Abstract
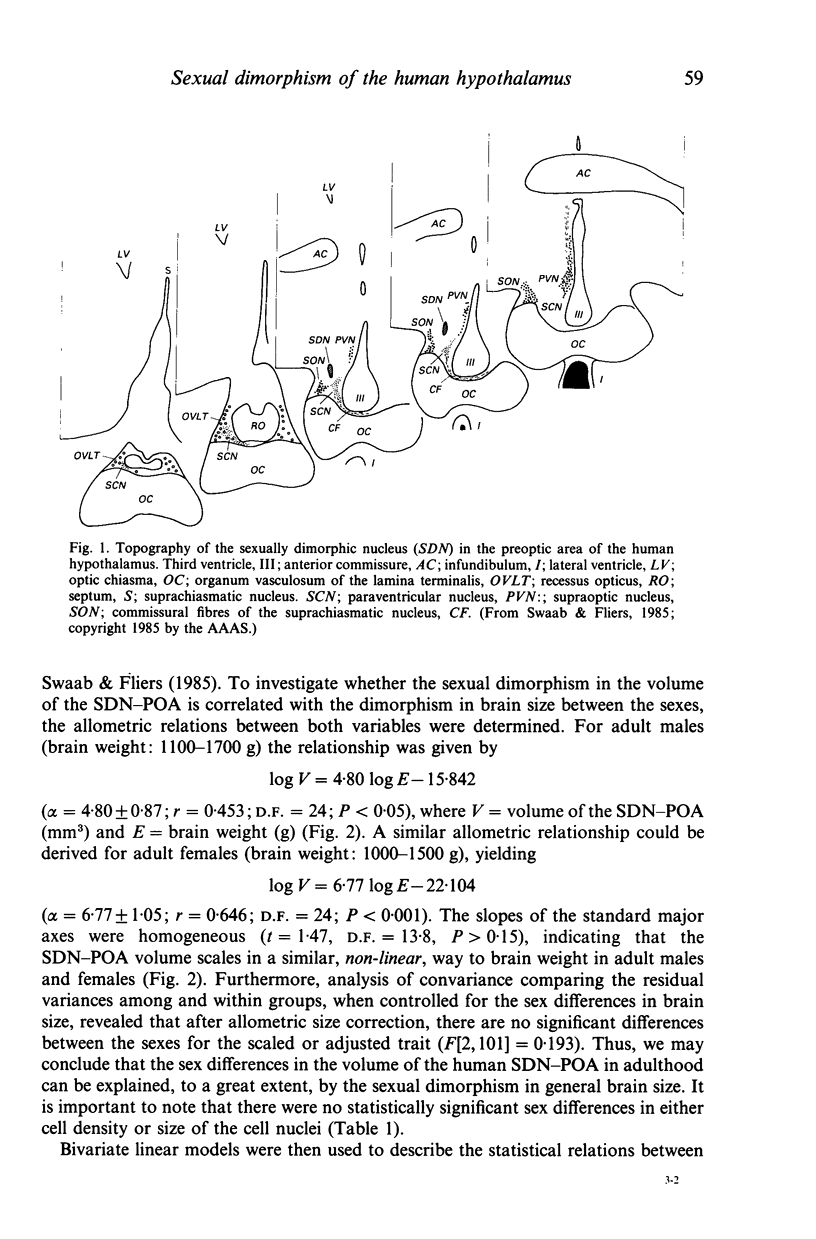
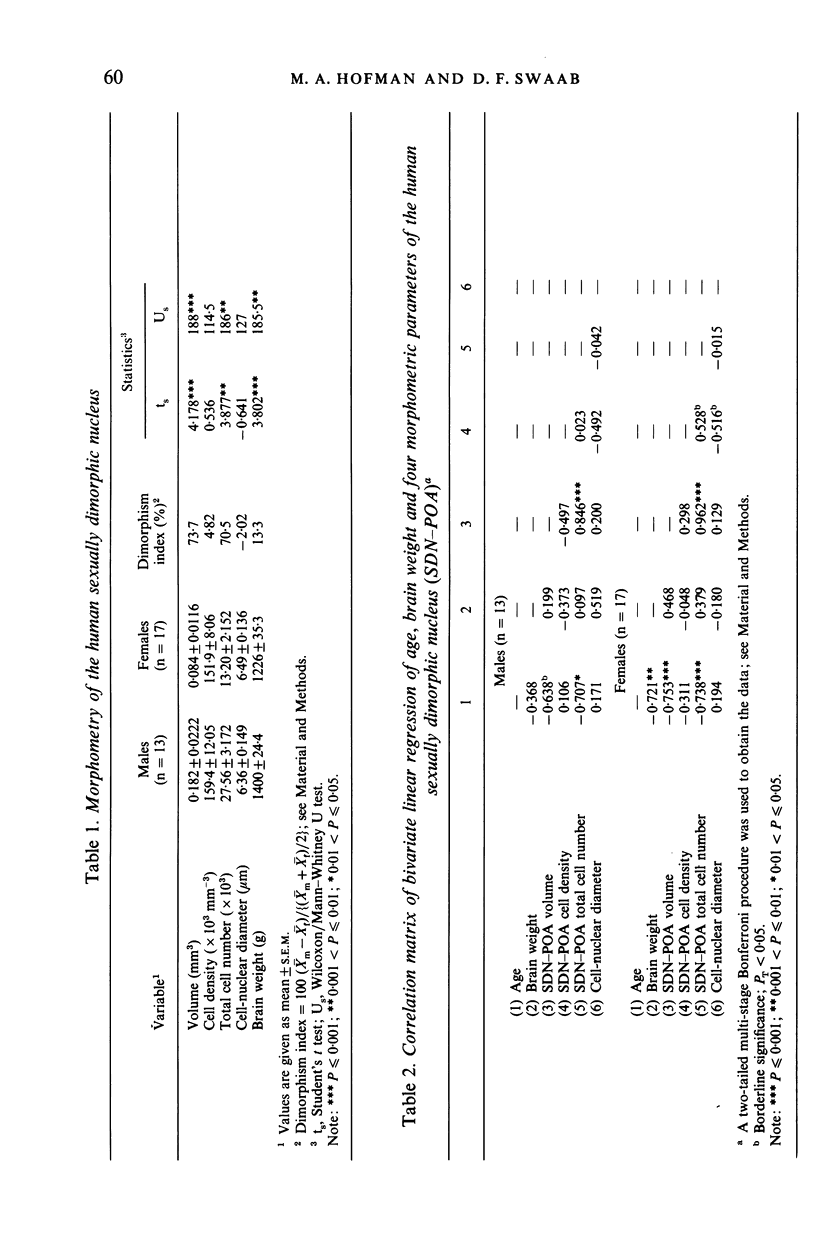
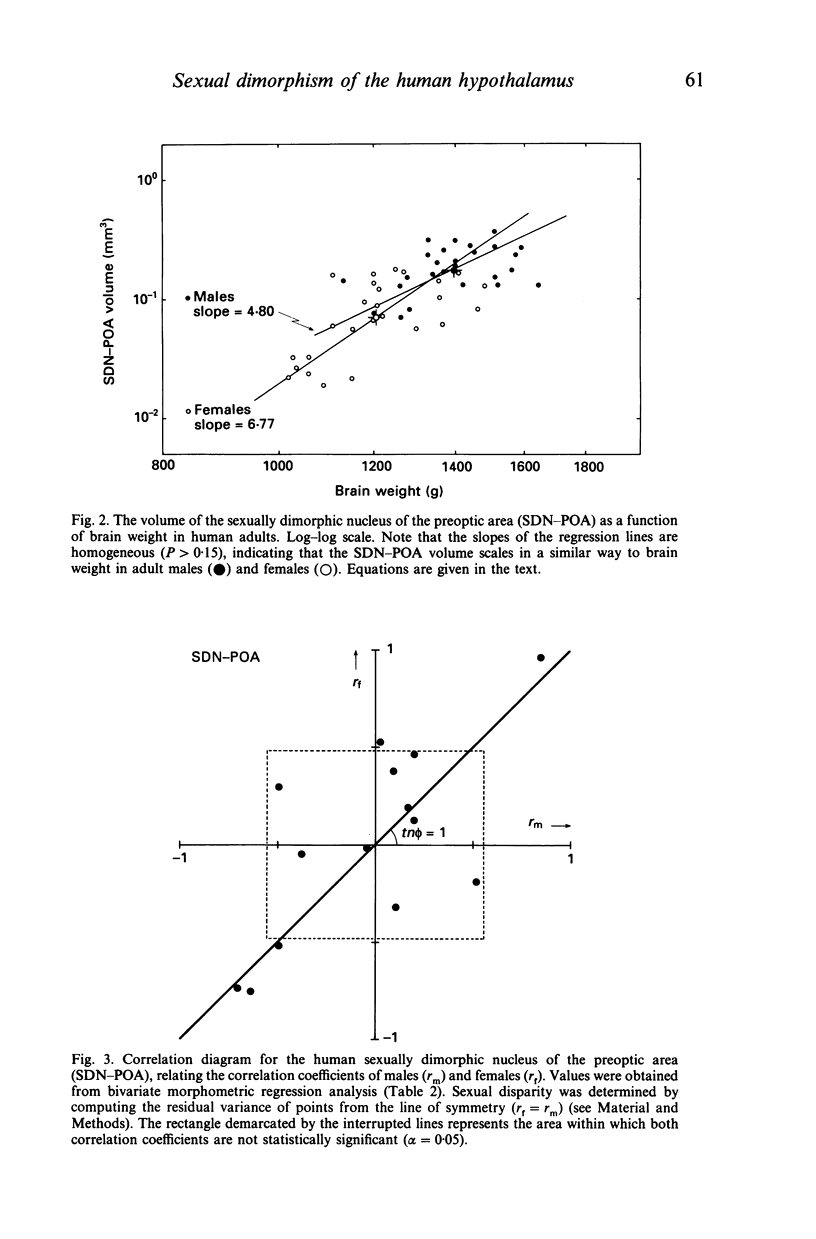
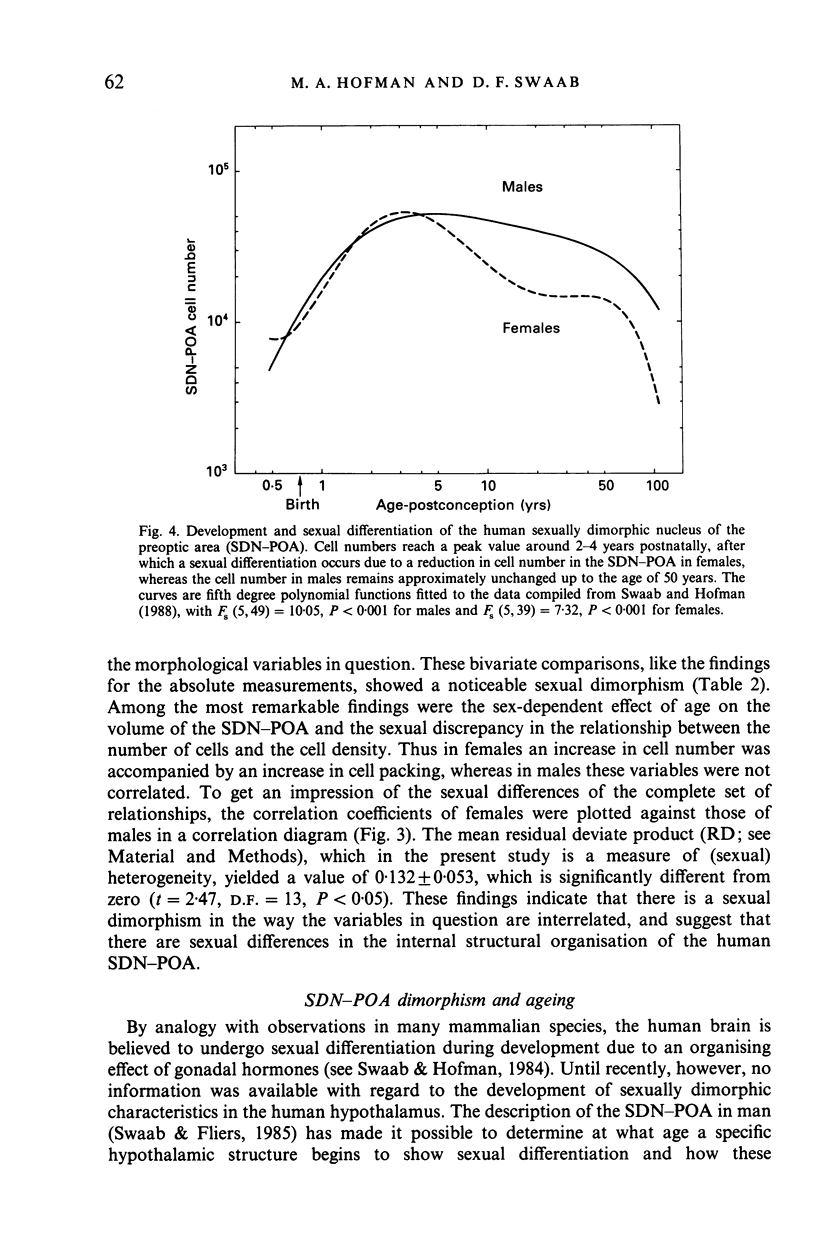
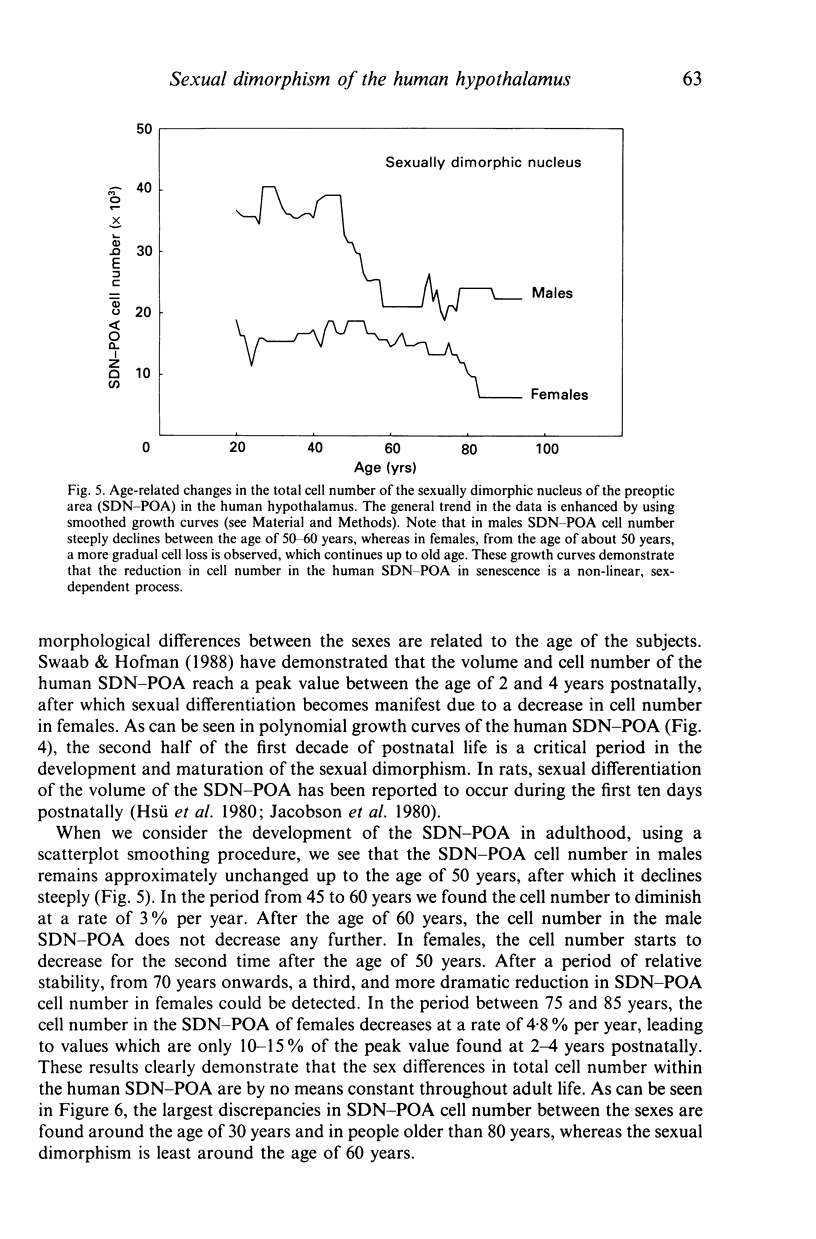
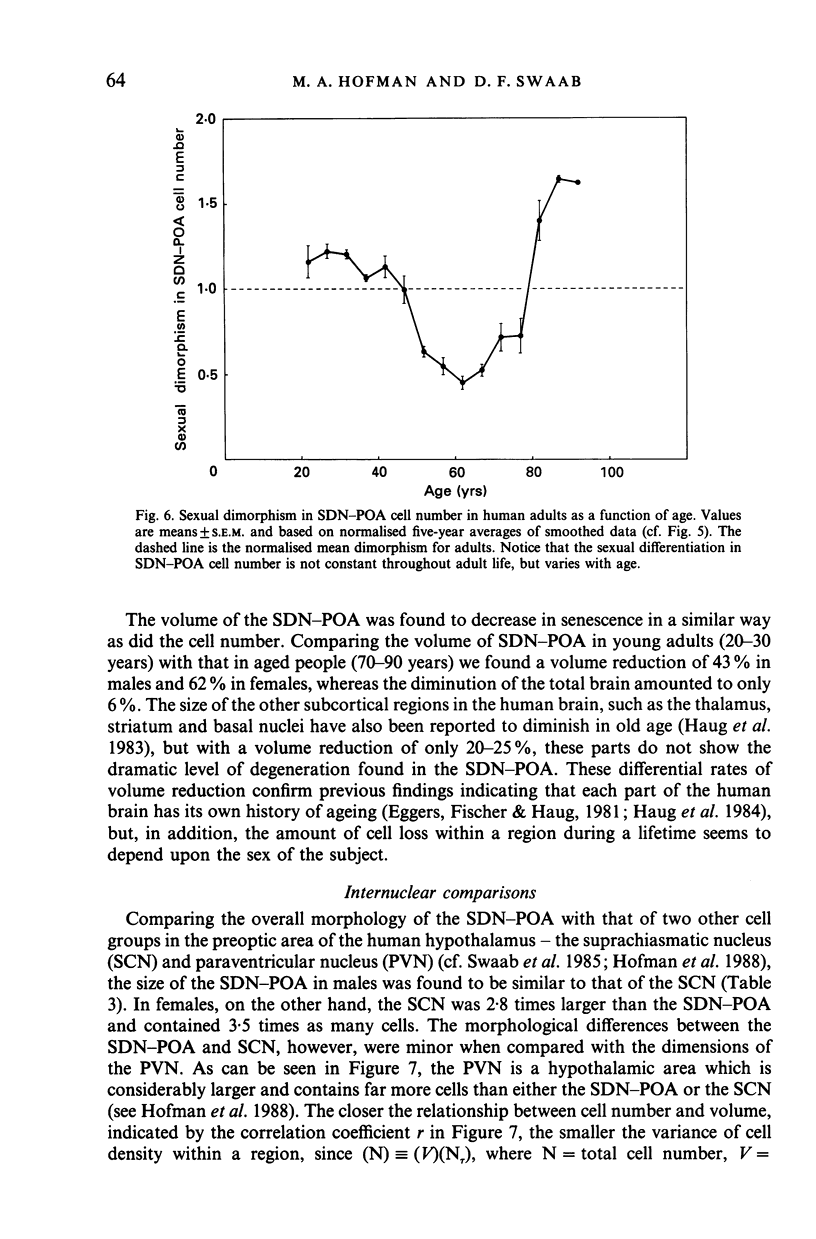
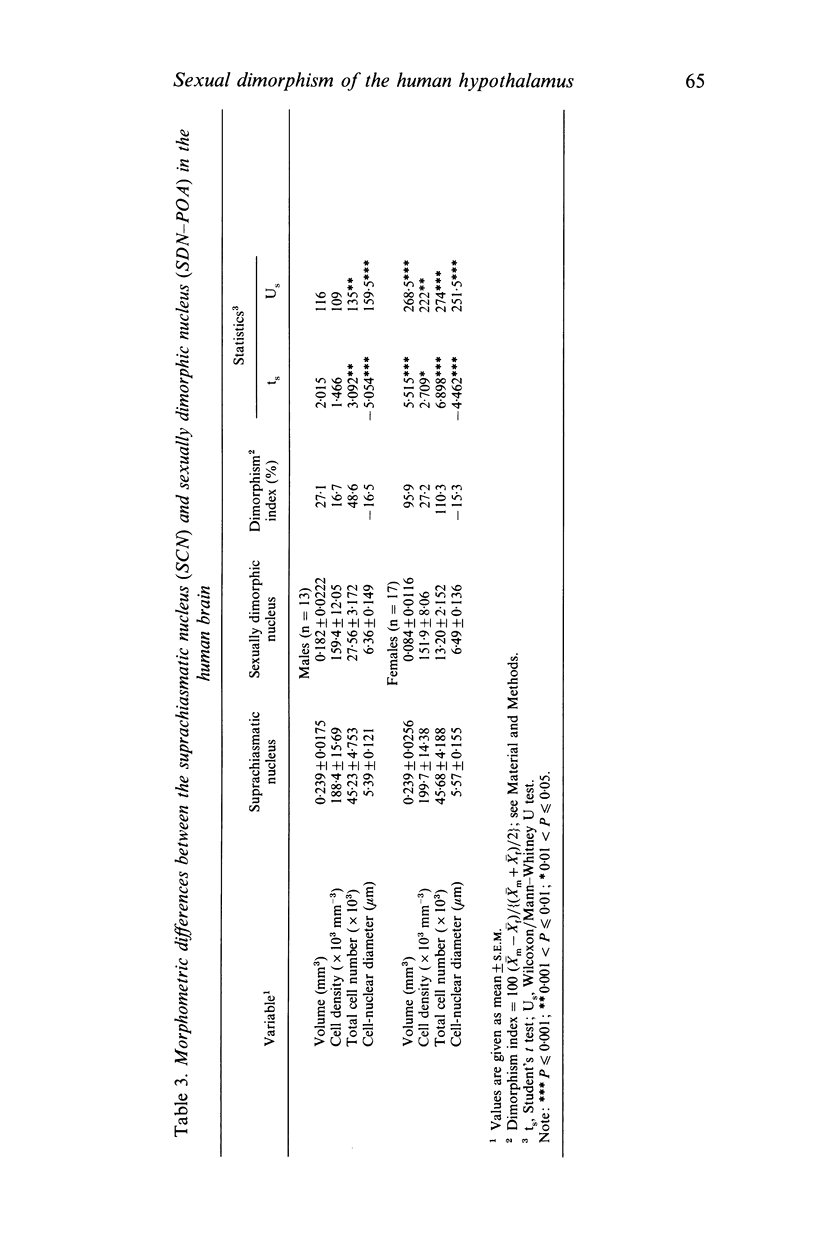
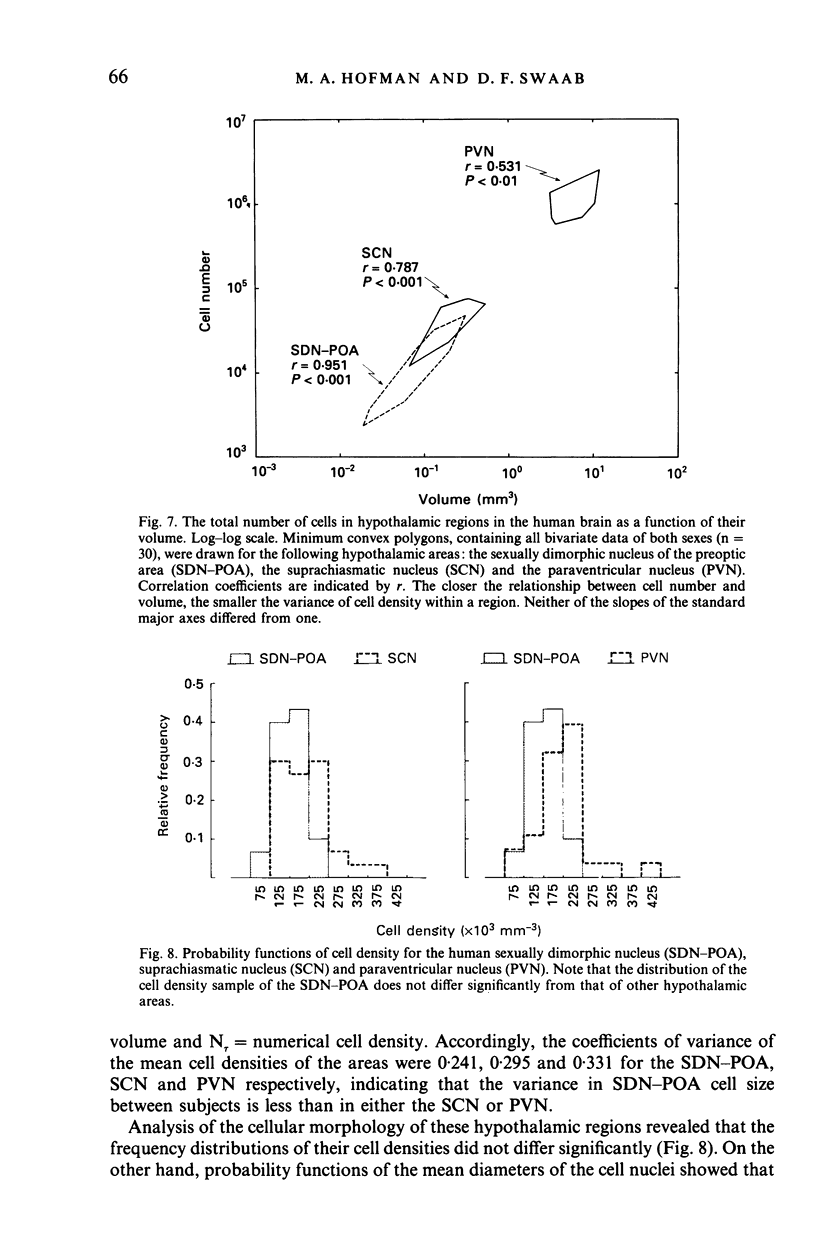
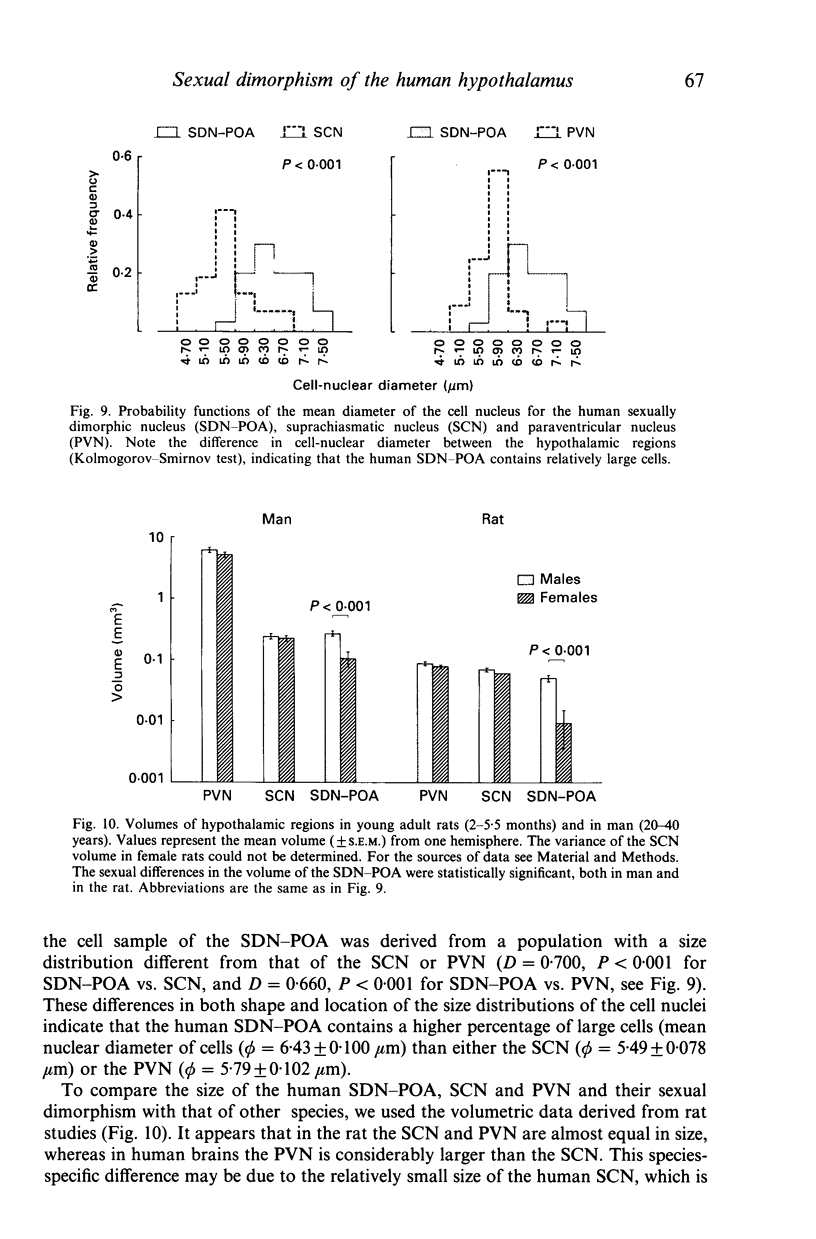
The sexually dimorphic nucleus of the preoptic area (SDN-POA) in the human hypothalamus is an ovoid, densely packed collection of large cells. The size, shape and cellular morphology of the SDN-POA was examined in relation to sex and age in adult human subjects. In this region the following parameters were measured: length of the rostrocaudal axis, maximum cross-sectional area, volume, numerical cell density, total number of cells, and the diameter of the cell nucleus. The SDN-POA was elongated in females and more spherical in males. The mean volume and total cell number were markedly sexually dimorphic: the volume of the SDN-POA was 2.2 times as large in males as in females and contained 2.1 times as many cells. No sex differences were