Abstract
The isolated glycine betaine uptake carrier BetP from Corynebacterium glutamicum was reconstituted in Escherichia coli phospholipid liposomes and its response to osmotic stress studied. The transport activity of BetP, which was previously shown to comprise both osmosensory and osmoregulatory functions, was used to identify the nature of the physicochemical stimulus related to hyperosmotic stress. Putative factors modulating transport activity in response to osmotic stress were dissected. These include type, osmolality and concentration of solutes in the internal and/or external compartment (cationic, anionic, zwitterionic, neutral), as well as membrane strain as a response to increased osmolality. Osmoresponsive activation of BetP was independent of any external factor and of physical alterations of the membrane, but was triggered by a change in the internal K+ concentration. Activation did not depend on the type of anion present and was K+ (or Cs+ and Rb+) specific, as choline and NH4+ did not trigger BetP activity. The half-maximal activation of BetP in E.coli phospholipid liposomes was correlated to an internal concentration of 221 ± 23 mM K+.
Keywords: Corynebacterium/osmoregulation/reconstitution/sensing/stress
Introduction
Osmotic stress is a common challenge encountered by eukaryotic and prokaryotic cells. Hyperosmotic stress causes instant water efflux, thereby changing the cell’s hydration, volume and/or turgor pressure. As a response, cells have developed a variety of mechanisms to overcome water stress. The most widely distributed strategy against hyperosmotic stress is the uptake and biosynthesis of osmoprotectants, so-called compatible solutes, e.g. glycine betaine, proline, ectoine or trehalose (Csonka, 1989; Wood, 1999).
In addition to the catalytic activity of carrier proteins, i.e. solute transport, osmoreactive uptake systems are characterized by two additional functions that are not necessarily confined to one polypeptide. They are able to regulate the catalytic activity to optimally adapt their function to the actual extent of osmotic stress (osmoregulation), and they have means to recognize the challenge quantitatively, i.e. they sense a so far unknown physicochemical signal related to osmostress (osmosensing).
Corynebacterium glutamicum, a GC-rich Gram-positive soil bacterium, is used extensively in amino acid production (Krämer, 1994). It is equipped with five secondary carriers for uptake of the compatible solutes betaine, proline and ectoine (Peter et al., 1998a; our unpublished results). At least two of them, BetP and ProP, are regulated in response to changes in external osmolality, both at the level of activity and expression. EctP is synthesized constitutively but osmoregulated at the activity level. The high-affinity, Na+-coupled glycine betaine-specific uptake system, BetP, was analyzed biochemically in detail (Farwick et al., 1995; Peter et al., 1998b), and the corresponding gene was characterized (Peter et al., 1996). BetP is predicted to be a typical 12-transmembrane segment transporter carrying N- and C-terminal extensions of 50–60 hydrophilic amino acids that face the cytoplasm. Specific deletions in both terminal domains resulted in modulation of its response to osmotic stress and finally in uncoupling of the sensory from the catalytic function of transport (Peter et al., 1998b). By isolation and reconstitution, we demonstrated that BetP comprises functions of both an osmosensor and an osmoregulator (Rübenhagen et al., 2000). The first osmoreactive carrier where this fact has been shown is ProP from Escherichia coli (Racher et al., 1999), followed later by the primary transporter OpuA from Lactobacillus lactis (van der Heide and Poolman, 2000).
Whereas both the direct response and the long-term adaptation to osmotic stress have been studied in several bacteria, e.g. E.coli, Salmonella typhimurium and Bacillus subtilis (Csonka, 1989; Kempf and Bremer, 1998; Wood, 1999), the mechanisms of osmosensing are not well understood. This refers in particular to the physical parameters that may be osmotically relevant stimuli as well as to their interaction with osmosensory proteins. In order to analyze these putative stimuli, a reduction in the complexity of the system used for their identification would be advantageous. The suitability of reconstituted systems for this purpose has been demonstrated recently (Racher et al., 1999; Rübenhagen et al., 2000; van der Heide and Poolman, 2000). When reconstituted into E.coli phospholipid vesicles, BetP has been shown to retain all functions known from intact cells with respect to both transport activity and regulatory response (Rübenhagen et al., 2000). Thus, this system seems to be an ideal tool to dissect and analyze the variety of factors putatively involved in osmosensing.
Over the course of time, a long list of physicochemical stimuli has been discussed as putative parameters triggering activation of osmoresponsive membrane proteins (Csonka, 1989; Csonka and Hanson, 1991; Poolman and Glaasker, 1991; Wood, 1999). Possible candidates are (i) cell turgor, (ii) the change in ion concentration or ion strength of the surrounding medium, (iii) transmembrane gradients of osmolality or of particular solutes, (iv) membrane strain as a consequence of transmem brane solute gradients, (v) macromolecular crowding and (vi) direct effects of osmolality changes on protein structure.
When discussing possible stimuli related to osmotic up-shifts, three different time windows with respect to the processes involved have to be considered (Wood, 1999). The time range of milliseconds to seconds after an up-shift is dominated by passive water flux and the consecutive shrinkage of the cytoplasm. Immediately after that, an active response of the cell by solute uptake, in general K+ and compatible solutes, as well as osmoprotectant biosynthesis take place, leading to a partial recovery in the minute to 1 h time scale. This is followed by long-term changes involving regulation of expression as well as structural remodeling of the cell. The time window relevant for the present study concerns the fast events. This does not necessarily mean that the results obtained will not be significant for the consecutive processes as well.
The aim of this study was to identify the nature of the physicochemical stimulus that is perceived by BetP in the process of osmosensing. In using a reconstituted system we demonstrate that the only parameter triggering efficiently the osmoresponsive activation of BetP from C.glutamicum reconstituted into vesicles of E.coli phospholipids is the increase in internal K+ concentration.
Results
In order to discriminate between the various possible osmotic stimuli that may actually be recognized by the membrane-embedded BetP protein from C.glutamicum, we used the extraordinary flexibility of the proteoliposomal system consisting of isolated and purified BetP integrated into E.coli lipid vesicles in functionally active form. A typical experiment for studying osmotic up-shifts is started by diluting proteoliposomes that carry BetP in an inactive state into buffer solutions of different composition and osmolality. Dilution into hyperosmolal buffer leads to instant activation of BetP and changes all relevant parameters at once, namely (i) external osmolality and solute composition, (ii) internal osmolality and solute concentration as a consequence of immediate water efflux and shrinkage of liposomes, and (iii) the shape of the proteoliposomes and the physical properties of the membrane (membrane strain). This list of possible triggering parameters will be dealt with one by one in the following sections, using the reconstituted system for dissecting crucial signals relevant for BetP activation from insignificant ones.
