could be reduced without compromising solubility of the [3H]DFP-labelled components.
Characterization and purification of the putative trypanosome inositol deacylase
Equal numbers of bloodstream- and procyclic-form T.brucei membranes were labelled with [3H]DFP and solubilized with nOG. The soluble fractions were adsorbed with anti-VSG–Sepharose and then with ConA–agarose. The ConA-binding fractions were analysed by SDS– PAGE and fluorography (Figure 2A). A [3H]DFP-labelled 50 kDa doublet was apparent in the bloodstream-form preparation but not in the procyclic-form preparation, suggesting that this glycoprotein is either absent or does not receive ConA-binding glycans in procyclic forms. The latter is unlikely since procyclic forms express ConA-binding oligomannose structures on both endogenous and heterologously expressed bloodstream-form glycoproteins (Paturiaux-Hanocq et al., 1997; Treumann et al., 1997). Treatment of the glycoprotein fraction from bloodstream forms with PNGase F converted the 50 kDa doublet to a single band of 43 kDa, demonstrating that the [3H]DFP-labelled protein was N-glycosylated (Figure 2B).
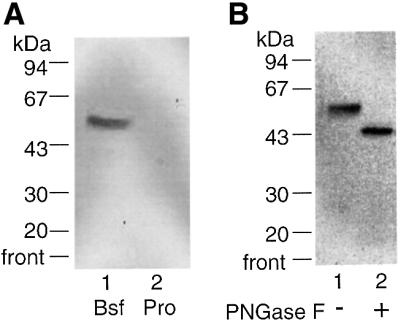
Fig. 2. Affinity labelling of the inositol deacylase glycoprotein in T.brucei membranes. (A) Bloodstream (Bsf)- and procyclic (Pro)-form T.brucei membranes were incubated with a cocktail of serine esterase/amidase inhibitors, labelled with [3H]DFP, washed, lysed and adsorbed with ConA–agarose. The adsorbed glycoproteins were subjected to SDS–PAGE and fluorography. (B) Bloodstream-form T.brucei membranes were labelled as described above and the glycoproteins were treated with (+) and without (–) PNGase F and subjected to SDS–PAGE and fluorography.
The properties of this glycoprotein with respect to (i) its lack of reactivity with serine esterase/protease inhibitors except DFP, (ii) its membrane association and (iii) its absence from procyclic forms, which do not utilize inositol deacylation in GPI biosynthesis (Field et al., 1992), suggested that it was worthy of further investigation as a putative inositol deacylase.
A purification procedure was developed to obtain peptide sequence from the putative inositol deacylase. The final purification strategy started from 4 × 1011 cell equivalents of [3H]DFP-labelled washed trypanosome membranes, stripped with sodium carbonate buffer and solubilized with 8% nOG and sonication. After ultracentrifugation, the supernatant was absorbed with anti-VSG– Sepharose and then with ConA–agarose. The bound glycoproteins were eluted with α-methyl-mannoside and fractionated on TMAE–Fractogel. Two peaks of radioactivity were obtained at 150 and 400 mM NaCl and aliquots were analysed by SDS–PAGE (both contained the labelled 50 kDa doublet). The first peak ran with an apparent molecular weight of 150–200 kDa on Superdex S-200 gel filtration whereas the second ran at ∼50 kDa, suggesting that the labelled 50 kDa protein in the first peak was complexed to itself and/or other proteins. The labelled protein from both peaks co-chromatographed on mono-Q and hydroxyapatite fast performance liquid chromatography (FPLC), suggesting that the labelled protein in the first peak can be dissociated into 50 kDa monomers under these conditions.
The labelled protein from the second TMAE–Fractogel peak was further purified by hydroxyapaptite FPLC. Aliquots from each step of the purification were analysed by SDS–PAGE and silver staining (Figure 3). The radiolabelled peak eluting from the hydroxyapatite column (Figure 3, lane 6) was highly enriched for a protein that co-migrated exactly with the [3H]DFP-labelled glycoprotein (Figure 3, lane 7). The remaining material was reduced and S-alkylated with vinylpyridine before SDS–PAGE and staining with Coomassie Blue. A band containing ∼0.5 µg protein, corresponding to the band marked by an arrow in Figure 3, lanes 6 and 7, was excised along with other gel slices of equivalent size and taken for in-gel trypsin digestion. The resulting tryptic peptides were applied to a 0.5 mm capillary microbore C18 reverse-phase HPLC column. The eluate was transferred directly to PVDF membrane using an on-line microblotter and strips containing seven peptides unique to the putative inositol deacylase band were taken for Edman microsequencing. Of these, five gave low-level peptide sequences containing several ambiguities (Figure 3).
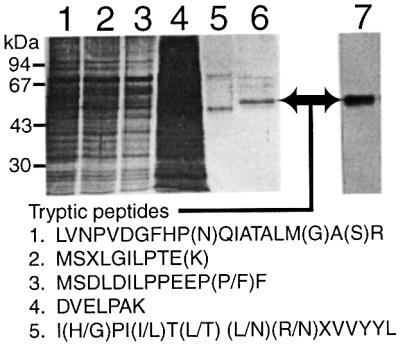
Fig. 3. Purification and microsequencing of the inositol deacylase. [3H]DFP-labelled bloodstream-form T.brucei membranes (4 × 1011 cell equivalents) were washed with high-pH buffer and extracted with nOG. The soluble fraction (lane 1) was adsorbed with anti-VSG–Sepharose and the supernatant (lane 2) was adsorbed with ConA–agarose. The unbound material (lane 3) was discarded and the α-methyl-mannoside eluate (lane 4) was fractionated by TMAE–Fractogel FPLC. The radioactive fractions eluting at 400 mM NaCl (lane 5) were diafiltered and applied to a hydroxyapatite FPLC column. An aliquot (1%) of the radioactive fraction eluting at 200 mM sodium phosphate (lane 6) was analysed by SDS–PAGE and fluorography (lane 7). The silver-stained gel (lanes 1–6) represents 0.0005% (lanes 1–3) or 1% (lanes 4–6) of the total material. A preparative gel of the material shown in lanes 6 and 7 was used to excise the band indicated by the arrow for in-gel trypsin digestion. The tryptic peptides were fractionated by capillary microbore HPLC and the Edman sequencing results of five peptides are indicated. Residues in parentheses were ambiguous.
Cloning of the GPIdeAc gene
Attempts to amplify segments of the gene encoding the putative inositol deacylase by PCR, using cDNA and degenerate forward and reverse oligonucleotide primers designed from peptides 1–4 (Figure 3), were unsuccessful. We used an intra-peptide PCR approach (Figure 4A, step 1) using genomic DNA as the template, degenerate 14mer primers corresponding to most of the LVNPV and QIATA segments of the peptide LVNPVDGFHP(N)QIATA LM(G)A(S)R, and temperature-gradient PCR, to generate a PCR product of the expected size (69 bp). The PCR product was purified from a polyacrylamide gel and ligated into the pGEMTeasy vector. The inserts of six clones were sequenced and all contained the same nucleotide sequence (encoding DGFHPS) between the degenerate primer sequences. An 18mer reverse primer based on this sequence, a forward primer based on the trypanosome 5′-spliced leader (a 35 bp sequence trans-spliced on to every trypanosome mRNA) and bloodstream-form T.brucei cDNA were used to amplify a large fragment of the gene by PCR (Figure 4A, step 2). This fragment was cloned and sequenced, revealing a continuous open reading frame (ORF) starting 82 bp downstream of the 5′-spliced leader and terminating in the sequence LVNPVDGFHPS. Two 400 bp probes (probes 1 and 2) corresponding to the 5′ and 3′ ends of this clone were made by PCR (Figure 4A, step 3) to assist in obtaining a genomic clone containing the entire coding region and flanking sequences.
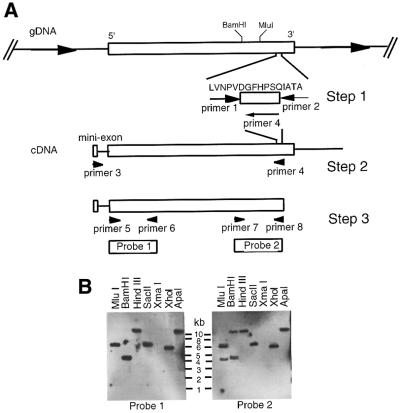
Fig. 4. Cloning strategy and Southern blot analysis of the inositol deacylase gene. (A) Degenerate PCR primers (primers 1 and 2) were used to amplify a short non-degenerate DNA sequence coding for the DGFHPS segment of the protein (step 1). Primers based on this sequence (primer 4) and the 5′-spliced leader (mini exon) (primer 3) were used to amplify a cDNA sequence spanning most of the ORF (step 2). Additional primers were used to amplify two 400 bp probes (probes 1 and 2) corresponding to the 5′ and 3′ regions of the ORF, respectively (step 3). (B) Probes 1 and 2 were used in Southern blot analyses of restriction endonuclease-digested T.brucei DNA. The digestion patterns indicated that the gene was single-copy per haploid genome and that SacII, XhoI and ApaI digests contained fragments encompassing the whole gene. XhoI-digested DNA was used to prepare the 6.0–6.5 kb size-selected library used to obtain the 6.2 kb genomic clone containing the gene.
