Abstract
Membrane fusion reactions have been considered to be primarily regulated by Rab GTPases. In the model system of homotypic vacuole fusion in the yeast Saccharomyces cerevisiae, we show that Cdc42p, a member of the Rho family of GTPases, has a direct role in membrane fusion. Genetic evidence suggested a relationship between Cdc42p and Vtc1p/Nrf1p, a central part of the vacuolar membrane fusion machinery. Vacuoles from cdc42 temperature-sensitive mutants are deficient for fusion at the restrictive temperature. Specific amino acid changes on the Cdc42p protein surface in these mutants define the putative interaction domain that is crucial for its function in membrane fusion. Affinity-purified antibodies to this domain inhibited the in vitro fusion reaction. Using these antibodies in kinetic analyses and assays for subreactions of the priming, docking and post-docking phase of the reaction, we show that Cdc42p action follows Ypt7p-dependent tethering, but precedes the formation of trans-SNARE complexes. Thus, our data define an effector binding domain of Cdc42p by which it regulates the docking reaction of vacuole fusion.
Keywords: actin/Rho GTPase/Saccharomyces cerevisiae/SNARE/tethering
Introduction
Small GTPases are key regulatory proteins in many cellular processes (Lazar et al., 1997; Matozaki et al., 2000; Zerial and McBride, 2001). They can bind and hydrolyze GTP and act as molecular switches by cycling between an active, GTP-bound state and an inactive, GDP-bound state. Depending on the activation state they can expose a lipid anchor and insert into membranes. In the active state, they associate with other proteins that mediate downstream effects. Three other factors regulate the different states of small GTPases. GTP hydrolysis is accelerated by GTPase-activating proteins (GAPs), exchange of GDP for GTP is regulated by guanine-nucleotide exchange factors (GEFs), and membrane association is controlled by guanine-dissociation inhibitors (GDIs), which extract the protein from the membrane in its GDP-bound state. This extraction is thought to help in recycling the proteins after a signaling event.
Small GTPases can be grouped into several families. Original classifications suggested that Ras proteins influence cell growth, Rho proteins control the actin cytoskeleton architecture, Rab and Arf proteins participate in intracellular trafficking and Ran directs nucleocytoplasmic transport. However, it has become increasingly apparent that a given GTPase can interact with a variety of targets and influence a diverse range of cellular responses. An example of this is Cdc42, a member of the Rho subfamily (Johnson, 1999; Erickson and Cerione, 2001).
The gene encoding Cdc42 was first identified in Saccharomyces cerevisiae as an essential element in the assembly of the bud site (Adams et al., 1990; Johnson and Pringle, 1990). Further studies in yeast suggested a role for Cdc42 in the establishment of cell polarity through effects on the actin cytoskeleton (Ziman et al., 1991, 1993; Li et al., 1995). Studies in mammalian cells revealed that Cdc42 influences cell shape and structure by initiating actin cytoskeleton remodeling (Kozma et al., 1995; Nobes and Hall, 1995). Recent work has shown a direct role of Cdc42 in stimulating actin polymerization (Ma et al., 1998a,b; Rohatgi et al., 1999, 2000). Cdc42 relieves the autoinhibition of a C-terminal region of Wiscott–Aldrich syndrome protein (WASP), which can then couple to the Arp2/3 complex (Kim et al., 2000; Prehoda et al., 2000). This complex promotes the incorporation of actin monomers into F-actin polymers. Cdc42 also appears to play a role in reactions of vesicular traffic. Cdc42 interacts with Golgi proteins involved in vesicle budding such as ARF (ADP ribosylation factor) (Erickson et al., 1996) and the γ-subunit of the COP1 coatomer complex (Wu et al., 2000). Other targets for activated Cdc42 are the ACKs (activated Cdc42-associated tyrosine kinases) (Manser et al., 1993; Yang and Cerione, 1997; Yang et al., 1999). One of these non-receptor tyrosine kinases, ACK2, competes with AP-2 (adaptor protein-2) for binding to clathrin, leading to an inhibition of AP-2-mediated transferrin receptor endocytosis (Erickson and Cerione, 2001). Previous studies in MDCK cells, dendritic cells and Schizosaccharomyces pombe support an involvement of Cdc42 in endocytosis (Kroschewski et al., 1999; Garrett et al., 2000; Murray and Johnson, 2001). A role for Cdc42 in exocytosis has been suggested based on studies on secretion in mast cells (Brown et al., 1998; Hong-Geller and Cerione, 2000). Cdc42 has also been implicated in the maintenace of tight junctions, as well as in the regulation of RNA processing (Erickson and Cerione, 2001). Thus, Cdc42 acts on a number of different targets in a variety of cellular processes.
We have investigated the role of Cdc42 in membrane fusion using the model system of homotypic vacuole fusion in the yeast S.cerevisiae (Wickner and Haas, 2000). This reaction occurs in sequential phases of priming, tethering, docking and membrane fusion. It can be reconstituted in vitro and the different kinetic stages of the reaction can be analyzed (Conradt et al., 1994; Mayer et al., 1996; Wickner and Haas, 2000). The priming event activates the machinery required for recognition and membrane attachment, including the SNARE proteins Vam3p, Nyv1p, Vam7p, Vti1p and Ykt6p, the Rab GTPase Ypt7p, and the HOPS complex of tethering factors (Vps11, 16, 18, 33, 39, 41) (Ungermann et al., 1999; Price et al., 2000a,b; Sato et al., 2000; Wurmser et al., 2000). Tethering factors and Rab GTPases can establish an initial SNARE-independent interaction of the membranes (Cao et al., 1998; Ungermann et al., 1998a; Waters and Pfeffer, 1999). The ATPase Sec18p/NSF and its cofactor Sec17p/α-SNAP disassemble cis-SNARE complexes, i.e. complexes of SNARE proteins on the same membrane (Ungermann et al., 1998b), and Sec17p/α-SNAP is released from the membrane (Mayer et al., 1996). LMA1 stabilizes the primed state of SNAREs (Xu et al., 1998), which is a prerequisite for the formation of trans-SNARE complexes, i.e. complexes between SNAREs on apposed membranes. Trans-SNARE pairing is believed to establish a very close attachment (docking) of the membranes, which activates a calcium efflux from the vacuolar lumen (Peters and Mayer, 1998). Calcium binds to calmodulin and enhances its association with the vacuolar membrane probably through its interaction with the V0 sector of the vacuolar H+-ATPase (Peters et al., 2001). V0 sectors from apposed membranes form trans-complexes that might result in a proteinaceous fusion pore. The maintenance of V0 trans-complexes is independent of trans-SNARE complexes (Peters et al., 2001), as is the completion of fusion (Ungermann et al., 1998a).