Turgor, vesicle volume, proteoliposome shrinkage and membrane strain
As membrane-related factors, in particular turgor and membrane strain, are accepted as being important triggers activating membrane-embedded proteins, e.g. BetP, in response to osmotic stress (Csonka, 1989; Wood, 1999), they will be dealt with first. Although turgor is still an attractive candidate for an osmostress-related signal, it seems unlikely that osmoregulated transport systems are triggered by changes in turgor in view of the fact that ProP of E.coli (Racher et al., 1999), BetP of C.glutamicum (Rübenhagen et al., 2000) and OpuA of L.lactis (van der Heide and Poolman, 2000) have been shown to be activated by osmotic up-shifts in proteoliposomes where no turgor is present.
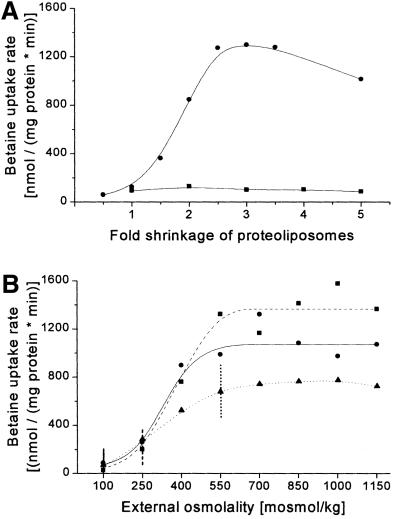
Membrane strain as a consequence of an osmotic shift, in contrast to turgor, is independent of the presence of a rigid cell wall and is thus a likely candidate for the signal perceived by a membrane-embedded protein (Epand, 1997; Cantor, 1999; Wood, 1999). Shrinkage of lipid vesicles after exposure to hyperosmotic stress has been analyzed in detail (White et al., 2000). When performing the simplest experiment (Rübenhagen et al., 2000), i.e. increasing external osmolality by diluting reconstituted proteoliposomes in NaCl-supplemented buffer with higher osmolality, BetP becomes activated (Figure 1A). For discriminating the effects of membrane strain from those related to changes in solute composition and concentration, experiments would be helpful in which the internal and/or external conditions are kept constant, whereas the membrane strain (shrinkage of liposomes) is varied. This type of experiment cannot be carried out in intact cells but only in proteoliposomes. As shrinkage necessarily leads to an increase in the internal solute concentration, the desired experiments have to be carried out in a different way as compared with the general type (see above) in order to control the internal concentration after the shift. In the experiment described in Figure 1A, proteoliposomes were subjected to hyperosmotic stress of various extents, which led to volume changes from 0.5 (2-fold increase) up to 5 (5-fold shrinkage). If, under the different osmotic conditions, one single set of proteoliposomes was used leading both to shrinkage and a concomitant increase in internal solute concentration, BetP became activated. On the other hand, when for each single experiment at a different osmolality a different set of proteoliposomes with decreasing internal K+ concentrations at starting conditions was used, which were chosen not to exceed a final 200 mosmol/kg internal KPi after shrinkage, BetP was not activated. It has to be emphasized that in the two different sets of experiments (Figure 1A) the extent of shrinkage of the proteoliposomes was essentially identical. Conse quently, shrinkage, i.e. membrane strain, cannot be a major factor triggering activation of BetP in proteoliposomes as a result of hyperosmotic shift.
Fig. 1. Activity regulation of BetP in dependence of liposomal shrinkage. The uptake of [14C]glycine betaine in proteoliposomes was measured. (A) In the two experiments, proteoliposomes shrink to an identical extent; however, they differ with respect to an increase in internal K+ (circles) or the lack of this increase (squares). The internal buffer contained 76 mM KPi pH 7.5 (circles) or 76, 38, 25, 19 and 15 mM KPi pH 7.5, respectively (squares, initial osmolality 200, 100, 67, 50 or 40 mosmol/kg). The lower line connects mean values of two independent experiments using different liposome preparations, whereas the upper curve is combined from five different sets of experiments, performed in duplicate, from 10 different preparations. The external buffer contained 20 mM NaPi pH 7.5, 25 mM NaCl (100 mosmol/kg) and was adjusted by NaCl to final osmolality values between 100 and 1000 mosmol/kg (circles) or of 200 mosmol/kg (squares). The abscissa indicates the extent of shrinkage (for calculation see Materials and methods). (B) The internal buffer contained 38 mM KPi pH 7.5 and was adjusted by K+-glutamate to an initial osmolality of 100 (circles), 250 (squares, dashed line) or 550 (triangles, dotted line) mosmol/kg. The external buffer contained 20 mM NaPi pH 7.5, 25 mM NaCl and was adjusted by NaCl to final values between 100 and 1150 mosmol/kg. The values of (identical) external and internal osmolality at the respective starting conditions of the three individual sets of experiments are indicated by vertical lines. Data points represent means of two independent determinations. The calculated final internal K+ concentrations, i.e. the concentrations after osmotic up-shift and volume change, reached values between 67.5 and 775 mM (circles), 57 and 655 mM (squares) or 53 and 610 mM (triangles).
As this experiment, designed to test the relevance of membrane strain as a stimulus in osmosensing, is of core significance in defining the correct signal related to osmotic stress, an alternative approach was applied in addition. We prepared proteoliposomes with different internal osmolalities and KPi concentrations, respectively (Figure 1B). Under iso-osmotic starting conditions, i.e. without a volume change, the proteoliposomes showed no (100 mosmol/kg), slight (250 mosmol/kg) and high activity (550 mosmol/kg). The external osmolality was then varied to lower and higher values. Although somewhat different maximum activities were reached, a fact that we found to be caused by a different extent of total reconstituted activity at differing ionic strength during vesicle extrusion (see also Figure 3), a very similar activation profile was found by either shrinkage or increase of the vesicles irrespective of the starting point. Taken together, the results of the experiments reported in Figure 1 argue for the fact that shrinkage per se cannot be a major trigger for activating BetP.