Southern blot analysis with probes 1 and 2 suggested that the gene was single-copy per haploid genome (Figure 4B) and, based on the blot, a 6.0–6.5 kb size-selected library of XhoI-digested genomic DNA fragments was prepared in the pUC18 vector. Duplicate colony blots of bacteria transformed with the library were hybridized with probes 1 and 2. After three rounds of selection, a representative clone was picked and the presence of the gene in the 6.1 kb insert was confirmed by PCR using primers 5 and 8. The entire insert was sequenced (accession number AJ344051) and found to contain a 1.7 kb ORF corresponding to the putative inositol deacylase (which we name GPIdeAc) flanked by 2.7 and 1.8 kb of 5′ and 3′ DNA, respectively. Four of the five tryptic peptide sequences (Figure 3, peptides 1, 2, 3 and 5), preceded by the expected R or K trypsin digestion sites, were found in the predicted ORF (Figure 5A), albeit with several errors due to the extremely low levels of peptide sequenced and with two of the sequences (Figure 3, peptides 2 and 3) representing overlapping peptides. A retrospective MALDI-Tof analysis of the in-gel tryptic digest also identified peptide 1 and peptide 2/3 as well as four other peptides belonging to GPIdeAc (Figure 5A). The other ions in the MALDI-Tof spectrum were either due to the matrix or trypsin autolysis peptides, suggesting that there were no other detectable proteins in the gel piece.
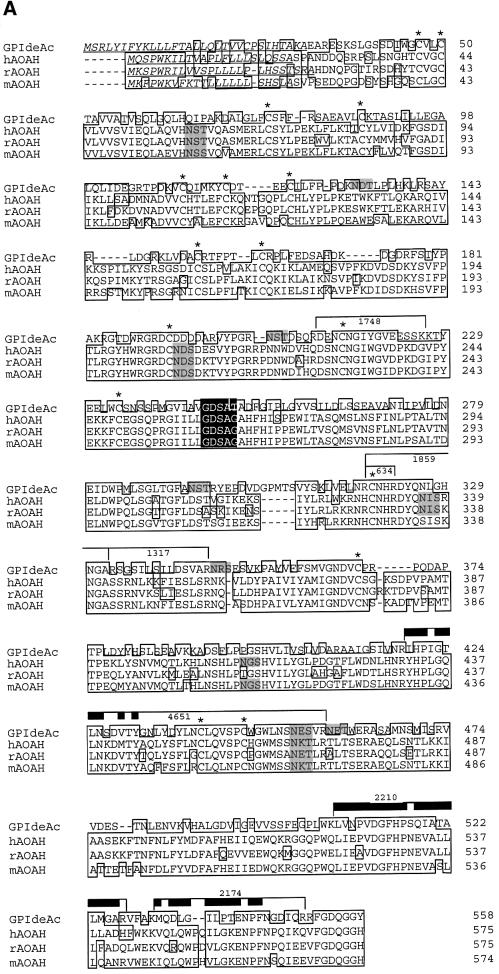
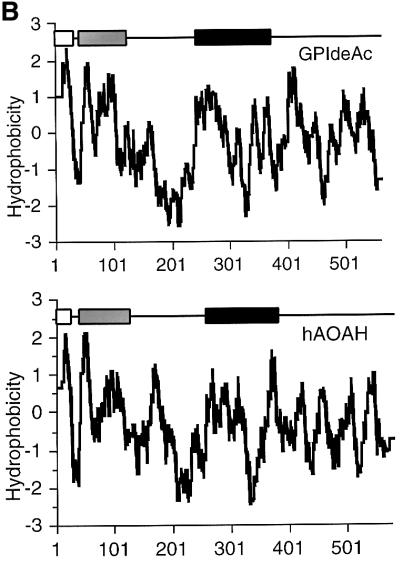
Fig. 5. Sequence analysis of GPIdeAc and comparison with mammalian AOAH. (A) Clustal W alignment of GPIdeAc and human (h) rabbit (r) and mouse (m) AOAH. Underlined residues indicate the N-terminal signal peptides, boxed residues the conserved sequences, black box the lipase motif, shaded residues the N-glycosylation sites and * conserved Cys residues. The bars indicate tryptic peptides identified in the MALDI-Tof spectrum (with the m/z values of those peptides indicated by the numbers) and the thick bars indicate residues identified by Edman sequencing. (B) Kite–Dolittle hydropathy plots of GPIdeAc (upper panel) and hAOAH (lower panel) together with their domain structures. The open boxes indicate the N-terminal signal sequences, the shaded boxes the saposin type-B domains and the black boxes the GDSL-lipase domains.
The putative inositol deacylase has homology with mammalian acyloxyacyl hydrolase
Translation of GPIdeAc predicts a protein of 61.4 kDa with a 31 amino acid N-terminal signal peptide, a saposin type-B domain (residues 43–122), a GDSL-lipase motif domain (residues 241–372) and six potential N-glycosyl ation sites. The presence of an N-terminal signal sequence, the occupancy of several of the potential glycosylation sites (Figure 2) and the absence of predicted transmembrane domains suggests that GPIdeAc is a lumenal glycoprotein. BLASTp analysis against the NCBI and SwissProt non-redundant protein databases revealed three related proteins with the same domain structure: human, mouse and rabbit acyloxyacyl hydrolase (AOAH) (Figure 5A). The lower apparent molecular weight of the [3H]DFP-labelled glycoprotein (50 kDa glycosylated and 43 kDa deglycosylated) suggests post-translational proteolytic processing that is more extensive than simple removal of the signal sequence. Such processing has been reported for human AOAH such that the 14 kDa saposin type-B N-terminal domain remains attached to the 52 kDa lipase-containing domain by a disulfide bridge that is broken in reducing SDS–PAGE (Staab et al., 1994). The sequence identities and similarities to human, mouse and rabbit AOAH are 35–36% and 53–54%, respectively; the three mammalian proteins are closely related to each other (∼77% identity and 89% similarity). Hydropathy plots for the trypanosome protein and the human AOAH are very similar (Figure 5B), suggesting that they have similar folds and that they are truly homologous. The conservation of 16 out of 17 Cys residues between the trypanosome and the mammalian sequences further supports the notion that these proteins have similar tertiary structure. Furthermore, like the T.brucei inositol deacylase, mammalian AOAH is inhibited by DFP but not by many other serine esterase inhibitors (Munford and Hunter, 1992) and both enzymes require detergent extraction despite the absence of transmembrane domains (Munford and Hall, 1989). Membrane association could be via tight binding to integral membrane components and/or interaction between the saposin type-B domain and the phospholipid bilayer (Liepinsh et al., 1997).
In summary, GPIdeAc is very similar to the mammalian AOAH described previously proteins. The AOAHs are involved in the breakdown and detoxification of bacterial lipopolysaccharides through the removal of fatty acids from lipid A (Munford and Hall, 1986; Hagen et al., 1991; Staab et al., 1994). Both GPIdeAc and the AOAHs contain a saposin type-B domain, a structure generally associated with glycosphingolipid binding for degradation (Bierfreund et al., 2000), and a GDSL-lipase domain, a serine hydroxyesterase motif associated with the removal of fatty acids. Thus, the putative trypanosome inositol deacylase contains all the hallmarks of a glycolipid-specific lipase. The absence of any glycolipids in T.brucei, other than GPI anchors and their biosynthetic intermediates, further suggests that the cloned gene encodes a GPI-specific deacylase.
Construction of GPIdeAc gene knockout and conditional mutant trypanosomes
In order to assess the function of the GPIdeAc gene, we performed a gene knockout. Sequences of 263 and 245 bp immediately flanking the 5′ and 3′ termini of GPIdeAc (avoiding adjacent potential ORFs) were used as targeting sequences for homologous recombination. The first GPIdeAc allele was replaced by a hygromycin resistance gene (HYG) and the second allele was replaced by a puromycin resistance gene (PAC) (Figure 6A). After several attempts, seven double knockout (GPIdeAc–/–) clones were obtained and the absence of GPIdeAc genes was confirmed by PCR (Figure 6B) and Southern blot (data not shown). All clones were viable in culture with growth characteristics similar to the wild type. Two GPIdeAc–/– clones tested were infective to mice and rats, with no gross growth phenotype in animals.