The proteins involved in this sequence of events are connected by physical interactions. Tethering factors regulate the nucleotide exchange on Ypt7p and, together with Ypt7p, are required for the formation of trans-SNARE complexes (Eitzen et al., 2000; Price et al., 2000a; Sato et al., 2000; Seals et al., 2000). Different subunits of the Vtc complex regulate priming and a post-docking event and link Sec18p/NSF activity to V0 trans-complex formation (O.Müller, M.Bayer, J.Anderson, M.Mann and A.Mayer, submitted). Genetic evidence suggested an interaction between Cdc42p and Vtc1p/Nrf1p (Murray and Johnson, 2000) and led us to investigate the involvement of Cdc42p in membrane fusion.
Results
Cdc42p is present on vacuoles
In a genetic screen, Vtc1p/Nrf1p, a protein of the Vtc complex (Nelson et al., 2000) that has recently been shown to link different steps of vacuole fusion (O.Müller, M.Bayer, J.Anderson, M.Mann and A.Mayer, submitted), was isolated as a negative regulator of Cdc42p in S.pombe (Murray and Johnson, 2000). Therefore, we asked whether Cdc42p is involved in the fusion reaction. As a tool for the analysis of Cdc42p, we used an antibody raised against a Cdc42p-specific peptide (Ziman et al., 1991). The antibodies were affinity purified on recombinantly expressed glutathione S-transferase (GST)–Cdc42p coupled to a Sepharose matrix. Western analysis showed that Cdc42p is present on isolated vacuoles (Figure 1, lane 1). The antibody recognized a single band at the expected molecular weight of ∼21 kDa. Previous studies showed that Cdc42p is a geranylgeranylated peripheral membrane protein mainly localized to sites of bud emergence where transport vesicles fuse with the plasma membrane (Ohya et al., 1993; Ziman et al., 1993). We show that, like the Rab GTPase Ypt7p, Cdc42p is also highly enriched on vacuolar membranes when compared with the the same protein amounts of cytosol (Figure 1A and B, lane 2) or whole-cell extract (Figure 1A and B, lane 3). As a control we also decorated for the cytosolic marker Pgk1p, which is highly enriched in the cytosolic fraction (Figure 1A).
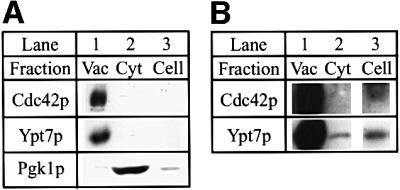
Fig. 1. Cdc42p is associated with the vacuolar membrane. (A) 70 µg of isolated vacuoles (Vac), cytosol (Cyt) or a whole-cell extract (Cell) from strain BJ3505 were analyzed by SDS–PAGE and western blotting for their content of Cdc42p, Ypt7p and Pgk1p. (B) Longer exposure of the blots for Cdc42p and Ypt7p in (A) with the bands visible in the whole-cell extract (lane 3).
Vacuoles from a specific cdc42ts mutant are thermolabile for fusion
Deletion of the CDC42 gene is lethal (Johnson and Pringle, 1990). Therefore, we tested the involvement of Cdc42p in vacuole fusion using different cdc42ts mutants (Kozminski et al., 2000). We also deleted either PEP4 or PHO8 in the mutants and the corresponding wild-type strain to make them suitable for the in vitro fusion assay.
In the in vitro fusion assay, two populations of vacuoles are used. One is lacking the proteinase Pep4p and therefore only bears the inactive pro-alkaline phosphatase (Pho8p), because Pep4p is needed for the maturation of pro-Pho8p to the active enzyme. The other population has Pep4p, but is lacking Pho8p. Upon fusion and contents mixing, pro-Pho8p is activated. It can be assayed colorimetrically as a quantitative readout of fusion (Haas, 1995).
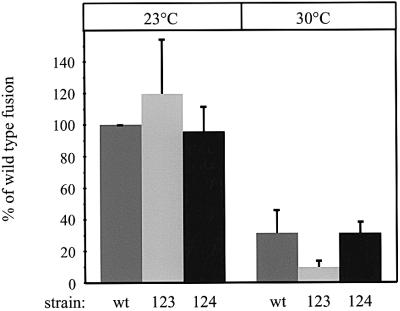
Vacuoles from a cdc42ts-123 mutant were thermolabile for fusion (Figure 2). At 23°C they fused even slightly better than wild-type vacuoles, but they showed a dramatic decrease in fusion activity compared with wild type at 30°C. The wild-type fusion activity was also lower at 30°C due to the narrow temperature optimum of the in vitro fusion reaction (T.Sattler and A.Mayer, unpublished observation). In contrast, vacuoles from the cdc42ts-124 mutant behaved like wild type (Figure 2). These cdc42 alleles have mutations in different functional domains (Kozminski et al., 2000). The cdc42ts-123 allele contains the R163A and K166A substitutions and the cdc42ts-124 allele has the K183A, K184A, K186A and K187A mutations. Both mutants are temperature sensitive for growth, but only cdc42ts-123 is temperature sensitive for membrane fusion. Since both sets of mutations map to different areas on the surface of Cdc42p (Kozminski et al., 2000), this suggests that Cdc42p uses a specific surface region to regulate membrane fusion.
Fig. 2. In vitro fusion of vacuoles from cdc42ts mutants. Standard fusion reactions with cytosol were incubated for 70 min at 23 or 30°C. The indicated tester strain combinations were used: OMY14/OMY15(wt), OMY16/OMY17 (cdc42ts-123) and OMY18/OMY19 (cdc42ts-124). Growth of cells and preparation of vacuoles was at 23°C instead of the 30°C used in the standard protocol. Four experiments were averaged. The 100% fusion activities of the wild type at 23°C were between 2.15 and 2.38 U.
In agreement with our findings, another temperature-sensitive mutant, namely cdc42ts-1 (Adams et al., 1990), also shows thermolability of vacuole fusion in vitro (see Eitzen et al., 2001). Furthermore, vacuoles in these mutants rapidly fragment into smaller vesicles upon shift to non-permissive temperature. These data provide in vivo evidence for an involvement of Cdc42p in vacuole fusion (see Eitzen et al., 2001).