observed in either cell density or mean diameter of the cell nuclei. Furthermore, multivariate regression analysis revealed that there are also sex-linked differences in the structural organisation of the human SDN-POA, finding expression in the way the morphometric parameters are interrelated. Of the parameters measured, only the volume and cell number of the SDN-POA showed a dramatic decrease with ageing. The reduction in cell number, however, was not constant throughout adulthood but was found to depend upon sex and age. In males, a major reduction in SDN-POA cell number was observed between the age of 50-60 years. In females, cell death was found to be more prominent than in males, especially among old people (t greater than 70 years), dropping to values which were only 10-15% of the cell number found in early childhood. In conclusion, the human SDN-POA has a sex-dependent pattern of ageing. Finally, the morphology of the SDN-POA was compared with that of other hypothalamic regions--the suprachiasmatic nucleus (SCN) and the paraventricular nucleus (PVN)--both in man and in rat. Species-specific differences in the dimensions of these nuclear regions are discussed in the light of their assumed functional significance.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. K., Rhees R. W., Fleming D. E. Effects of prenatal stress on differentiation of the sexually dimorphic nucleus of the preoptic area (SDN-POA) of the rat brain. Brain Res. 1985 Apr 15;332(1):113–118. doi: 10.1016/0006-8993(85)90394-4. [DOI] [PubMed] [Google Scholar]
- Anderson R. H., Fleming D. E., Rhees R. W., Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986 Apr 2;370(1):1–10. doi: 10.1016/0006-8993(86)91098-x. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. The hypothalamus of the human adult: chiasmatic region. Anat Embryol (Berl) 1987;175(3):315–330. doi: 10.1007/BF00309845. [DOI] [PubMed] [Google Scholar]
- Byne W., Bleier R. Medial preoptic sexual dimorphisms in the guinea pig. I. An investigation of their hormonal dependence. J Neurosci. 1987 Sep;7(9):2688–2696. doi: 10.1523/JNEUROSCI.07-09-02688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. W., Nance D. M., Gorski R. A. Effects of hypothalamic and preoptic lesions on reproductive behavior in male rats. Brain Res Bull. 1977 Mar-Apr;2(2):137–141. doi: 10.1016/0361-9230(77)90010-7. [DOI] [PubMed] [Google Scholar]