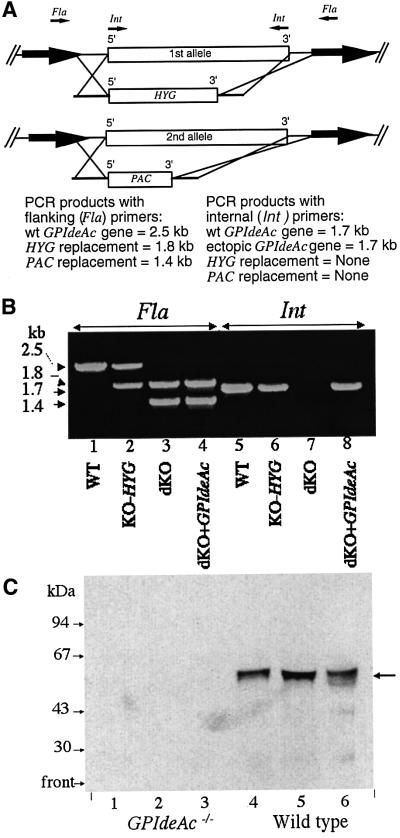
Fig. 6. Construction and characterization of GPIdeAc–/– double knockout and conditional mutants. (A) The first GPIdeAc allele was replaced by HYG and the second by PAC. The targeting sequences used for homologous recombination were designed to avoid two adjacent potential ORFs (bold arrows). PCR primers were designed to DNA regions flanking (Fla) the recombination regions and internal (Int) to the GPIdeAc ORF. (B) PCR products from DNA extracted from wild-type cells (WT; lanes 1 and 5), single knockout cells (KO-HYG; lanes 2 and 6), double knockout cells (dKO; lanes 3 and 7) and double knockout cells with a tetracycline-inducible HA-tagged ectopic copy of GPIdeAc (dKO + GPIdeAc; lanes 4 and 8) using flanking (Fla; lanes 1–4) and internal (Int; lanes 4–8) primers, as indicated. (C) Membranes from wild-type and GPIdeAc–/– double knockout mutants were labelled with [3H]DFP and processed as described in Figure 2A. The ConA–agarose beads were eluted twice with α-methyl-mannoside (lanes 1 and 2, and 4 and 5) and finally boiled in SDS sample buffer (lanes 3 and 6). The samples were subjected to SDS–PAGE and fluorography.
Washed trypanosome membranes prepared from wild-type and GPIdeAc–/– cells were labelled with [3H]DFP, solubilized with nOG, and the ConA-binding fractions were analysed by SDS–PAGE and fluorography. The [3H]DFP-labelled 50 kDa doublet seen in the wild-type sample was absent from the GPIdeAc–/– sample (Figure 6C), confirming that GPIdeAc does indeed encode the DFP-reactive protein.
A tetracycline-inducible HA-tagged ectopic copy of the GPIdeAc gene was also introduced into a GPIdeAc–/– clone using the pLEW82 vector (Wirtz et al., 1999) (Figure 6A and B).
GPIdeAc–/– trypanosome membranes show reduced inositol deacylase activity
Several preparations of wild-type and GPIdeAc–/– cell-free systems were labelled with GDP-[3H]mannose in the presence of UDP-GlcNAc. Both wild-type and GPIdeAc–/– membranes produced glycolipids A′ and Θ, showing that inositol deacylase activity was still present in the GPIdeAc–/– cells. However, the GPIdeAc–/– membranes showed a reproducible accumulation of glycolipid C′, as compared with wild-type membranes (Figure 7, lanes 2 and 4). Digestion of these intermediates with PI-PLC, to reveal only PI-PLC-resistant (inositol-acylated) species, further emphasized the accumulation of glycolipid C′ in the GPIdeAc–/– membranes relative to wild type. These data are consistent with a reduction in, but not a total loss of, inositol deacylase activity.
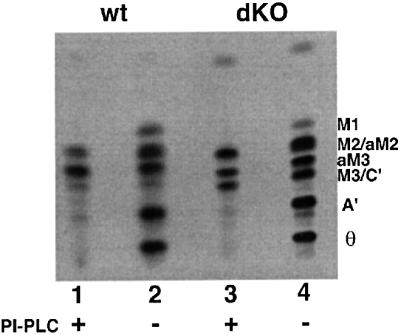
Fig. 7. The cell-free system prepared from GPIdeAc–/– cells accumulates inositol-acylated GPI intermediates. Cell-free system from wild-type (wt) and GPIdeAc–/– double knockout (dKO) cells were labelled with GDP-[3H]Man in the presence of UDP-GlcNAc. Labelled glycolipids were extracted and analysed by HPTLC before (–) and after (+) digestion with B.thuringiensis PI-PLC, as indicated.
Recombinant GPIdeAc has inositol deacylase activity
Expression of GPIdeAc in Escherichia coli produced recombinant protein in inclusion bodies that was inappropriate for enzyme assays, although this material was used to successfully raise rabbit polyclonal antibodies for localization studies. We used GPIdeAc–/– cells expressing HA-tagged inositol deacylase from an ectopic copy of GPIdeAc–HA as a source of recombinant inositol deacylase. Cell lysates from 109 GPIdeAc–/– and GPIdeAc–/–/ectopic GPIdeAc–HA trypanosomes were immunoprecipitated with anti-HA antibody beads. The washed beads were incubated with purified [3H]myristate-labelled glycolipid C for 1 h at 37°C and the products analysed by HPTLC and fluorography (Figure 8). The immunoprecipitate from the cells expressing GPIdeAc–HA showed conversion of glycolipid C to glycolipid A (i.e. removal of the fatty acid from the inositol ring) whereas that from the GPIdeAc–/– cells did not. The Rf value of the glycolipid A product is distinct from that of lyso-glycolipid C, showing that the reaction removed exclusively the fatty acid [predominantly palmitate and stearate (Güther et al., 1996)] from the 2-hydroxyl of the inositol ring and not myristate from the sn-1 or sn-2 position of the glycerolipid. Furthermore, incubation of identical aliquots of the immunoprecipitate beads with [3H]glycolipid A did not result in the formation of any glycolipid Θ-like species due to removal of myristate from the sn-1 or sn-2 positions of the glycerolipid (data not shown). These data directly demonstrate a specific inositol deacylase activity for GPIdeAc–HA.
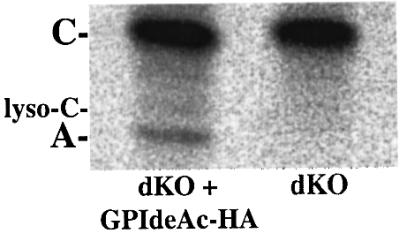
Fig. 8. Affinity-purified recombinant GPIdeAc expressed in T.brucei can convert radiolabelled glycolipid C to glycolipid A. GPIdeAc–/– double knockout (dKO) cells and GPIdeAc–/– double knockout cells expressing HA-tagged GPIdeAc (dKO + GPIdeAc–HA) were lysed in detergent and immunoprecipitated with anti-HA–agarose beads. The washed beads were incubated with freshly prepared [3H]myristate-labelled glycolipid C and the products were analysed by HPTLC and fluorography. The positions of glycolipid C, glycolipid A and lyso-glycolipid C, made by phospholipase A2 digestion of glycolipid C (Güther et al., 1995), are indicated by A, C and lyso-C, respectively.
GPIdeAc–/– trypanosomes show an accumulation of galactosylated GPI precursors
The labelling of living trypanosomes with [3H]mannose showed that sufficient inositol deacylation was occurring to give rise to fully remodelled glycolipid A. However, we observed a significant and highly reproducible increase in the level of labelled polar GPI species in the GPIdeAc–/– clones (Figure 9A). This increase was reversed when an ectopic copy of the trypanosome gene was expressed in the double knockout cells (Figure 9B), showing that the increase in polar GPIs was a direct consequence of the targeted gene replacement and not an artefact of the trans formation. The polar glycolipids could be labelled with [3H]myristate (data not shown), showing that they have undergone fatty acid remodelling. Their sensitivity to PI-PLC and resistance to jack bean α-mannosidase (Figure 9C) further suggest that they are not inositol acylated and most likely represent modified versions of glycoplid A. Low-levels of galactosylated versions of glycolipid A have been described previously in wild-type trypanosomes (Mayor et al., 1992) and their probable structures have been discussed (Mehlert et al., 1998). The GPI galactose side chains in T.brucei are unusual. Exhaustive digestion of VSG221 GPIs with coffee bean α-galactosidase produced the GPI core structures Manα1– 2Manα1–6Manα1–4GlcN-, Galα1–2Manα1–2Manα1– 6Manα1–4GlcN- and Gal1-2αManα1–2(Galβ1–3) Manα1–6Manα1–4GlcN- where the unusual conformation of the glycan precludes the removal of the terminal αGal and βGal residues by α- and β-galactosidases, respectively (Mehlert et al., 1998). Thus, the data shown in Figure 9D are consistent with the polar GPIs being galactosylated versions of glycolipid A.