Cdc42p functions up to the docking stage of membrane fusion
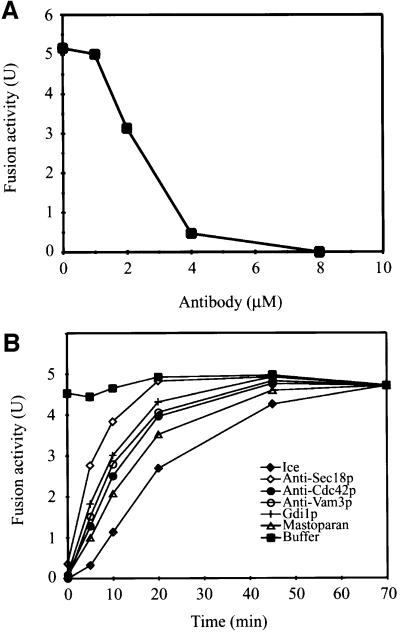
For kinetic analyses we used a Cdc42p-specific antibody directed against a peptide (residues 167–183; Ziman et al., 1991) encompassing a putative interaction domain with no known function (Kozminski et al., 2000). The affinity-purified antibody inhibited fusion in a concentration-dependent manner (Figure 3A; IC50 ≈ 2.4 µM). In a kinetic experiment, we monitored when the reaction became resistant to anti-Cdc42p (Figure 3B). The bulk of vacuoles complete distinct reaction steps within defined intervals and become resistant to inhibitors of these steps (Conradt et al., 1994; Mayer et al., 1996), revealing the sequence of events in vacuole fusion. Inhibitors or control buffer were added into an ongoing fusion reaction at different times and the samples were incubated further until the end of a standard fusion period (70 min). Another aliquot was chilled to stop fusion at that time and monitor progression of the reaction. All inhibitors abolished fusion when added at the start of the reaction (Figure 3B). After 15 min the reaction was resistant to anti-Sec18p, i.e. priming was completed. After 35 min, the reaction was resistant to Gdi1p, the GDI that extracts Ypt7p from the vacuolar membrane (Wickner and Haas, 2000), and to antibodies to the SNARE Vam3p. Resistance to these two reagents indicates the completion of docking (Mayer and Wickner, 1997; Nichols et al., 1997; Ungermann et al., 1998a). The inhibition curve for anti-Cdc42p overlapped with those of Gdi1p and anti-Vam3p, indicating that Cdc42p acts at least up to the docking stage of vacuole fusion.
Fig. 3. Cdc42p functions up to the docking stage. (A) Antibody inhibition. The indicated amounts of affinity-purified antibodies to Cdc42p were added to standard fusion reactions without cytosol. After pre-incubation for 10 min on ice, ATP was added and fusion was measured after 70 min at 27°C. (B) Kinetic analysis. Standard fusion reactions without cytosol were started. After the indicated times at 27°C inhibitors or control buffer were added. The samples were left on ice for 10 min. Then they were transferred to 27°C or left on ice for the remainder of the 70 min reaction period. Finally, fusion activity was assayed. Inhibitors used were: anti-Sec18p, 2 µM; Gdi1p, 5 µM; anti-Vam3p, 2 µM; Mastoparan, 20 µM; anti-Cdc42p, 8 µM.
An independent approach by Eitzen et al. (2001) further supports this conclusion. In that study, Rdi1p, the RhoGDI that specifically extracts Cdc42p from the membrane, was used as a tool to investigate Cdc42p function in vacuole fusion. Like the specific antibodies to Cdc42p, Rdi1p inhibits the fusion reaction in a concentration-dependent manner and shows inhibition up to the docking stage in a kinetic experiment.
Cdc42p is not required for the priming reaction
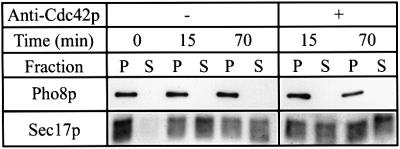
Sensitivity of the reaction to anti-Cdc42p in a kinetic experiment up to the docking stage does not exclude a further involvement of Cdc42p in the preceding priming phase. For example, Vtc4p, a component of the Vtc complex involved in vacuole fusion (O.Müller et al., submitted), controls priming, but has further activities required up to the docking stage. Therefore, we asked whether priming is affected by anti-Cdc42p. We tested this by monitoring Sec17p/α-SNAP release from the vacuolar membrane, which is a molecularly defined subreaction occurring as a consequence of priming (Mayer et al., 1996). At concentrations sufficient to block overall fusion, anti-Cdc42p did not compromise Sec17p/α-SNAP release (Figure 4), indicating that Cdc42p is not required for priming.
Fig. 4. Sec17p/α-SNAP release is not compromised by anti-Cdc42p. Standard fusion reactions (3-fold volume) without cytosol were incubated for 70 min without ATP on ice (0 min) or with ATP at 27°C for 15 or 70 min. Where indicated, 6 µM anti-Cdc42p was added. Reactions were chilled on ice and centrifuged (10 000 g, 10 min, 4°C). The supernatants (S) were recovered and the pellets (P) resuspended in 90 µl of PS buffer. All samples were trichloroacetic acid precipitated and processed for non-reducing SDS–PAGE and western blotting with the indicated antibodies. Alkaline phosphatase (Pho8p) serves as a membrane integral vacuolar marker.
Cdc42p acts after Ypt7p but before trans-SNARE pairing
Several molecular events kinetically map to the docking step, such as Ypt7p action, trans-SNARE pairing and the triggering of a calcium efflux from the lumen of the organelles. In order to determine more precisely when Cdc42p acts within the sequence of these different subreactions of the docking stage, we used anti-Cdc42p in specific assays for these subreactions.
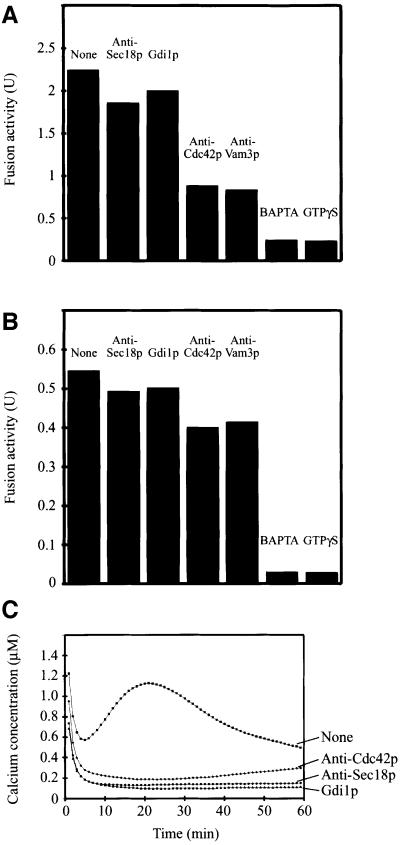
The in vitro reaction can be blocked at distinct stages by the use of reversible inhibitors. GTPγS, a poorly hydrolyzable analog of GTP, locks Ypt7p in its GTP-bound state (Eitzen et al., 2000). A GTPγS block can be released by re-isolating the vacuoles and resuspending them in fresh fusion buffer without GTPγS (Mayer et al., 1996). After release of the GTPγS block, different inhibitors worked with different efficiencies, indicating the sequence of events (Figure 5A). BAPTA, a calcium chelator that inhibits fusion by quenching the calcium efflux triggered by docking (Peters and Mayer, 1998), inhibited the released reaction completely, indicating that calcium efflux from the vacuolar lumen coincides with or follows a GTPγS-sensitive step (cf. Eitzen et al., 2000). Antibodies to Sec18p/NSF, which is involved in priming, i.e. in a subreaction preceding docking, did not inhibit the released reaction. Gdi1p did not inhibit the released reaction (Figure 5A; cf. Eitzen et al., 2001), probably because the GTPγS bound state of Ypt7p is resistant to GDI extraction. Anti-Cdc42p still inhibited the released reaction to ∼50% (Figure 5A), as did antibodies to the SNARE Vam3p. This is consistent with the fact that formation of trans-SNARE complexes requires Ypt7p (Sato et al., 2000) and that Ypt7p function is necessary to pass the Cdc42p-dependent step. Further data by Eitzen et al. (2001) show that Rdi1p—by extracting Cdc42p—still inhibits after a reversible block by Ypt7p antibodies. Collectively, these data strongly suggest that Cdc42p and Vam3p act after Ypt7p.