- Commins D., Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J Comp Neurol. 1984 Mar 20;224(1):132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- Deslypere J. P., Vermeulen A. Leydig cell function in normal men: effect of age, life-style, residence, diet, and activity. J Clin Endocrinol Metab. 1984 Nov;59(5):955–962. doi: 10.1210/jcem-59-5-955. [DOI] [PubMed] [Google Scholar]
- Döhler K. D., Coquelin A., Davis F., Hines M., Shryne J. E., Gorski R. A. Aromatization of testicular androgens in physiological concentrations does not defeminize sexual brain functions. Monogr Neural Sci. 1986;12:28–35. doi: 10.1159/000412729. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Gordon J. H., Shryne J. E., Southam A. M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978 Jun 16;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Harlan R. E., Jacobson C. D., Shryne J. E., Southam A. M. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980 Sep 15;193(2):529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Haug H., Kühl S., Mecke E., Sass N. L., Wasner K. The significance of morphometric procedures in the investigation of age changes in cytoarchitectonic structures of human brain. J Hirnforsch. 1984;25(4):353–374. [PubMed] [Google Scholar]
- Hines M., Davis F. C., Coquelin A., Goy R. W., Gorski R. A. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985 Jan;5(1):40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman M. A. A biometric analysis of brain size in micrencephalics. J Neurol. 1984;231(2):87–93. doi: 10.1007/BF00313723. [DOI] [PubMed] [Google Scholar]
- Hofman M. A., Fliers E., Goudsmit E., Swaab D. F. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J Anat. 1988 Oct;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Hofman M. A., Laan A. C., Uylings H. B. Bivariate linear models in neurobiology: problems of concept and methodology. J Neurosci Methods. 1986 Oct;18(1-2):103–114. doi: 10.1016/0165-0270(86)90114-7. [DOI] [PubMed] [Google Scholar]
- Hsu H. K., Peng M. T. Hypothalamic neuron number of old female rats. Gerontology. 1978;24(6):434–440. doi: 10.1159/000212283. [DOI] [PubMed] [Google Scholar]