Fig. 3. Activation of BetP is dependent on the internal osmolality. The uptake of [14C]glycine betaine in proteoliposomes was measured. The values are means of three independent experiments each. (A) The internal buffer contained 38 mM KPi pH 7.5 (100 mosmol/kg) and was adjusted by KPi pH 7.5 (solid circles) or proline (open circles) to final values between 100 and 650 or 500 mosmol/kg, respectively. The external buffer contained 20 mM NaPi pH 7.5, 25 mM NaCl and was adjusted by NaCl to final values between 100 and 650 or 500 mosmol/kg, respectively. (B) The internal buffer (250 mM KPi pH 7.5) had an initial osmolality of 650 mosmol/kg. The external buffer contained 20 mM NaPi pH 7.5, 25 mM NaCl and was adjusted by NaCl to final values between 500 and 1000 mosmol/kg. The abscissa indicates the extent of shrinkage (for calculation see Materials and methods).
External and internal solute composition
In previous experiments, BetP was found to be activated in a time scale of seconds after raising the external osmolality, both in intact cells of C.glutamicum (Farwick et al., 1995; Peter et al., 1998b) and E.coli (Peter et al., 1996), as well as in proteoliposomes (Rübenhagen et al., 2000). In this type of experiment, solute concentrations are changed on both sides of the membrane simultaneously, as external addition instantly increases the internal solute concentration due to the fast water outflow.
In Figure 2, experiments are shown by which we tested whether changes in external and/or internal solute concentration are both relevant for BetP activation. BetP in liposomes prepared in KPi buffer was activated equally well by addition of either NaCl or proline, strictly correlated to the extent of increase in external osmolality. Instead of proline, we also used sorbitol, which led to exactly the same result (not shown). The slightly more efficient activation of BetP by NaCl in comparison to proline is explained by the fact that an increased Na+ gradient contributes favorably to the driving force of Na+-coupled transport by BetP. If, however, KPi in the internal lumen was largely substituted by proline, BetP could not be activated by an osmotic up-shift. Instead of proline, we also tested internal ectoine, carnitine, glucose or urea. None of these solutes was able to trigger BetP activation (data not shown).
Fig. 2. Activity regulation of BetP dependent on internal and external solute composition. The uptake of [14C]glycine betaine in proteoliposomes was measured. Each line connects mean values of two independent experiments based on two different proteoliposome preparations. Circles: the internal buffer had an initial osmolality of 200 mosmol/kg (76 mM KPi pH 7.5). The calculated final K+ concentration in the different experiments reached values between 67.5 and 675 mM. Squares: the internal buffer had an initial osmolality of 250 mosmol/kg (38 mM KPi pH 7.5, 150 mM proline); after osmotic up-shift, final K+ concentrations between 27 and 270 mM were reached. Triangles: the internal buffer had an initial osmolality of 550 mosmol/kg (38 mM KPi pH 7.5, 450 mM proline), the final K+ concentration reached values between 13.5 and 135 mM. The external buffer contained 20 mM NaPi pH 7.5, 25 mM NaCl. It was adjusted by NaCl (solid symbols) or proline (open circles) to final values between 100 and 1000 mosmol/kg. The experiment indicated by the solid circles is identical to that shown in the upper curve of Figure 1A.
For studying the influence of internal ion conditions, the initial internal K+ concentration had to be varied in several experiments, which in principle may lead to problems due to the fact that the K+ diffusion potential is necessary as a driving force. The K+ diffusion potential, however, created by diluting liposomes into K+-free buffer was kept virtually constant during the measurement of initial uptake rates as an identical dilution ratio and thus an identical K+ gradient was applied. Moreover, in experiments where an identical extent of osmotic stress was applied, the chemical Na+ potential was kept constant. In order to corroborate this argument, we performed experiments starting with an intermediate internal K+ concentration (Figure 2). Consequently, this led to full activation only if an increased hyperosmotic shift was applied; however, BetP reached similar maximum uptake rates irrespective of the decreased initial internal K+ concentration. Taken together, only the internal solute composition seems to matter for BetP activation, whereas the composition of external solutes was irrelevant, as long as an appropriate osmotic up-shift was provided, i.e. the signal triggering BetP activation must be common for all types of external solutes.
Internal osmolality
The results described above indicate that for activation of BetP, a shift of internal and not of external conditions is essential. But these experiments always involved manipulation of both external and, as a consequence of shrinkage, also internal osmolality and solute conditions. In order to discriminate whether, besides internal ion composition, internal osmolality may also be a factor triggering BetP, we studied the effect of changing internal osmolality in proteoliposomes. Various sets of proteoliposomes were prepared in which the external and internal osmolality were set to increasing absolute values, which were identical with respect to the two compartments (internal = external), thereby avoiding any osmotic gradient or vesicle shrinkage (Figure 3A). If the conditions were changed by using increasing proline concentrations, BetP never became active even at high osmolalities, whereas BetP was activated in the absence of any osmotic gradient, provided KPi was increased on both sides. Again, it was the internal solute composition that mattered, and an increase in internal KPi alone was sufficient to activate BetP.
The values of transport activity in the experiments reported in Figure 3 were somewhat lower than those observed in the previous experiments (Figures 1A and 2). In control experiments, we found that the extent of reconstituted BetP depends on the solute concentration in the reconstitution buffer, high concentrations leading to decreased efficiency of reconstitution (not shown). That this may in fact be the reason for the decreased maximum uptake activity in Figure 3A was further shown by a control experiment. In order to rule out that at identical internal and external osmolalities (Figure 3A) only partial activation may be reached, we selected one particular experimental set-up of those described in the upper curve of Figure 3A (650 mosmol/kg) and varied the external osmolality by dilution or by addition of NaCl (Figure 3B). This manipulation, in contrast to the previous experiment (Figure 3A), led to shrinkage or slight extension of the liposomes. No stimulation of BetP could be observed as a consequence of vesicle shrinkage. In contrast, a similar, although small increase in activity was observed when slightly extending the liposomes (500 mosmol/kg), as also determined in the previous experiment (Figure 3A).