Fig. 5. Kinetic analysis of Cdc42p action. (A and B) Standard fusion reactions (10-fold volume) without cytosol were incubated at 27°C in the presence of (A) 2 mM GTPγS or (B) 2 mM BAPTA. After 30 min, reactions were chilled on ice and vacuoles were re-isolated (6800 g, 2 min, 4°C). Vacuoles were resuspended in fresh fusion buffer with cytosol. In the case of the BAPTA block, 200 µM CaCl2 was added. Aliquots were supplemented with the indicated inhibitors and incubated for another 60 min at 27°C. Finally, fusion activity was measured. Fusion without re-isolation was 3.94 U. Inhibitors used were: anti-Sec18p, 2 µM; Gdi1p, 3.7 µM; anti-Cdc42p, 6 µM; anti-Vam3p, 2 µM; BAPTA, 2 mM; GTPγS, 2 mM. (C) Ca2+ release from vacuoles in the course of the fusion reaction was assayed as described in Materials and methods. Inhibitors used were: Gdi1p, 4 µM; anti-Sec18p, 1 µM; anti-Cdc42p, 6 µM.
After a BAPTA block, the reaction was resistant to anti-Cdc42p (Figure 5B), as it was to anti-Sec18p, Gdi1p and anti-Vam3p. Only BAPTA itself and GTPγS still inhibited fusion. The same effect as with anti-Cdc42p could be observed with Rdi1p (Eitzen et al., 2001). These results show that Cdc42p and Vam3p act before the BAPTA-sensitive step, i.e. before calcium efflux from the lumen of the vacuole. This could be tested by direct measurements of calcium efflux (Peters and Mayer, 1998). Anti-Cdc42p inhibited this calcium efflux, as did anti-Sec18p and Gdi1p (Figure 5C).
Trans-SNARE pairing is thought to establish the docked state of the membranes that precedes the calcium efflux. Since trans-SNARE complex formation was also inhibited by anti-Cdc42p (Figure 6), Cdc42p must act after Ypt7p has fulfilled its function, but before trans-SNARE pairing occurs, and before the efflux of lumenal calcium is triggered.
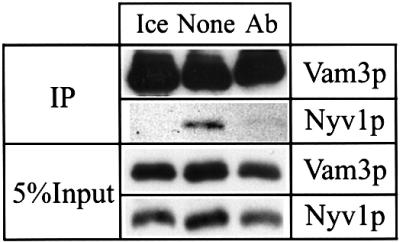
Fig. 6. Cdc42p acts before trans-SNARE pairing. Standard fusion reactions (5-fold volume) with cytosol containing vacuoles from strains BJ3505Δvam3 and BJ3505Δnyv1 were incubated without ATP on ice or with ATP at 27°C. One of the samples at 27°C received buffer only (None) and another one 6 µM affinity-purified antibodies to Cdc42p (Ab). After 45 min, trans-SNAREs were assayed as described in Materials and methods by the amount of Nyv1p that co-immuno precipitates with Vam3p. As can be seen by comparison of co-precipitated Nyv1p with the corresponding input, formation of trans-SNARE pairs in the control reaction is in the range of literature values (Ungermann et al., 1998a).
Discussion
To date, mainly Rab GTPases have been implicated as direct players in membrane fusion reactions. In this study we identify another class of GTPase as a new factor in vacuole fusion. The Rho GTPase Cdc42p functions at the docking stage of vacuole fusion. Our kinetic analysis places Cdc42p between Rab (Ypt7p) action and trans-SNARE pairing. This is surprising because Rab and SNARE action are intimately connected, as illustrated by the fact that Rab and SNAREs are part of a large complex (McBride et al., 1999). Furthermore, Rab activity is required for trans-SNARE pairing (Eitzen et al., 2000; Sato et al., 2000; Seals et al., 2000; Wurmser et al., 2000), and SNARE overexpression can compensate for loss of Rab function (Dascher et al., 1991). The observation that Cdc42p acts between Rab and SNAREs suggests that Rab proteins and associated tethering factors may not be the sole controllers of trans-SNARE pairing, but require other GTPases as mediators. In agreement with this view, Cdc42p and Rho1p, but not Rho2p, Rho3p or Rho4p, are required in vacuole fusion (cf. Eitzen et al., 2001). Rho1 is also required for spatial regulation of the exocyst, a complex of tethering factors involved in exocytosis in yeast (Guo et al., 2001). Rho3p has a direct role in exocytosis that is distinct from its role in actin polarity (Adamo et al., 1999). It was suggested that it may coordinate several different events necessary for delivery of proteins to specific sites on the cell surface. In addition, Ran, a small GTPase well known for its role in nuclear protein import and RNA export, has been implicated in a fusion process, the reassembly of the nuclear envelope from mitotic vesicles (Hetzer et al., 2000).
