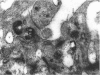Abstract
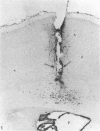
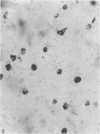
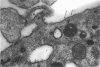
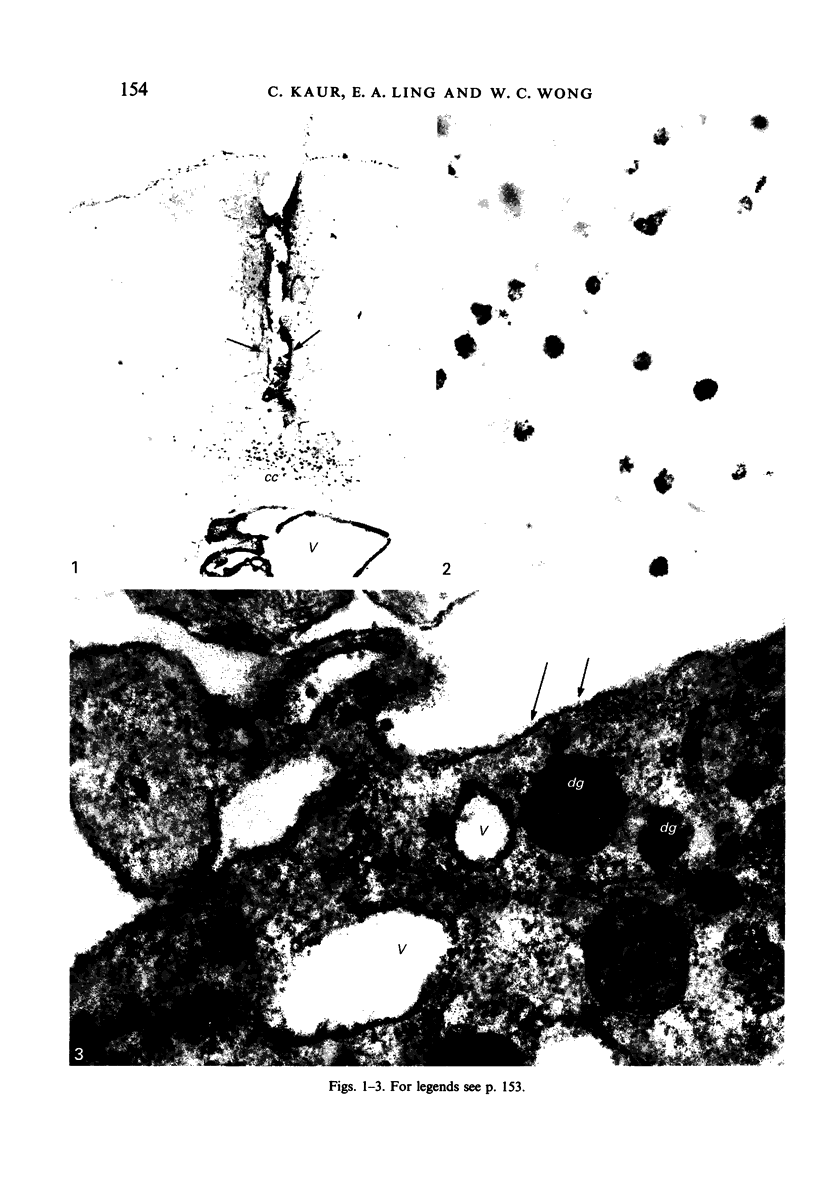
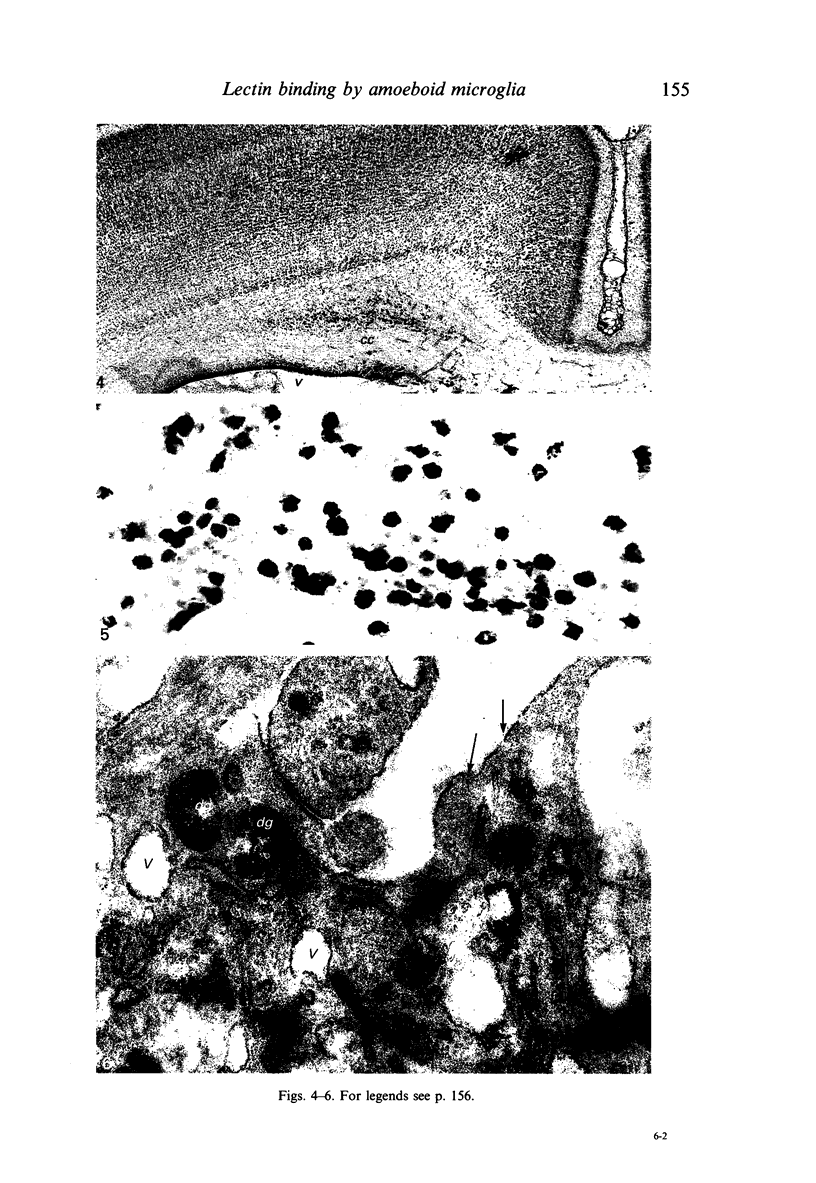
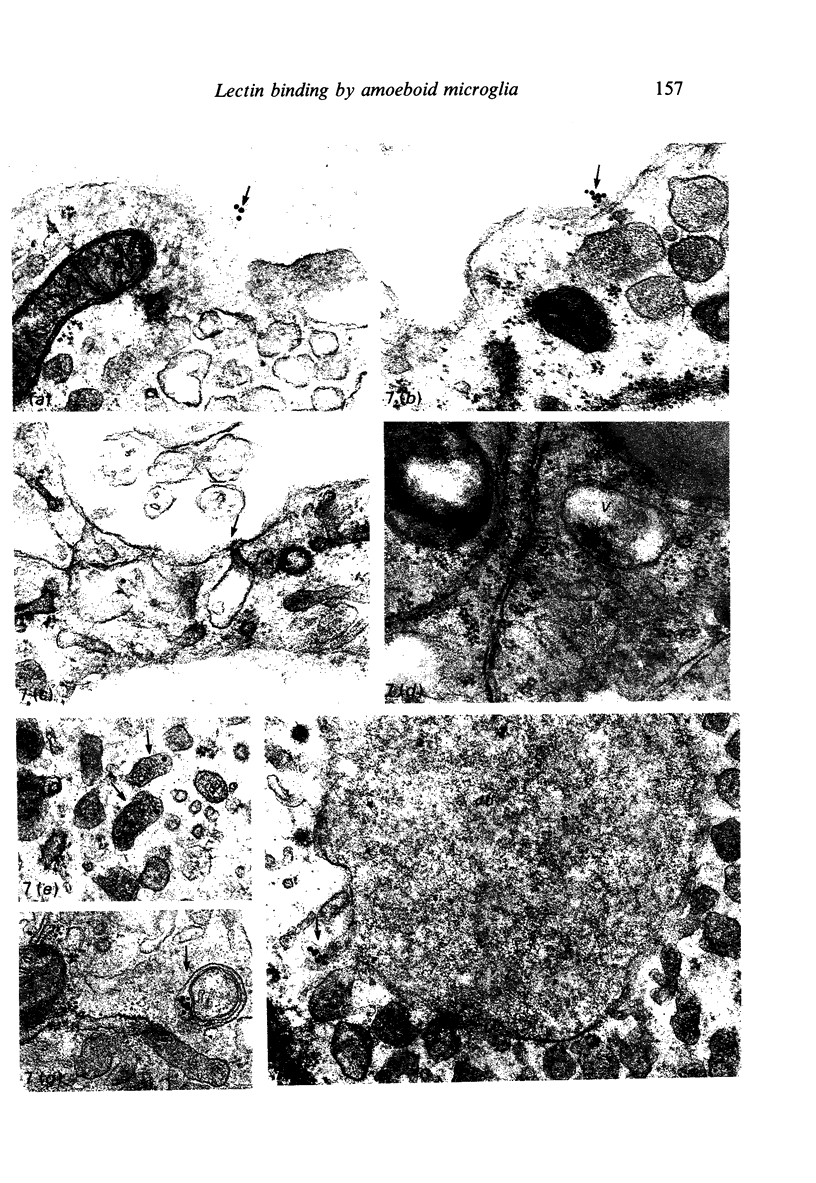
The labelling of amoeboid microglial cells in the postnatal (2-10 days old) rat brain was studied by intracerebral injection of various lectins, including peroxidase-labelled Ricinus communis agglutinin (RCA), peroxidase-labelled isolectin Griffonia simplicifolia (GSA1-B4) and gold-labelled concanavalin A (Con A). Three to six hours after the injection of RCA and GSA1-B4, the amoeboid microglial cells in the supraventricular corpus callosum were selectively labelled. Most of the labelled cells were round, showing dense black reaction products. With the electron microscope the reaction of the binding sites for RCA and GSA1-B4 was localised on the plasma membrane, in the plasmalemmal invaginations, in the limiting membrane of the cytoplasmic vacuoles and in the dense granules identified as lysosomes. The binding sites for gold-labelled Con A were initially (one hour) observed at the plasma membrane. With time (3-6 hours) the gold particles occurred in the invaginations of the plasma membrane and consequently in the cytoplasmic vacuoles and in the dense granules. It appears therefore that the lectins first bind to their specific carbohydrate receptors on the plasma membrane and are later internalised by the cells. It is suggested that the receptors play an active role in phagocytic function. Furthermore, the fact that the amoeboid microglial cells show similar membrane lectin receptors as the monocyte-derived tissue macrophage supports the hypothesis of their origin from blood monocytes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Adams J. C. Stabilizing and rapid thionin staining of TMB-based HRP reaction product. Neurosci Lett. 1980 Apr;17(1-2):7–9. doi: 10.1016/0304-3940(80)90052-x. [DOI] [PubMed] [Google Scholar]