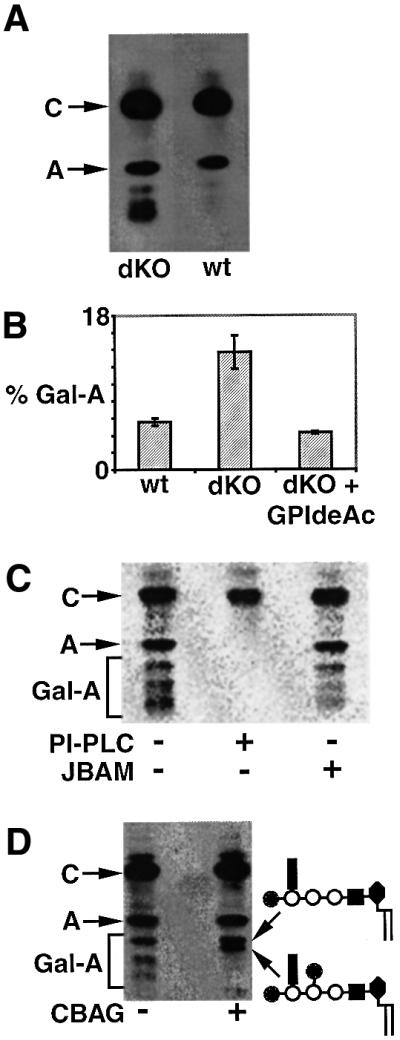
Fig. 9. GPIdeAc–/– cells accumulate polar galactosylated GPI species. (A) Wild-type (wt) and GPIdeAc–/– double knockout (dKO) cells were metabolically labelled with [3H]mannose and the radiolabelled glycolipids were analysed by HPTLC and fluorography. The dKO cells produced glycolipid A and substantial amounts of more polar glycolipids running between glycolipid A and the origin. (B) wt, dKO and dKO cells expressing GPIdeAc–HA (dKO + GPIdeAc–HA) were labelled and the glycolipids analysed as described above, except that a phosphorimager was used to quantify the proportion of polar glycolipids running between glycolipid A and the origin (mean ± SEM for triplicate measurements are shown). (C) Glycolipids labelled in dKO cells were analysed by HPTLC and phosphorimager with (+) and without (–) PI-PLC and jack bean α-mannosidase (JBAM) digestion. (D) Glycolipids labelled in dKO cells were analysed by HPTLC and fluorography with (+) and without (–) coffee bean α-galactosidase (CBAG) digestion. The terminal digest produced glycolipid A and, most likely, the two α-galactosidase-resistant galactosylated glycolipid A products (Mehlert et al., 1998) shown on the right.
GPIdeAc–/– trypanosomes produce normal VSG-linked GPI anchors
We wanted to assess whether reduced inositol deacylase activity and/or the accumulation of galactosylated glycolipid A GPIs in the GPIdeAc–/– cells might affect the structures of the mature GPI anchors attached to VSG on the cell surface. We isolated bulk mfVSG221 from wild-type and GPIdeAc–/– cells by reverse-phase HPLC and compared their masses with wild-type sVSG221 by electrospray mass spectrometry (ES-MS) (Figure 10A–C). All three VSG preparations showed a similar envelope of at least six glycoforms arising from microheterogeneity in the two N-linked glycosylation sites (Zamze et al., 1991) and in the GPI anchor (Mehlert et al., 1998). Both of the mfVSG221 profiles were shifted ∼510 Da from the sVSG221 profile, consistent with both mfVSG221 preparations containing dimyristoylglycerol. The theoretical mass difference between mfVSG221, containing dimyristoylglycerol, and GPI-PLC-cleaved sVSG221, terminating in inositol-1,2-cyclic phosphate, is 511 Da. Thus, at the resolution afforded by ES-MS for 50 kDa glycoproteins, the mature mfVSG221 made by the GPIdeAc–/– trypanosomes is indistinguishable from wild-type mfVSG221.
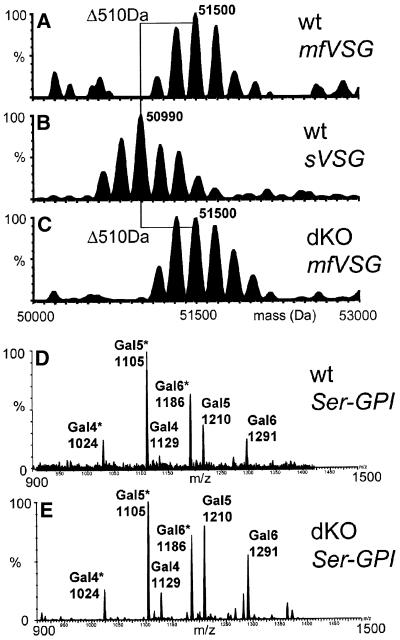
Fig. 10. GPIdeAc–/– cells produce normal GPI anchors attached to mature mfVSG221. (A–C) Mature mfVSG221 and sVSG221 from wild-type (wt) cells and mfVSG221 from GPIdeAc–/– (dKO) cells were analysed by positive-ion ES-MS. (D and E) wt and dKO mfVSG221 was exhaustively digested with Pronase and the C-terminal GPI peptide fragments were enriched on a C4 reverse-phase cartridge and analysed on line by negative ion ES-MS. The number of Gal residues attached to the common Ser-EtN-P-Man3GlcN-PI core is indicated above the m/z values of the [M-2H]2– ions. Ions indicated with * are lyso-species that have lost one myristic acid.
To analyse the GPI anchor structures with greater resolution, the mfVSG221 preparations were exhaustively digested with Pronase and the hydrophobic C-terminal GPI peptides were trapped on a C4 reverse-phase micro-cartridge. The cartridge was washed and then eluted with 60% 1-propanol in line with the source of the ES-MS mass spectrometer. This proved to be an efficient way to analyse GPI peptides by negative ion ES-MS. Both samples gave similar profiles of [M-2H]2– pseudomolecular ions that were readily assigned as Ser-EtN-P-Gal5–7Man3GlcN-Ino-P-dimyristoylglycerol and Ser-EtN-P-Gal5–7Man3HexN-Ino-P-myristoyl-lyso-glycerol species (Figure 10D and E). The latter presumably arise from traces of esterase activity in the Pronase since they were present in the wild-type mfVSG digest as well as the GPIdeAc–/– mfVSG digest and since there was no evidence of lyso-GPI anchors in the spectra of the intact mfVSGs (Figure 10A and C). The relative ion intensities for the different glycoforms were indistinguishable, suggesting that the accumulation of galactosylated glycolipid A species in the GPIdeAc–/– cells does not lead to hyper-galactosylation of the VSG221-linked GPI anchor.
Discussion
Components of the GPI biosynthetic machinery have thus far been identified by expression cloning of genes that rescue GPI– mammalian cell lines, or in temperature-sensitive yeast mutants. Cross-complementation has been used to confirm that yeast, mammalian or parasite homologues are the functional counterparts of these genes. In four cases, enzymic activity of recombinant proteins or purified complexes has been obtained [for the UDP-GlcNAc: PI α1–6 GlcNAc-transferase (Watanabe et al., 1998), the GlcNAc-PI de-N-acetylase (Watanabe et al., 1999), the GPI transamidase (Sharma et al., 2000) and the Dol-P-Man: GlcN-(acyl)PI α1–4 mannosyltransferase (MT-1) (Maeda et al., 2001)]. In the absence of any mutants with a defect in the inositol deacylation step (Figure 1), we have taken a biochemical approach to the purification and molecular cloning of the enzyme and its gene. We then used gene knockout and expression techniques to confirm the biochemical activity of the enzyme and to analyse the phenotype of cells lacking the enzyme.
Initial attempts to establish an enzymic assay to fractionate T.brucei membrane proteins for GPI inositol deacylase activity were unsuccessful. Radiolabelled glycolipid C substrate either remained undigested or was non-specifically degraded, losing fatty acids from the glycerolipid as well as from the inositol ring. Consequently, we tried to exploit the sensitivity of the trypanosome inositol deacylase activity to DFP and its insensitivity to a cocktail of other potent serine esterase and protease inhibitors (Güther and Ferguson, 1995). The labelling of a membrane-bound 50 kDa glycoprotein doublet with [3H]DFP in inhibitor cocktail-treated membranes encouraged us to pursue this material as a potential inositol deacylase. The glycoprotein was purified to near homogeneity by lectin affinity, ion exchange and hydroxyapatite chromatography, and in-gel tryptic digestion produced peptides that were sequenced by Edman chemistry. A degenerate intra-peptide PCR approach was used to generate a short non-degenerate nucleotide sequence from within the gene that, together with a primer from the 35 bp 5′-spliced leader, was used to amplify most of the coding region. This, in turn, facilitated the isolation and sequencing of a 6.2 kb genomic clone that contained the entire gene.