Our findings and those of Eitzen et al. (2001) now present a detailed kinetic analysis of Cdc42p function in vacuole fusion, which allows us to map the requirement for this protein within the reaction sequence leading to fusion. To investigate Cdc42p function, two specific and independent reagents were used. We used an affinity-purified antibody to Cdc42p, while Eitzen et al. (2001) used Rdi1p, the RhoGDI that specifically extracts Cdc42p from the membrane. The biochemical data for Cdc42p involvement in membrane fusion were reinforced by data on vacuole fusion in cdc42 temperature-sensitive mutants. Eitzen et al. (2001) show in vitro and in vivo defects in vacuole fusion in a cdc42ts-1 mutant and we demonstrate fusion defects in a cdc42ts-123 mutant. This mutant originated from a site-directed mutagenesis approach to explore the functions and interactions of Cdc42p in S.cerevisiae (Kozminski et al., 2000). Groups of charged residues expected to be at the protein surface were mutated and the mutants were tested for functional consequences. This approach not only confirmed the importance of domains that were already predicted to be interaction sites, but also defined a new putative protein interaction domain. The R163A and K166A mutations in the cdc42ts-123 mutant, for which we now show defects in membrane fusion, are in this new putative interaction domain. They are in a region (α5-helix, residues 166–177) predicted by structural models to be a specificity determinant for Cdc42p–protein interactions (Guo et al., 1998; Abdul-Manan et al., 1999; Mott et al., 1999; Kozminski et al., 2000). The cdc42ts-124 mutant, which has mutations in another interaction domain, is also temperature sensitive for growth, but behaves like wild type in vacuole fusion. The inhibitory antibody we used was raised against residues 167–183 of Cdc42p, which contains the α5-helix region. Thus, two independent approaches pointed to the same domain. This strongly suggests that the new putative interaction domain altered in the cdc42ts-123 mutant is responsible for Cdc42p action in membrane fusion. This domain binds proteins with a CRIB (Cdc42/Rac interactive binding) motif (Burbelo et al., 1995; Guo et al., 1998; Abdul-Manan et al., 1999; Mott et al., 1999; Kozminski et al., 2000). Yeast proteins with such a CRIB motif are Cla4p, Gic1p, Gic2p and Ste20p (Burbelo et al., 1995). Cla4p is a Cdc42p-regulated serine/threonine kinase involved in the control of the actin cytoskeleton (Holly and Blumer, 1999). A direct interaction of Cla4p with the GTP-bound form of Cdc42p was shown by overlay and two-hybrid assays (Cvrcková et al., 1995; Richman et al., 1999). Strikingly, cla4 deletion mutants have a phenotype of fragmented vacuoles (S.Seeley, M.Kato, G.Eitzen and W.Wickner, personal communication), suggesting an involvement of Cla4p in vacuole fusion.
Could the actin cytoskeleton play a role in vacuole fusion and explain the function of Rho GTPases like Cdc42p and Rho1p in this process? This is likely for several reasons. First, actin is involved in the process of vacuole fusion, as drugs that affect actin inhibit the in vitro reaction (see Eitzen et al., 2001). Secondly, phosphatidylinositol 4,5-bisphosphate (PIP2) is required at two steps in vacuole fusion, in priming and in the subsequent docking reaction (Mayer et al., 2000). PIP2 is involved in the regulation of Rho GTPase targets and in actin dynamics (Higgs and Pollard, 2000; Kim et al., 2000; Prehoda et al., 2000; Rohatgi et al., 2000). Thirdly, vacuole inheritance, i.e. transmission of vacuolar fragments into the nascent daughter cells of yeast, requires myosin-dependent transport of vacuoles along actin filaments (Hill et al., 1996) and illustrates the capacity of vacuoles to bind actin. Actin binding may involve Vac8p, an armadillo repeat protein required for vacuole inheritance and fusion (Wang et al., 1998, 2001; Veit et al., 2001). In other trafficking pathways Rho GTPases are believed to act by regulating actin remodeling too. One possibility is that a layer of F-actin might shield the membrane of organelles, making it necessary to locally remove it by depolymerization before a vesicle can dock or fuse (Orci et al., 1972; Trifaro and Vitale, 1993; Vitale et al., 1995). In line with this possibility, actin depolymerization was shown to stimulate exocytosis in pancreatic acinar cells (Muallem et al., 1995). However, the polymerization of actin was also shown to facilitate phagosome fusion in vitro (Jahraus et al., 2001). Thus, proper dynamics of dis- and re assembly of F-actin may be needed for fusion on intra cellular compartments as well as for endo- (Ayscough, 2000) and exocytosis (Bernstein et al., 1998). F-actin may also provide tracks for vesicles in endo- and exocytosis, facilitating mechanical movement of the vesicles inside cells (Govindan et al., 1995; Murphy et al., 1996; Lamaze et al., 1997; Lang et al., 2000).
The role of Cdc42p in vacuole fusion, which we define here, might involve actin regulation, e.g. to support actin-dependent interaction of vesicles over longer distances. This could facilitate clustering of vacuoles by transport along actin filaments and promote membrane tethering. But this can not be the sole function of Cdc42p in fusion because Cdc42p acts after Ypt7p, which mediates vacuole tethering (Mayer and Wickner, 1997; Ungermann et al., 1998a). Furthermore, priming, i.e. Sec18p/NSF-dependent SNARE activation, must occur before the Cdc42p requirement can be satisfied (see Eitzen et al., 2001). If Cdc42p were just needed to facilitate non-specific actin-dependent vacuole clustering by vesicle movement along polymerizing actin filaments, it would be hard to understand why Cdc42p is so tightly integrated into the reaction sequence. In this case we would expect that the Cdc42p requirement should precede Ypt7p-mediated tethering and be independent of Sec18p/NSF and priming. A further argument against a pure clustering function is the fact that vacuoles fuse well without the addition of cytosol (Mayer et al., 1996). Therefore, an external reservoir of G-actin is not required and a function for actin cables as vesicle tracks is unlikely in our in vitro reaction.
The view of actin as a barrier to fusion, however, is consistent with the data available so far. There is actin on the surface of isolated vacuoles that polymerizes under conditions of the in vitro reaction (P.Sluszarewicz, A.Merz and W.Wickner, personal communication). Local actin disassembly may be required to facilitate docking, i.e. a close and tight apposition of the membranes involving trans-SNARE pairing. An actin meshwork on the membranes would be strongly expected to interfere with such close apposition. Due to the topology and dimensions of the SNARE complex, its formation can be expected to bring the membranes into a distance of ∼2–3 nm (Sutton et al., 1998), leaving barely any space for actin filaments, which have diameters of 5–9 nm (Holmes et al., 1990). Finally, the available data still leave the possibility of actin being an intrinsic component of the fusion machinery. The exact role of actin in the fusion process remains to be elucidated.
Cdc42p could also be connected to the fusion machinery in a more direct way. Its function in vacuole fusion could be related to the Vtc complex. This complex regulates the capacity of Sec18p/NSF to prime SNAREs and links it to the late reaction step of trans-complex formation of V0 sectors of the vacuolar H+-ATPase (O.Müller, M.Bayer, J.Anderson, M.Mann and A.Mayer, submitted). It is striking that Vtc1p/Nrf1p, a subunit of the Vtc complex, was isolated in a genetic screen as a regulator of Cdc42p and co-localizes with Cdc42p to vacuolar membranes in S.pombe (Murray and Johnson, 2000, 2001). In view of these connections, it may also be relevant that mutations in VMA5, the gene encoding subunit C of the vacuolar H+-ATPase, show synthetic lethality with mutations in CDC24 (White and Johnson, 1997), the GEF for Cdc42p. The same study also suggested a role for Cdc24p in vacuole function because one of the csl (CDC24 synthetic-lethal) mutants displayed phenotypes similar to those observed with calcium-sensitive, Pet– V-ATPase mutants defective in vacuole function.