- Ashwell K. W., Holländer H., Streit W., Stone J. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2(5):437–448. doi: 10.1017/s0952523800012335. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., McGee J. O. Monoclonal antibody to macrophages (EMB/11) labels macrophages and microglial cells in human brain. J Clin Pathol. 1986 Jun;39(6):615–621. doi: 10.1136/jcp.39.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci. 1987 Aug;80(1):25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Ibrahim M. Z., Khreis Y., Koshayan D. S. The histochemical identification of microglia. J Neurol Sci. 1974 Jun;22(2):211–233. doi: 10.1016/0022-510x(74)90247-0. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Fujiwara R., Nagai T., Maeda T. Distribution and fate of macrophagic ameboid cells in the rat brain. Arch Histol Jpn. 1982 Dec;45(5):505–518. doi: 10.1679/aohc.45.505. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in rats of various ages following an intravenous injection of horseradish peroxidase. Acta Anat (Basel) 1986;125(2):132–137. doi: 10.1159/000146150. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Transformation of amoeboid microglial cells into microglia in the corpus callosum of the postnatal rat brain. An electron microscopical study. Arch Histol Jpn. 1985 Feb;48(1):17–25. doi: 10.1679/aohc.48.17. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W., Barron K. D. 5'-Nucleotidase of microglial cells in the facial nucleus during axonal reaction. J Neurocytol. 1978 Oct;7(5):601–610. doi: 10.1007/BF01260892. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Light and electron microscopic demonstration of non-specific esterase in amoeboid microglial cells in the corpus callosum in postnatal rats: a cytochemical link to monocytes. J Anat. 1982 Sep;135(Pt 2):385–394. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Light and electron microscopic demonstration of some lysosomal enzymes in the amoeboid microglia in neonatal rat brain. J Anat. 1977 Jul;123(Pt 3):637–648. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Maddox D. E., Shibata S., Goldstein I. J. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci U S A. 1982 Jan;79(1):166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoji H., Yeger H., Becker L. E. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71(3-4):341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969 Jan;135(1):57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Thiaminepyrophosphatase activity in the plasma membrane of microglia. Histochemistry. 1981;71(1):45–52. doi: 10.1007/BF00592569. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Franz H., Yamamoto T., Iwasaki Y., Konno H. Identification of the normal microglial population in human and rodent nervous tissue using lectin-histochemistry. Neuropathol Appl Neurobiol. 1988 May-Jun;14(3):221–227. doi: 10.1111/j.1365-2990.1988.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Woodroofe M. N., Bellamy A. S., Feldmann M., Davison A. N., Cuzner M. L. Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. Possible role for microglia in lesion growth. J Neurol Sci. 1986 Jul;74(2-3):135–152. doi: 10.1016/0022-510x(86)90100-0. [DOI] [PubMed] [Google Scholar]
- de Water R., van Blitterswijk C. A., Daems W. T., Ginsel L. A. Heterogeneity of concanavalin A binding by mouse peritoneal macrophages. Histochemistry. 1982;74(3):301–307. doi: 10.1007/BF00493429. [DOI] [PubMed] [Google Scholar]