Internal ion composition in proteoliposomes
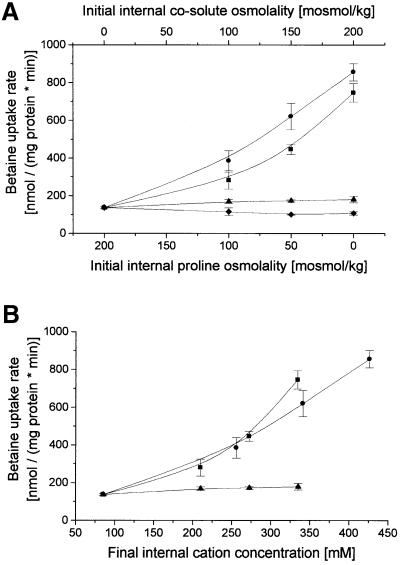
In the previous experiments, all external parameters, as well as membrane strain and internal osmolality, were ruled out as being relevant signals for BetP activation; consequently, we now studied the influence of internal solute composition in more detail. Previous results (Figure 2) demonstrated that internal KPi buffer is an appropriate condition for BetP to become activated after hyperosmotic shift, whereas internal proline is not. In the next experiment (Figure 4A), identical hyperosmotic shifts leading to 2.5-fold shrinkage were applied in a set of liposomes which were identical with respect to internal osmolality, whereas the internal solute composition was varied widely. A basic KPi concentration was provided as a source of the K+ diffusion potential as a driving force for betaine uptake. An increasing concentration of internal KPi and K+-glutamate, respectively, activated BetP, whereas choline chloride, another ionic solute, or glucose, a non-ionic solute, at identical osmolalities did not. In previous experiments (Figure 2), it had already been shown that besides proline, ectoine (another compatible solute in C.glutamicum), carnitine (a compatible solute in other bacteria) and urea were unable to trigger BetP activation. To analyze the slight difference in the activation profile of KPi and K+-glutamate in more detail, the data were replotted in terms of the absolute internal K+ concentration (Figure 4B). The result argues for a direct correlation of activation to the final internal K+ concentration reached. Furthermore, in combining one of the experiments reported in Figure 5, where KCl was used instead of KPi, with the results shown in Figure 4, it becomes evident that the type of anion used is irrelevant for activation.
Fig. 4. Activity regulation of BetP dependent on the type of internal solute. The uptake of [14C]glycine betaine in proteoliposomes was measured. (A) The internal buffer contained 19 mM KPi pH 7.5, and 200, 150, 100, 50 or 0 mM proline and was adjusted with KPi pH 7.5 (circles; 0, 19, 38, 57 or 76 mM), K+-glutamate (squares; 0, 25, 50, 75 or 100 mM), choline chloride (triangles; 0, 25, 50, 75 or 100 mM) or glucose (diamonds; 0, 50, 100, 150 or 200 mM) to an initial osmolality of 250 mosmol/kg. The external buffer (20 mM NaPi pH 7.5, 287.5 mM NaCl) had an osmolality of 625 mosmol/kg. Thus, a constant 2.5-fold shrinkage was obtained in each experiment. The values are means of three independent experiments each. (B) The same data plotted against the calculated final internal cation concentration.
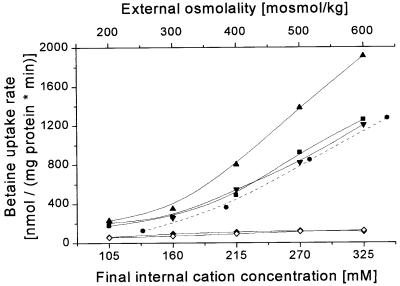
Fig. 5. Activity regulation of BetP dependent on the type of internal cation. The uptake of [14C]glycine betaine in proteoliposomes was measured. The internal buffer contained 19 mM KPi pH 7.5, and was adjusted by KPi pH 7.5 (circles, dashed line; same data as in Figure 1), to an initial internal osmolality of 200 mosmol/kg, or by KCl (squares), RbCl (upward pointing triangles), CsCl (downward pointing triangles), or NH4Cl (diamonds) to an initial internal osmolality of 250 mosmol/kg. The external buffer contained 20 mM NaPi pH 7.5, 25 mM NaCl and was adjusted by NaCl (solid symbols) or NH4Cl (open diamonds) to final values between 200 and 600 mosmol/kg. The external osmolality and the final solute, resp. K+ concentration in the liposomal lumen is shown. The external osmolality was adjusted to values between 200 and 500 mosmol/kg. Data points represent means of two independent determinations.
To study the specificity of BetP for activating ions in more detail, we varied the internal cations systematic ally. It was not possible to investigate Na+ or related monovalent cations in significant concentrations because of a counteracting electrochemical Na+ potential under these conditions (Rübenhagen et al., 2000). Besides choline, which had already been shown not to activate BetP, we tested further monovalent cations (Figure 5). Among these, Cs+ seemed to be similarly efficient as K+ in activation, while Rb+ was found to stimulate BetP to the greatest extent. No influence of the type of anion was observed. Interestingly, ammonium did not lead to activation of BetP. In view of the fact that, due to the permeability of uncharged NH3, transmembrane ammonium gradients might give rise to a secondary proton gradient, which could lead to inhibition of BetP activity, we modified this experiment. To avoid significant transmembrane ammonium gradients, external NaCl added to induce the osmotic up-shift was replaced by NH4Cl (Figure 5). Nevertheless, BetP was not activated, indicating that ammonium was not able to replace K+ in this respect. In control experiments, we proved that NH4Cl was basically efficient in leading to the activation of BetP when applied externally, provided that appropriate internal K+ concentrations were present (not shown).
Membrane topology of BetP
The preceding experiments proved that membrane- inserted BetP does not sense transmembrane gradients or membrane strain as a stimulus, but instead perceives the side-specific change in the concentration of a particular solute, namely K+ or closely related cations. Because of this side-specific activation, it is important to know the direction of BetP insertion in the bilayer membrane. This is of particular significance as we have previously shown in intact cells of C.glutamicum that BetP, when truncated by a small part of the C-terminal domain, loses its sensitivity to osmotic stress (Peter et al., 1998b). This result indicates that the terminal domains of BetP are putative signal input sites in osmosensing.
To study the topology of BetP, recombinant forms of the carrier with terminal tags or with strategically located cysteines were expressed in intact E.coli cells. Both N- and C-terminally Strep-tagged BetP was synthesized in E.coli, and spheroplasts (right-side out) or sonic vesicles (inside out) were prepared (Figure 6A). As a control, E.coli cells without BetP were used. Care was taken to provide conditions for a correct comparison of spheroplasts and vesicles by using the complete sample volume without further separation or treatment in both cases, starting from an identical batch of cells. Thus, an identical amount of immunoreactive material should be present in the two preparations. The reactivity in ELISA tests of both N- and C-terminal domains with the applied anti-Strep antibody was significantly higher in vesicles than in spheroplasts, whereas the particles without BetP did not react at all (Figure 6A). The somewhat higher reactivity of the spheroplasts with the C-terminal Strep tag as compared with the N-terminal construct was due to the fact that E.coli cells synthesizing this construct showed a severe growth defect, which led to partial cell lysis after expression of the betP gene (data not shown).