Thus, Cdc42p has several relations to proteins involved in vacuole fusion, supporting our conclusion of a direct role of Cdc42p in this reaction. New assays will have to be developed to monitor actin dynamics in the fusion reaction and explore the function of Rho proteins in detail.
Materials and methods
Strains
Standard fusion strains were Saccharomyces cerevisiae BJ3505 and DKY6281 (Mayer et al., 1996). BJ3505Δvam3 and BJ3505Δnyv1 were described previously (Nichols et al., 1997).
For the analysis of cdc42 ts mutants, strains DDY1300 (CDC42, wt), DDY1336 (cdc42ts-123) and DDY1338 (cdc42ts-124) were used, which were described previously (Kozminski et al., 2000). To make these strains suitable for the in vitro fusion assay, either PEP4 or PHO8 was deleted by replacing the gene with a loxP-kanMX-loxP cassette from plasmid pUG6 (Güldener et al., 1996). The resulting strains were OMY14 (CDC42 Δpep4), OMY15 (CDC42 Δpho8), OMY16 (cdc42ts-123 Δpep4), OMY17 (cdc42ts-123 Δpho8), OMY18 (cdc42ts-124 Δpep4) and OMY19 (cdc42ts-124 Δpho8). The oligonucleotides used for generation of deletion cassettes by PCR were: pep4.5′.Del.kan, 5′-TGGTCAGCGCCAACCAAGTTGCTGCAAAAGTCCACAAGGCCAGCTGAAGCTTCGTACGC-3′; pep4.3′.Del.kan, 5′-AATCGTAAATAGAATAGTATTTACG CAAGAAGGCATCACCGCATAGGCCACTAGTGGATCTG-3′; pho 8.5′.Del.kan, 5′-GGCTACATAAACATTTACATATCAGCATACGGGACATTATCAGCTGAAGCTTCGTACGC-3′; and pho8.3′.Del.kan, 5′-CGTATTAAATAATATGTGAAAAAAGAGGGAGAGTTAGATAGCATAGGCCACTAGTGGATCTG-3′. Deletions were verified by western blotting.
General procedures
Vacuole isolation, preparation of cytosol, Gdi1p, inhibitory antibodies and other reagents, as well as fusion assays, were essentially performed as described (Peters et al., 1999). PS buffer is 10 mM PIPES–KOH pH 6.8, 200 mM sorbitol. 1× PIC is a protease inhibitor cocktail: 100 µM pefabloc SC, 100 ng/ml leupeptin, 50 µM o-phenanthroline, 500 ng/ml pepstatin A.
GST–Cdc42p and affinity purification of antibodies
The plasmid pGEX-Cdc42 (M.Ziman and D.I.Johnson, unpublished results), which encodes a GST–Cdc42p fusion protein for expression in Escherichia coli, was generated by inserting the CDC42 coding region on a blunt-ended NdeI fragment into HindIII-cleaved, blunt-ended pGEX-KG (Guan and Dixon, 1991). Escherichia coli BL21 cells containing pGEX-Cdc42 were grown in LB/ampicillin medium (37°C, 225 r.p.m.) to an OD600 of ∼0.6. Expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside at 27°C for 4 h. Cells were broken by a freeze–thaw cycle and sonification in buffer A [20 mM Tris–HCl pH 6.8, 150 mM KCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1× PIC]. Triton X-100 was added to a final concentration of 1% (w/v) and the lysate was mixed for 30 min at 4°C to complete solubilization. The lysate was cleared (100 000 g, 20 min, 4°C) and the supernatant was repeatedly passed over 2 ml of GSH–Sepharose (Pharmacia) in a column for 4 h at 4°C. The column was washed with 20 column vols of buffer B (20 mM Tris–HCl pH 6.8, 150 mM NaCl). The fusion protein was cleaved on the column by adding thrombin (Pharmacia; 25 U in 1 ml of buffer B) at 4°C for 4 h. The eluate was diluted with 20 mM Tris–HCl pH 6.8 to a final KCl concentration of 50 mM and run over a MiniQ column (PE 4.6/50; Pharmacia; 0.5 ml/min) pre-equilibrated with buffer C (20 mM Tris–HCl pH 6.8, 50 mM KCl). Under these conditions, all contaminating proteins bound to the column while the pure, cleaved Cdc42p was in the flow through. The protein was dialyzed into PS buffer (10 mM PIPES–KOH pH 6.8, 200 mM sorbitol) containing 150 mM KCl, concentrated by ultrafiltration in centricon-10 (Amicon), and stored at –80°C.
Antibodies were affinity purified with the recombinant protein immobilized on activated CH-Sepharose 4B (Pharmacia) in coupling buffer (0.1 M NaHCO3 pH 8.0, 0.5 M NaCl). Remaining active groups were blocked with 0.1 M Tris–HCl pH 8.0. Antibodies were eluted with 0.1 M glycine pH 2.75/150 mM NaCl and the pH was immediately neutralized by addition of 1 M Tris–HCl pH 8.8. The antibodies were dialyzed into PS buffer (10 mM PIPES–KOH pH 6.8, 200 mM sorbitol) containing 150 mM KCl, concentrated by ultrafiltration in centricon-30 (Amicon), and stored at –20°C.
Assay for trans-SNARE pairing
Trans-SNARE pairing was analyzed with a modified method according to Ungermann et al. (1998a). Five standard fusion reactions with cytosol containing 20 µg of vacuoles from strain BJ3505Δvam3 and 20 µg of vacuoles from BJ3505Δnyv1 and 10 µM microcystin LR, were incubated without ATP-regenerating system on ice or with ATP at 27°C. After 45 min, the reactions were chilled on ice and centrifuged (20 000 g, 5 min, 4°C). The vacuole pellet was solubilized in 300 µl of buffer A (0.5% Triton X-100, 2 mM EDTA, 1 mM PMSF in phosphate-buffered saline) and incubated for 10 min at 4°C on a mixer. The lysate was cleared (100 000 g, 10 min, 4°C) and 5% of the lysate was extracted with chloroform/methanol (Wessel and Flügge, 1984) to serve as an input control. The rest of the lysate was rotated for 45 min at 4°C with 10 µg of affinity-purified antibodies to Vam3p and 20 µl of protein A–Sepharose (Pharmacia). The beads were washed four times with 1 ml of buffer A, transferred to a fresh tube and eluted with non-reducing sample buffer. Proteins were resolved by SDS–PAGE and analyzed by western blotting for immunoprecipitated Vam3p and co-immunoprecipitated Nyv1p.