- Hsü H. K., Chen F. N., Peng M. T. Some characteristics of the darkly stained area of the medial preoptic area of rats. Neuroendocrinology. 1980 Nov;31(5):327–330. doi: 10.1159/000123096. [DOI] [PubMed] [Google Scholar]
- Jacobson C. D., Shryne J. E., Shapiro F., Gorski R. A. Ontogeny of the sexually dimorphic nucleus of the preoptic area. J Comp Neurol. 1980 Sep 15;193(2):541–548. doi: 10.1002/cne.901930215. [DOI] [PubMed] [Google Scholar]
- Leibnitz L. Untersuchungen zur Optimierung der Gewichts- und Volumenänderungen von Hirnen während der Fixierung, Dehydrierung und Aufhellung sowie über Rückschlüsse vom Gewicht des behandlten auf das Volumen des frischen Gehirns. J Hirnforsch. 1971;13(4):320–329. [PubMed] [Google Scholar]
- Lisk R. D. Copulatory activity of the male rat following placement of preoptic-anterior hypothalamic lesions. Exp Brain Res. 1968;5(4):306–313. doi: 10.1007/BF00235905. [DOI] [PubMed] [Google Scholar]
- Peng M. T., Hsü H. K. No neuron loss from hypothalamic nuclei of old male rats. Gerontology. 1982;28(1):19–22. doi: 10.1159/000212507. [DOI] [PubMed] [Google Scholar]