Fig. 6. Determination of the orientation of Strep-tagged BetP in intact cells. (A) Localization of N- and C-terminal domains of Strep-tagged BetP by ELISA. Spheroplasts (open symbols) or inverted membrane vesicles (sonic vesicles, solid symbols) were prepared from isopropyl-β-d-thiogalactopyranoside (IPTG)-induced cells of DH5αmcr pASK-IBA5 betP (circles, N-terminal Strep-tagII), DH5αmcr pASK-IBA3 betP (squares, C-terminal Strep-tagII) and DH5αmcr pASK-IBA5 (triangles, empty vector). The highest absorption of the sonic vesicles derived from each Strep–BetP-expressing strain was set to 100%. Streptavidin–alkaline phosphatase conjugate was used for the immuno detection of Strep–BetP. The amount of spheroplasts and vesicles was standardized as described in the text; the abscissa refers to identical amounts of protein from intact cells. (B) External localization of cysteines at positions 89 and 516 in Cys-less BetP. The proteins were expressed in E.coli and incubated with membrane-impermeable stilbene maleimide (SM). After solubilization and purification, the proteins were incubated with biotin maleimide (BM); the extent of BM reaction with unmodified cysteines was detected by western blotting. The application of equal amounts of BetP protein was controlled by Coomassie Blue staining.
In a different approach we expressed BetP constructs in which cysteines had been introduced at positions 89 and 516 in place of the native serine residues at these positions, on the basis of an engineered Cys-less form of BetP. The computer-predicted topology places position 89 in the first periplasmic loop between transmembrane segments I and II, whereas position 516 is predicted to be located in the last periplasmic loop preceding segment XII. The two cysteines should thus face the side opposite to that of the N- and C-terminal domains. As seen in Figure 6B, both cysteines are fully accessible in intact E.coli cells from the outside, as proven by membrane-permeable and -impermeable maleimide reagents. Unfortunately, engineered cysteines in both terminal domains showed only low accessibility to SH reagents in general, independently of whether membrane-impermeable or -permeable reagents were used. As a control, we proved that all constructs, i.e. Cys-less BetP, the proteins with Ser/Cys replacements and the terminally tagged proteins, were active in transport and in osmosensing (not shown).
In contrast to the clear data obtained for BetP in intact cells, the situation turned out to be more difficult in proteoliposomes. Application of the antibody approach was not possible as all detergents used for destroying the liposomes, thereby randomizing the access to BetP, quenched the antibody and/or the indication reaction. Consequently, a quantitative comparison of the reaction in the absence (intact proteoliposomes) or presence of detergent (solubilized protein) was not possible. Application of the accessibility assay led to somewhat undefined results in spite of the fact that a series of BetP constructs with cysteines at different loop positions was used. Specifically located cysteines in BetP were either not accessible at all (terminal domains) or accessible from both sides to some extent (hydrophilic loops). Although both the immunological as well as the reagent-accessibility approach were always in favor for a right-side out orientation of BetP in proteoliposomes, the results were not really convincing. We conclude from these experiments that BetP in proteoliposomes is either (i) not oriented fully unidirectionally or (ii) is, in addition to being correctly inserted and active, in part also incorrectly and non-functionally inserted, or finally (iii) that membrane-attached and not fully inserted BetP protein is present, which interferes with the experiments testing the orientation.
Discussion
In previous studies using proteoliposomes, which consisted of E.coli phospholipids and purified BetP protein from C.glutamicum, we have demonstrated that reconstituted BetP has retained its full functional (catalytic) and regulatory competence. This was one of the reasons for arguing that BetP acts both as osmosensor and osmoregulator without the need for further factors (Rübenhagen et al., 2000). In this study, we used proteoliposomes for elucidating the type of stimulus sensed by BetP in response to a hyperosmotic shift. The results obtained define internal K+ as the relevant signal. This finding, however, does not rule out other factors as being possibly relevant in addition, in particular in intact cells.
Although not essential for defining the nature of the stimulus involved in osmosensing, the orientation of the reconstituted BetP is in fact relevant for a correct interpretation. We provide strong evidence for an orientation of BetP in intact cells that coincides with the computer-based secondary structure prediction (Peter et al., 1998b), namely both terminal domains facing the cytoplasm. Unfortunately, an equally clear result was not obtained for reconstituted BetP. However, a series of facts argue in favor of a right-side out orientation, identical to intact cells. (i) All catalytic properties tested so far in proteoliposomes, including vectorial energy dependence, were found to be identical to intact cells (Rübenhagen et al., 2000). (ii) The osmoregulatory properties are identical as well. (iii) As found in intact cells, BetP seems to be fairly unidirectional in its transport activity (Peter et al., 1998b), i.e. inversely oriented BetP protein in proteoliposomes would presumably not be active. (iv) Specific truncations in the terminal domains of BetP led to specific changes in its regulatory properties (Peter et al., 1998b). Consequently, the signal input site is most probably located in or close to these domains, which face the cytoplasm in cells. The conserved side specificity of signal input and intramolecular signal transduction thus argues for an identical orientation. (v) Most convincingly, in both proteoliposomes and cells the osmoreactive properties of BetP were found to be independent of the externally applied solutes, which coincides perfectly with the side-specific effect of ions only from the lumen of the proteoliposomes. Although there is still no definite proof available for a right-side out orientation of BetP in proteoliposomes, in view of the arguments summarized above we feel safe to conclude that at least the particular share of BetP that is functionally active in liposomes, and which is thus detected in transport studies, has the same orientation as shown for BetP in intact cells, i.e. both terminal domains facing the inside.
A fundamental aspect of BetP structure and function, which is highly relevant for interpreting the results obtained in this study, has to be discussed in more detail. We have demonstrated by truncating short segments of the N- as well as the C-terminal domain of BetP that the structural integrity of these domains is essential for the correct response of BetP to osmotic shifts (Peter et al., 1998b). Deletion of short, 12- or 23-amino-acid-long segments of the C-terminal domain of BetP led to deregulation of its activity. The truncated protein was permanently active and insensitive to the changes in osmolality. In addition, truncations of the N-terminal domain led to a shift in the set point of the activation profile (Peter et al., 1998b). These results indicate that osmosensing as well as the signal input to the BetP protein are mediated predominantly, if not exclusively, via the side where the terminal domains are located.