The replacement of both alleles of the GPIdeAc gene did not grossly affect the growth of the parasite in vitro or in vivo. Labelling of GPI intermediates in living trypanosomes and cell-free system, as well as analysis of mature mfVSG GPI anchor, revealed that inositol deacylation still occurs in GPIdeAc–/– cells. However, the accumulation of inositol acylated GPI intermediates in these cells, relative to wild type, and the ability of HA-tagged GPIdeAc to catalyse the conversion of glycolipid C to glycolipid A, clearly demonstrate GPI inositol deacylase activity. Thus, we conclude that there must be redundancy with respect to inositol deacylase activity in T.brucei bloodstream forms. Low stringency Southern blots and searches in the T.brucei genome project database have failed to identify GPIdeAc homologues, and we conclude that the residual deacylase activity in the GPIdeAc–/– cells most likely comes from distinct enzyme(s). Careful analysis of [3H]DFP-labelled membranes from GPIdeAc–/– cells has revealed a faint 30 kDa labelled protein that is a candidate second inositol deacylase that we intend to identify and characterize.
The predicted amino acid sequence of GPIdeAc shows a striking similarity to human, mouse and rabbit AOAH, a lipase with specificity for the lipid A component of bacterial lipopolysaccharide (Munford and Hall, 1986; Hagen et al., 1991; Staab et al., 1994). Homology with this known glycoconjugate-degrading, serine-dependent, lipase is comforting but, at the same time, perplexing. Mammalian AOAH is believed to be involved in the detoxification of lipid A and, therefore, to be a part of a protection mechanism against excessive inflammatory responses during Gram-negative bacterial infections. T.brucei, a protozoan parasite that neither produces nor (presumably) responds to bacterial endotoxin, has no obvious need for AOAH activity. This means that either the physiological function of mammalian AOAH has been mis-assigned, and that it is also a GPI inositol deacylase, or that the enzyme has evolved to perform different lipase functions in different organisms. It is striking that there are no other obvious GPIdeAc (or AOAH) homologues in the database since many organisms carry out the inositol deacylation reaction, including the fully sequenced organism Saccharomyces cerevisiae. However, there are clearly other inositol deacylases in T.brucei and this organism is unusual in that it performs inositol acylation and deacylation cycles throughout the GPI pathway, whereas mammals (and yeast) appear to utilize these reactions just once at a discrete stage of the pathway (Figure 1). It seems likely that the GPIdeAc gene encodes an inositol deacylase unique to trypanosomes and that the other inositol deacylase(s) will be common to other organisms.
The GPIdeAc–/– cells exhibited a curious, highly reproducible, phenotype with respect to GPI metabolism; namely the production of relatively large amounts of galactosylated GPI intermediates (i.e. galactosylated versions of glycolipid A) (Figure 9). This phenotype is reversed by expression of an ectopic copy of the gene, demonstrating that this is a genuine GPIdeAc–/– phenotype. It is known that up to three Gal residues are normally added to the VSG-linked GPI anchor in the ER (Mehlert et al., 1998), with up to three more added in the Golgi (Bangs et al., 1988). Furthermore, a minor fraction of excess glycolipid A normally becomes galactosylated in the ER (Mayor et al., 1992). In the GPIdeAc–/– cells, the proportion of galactosylated glycolipid A is increased 2- to 3-fold, suggesting that the excess glycolipid A molecules have greater access to the ER GPI galactosyltransferases in the absence of this particular inositol deacylase. Thus, it is conceivable that the rate of exit of surplus glycolipid A molecules from the ER is reduced in the absence of the deacylase. A role for inositol acylation and deacylation in transbilayer/membrane subcompartment movement has been postulated previously (Güther and Ferguson, 1995) and the observed GPIdeAc–/– phenotype could be consistent with such a role.
Materials and methods
Trypanosomes
Bloodstream-form T.brucei (strain 427, variant 117) were isolated from infected rats and purified over DEAE–cellulose. Genetically modified bloodstream-form trypanosomes (strain 427, variant 221) were isolated from cultures or from rat blood. Procyclic-form T.brucei (strain 427) was grown as described (Hesse et al., 1995).
Affinity labelling with [3H]DFP and PNGase F digestion
Bloodstream and procyclic-form trypanosome membranes (2.5 × 109 cell equivalents) were washed twice with 15 ml ice-cold buffer A (50 mM HEPES–NaOH pH 7.4, 25 mM KCl, 5 mM MgCl2, 0.1 mM TLCK, 1 µg/ml leupeptin) containing 0.8 mM phenylmethylsulfonyl fluoride (PMSF), 4-amidinophenylmethylsulfonyl fluoride (APMSF), 4-(2-aminoethyl)benzene sulfonyl fluoride (AEBSF), iodoacetamide and iodoacetic acid. The pellet was resuspended at 109 cell equivalents/ml in buffer A plus 5 mM MnCl2, 0.5 mM dithiothreitol (DTT) and labelled for 40 min at 37°C with 25 µCi [1,3-3H]DFP (8.4 Ci/mmol, NEN). Non-radioactive DFP was added (1 mM final) and unincorporated radioactivity was removed by washing three times with 26 ml buffer A. The washed membranes were solubilized with 2.5 ml of 8% (w/v) nOG in buffer B (20 mM Tris–HCl pH 7.2, 0.1 M NaCl containing the cocktail of inhibitors described above). After four cycles of sonication, the mixture was diluted 4-fold with buffer B and centrifuged (30 000 g, 30 min). The supernatant was incubated end-over-end with 0.25 ml of packed anti-VSG–Sepharose beads containing 6 mg/ml affinity-purified anti-VSG117 antibodies (2 h, 4°C). The unbound material was incubated with 25 µl of packed ConA–agarose beads (Sigma) that had been washed with 6 M urea (2 × 1 h, 22°C) to remove non-covalently bound ConA monomers, renatured in buffer A containing 10 mM α-methyl-mannoside and 1 mM CaCl2, MgCl2 and MnCl2 and washed five times with 10 vol of buffer C (2% nOG, 20 mM Tris–HCl pH 7.2, 0.1 M NaCl). The ConA beads were washed twice with 1 ml of buffer C, boiled in SDS sample buffer, 0.1 M DTT and analysed by 10% SDS–PAGE and fluorography using En3Hance (NEN). For PNGase F digestions, partially purified [3H]DFP-labelled protein was precipitated overnight with cold 10% (w/v) trichloroacetic acid, washed twice in cold ethanol, boiled in 14 µl of 1% SDS, 0.1 M DTT and mixed with 50 µl 2% NP-40. Aliquots (10 µl) were mixed with 3 µl of water and 5 µl of 0.2 M Tris–acetate pH 8.6, 40 mM EDTA, 0.2 M DTT and incubated overnight at 37°C with or without 2 µl of PNGase F (106 U/ml; New England Biolabs) before SDS–PAGE and fluorography.
Protein purification
Bloodstream-form trypanosome membranes (4 × 1011 cell equivalents) were washed with 250 ml buffer A plus inhibitors and labelled with 2 mCi [3H]DFP, as described above. Non-radioactive DFP was added (1 mM final) and unincorporated radioactivity was removed by washing three times with 160 ml buffer A. The membranes were stripped of peripheral membrane proteins and ER lumen contents by washing with 112 ml 0.125 M NaHCO3/Na2CO3 buffer pH 10 (10 min, 0°C), washed further with 300 ml buffer A and solubilized with 70 ml 8% nOG, 1 mM N-ethyl maleimide, 2 µg/ml aprotinin, 0.8 mM benzamidine in buffer B with sonication (four bursts of 20 s). After ultracentrifugation (100 000 g, 1 h, 4°C) the supernatant was diluted 4-fold with buffer B and VSG was removed by incubation with 5 ml anti-sVSG–Sepharose (4 h, 4°C). The supernatant was incubated overnight at 4°C with 2 ml urea-treated ConA–agarose, washed five times with 15 ml of buffer C and eluted overnight at 4°C with 6 ml buffer C containing 1 M α-methyl-mannoside, 10 mM EDTA. Two further elutions were performed for 5 h at 4°C and the combined eluates were filtered through 0.45 µ Millex-HV (Millipore). The total radioactivity recovered from the beads was ∼2 × 106 c.p.m. This material was applied to a TMAE–Fractogel FPLC column (1.5 × 15 cm; Merck) pre-equilibrated with buffer D (20 mM Tris–HCl pH 7.2, 0.1% nOG, 50 mM NaCl, 2 mM EDTA, 0.25 M α-methyl-mannoside). The column was washed with 5 ml buffer D and eluted with a two-step salt gradient to 0.5 M NaCl (50 min) and 1 M NaCl (80 min) at 1 ml/min. Some radioactivity eluted in the wash, most likely corresponding to free [3H]DFP, followed by two peaks at ∼150 and 400 mM NaCl. The recovery of radioactivity from the second peak was ∼25% and this material was diafiltered into 0.5 ml 5 mM sodium phosphate pH 7.2, containing 0.1% nOG using a Microcon-30 (Amicon). The sample was applied to a ceramic hydroxyapatite FPLC column (CHT2-1; Bio-Rad), washed with 6 ml starting buffer and eluted with a gradient to 500 mM sodium phosphate pH 7.2, 0.1% nOG over 30 min at 1 ml/min. A single radioactive peak was eluted at 200 mM sodium phosphate and the recovery of radioactivity for this step was 50%. This material was diafiltered into 35 µl of 20 mM Tris–HCl pH 7.2, 0.1% nOG using a Microcon-30 and combined with 20 µl of the same buffer used to wash the filter.