Measurement of Ca2+ efflux
Ca2+ release from vacuoles in the course of the fusion reaction was assayed with a modified method according to Peters and Mayer (1998). Ca2+ was directly measured with aequorin in ongoing fusion reactions running in parallel in a microtiter plate luminometer (EG&G Berthold, Germany). Reactions (30 µl) contained 10 µg of vacuoles from strain BJ3505 in PS buffer with 150 mM KCl and 0.5 µl of cytosol (30 mg/ml). Respective inhibitors were included. After 2 min of pre-incubation on ice, 2 µl of ATP-regenerating system (same composition as in the fusion assay) and 3 µl of a freshly prepared 2:2:1 mix of the following stock solutions were added: 2.5 µM aequorin (Aqualite, Molecular Probes), 1 mM EGTA–KOH pH 7.5 and 500 µM native coelenterazine (Molecular Probes) in EtOH. Samples were pipetted into a microtiter plate and light emission of aequorin was measured with the luminometer for 60 min at 27°C. The assay was calibrated with solutions of different calcium concentrations as described (Peters and Mayer, 1998).
Acknowledgments
Acknowledgements
We thank Susanne Bühler, Tanja Baader, Christa Baradoy and Kurt Toenjes for assistance, and David Drubin for providing strains. This work was supported by the Deutsche Forschungsgemeinschaft (SFB446, A.M.), the Boehringer-Ingelheim-Foundation (A.M.), the Boehringer-Ingelheim-Fonds (O.M.) and National Science Foundation grants MCB-9728218 and MCB-0076826 (D.I.J.).
References
- Abdul-Manan N., Aghazadeh,B., Liu,G.A., Majumdar,A., Ouerfelli,O., Siminovitch,K.A. and Rosen,M.K. (1999) Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott–Aldrich syndrome’ protein. Nature, 399, 379–383. [DOI] [PubMed] [Google Scholar]
- Adamo J.E., Rossi,G. and Brennwald,P. (1999) The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell, 10, 4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A.E., Johnson,D.I., Longnecker,R.M., Sloat,B.F. and Pringle,J.R. (1990) CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae.J. Cell Biol., 111, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K.R. (2000) Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol., 10, 1587–1590. [DOI] [PubMed] [Google Scholar]
- Bernstein B.W., DeWitt,M. and Bamburg,J.R. (1998) Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Brain Res. Mol. Brain Res., 53, 236–251. [DOI] [PubMed] [Google Scholar]
- Brown A.M., O’Sullivan,A.J. and Gomperts,B.D. (1998) Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol. Biol. Cell, 9, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P.D., Drechsel,D. and Hall,A. (1995) A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem., 270, 29071–29074. [DOI] [PubMed] [Google Scholar]
- Cao X., Ballew,N. and Barlowe,C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B., Haas,A. and Wickner,W. (1994) Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J. Cell Biol., 126, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrcková F., De Virgilio,C., Manser,E., Pringle,J.R. and Nasmyth,K. (1995) Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev., 9, 1817–1830. [DOI] [PubMed] [Google Scholar]
- Dascher C., Ossig,R., Gallwitz,D. and Schmitt,H.D. (1991) Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol., 11, 872–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G., Will,E., Gallwitz,D., Haas,A. and Wickner,W. (2000) Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J., 19, 6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G., Thorngren,N. and Wickner,W. (2001) Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J., 20, 5650–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.W. and Cerione,R.A. (2001) Multiple roles for Cdc42 in cell regulation. Curr. Opin. Cell Biol., 13, 153–157. [DOI] [PubMed] [Google Scholar]
- Erickson J.W., Zhang,C.J., Khan,R.A., Evans,T. and Cerione,R.A. (1996) Mammalian Cdc42 is a brefeldin-A sensitive component of the Golgi apparatus. J. Biol. Chem., 271, 26850–26854. [DOI] [PubMed] [Google Scholar]
- Garrett W.S., Chen,L.M., Kroschewski,R., Ebersold,M., Turley,S., Trombetta,S., Galan,J.E. and Mellman,I. (2000) Developmental control of endocytosis in dendritic cells by Cdc42. Cell, 102, 325–334. [DOI] [PubMed] [Google Scholar]
- Govindan B., Bowser,R. and Novick,P. (1995) The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol., 128, 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. and Dixon,J.E. (1991) Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem., 192, 262–267. [DOI] [PubMed] [Google Scholar]
- Güldener U., Heck,S., Fiedler,T., Beinhauer,J. and Hegemann,J.H. (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res., 24, 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Sutcliffe,M.J., Cerione,M.A. and Oswald,R.E. (1998) Identification of the binding surface on Cdc42Hs for p21-activated kinase. Biochemistry, 37, 14030–14037. [DOI] [PubMed] [Google Scholar]
- Guo W., Tamanoi,F. and Novick,P. (2001) Spatial regulation of the exocyst complex by Rho1 GTPase. Nature Cell Biol., 3, 353–360. [DOI] [PubMed] [Google Scholar]
- Haas A. (1995) A quantitative assay to measure homotypic fusion in vitro. Methods Cell Sci., 17, 283–294. [Google Scholar]
- Hetzer M., Bilbao-Cortes,D., Walther,T.C., Gruss,O.J. and Mattaj,I.W. (2000) GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol. Cell, 5, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Higgs H.N. and Pollard,T.D. (2000) Activation by Cdc42 and PIP(2) of Wiskott–Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol., 150, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K.L., Catlett,N.L. and Weisman,L.S. (1996) Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol., 135, 1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly S.P. and Blumer,K.J. (1999) PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae. J. Cell Biol., 147, 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.C., Popp,D., Gebhard,W. and Kabsch,W. (1990) Atomic model of the actin filament. Nature, 347, 44–49. [DOI] [PubMed] [Google Scholar]
- Hong-Geller E. and Cerione,R.A. (2000) Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP3/calcium pathway in RBL-2H3 mast cells. J. Cell Biol., 148, 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahraus A. et al. (2001) ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol. Biol. Cell, 12, 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.I. (1999) Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev., 63, 54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.I. and Pringle,J.R. (1990) Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol., 111, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A.S., Kakalis,L.T., Abdul-Manan,M., Liu,G.A. and Rosen,M.K. (2000) Autoinhibition and activation mechanisms of the Wiskott–Aldrich syndrome protein. Nature, 404, 151–158. [DOI] [PubMed] [Google Scholar]
- Kozma R., Ahmed,S., Best,A. and Lim,L. (1995) The ras-related protein Cdc42Hs and Bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol., 15, 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski K.G., Chen,A.J., Rodal,A.A. and Drubin,D.G. (2000) Functions and functional domains of the GTPase Cdc42p. Mol. Biol. Cell, 11, 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R., Hall,A. and Mellman,I. (1999) Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nature Cell Biol., 1, 8–13. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Fujimoto,L.M., Yin,H.L. and Schmid,S.L. (1997) The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem., 272, 20332–20335. [DOI] [PubMed] [Google Scholar]