In a series of experiments in proteoliposomes we tested a large variety of possible signals triggering BetP activation in response to hyperosmotic stress. The only signal, which was perceived by reconstituted BetP, was the luminal K+ concentration, i.e. at the side where the N- and C-terminal domains are most probably located. In view of the current hypotheses, the most important factor to be investigated was membrane strain due to shrinkage of the vesicles (Cantor, 1999; Wood, 1999; White et al., 2000). We demonstrated that reconstituted BetP does not directly react to changes in liposomal shape and structure, but perceives the rise in external osmolality by its effect on the concentration of internal K+. By excluding a direct influence of the physical state of the membrane and by positively identifying internal K+ as the trigger of BetP activation, a putative mechanosensor has been found to actually function as a chemosensor (J.M.Wood, personal communication). Besides membrane effects, a long list of further possible candidates for stimuli involved in osmosensing could be excluded. Notably, this also holds for direct influences on BetP protein structure by changes in solute concentration in its surroundings, which is an intensively discussed topic related to osmosensing (Qu and Bolen, 1998; Parsegian et al., 2000). Furthermore, molecular crowding, another candidate of osmostress-related stimuli, although not directly studied here, can be excluded as a major factor, as proteoliposomes do not contain macromolecules in their luminal space.
In view of previous results from our own group, we would, however, prefer to be careful with respect to a broad generalization of this finding. First, the efficiency of triggering BetP by osmotic stress was found to depend on the type of phospholipid in liposomes (Rübenhagen et al., 2000), as well as on the type of membrane in which it is inserted, namely in cells of E.coli or C.glutamicum (Peter et al., 1998b; Rübenhagen et al., 2000). Evidence for a direct influence of the membrane surrounding was also provided for the ABC-type betaine transporter in L.lactis (van der Heide and Poolman, 2000). Moreover, we observed a modulating influence of tetracaine when added to cells (Peter et al., 1998b) and to liposomes, respectively (Rübenhagen et al., 2000). Tetracaine is supposed to alter the physical state of the membrane (Auger et al., 1988; Shimooka et al., 1992). One may hypothesize, however, that both negatively charged phospholipid species and positively charged tetracaine molecules influence a signal based on protein membrane interaction without necessarily being connected to a signal input pathway via the hydrophobic part of the bilayer.
We found a pronounced specificity of the sensing mechanism of BetP. This specificity was two-fold with respect to sidedness and with respect to the type of signal perceived. The former is most probably explained by the exclusive involvement of the N- and C-terminal domains in sensing. The specificity with respect to the triggering solute is convincing, yet not fully elaborated. Based on the variation of solutes in the liposomal lumen, general parameters like osmolality or ionic strength could be ruled out. The same holds true for uncharged and for zwitterionic molecules, as well as for anions in general. Only cations similar to K+, i.e. Rb+ and Cs+, were recognized by BetP. As the reconstituted system requires an electrochemical Na+ potential as the driving force for betaine uptake, it has not been possible so far to test Na+ for its efficiency in activation. However, the larger organic cation choline was completely inactive and the same was found for NH4+. Although we tried to circumvent well known problems due to the high membrane permeability of NH3 resulting in the formation of a pH gradient, the definite exclusion of NH4+ as an activating cation has to be taken with some caution.
Once the signaling by internal K+ has been demonstrated, we can combine various experiments of this study in terms of calculating an internal K+ concentration that leads to half-maximal stimulation of betaine uptake activity. For the experiments shown in Figures 1B and 2, which comprise different experimental set-ups and widely varying internal K+ concentrations, a common value of 221 ± 23 mM for the internal K+ concentration leading to half-maximal activity was obtained. The value taken from the standard experiment, as shown in the upper curve of Figure 2, was 243 mM. This is an additional argument for K+ being the common signal in all these experiments.
What type of mechanism could be responsible for the observed K+-specific signal perception of BetP? Based on the finding that the terminal 12–23 amino acids are essential for osmosensing (Peter et al., 1998b) and on the right-side out orientation of functionally active BetP, one may speculate that this segment of the C-terminal domain specifically interacts with cytoplasmic K+ ions. To prove this hypothesis, however, detailed experiments using recombinant forms of BetP, ligand interaction studies with isolated BetP, as well as studies on the structural dynamics of the C-terminal domain are necessary.
It is interesting to relate these findings to the physiological situation in intact cells. As mentioned in the Introduction, it is well known that the first response of many bacteria to osmotic up-shifts consists of rapid uptake of K+ (Csonka, 1989; Wood, 1999). Preliminary studies have revealed that C.glutamicum reacts in a similar way to a sudden hyperosmotic shock by increasing cytoplasmic K+ via active uptake (our unpublished data). For an appropriate evaluation of the proteoliposome data, however, a shorter time scale of milliseconds to seconds is significant, which represents instant volume changes due to the fast water efflux after an osmotic up-shift before the onset of K+ uptake. Consequently, for this correlation, exact values of the internal K+ concentration in non-stressed and osmotically challenged cells are necessary. These data are not yet available. Preliminary studies led to values of cytoplasmic K+ significantly higher than 200–250 mM, as was found for the threshold in liposomes (not shown). In view of the known influence of phospholipids on BetP activation (Rübenhagen et al., 2000), it should be noted that this value refers to E.coli lipids only and that in liposomes with a more C.glutamicum-like lipid composition the activation threshold was significantly increased to higher values.
In view of the fact that the stimulus responsible for osmosensing had not been resolved so far for any of the osmoreactive carriers, this study adds fundamentally new knowledge in this respect. It had been noted previously that K+ may actually be a trigger for the osmoregulated proline carrier ProP in E.coli; however, other possible factors could not be ruled out due to the complexity of intact cells (Sutherland et al., 1986). Also, in the case of the E.coli Kdp system, high internal K+ was identified as one of the factors inhibiting this transport and signal transduction system, the regulation of which is much more complicated than that of BetP (Jung et al., 2000). Taken together, to our knowledge, the present study is the first to directly correlate the osmoresponsive regulation of a transport system to cytoplasmic K+.