Peptide sequencing
The purified protein in 55 µl 20 mM Tris–HCl pH 7.2, 0.1% nOG was mixed with 12 mg urea and concentrated to 25 µl in a Speed-vac. The protein was reduced with 2 µl of 10% (v/v) 2-mercaptoethanol under argon (15 min, 50°C) and alkylated with 1 µl 4-vinylpyridine (Fluka) (30 min, 22°C). The sample was mixed with 7 µl of 5 × SDS sample buffer, heated to 50°C for 10 min and applied to an 10% SDS–PAGE gel. The gel was stained with Coomassie Blue and a gel piece containing the purified 50 kDa protein (and two control pieces, one below the band and one next to the band) were washed twice with 1 ml water for 1 h and dried in the Speed-vac. Each gel piece was digested overnight with 25 ng of modified trypsin (Promega sequence grade) in 100 µl of 20 mM NH4HCO3, 0.05% Zwittergent 3-16 (Calbiochem) at 37°C. The supernatant was removed, combined with two 200 µl washes and concentrated to ∼40 µl in a Speed-vac. The digests were mixed with an internal standard dye mix and applied to an ABI C18-reverse-phase capillary microbore column (5 µ 300 Å; 0.5 mm × 15 cm) using an ABI 140D microbore HPLC and microblotter system. The column was eluted with 2% acetonitrile, 0.1% trifluoroacetic acid (TFA) for 10 min followed by a linear gradient to 50% acetonitrile, 0.93% TFA over 100 min at a flow rate of 7 µl/min. Peptide and dye elution was monitored at 210 nm (0.1 AUFS) and the chromatograms were aligned to the microblotter PVDF membrane strips via the dye spots. Regions of the PVDF strip corresponding to UV-absorbing peaks unique to the 50 kDa protein digest were taken for Edman sequencing using a modified ABI 476A sequenator. PTH amino acids were separated on an ABI Procise cLC PTH amino acid analysis column (0.8 × 250 mm) developed with a discontinuous gradient of 3.5% tetrahydrofuran versus acetonitrile at 50 µl/min.
Gene cloning
Genomic DNA and cDNA were prepared from bloodstream-form trypanosomes by standard methods. In the oligonucleotide primers described below, lower-case letters indicate restriction site extensions. Degenerate primer 1 [5′-ggccaggatccT(C)T(TCGA)GT(TCGA)AA(TC)CC(TCGA)GT-3′] and primer 2 [5′-ggccgaattcGC(TCGA)GT(TCGA)GC(AGT)AT(CT)TG-3′] were used to PCR amplify a small DNA fragment with AmpliTaq-Gold (Perkin-Elmer); 95°C, 5 min; 47°C, 2.5 min; 72°C, 2.5 min for 35 cycles. The product was resolved on a 20% polyacrylamide gel in TAE buffer, stained with ethidium bromide, excised, isolated (Qiaex II kit) and cloned into pGEMTeasy. Several clones were sequenced and a non-degenerate primer (primer 4; 5′-ggccgaattcAGAAGGATGGAAACCATC-3′) corresponding to the central region of the insert was prepared. Primer 4 and primer 3 (5′-ggcccgcTATTATTAGAACAGTTTCTGTA-3′), based on the T.brucei 5′-spliced leader, were used to amplify a cDNA fragment with AmpliTaq-Gold; 95°C, 2 min; 60°C, 2.5 min; 72°C, 2.5 min for 35 cycles. The product was isolated from an agarose gel, cloned and sequenced. Primer 5 (5′-AGTCGACTCTATATATTTTAC-3′), primer 6 (5′-TCGTAGCTTATGTAAAGGCAA-3′), primer 7 (5′-GCACCCACCCCACTTGATTAC-3′) and primer 8 (5′-AGGATGGAAACCATCAACCGG-3′) were designed to PCR amplify two ∼400 bp fragments (probes 1 and 2) of the cDNA clone, corresponding to the 5′ and 3′ ends of the ORF, respectively. The probes were labelled with fluorescein–dUTP by random priming (Gene Images kit, Amersham) for use in Southern blotting.
A size-selected genomic DNA library was constructed by isolating 6.0–6.5 kb XhoI-digested fragments from an agarose gel and cloning these into SalI-digested pUC18. Duplicate colony blots of this library were probed with probes 1 and 2 and colonies that were positive with both probes were grown and screened for the presence of the ORF by PCR using primers 5 and 8. Positive cultures were subcloned three times to obtain a clone containing a 6.2 kb insert for sequencing.
Genetic modification of trypanosomes
All genetic modifications were performed using strain 427 (variant 221) bloodstream forms stably transfected to express T7 RNA polymerase and tetracycline repressor (TetR) protein under G418 selection (Wirtz et al., 1999). For convenience, these are referred to as ‘wild-type’ cells. The cultivation and transfection of these cells is described in Wirtz et al. (1999) with minor modifications described in Milne et al. (2001).
The 5′- and 3′-UTR sequences immediately adjacent to the start and stop codons were PCR amplified using the Expand long-template PCR System (Roche) with primer 9 (5′-ataagtatgcggccgcTACATGATG TGGTGTA-3′) and primer 10 (5′-GTTTAAACTTACGGACCGTCAAGCTTTTTTCCAGGCTGTGGA-3′) for the 5′-UTR and primer 11 (5′-ataagtaagcggccgcACCCCCTCGCGGCTAT-3′) and primer 12 (5′-GACGGTCCGTAAGTTTAAACGGATCCTGCGTTAGAGTACATC-3′) for the 3′-UTR. The two PCR products were used together in a further PCR to yield a product containing the 5′-UTR linked to the 3′-UTR by a short HindIII, PmeI and BamHI cloning site (italic sequences) and NotI restriction sites at each end (lower-case sequences). The PCR product was cloned into the NotI site of pGEM-5Zf(+) vector (Promega) and the HYG and PAC drug resistance genes were introduced into the targeting vector via the HindIII and BamHI cloning sites. Plasmids were prepared using Qiagen Maxi-Prep kits, digested with NotI, precipitated and washed with ethanol, redissolved in sterile water and used for electroporation of the parasites (Wirtz et al., 1999).
For overexpression, the GPIdeAc gene was PCR amplified using Pfu-Turbo (Stratagene). The gene was amplified in two segments. The 5′ end of the ORF was amplified using primer 13 (5′-cccaagcttCATATGAGTCGACTCTATATATTTTAC-3′) and primer 14 (5′-ATGGGATGTATCCGATTAACGATGCTGCCA-3′) and the 3′ end of the ORF was amplified using primer 15 (5′-TGGCAGCATCGTTAATCGGATACATCCCAT-3′) and primer 16 (5′-cgcggatccTCATGCGTAATCAGGGACGTCATAAGGATAGTTAATTAAGTAGCCCCCCTGGTC-3′). The two PCR products were used together in a further PCR to yield a product containing a silent mutation that removed a BamHI site, an HA epitope tag (underlined letters) fused to the C-terminus of GPIdeAc and 5′–HindIII and 3′–BamHI restriction sites for cloning into the trypanosome expression vector pLEW82 (Wirtz et al., 1999) to yield the plasmid pLEW82-GPIdeAc–HA.