- Robinson S. M., Fox T. O., Dikkes P., Pearlstein R. A. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Res. 1986 Apr 23;371(2):380–384. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979 Jul;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Krey L. C., Zimmerman E. A. A comparative study of the luteinizing hormone releasing hormone (LHRH) neuronal networks in mammals. Biol Reprod. 1979 Feb;20(1):98–110. doi: 10.1093/biolreprod/20.1.98. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985 May 31;228(4703):1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Fliers E., Partiman T. S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985 Sep 2;342(1):37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Tobet S. A., Zahniser D. J., Baum M. J. Sexual dimorphism in the preoptic/anterior hypothalamic area of ferrets: effects of adult exposure to sex steroids. Brain Res. 1986 Feb 5;364(2):249–257. doi: 10.1016/0006-8993(86)90837-1. [DOI] [PubMed] [Google Scholar]
- Uylings H. B., Van Eden C. G., Verwer R. H. Morphometric methods in sexual dimorphism research on the central nervous system. Prog Brain Res. 1984;61:215–222. doi: 10.1016/s0079-6123(08)64437-4. [DOI] [PubMed] [Google Scholar]
- Uylings H. B., van Eden C. G., Hofman M. A. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods. 1986 Oct;18(1-2):19–37. doi: 10.1016/0165-0270(86)90111-1. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976 Feb;42(2):247–253. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- Viglietti-Panzica C., Panzica G. C., Fiori M. G., Calcagni M., Anselmetti G. C., Balthazart J. A sexually dimorphic nucleus in the quail preoptic area. Neurosci Lett. 1986 Feb 28;64(2):129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- Watson R. E., Jr, Hoffmann G. E., Wiegand S. J. Sexually dimorphic opioid distribution in the preoptic area: manipulation by gonadal steroids. Brain Res. 1986 Nov 19;398(1):157–163. doi: 10.1016/0006-8993(86)91261-8. [DOI] [PubMed] [Google Scholar]
- Zilles K. Biometrische Analyse der Frischvolumina verschiedener prosencephaler Hirnregionen von 78 menschlichen, adulten Gehirnen. Gegenbaurs Morphol Jahrb. 1972;118(2):234–273. [PubMed] [Google Scholar]