Materials and methods
Bacterial strains, plasmids and growth conditions
Escherichia coli MKH13 (Kempf and Bremer, 1998) was used for betaine uptake measurements, and E.coli DH5αmcr (Grant et al., 1990) for all other purposes. The plasmids used were pASK-IBA5 betP (Rübenhagen et al., 2000) or derivatives of it, and pASK-IBA3 betP, in which strepbetP is under the control of the tet promoter. Escherichia coli cells were grown at 37°C in Luria–Bertani medium with carbenicillin (50 µg/ml). For K+-limited conditions, cells were grown in citrate–phosphate buffer pH 6.0 (Roe et al., 2000), modified for E.coli MKH13: 9.16 g/l NaHPO4, 3.55 g/l citrate, 1 g/l (NH4)2SO4, 0.1 g/l MgSO4, 25 mg/l FeSO4, 5 mg/l thiamine, 0.2% glucose, 0.004% arginine, 0.004% isoleucine, 0.004% valine, 1/100 volumes of vitamin solution (1080 mg/l pyridoxine–HCl, 300 mg/l nicotine acid, 72 mg/l Ca pantothenate, 56 mg/l inositol, 22 mg/l folic acid, 20 mg/l nicotinamide, 16 mg/l p-aminobenzoate, 8 mg/l thiamine, 2.2 mg/l biotin, 2 mg/l riboflavin) and 1/1000 volumes of trace element solution [200 mg/l CoCl2, 100 mg/l MnCl2, 100 mg/l NiCl2, 70 mg/l ZnCl2, 50 mg/l Na(NH4)2MoO4, 20 mg/l CuCl2].
DNA techniques and construction of plasmids
Plasmid DNA isolation, E.coli transformation, DNA sequencing and PCR were carried out as described previously (Rübenhagen et al., 2000). pASK-IBA3 betP encoding BetP with a C-terminal Strep-tag II was constructed as described for pASK-IBA5 betP (Rübenhagen et al., 2000), but using pASK-IBA3 (Skerra, 1994) as ligation vector, pASK-IBA5 betP as PCR template, the PCR sense primer 5′-AAAAAGGGTCTCAAATGACTACATCTGACCCAAATCC-3′ and the antisense primer 5′-TCACAGGGTCTCCGCGCTTCGACGCTTCCCCGCGCCAC-3′. For construction of pAcl1 encoding Cys-free BetP protein pASK-IB5 betP was used as ligation vector and PCR template. The single Cys of wild-type BetP was exchanged for Thr using the mutagenic PCR sense primer 5′-GGCACGGCAACTTCCCTTGGCCTTGGTGCCTTGCAG-3′ and the antisense primer 5′-CTGCAAGGCACCAAGGCCAAGGGAAGTTGCCGTGCC-3′. For the construction of pAcl1 S89C and pAcl1 S516C, which encode single-Cys BetP proteins, pAcl1 was used as ligation vector and PCR template. Single serine residues of Cys-free BetP were replaced using the mutagenic PCR sense primer 5′-AACTTTGCTTGTTCTGCGTTG-3′ and the mutagenic antisense primer 5′-CAACGCAGAACAAGCAAAGTT-3′ for the construction of pAcl1 S89C, as well as 5′-AATGCCTTGTGCAACTTGCAA-3′ and 5′-TTGCAAGTTGCACAAGGCATT-3′ for the construction of pAcl1 S516C.
Protein purification, preparation of proteoliposomes and transport assay
Preparation of liposomes and inverted vesicles, purification and reconstitution of Strep–BetP, synthesis of [14C]glycine betaine and transport assays were carried out as described previously (Peter et al., 1998b; Rübenhagen et al., 2000). For reconstitution procedures, E.coli polar total lipid extract (Avanti Polar Lipids, Alabaster, USA) was used. In experiments in which internal solutes were varied, frozen proteoliposomes from stock preparations (10–50 µl containing 15–100 µg of protein and 0.6–4 mg of phospholipid) were diluted in the desired buffer solutions (in 500 µl portions), extruded (13 times) and sedimented by ultracentrifugation. After dilution, as indicated in the particular experiments, liposomes were used directly for transport assays. The efficiency of internal buffer exchange by the extrusion procedure was controlled by comparison with experiments using proteoliposomes that were frozen and thawed several times before extrusion, which led to the same results. Shrinkage of vesicles was calculated by assuming volume changes of proteoliposomes behaving as ideal osmometers, i.e. directly proportional to the changes in external osmolality.
Analytical procedures
SDS–PAGE, western blotting and determination of solubilized and of reconstituted protein was carried out as described previously (Rübenhagen et al., 2000). For western blot analysis, avidin–alkaline phosphatase conjugate (1:2500; Bio-Rad, Munich, Germany) was used. Proteins were visualized by staining with Coomassie Blue.
Labeling of Strep–BetP derivatives with thiol-specific reagents
Synthesis of single-Cys Strep–BetP in E.coli DH5αmcr pAcl1 S89C or S516C was initiated by adding 200 µg/l anhydrotetracycline to growth cultures at an OD600 of 1.5. After 3 h, cells were harvested, washed with 100 mM KPi pH 7.5 containing 1 mM Pefabloc® (Roche, Mannheim, Germany) and suspended in the same buffer adjusted to an OD600 of 200. For blocking periplasmic thiol groups (Jung et al., 1998), a 1.5 ml aliquot of the cell suspension was incubated for 30 min with 400 µM membrane-impermeant thiol reagent 4-acetamino-4′-maleimidylstilbene-2,2′-disulfonate (SM; Molecular Probes, Eugene, OR) at room temperature, and washed twice in KPi buffer. Strep–BetP protein from both untreated and SM-blocked cells was purified in mercaptoethanol-free buffer and incubated at room temperature with 200 µM 3-(N-maleimidylpropionyl)-biocytin (BM; Molecular Probes) for 30 min. Aliquots were subjected to SDS–PAGE and Coomassie Blue staining. Reaction of single-Cys Strep–BetP with BM was determined by western blot analysis using an avidin–alkaline phosphatase conjugate.