GPIdeAc gene replacement and GPIdeAc–HA gene insertion events were monitored by PCR. Two sets of PCR primers were used. Flanking (Fla) primers were designed to chromosomal sequences outside of the regions of homologous recombination such that PCR products of the wild-type alleles and the HYG and PAC gene replacements would be of different sizes (2.5, 1.8 and 1.4 kb, respectively). Internal (Int) primers were designed to sequences within the ORF such that any copy of GPIdeAc (endogenous or ectopic) would yield a 1.7 kb product. The forward and reverse Fla primers were 5′-ATCTTCGGCAACACTGTC-3′ and 5′-TTGGACGTGGTGAAATGA-3′, respectively, and the forward and reverse Int primers were 5′-cccaagcttGGGATGAGTCGACTCTATATATTTTACAAA-3′ and 5′-cgcggatccTCAGTAGCCCCCCTGGTCCCCAAATCGCCT-3′, respectively.
In vitro and in vivo GPI radiolabelling studies
Cell-free systems (washed membranes) were prepared from wild-type and GPIdeAc–/– cells and radiolabelled for 20 min with GDP-[3H]mannose (22 Ci/mmol, NEN) in the presence of UDP-GlcNAc and tunicamycin (Güther and Ferguson, 1995). The washed glycolipid extracts were analysed by silica HPTLC using chloroform/methanol/1 M ammonium acetate/13 M ammonia/water (180:140:9:9:23 v/v) as the solvent. The plates were sprayed with En3Hance (NEN) before fluorography.
Living wild type, GPIdeAc–/– cells and GPIdeAc–/– cells expressing ectopic GPIdeAc–HA were metabolically labelled for 1 h with [2-3H]mannose (21 Ci/mmol, NEN) (Güther and Ferguson, 1995). Washed glycolipid extracts were analysed as described above except that some HPTLC plates were imaged using a Fuji FLA-2000 phosphorimager to allow accurate quantification.
Digestions of glycolipid extracts with Bacillus thuringiensis PI-PLC and jack bean α-mannosidase were as described (Güther et al., 1994). Digestions with coffee bean α-galactosidase (Glyko) were performed with 0.5 U of enzyme in 20 µl of 0.1 M sodium acetate pH 5.0, 0.1% sodium taurodeoxycholate for 16 h at 37°C.
Inositol deacylase assay
Trypanosomes isolated from rat blood were metabolically labelled for 1 h with [9,10-3H]myristate (49 Ci/mmol) (Güther et al., 1994) and glycolipid C was purified by solvent extraction, solvent partitioning and amino-bonded normal-phase chromatography (Güther et al., 1996).
Cell-free systems of 109 GPIdeAc–/– cells (unable to express GPIdeAc) and 109 GPIdeAc–/–/GPIdeAc–HA cells (continuously induced with tetracycline to express GPIdeAc–HA) were solubilized in 5 ml 20 mM Tris–HCl pH 7.2, 0.15 M NaCl, 1% NP-40, 0.1 mM TLCK, 0.8 mM PMSF, 0.8 mM APMSF, 0.8 mM AEBSF, 1 µg/ml leupeptin (buffer E) for 15 min at 0°C and centrifuged (30 000 g, 30 min, 4°C). The supernatant was mixed with 25 µl of packed anti-HA–agarose beads (Roche) for 2.5 h at 4°C. The beads were washed three times with 200 µl buffer E and resuspended in 160 µl buffer E. Aliquots of 20 µl (enzyme concentrated from 1.25 × 108 cells) were added to freshly prepared and dried [3H]glycolipid C (50 000 c.p.m.) and incubated for 1 h at 37°C. The glycolipid products were extracted (three times) with 50 µl butan-1-ol saturated with water and the combined butan-1-ol phases were washed twice with 100 µl water saturated with butan-1-ol. The washed butan-1-ol phase was concentrated and analysed by HPTLC and phosphorimaging, as described above.
Isolation of mfVSG and mass spectrometry
Wild-type and GPIdeAc–/– cells (isolated from rat blood and both expressing VSG variant 221) were extracted with ice-cold 0.1% TFA (109 cells/ml) and mfVSG221 was purified from the supernatant by reverse-phase HPLC (Clarke et al., 1985), except that a 5 µ 300 Å C4 Vydac column (0.46 × 25 cm) was used. Aliquots of ∼10 µg of the mfVSG221 preparations and of sVSG221 from wild-type cells were mixed with 100 µl 50% acetonitrile, 0.2% formic acid and infused into the electrospray source of a Micromass Ultima mass spectrometer at 5 µl/min. Positive ion mass spectra of these mfVSG221 were recorded, averaged and processed by maximum-entropy deconvolution using MassLynx software.
For the analysis of the C-terminal GPI peptide fragments, 400 µg of wild-type and GPIdeAc–/– mfVSG221 were dissolved in 100 µl ammonium bicarbonate and treated with 25 µl 1 mg/ml Pronase (Roche) in 50 mM calcium acetate (16 h, 37°C) and a further 5 µl Pronase (4 h, 37°C). Aliquots of 6.5 µl (corresponding to 20 µg of original mfVSG) were mixed with 100 µl 100 mM ammonium acatate, 5% propan-1-ol and loaded on to a 300 Å C4 reverse-phase micro-cartridge (1 × 5 mm; LC Packings) placed in the sample loop position of a Rheodyne HPLC injector. The cartridge was then washed with 200 µl of the same buffer and 100 µl of 10 mM ammonium acetate, 5% propan-1-ol. The cartridge was then placed in-line with the electrospray source of a Micromass Ultima mass spectrometer in a stream of 5 mM ammonium acetate, 60% propan-1-ol at 5 µl/min. Negative ion mass spectra were recorded, averaged and processed with MassLynx software.
Tryptic digests were analysed in positive ion reflectron mode using a PerSeptive Biosystems Voyager DE-STR MALDI-Tof mass spectrometer using α-cyano-4-hydroxycinnaminic acid matrix and internal calibration with trypsin autolysis ions.
Acknowledgments
Acknowledgements
We thank Angela Mehlert for supplying sVSGs, Tunhan Chang for assistance with the MALDI work, David Martin for assistance with Figure 5 and Ken Milne and Janine Roper for helpful discussions. This work was supported by a Wellcome Trust Programme Grant (054491).
References
- Bangs J.D., Doering,T.L., Englund,P.T. and Hart,G.W. (1988) Biosynthesis of a variant surface glycoprotein of Trypanosoma brucei. J. Biol. Chem., 263, 17697–17705. [PubMed] [Google Scholar]
- Benghezal M., Benachour,A., Rusconi,S., Aebi,M. and Conzelmann,A. (1996) Yeast Gpi8p is essential for GPI anchor attachment onto proteins. EMBO J., 15, 6575–6583. [PMC free article] [PubMed] [Google Scholar]
- Bierfreund U., Kolter,T. and Sandhoff,K. (2000) Sphingolipid hydrolases and activator proteins. Methods Enzymol., 311, 255–276. [DOI] [PubMed] [Google Scholar]
- Chen R., Walter,E.I., Parker,G., Lapurga,J.P., Millan,J.L., Ikehara,Y., Udenfriend,S. and Medof,M.E. (1998) Mammalian glycophos phatidylinositol anchor transfer to proteins and posttransfer deacylation. Proc. Natl Acad. Sci. USA, 95, 9512–9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.W., Olafson,R.W. and Pearson,T.W. (1985) Rapid preparative scale purification of myristylated variant surface glycoproteins from African trypanosomes. Mol. Biochem. Parasitol., 17, 19–34. [DOI] [PubMed] [Google Scholar]
- Cross G.A.M. (1996) Antigenic variation in trypanosomes: secrets surface slowly. Bioessays, 18, 283–291. [DOI] [PubMed] [Google Scholar]
- Doerrler W.T., Ye,J., Falck,J.R. and Lehrman,M.A. (1996) Acylation of glucosaminyl phosphatidylinositol revisited. Palmitoyl-CoA dependent palmitoylation of the inositol residue of a synthetic dioctanoyl glucosaminyl phosphatidylinositol by hamster membranes permits efficient mannosylation of the glucosamine residue. J. Biol. Chem., 271, 27031–27038. [DOI] [PubMed] [Google Scholar]
- Ferguson M.A.J. (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors and the contributions of trypanosome research. J. Cell Sci., 112, 2799–2808. [DOI] [PubMed] [Google Scholar]
- Ferguson M.A.J. et al. (1999) The GPI biosynthetic pathway as a therapeutic target for African sleeping sickness. Biochim. Biophys. Acta, 1455, 327–340. [DOI] [PubMed] [Google Scholar]
- Field M.C., Menon,A.K. and Cross,G.A. (1992) Developmental variation of glycosylphosphatidylinositol membrane anchors in Trypanosoma brucei. In vitro biosynthesis of intermediates in the construction of the GPI anchor of the major procyclic surface glycoprotein. J. Biol. Chem., 267, 5324–5329. [PubMed] [Google Scholar]
- Güther M.L. and Ferguson,M.A.J. (1995) The role of inositol acylation and inositol deacylation in GPI biosynthesis in Trypanosoma brucei. EMBO J., 14, 3080–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güther M.L., Masterson,W.J. and Ferguson,M.A.J. (1994) The effects of phenylmethylsulfonyl fluoride on inositol-acylation and fatty acid remodeling in African trypanosomes. J. Biol. Chem., 269, 18694–18701. [PubMed] [Google Scholar]
- Güther M.L.S., Treumann,A. and Ferguson,M.A.J. (1996) Molecular species analysis and quantification of the glycosylphosphatidylinositol intermediate glycolipid C from Trypanosoma brucei. Mol. Biochem. Parasitol., 77, 137–145. [DOI] [PubMed] [Google Scholar]
- Hagen F.S., Grant,F.J., Kuijper,J.L., Slaughter,C.A., Moomaw,C.R., Orth,K., O’Hara,P.J. and Munford,R.S. (1991) Expression and characterization of recombinant human acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides. Biochemistry, 30, 8415–8423. [DOI] [PubMed] [Google Scholar]
- Hamburger D., Egerton,M. and Riezman,H. (1995) Yeast Gaa1p is required for attachment of a completed GPI anchor onto proteins. J. Cell Biol., 129, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse F., Selzer,P.M., Mühlstädt,K. and Duszenko,M. (1995) A novel cultivation technique for long-term maintenance of bloodstream form trypanosomes in vitro. Mol. Biochem. Parasitol., 70, 157–166. [DOI] [PubMed] [Google Scholar]
- Kinoshita T. and Inoue,N. (2000) Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol., 4, 632–638. [DOI] [PubMed] [Google Scholar]
- Leidich S.D., Kostova,Z., Latek,R.R., Costello,L.C., Drapp,D.A., Gray,W., Fassler,J.S. and Orlean,P. (1995) Temperature-sensitive yeast GPI anchoring mutants gpi2 and gpi3 are defective in the synthesis of N-acetylglucosaminyl phosphatidylinositol. Cloning of the GPI2 gene. J. Biol. Chem., 270, 13029–13035. [DOI] [PubMed] [Google Scholar]
- Liepinsh E., Andersson,M., Ruysschaert,J.-M. and Otting,G. (1997) Saposin fold revealed by the NMR structure of NK-lysin. Nat. Struct. Biol., 4, 793–795. [DOI] [PubMed] [Google Scholar]
- McConville M.J. and Menon,A.K. (2000) Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids. Mol. Membr. Biol., 17, 1–16. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Watanabe,R., Harris,C.L., Hong,Y., Ohishi,K., Kinoshita,K. and Kinoshita,T. (2001) PIM-M transfers the first mannose to glycosylphosphatidylinositol on the lumenal side of the ER. EMBO J., 20, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson W.J., Raper,J., Doering,T.L., Hart,G.W. and Englund,P.T. (1990) Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl-phosphatidylinositol membrane anchors. Cell, 62, 73–80. [DOI] [PubMed] [Google Scholar]
- Mayor S., Menon,A.K. and Cross,G.A.M. (1992) Galactose-containing glycosylphosphatidylinositols in Trypanosoma brucei. J. Biol. Chem., 267, 754–761. [PubMed] [Google Scholar]
- Mehlert A., Richardson,J.M. and Ferguson,M.A.J. (1998) Structure of the glycosylphosphatidylinositol membrane anchor of a class-2 variant surface glycoprotein from Trypanosoma brucei. J. Mol. Biol., 277, 379–392. [DOI] [PubMed] [Google Scholar]
- Milne K.G, Güther,M.L.S. and Ferguson,M.A.J. (2001) Acyl-CoA binding protein is essential in bloodstream form Trypanosoma brucei. Mol. Biochem. Parasitol., 112, 301–304. [DOI] [PubMed] [Google Scholar]
- Miyata T., Takeda,J., Iida,Y., Yamada,N., Inoue,N., Takahashi,M., Maeda,K., Kitani,T. and Kinoshita,T. (1993) The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science, 259, 1318–1320. [DOI] [PubMed] [Google Scholar]
- Morita Y.S., Acosta-Serrano,A. and Englund,P.T. (2000) The biosynthesis of GPI anchors. In Sinay,E.P. and Hart,G. (eds), Oligosaccharides in Chemistry and Biology: A Comprehensive Handbook. Wiley-VCH, Weinheim, Germany, pp. 417–433.
- Munford R.S. and Hall,C.L. (1986) Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science, 234, 203–205. [DOI] [PubMed] [Google Scholar]
- Munford R.S. and Hall,C.L. (1989) Purification of acyloxyacyl hydrolase, a leukocyte enzyme that removes secondary acyl chains from bacterial lipopolysaccharides. J. Biol. Chem., 264, 15613–15619. [PubMed] [Google Scholar]
- Munford R.S. and Hunter,J.P. (1992) Acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides, has phospholipase, lysophospholipase, diacylglycerollipase, and acyltransferase activities in vitro. J. Biol. Chem., 267, 10116–10121. [PubMed] [Google Scholar]
- Nagamune K. et al. (2000) Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei. Proc. Natl Acad. Sci. USA, 97, 10336–10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Inoue,N., Watanabe,R., Takahashi,M., Takeda,J., Stevens,V.L. and Kinoshita,T. (1997) Expression cloning of PIG-L, a candidate N-acetylglucosaminyl-phosphatidylinositol deacetylase. J. Biol. Chem., 272, 15834–15840. [DOI] [PubMed] [Google Scholar]
- Paturiaux-Hanocq F., Zitzmann,N., Hanocq-Quertier,J., Vanhamme,L., Rolin,S., Geuskens,M., Ferguson,M.A.J. and Pays,E. (1997) Expression of a variant surface glycoprotein of Trypanosoma gambiense in procyclic forms of Trypanosoma brucei shows that the cell-type dictates the nature of the glycosylphosphatidylinositol membrane anchor attached to the glycoprotein. Biochem. J., 324, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A. and Conzelmann,A. (1993) Characterization of abnormal free glycophosphatidylinositols accumulating in mutant lymphoma cells of classes B, E, F and H. J. Biol. Chem., 268, 7215–7224. [PubMed] [Google Scholar]
- Sharma D.K., Hilley,J.D., Bangs,J.D., Coombs,G.H., Mottram,J.C. and Menon,A.K. (2000) Soluble GPI8 restores glycosylphosphatidyl inositol anchoring in a trypanosome cell-free system depleted of lumenal endoplasmic reticulum proteins. Biochem. J., 351, 717–722. [PMC free article] [PubMed] [Google Scholar]
- Simons K. and Ikonen,E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Staab J.F., Ginkel,D.L., Rosenberg,G.B. and Munford,R.S. (1994) A saposin-like domain influences the intracellular localization, stability, and catalytic activity of human acyloxyacyl hydrolase. J. Biol. Chem., 269, 23736–23742. [PubMed] [Google Scholar]
- Treumann A., Zitzmann,N., Hülsmeier,A., Prescott,A.R., Almond,A., Sheehan,J. and Ferguson,M.A.J. (1997) Structural characterisation of two forms of procyclic acidic repetitive protein expressed by procyclic forms of Trypanosoma brucei. J. Mol. Biol., 269, 529–547. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Inoue,N., Westfall,B., Taron,C.H., Orlean,P., Takeda,J. and Kinoshita,T. (1998) The first step of glycosylphosphatidylinositol biosynthesis is mediated by a complex of PIG-A, PIG-H, PIG-C and GPI1. EMBO J., 17, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Ohishi,K., Maeda,Y., Nakamura,N. and Kinoshita,T. (1999) Mammalian PIG-L and its yeast homologue Gpi12p are N-acetylglucosaminylphosphatidylinositol de-N-acetylases essential in glycosylphosphatidylinositol biosynthesis. Biochem J., 339, 185–192. [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Murakami,Y., Marmor,M.D., Inoue,N., Maeda,Y., Hino,J., Kangawa,K., Julius,M. and Kinoshita,T. (2000) Initial enzyme for glycosylphosphatidylinositol biosynthesis requires PIG-P and is regulated by DPM2. EMBO J., 19, 4402–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E., Leal,S., Ochatt,C. and Cross,G.A. (1999) A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol., 99, 89–101. [DOI] [PubMed] [Google Scholar]
- Wong Y.W. and Low,M.G. (1992) Phospholipase resistance of the glycosyl-phosphatidylinositol membrane anchor on human alkaline phosphatase. Clin. Chem., 38, 2517–2525. [PubMed] [Google Scholar]
- Zamze S.E., Ashford,D.A., Wooten,E.W., Rademacher,T.W. and Dwek,R.A. (1991) Structural characterization of the asparagine-linked oligosaccharides from Trypanosoma brucei type II and type III variant surface glycoproteins. J. Biol. Chem., 266, 20244–20261. [PubMed] [Google Scholar]