Determination of Strep–BetP orientation by ELISA
Strep–BetP synthesis was induced by 200 µg/l anhydrotetracycline at an OD600 of 0.5 and incubation for 1–2 h. After washing with 650 mM saccharose, 33 mM Tris–HCl pH 8.0, the cells were resuspended in the same buffer (80 ml/g wet cell weight) and 1/5 volume of 100 mM EDTA was added. For preparation of spheroplasts, one-half of the cell suspension was incubated with 0.05 mg/ml lysozyme for 45 min at room temperature; the preparation was verified by light microscopy. The second half (3 ml) was sonicated four times using a tip-type sonicator (Branson W250, Andover, USA) in a plastic tube at 4°C for 20 s (output level 5), resulting in inverted vesicles. Aliquots (200 µl) of both spheroplasts and inverted vesicles were transferred into wells of MaxiSorp immuno plates (Nunc, Roskilde, Denmark) (Burkovski, 1997). After 1 h incubation at room temperature, remaining binding sites were blocked with 3% bovine serum albumin in 100 mM Tris–HCl pH 7.5, 0.9% NaCl (wash buffer) for 1 h at room temperature. Incubation with streptavidin–alkaline phosphatase conjugate (1:5000 in wash buffer) was carried out overnight at 4°C. The wells were washed five times with wash buffer, followed by incubation with 200 µl of substrate (1 mg/ml p-nitrophenyl phosphate in 100 mM glycine–NaOH pH 10.4, 1 mM ZnCl2, 1 mM MgCl2) for 20–60 min at room temperature. Absorption at 405 nm was measured in the microtiter plate wells (1420 VICTOR multilabel counter, Wallac, Turku, Finland).
Acknowledgments
Acknowledgements
We are indebted to Janet Wood for stimulating discussions. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Schwerpunkt 1070) and by the Fonds der Chemischen Industrie.
References
- Auger M., Jarell,H.C., Smith,I.C.P., Siminovitch,D.J., Mantsch,H.H. and Wong,P.T.T. (1988) Effects of the local anesthetic tetracaine on the structural and dynamic properties of lipids in model membranes: a high-pressure Fourier transform infrared study. Biochemistry, 27, 6086–6093. [DOI] [PubMed] [Google Scholar]
- Burkovski A. (1997) Rapid detection of bacterial surface proteins using an enzyme-linked immunosorbent assay system. J. Biochem. Biophys. Methods, 34, 69–71. [DOI] [PubMed] [Google Scholar]
- Cantor R.S. (1999) Lipid composition and the lateral pressure profile in bilayers. Biophys. J., 76, 2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L.N. (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev., 53, 121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L.N. and Hanson,A.D. (1991) Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol., 45, 569–606. [DOI] [PubMed] [Google Scholar]
- Epand R.M. (1997) Studies of membrane physical properties and their role in biological function. Biochem. Soc. Trans., 25, 1073–1079. [DOI] [PubMed] [Google Scholar]
- Farwick M., Siewe,R.M. and Krämer,R. (1995) Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J. Bacteriol., 177, 4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S.G.N., Jessee,J., Bloom,F.R. and Hanahan,D. (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl Acad. Sci. USA, 87, 4645–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Rübenhagen,R., Tebbe,S., Leifker,K., Tholema,N., Quick,M. and Schmid,R. (1998) Topology of the Na+/proline transporter of Escherichia coli. J. Biol. Chem., 273, 26400–26407. [DOI] [PubMed] [Google Scholar]
- Jung K., Veen,M. and Altendorf,K. (2000) K+ and ionic strength directly influence the autophosphorylation activity of the putative turgor sensor KdpD of Escherichia coli. J. Biol. Chem., 275, 40142–40147. [DOI] [PubMed] [Google Scholar]
- Kempf B. and Bremer,E. (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol., 170, 319–330. [DOI] [PubMed] [Google Scholar]
- Krämer R. (1994) Secretion of amino acids by bacteria—physiology and mechanism. FEMS Microbiol. Rev., 13, 75–94. [Google Scholar]
- Parsegian V.A., Rand,R.P. and Rau,D.C. (2000) Osmotic stress, crowding, preferential hydration, and binding: a comparison of perspectives. Proc. Natl Acad. Sci. USA, 97, 3987–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H., Burkovski,A. and Krämer,R. (1996) Isolation, characterisation, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J. Bacteriol., 178, 5229–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H., Weil,B., Burkovski,A., Krämer,R. and Morbach,S. (1998a) Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterisation of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J. Bacteriol., 180, 6005–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H., Burkovski,A. and Krämer,R. (1998b) Osmosensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J. Biol. Chem., 273, 2567–2574. [DOI] [PubMed] [Google Scholar]
- Poolman B. and Glaasker,E. (1998) Regulation of compatible solute accumulation in bacteria. Mol. Microbiol., 29, 397–407. [DOI] [PubMed] [Google Scholar]
- Qu Y., Bolen,C.L. and Bolen,D.W. (1998) Osmolyte-driven contraction of a random coil protein. Proc. Natl Acad. Sci. USA, 95, 9268–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racher K.I. et al. (1999) Purification and reconstitution of an osmosensor: transporter ProP of Escherichia coli senses and responds to osmotic shifts. Biochemistry, 38, 1676–1684. [DOI] [PubMed] [Google Scholar]
- Roe A.J., McLaggan,D., O’Byrne,C.P. and Booth,I.R. (2000) Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol. Microbiol., 35, 1235–1243. [DOI] [PubMed] [Google Scholar]
- Rübenhagen R., Rönsch,H., Jung,H., Krämer,R. and Morbach,S. (2000) Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J. Biol. Chem., 275, 735–741. [DOI] [PubMed] [Google Scholar]
- Shimooka T., Shibata,A. and Terada,H. (1992) The local anesthetic tetracaine destabilizes membrane structure by interaction with polar headgroups of phospholipids. Biochim. Biophys. Acta, 1104, 261–268. [DOI] [PubMed] [Google Scholar]
- Skerra A. (1994) Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene, 151, 131–135. [DOI] [PubMed] [Google Scholar]
- Sutherland L., Cairney,J., Elmore,M.J., Booth,I.R. and Higgins,C.F. (1986) Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J. Bacteriol., 168, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide T. and Poolman,B. (2000) Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl Acad. Sci. USA, 97, 7102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G.F., Racher,K.I., Lipski,A., Hallett,F.R. and Wood,J.M. (2000) Physical properties of liposomes and proteoliposomes prepared from Escherichia coli polar lipids. Biochim. Biophys. Acta, 1468, 175–186. [DOI] [PubMed] [Google Scholar]
- Wood J.M. (1999) Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev., 63, 230–262. [DOI] [PMC free article] [PubMed] [Google Scholar]