- Lang T., Wacker,I., Wunderlich,I., Rohrbach,A., Giese,G., Soldati,T. and Almers,W. (2000) Role of actin cortex in the subplasmalemal transport of secretory granules in PC-12 cells. Biophys. J., 78, 2863–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar T., Gotte,M. and Gallwitz,D. (1997) Vesicular transport—how many Ypt/Rab-GTPases make a eukaryotic cell. Trends Biochem. Sci., 22, 468–472. [DOI] [PubMed] [Google Scholar]
- Li R., Zheng,Y. and Drubin,D.G. (1995) Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J. Cell Biol., 128, 599–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Cantley,L.C., Janmey,P.A. and Kirschner,M.W. (1998a) Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J. Cell Biol., 140, 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Rohatgi,R. and Kirschner,M.W. (1998b) The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42. Proc. Natl Acad. Sci. USA, 95, 15362–15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E., Leung,T., Salihuddin,H., Tan,L. and Lim,L. (1993) A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature, 363, 364–367. [DOI] [PubMed] [Google Scholar]
- Matozaki T., Nakanishi,H. and Takai,Y. (2000) Small G-protein networks, their crosstalk and signal cascades. Cell. Signal., 12, 515–524. [DOI] [PubMed] [Google Scholar]
- Mayer A. and Wickner,W. (1997) Docking of yeast vacuoles is catalyzed by the ras-like GTPase Ypt7 after symmetric priming by Sec18 (NSF). J. Cell Biol., 136, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18 (NSF) driven release of Sec17 (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Mayer A., Scheglmann,D., Dove,S., Glatz,A., Wickner,W. and Haas,A. (2000) Phosphatidylinositol-4,5-bisphosphate controls two steps of homotypic vacuole fusion. Mol. Biol. Cell, 11, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Rybin,V., Murphy,C., Giner,A., Teasdale,R. and Zerial,M. (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell, 98, 377–386. [DOI] [PubMed] [Google Scholar]
- Mott H.R., Owen,D., Nietlispach,D., Lowe,P.N., Manser,E., Lim,L. and Laue,E.D. (1999) Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature, 399, 384–388. [DOI] [PubMed] [Google Scholar]
- Muallem S., Kwiatkowska,K., Xu,X. and Yin,H.L. (1995) Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol., 128, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. et al. (1996) Endosome dynamics regulated by a Rho protein. Nature, 384, 427–432. [DOI] [PubMed] [Google Scholar]
- Murray J.M. and Johnson,D.I. (2000) Isolation and characterization of Nrf1, a novel negative regulator of the Cdc42p GTPase in Schizosaccharomyces pombe. Genetics, 154, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M. and Johnson,D.I. (2001) The Cdc42p GTPase and its regulators Nrf1p and Scd1p are involved in endocytic trafficking in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem., 276, 3004–3009. [DOI] [PubMed] [Google Scholar]
- Nelson N., Perzov,N., Cohen,A., Hagai,K., Padler,V. and Nelson,H. (2000) The cellular biology of proton-motive force generation by V-ATPases. J. Exp. Biol., 203, 89–95. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann,C., Pelham,H.R.B., Wickner,W. and Haas,A. (1997) Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature, 387, 199–202. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Qadota,H., Anraku,Y., Pringle,J.R. and Botstein,D. (1993) Suppression of yeast geranylgeranyl transferase I defect by alternative prenylation of two target GTPases, Rho1p and Cdc42p. Mol. Biol. Cell, 4, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Gabbay,K.H. and Malaisse,W.J. (1972) Pancreatic β cell web: its possible role in insulin secretion. Science, 175, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews,P.D., Stark,M.J.R., Cesaro-Tadic,S., Glatz,A., Podtelejnikov,A., Mann,M. and Mayer,A. (1999) Control of the terminal step of membrane fusion by protein phosphatase 1. Science, 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Bühler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation of proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–588. [DOI] [PubMed] [Google Scholar]
- Prehoda K.E., Scott,J.A., Dyche Mullins,R. and Lim,W.A. (2000) Integration of multiple signals through cooperative regulation of the N-WASP–Arp2/3 complex. Science, 290, 801–806. [DOI] [PubMed] [Google Scholar]
- Price A., Wickner,W. and Ungermann,C. (2000a) Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J. Cell Biol., 148, 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A., Seals,D., Wickner,W. and Ungermann,C. (2000b) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol., 148, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman T.J., Sawyer,M.M. and Johnson,D.I. (1999) The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical–isotropic switch and nuclear division in yeast. J. Biol. Chem., 274, 16861–16870. [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Ma,L., Miki,H., Lopez,M., Kirchhausen,T., Takenawa,T. and Kirschner,M.W. (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell, 97, 221–231. [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Ho,H.Y. and Kirschner,M.W. (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol., 150, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.K., Rehling,P., Peterson,M.R. and Emr,S.D. (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Eitzen,G., Margolis,N., Wickner,W. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brunger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Trifaro J.M. and Vitale,M.L. (1993) Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci., 16, 466–472. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Sato,K. and Wickner,W. (1998a) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Nichols,B.J., Pelham,H.R.B. and Wickner,W. (1998b) A vacuolar v–t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol., 140, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., von Mollard,G.F., Jensen,O.N., Margolis,N., Stevens,T.H. and Wickner,W. (1999) Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol., 145, 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M., Laage,R., Dietrich,L., Wang,L. and Ungermann,C. (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J., 20, 3145–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M.L., Seward,E.P. and Trifaro,J.M. (1995) Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron, 14, 353–363. [DOI] [PubMed] [Google Scholar]
- Wang Y.X., Catlett,N.L. and Weisman,L.S. (1998) Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol., 140, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Kauffman,E.J., Duex,J.E. and Weisman,L.S. (2001) Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Waters M.G. and Pfeffer,S.R. (1999) Membrane tethering in intracellular transport. Curr. Opin. Cell Biol., 11, 453–459. [DOI] [PubMed] [Google Scholar]
- Wessel D. and Flügge,U.I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem., 138, 141–143. [DOI] [PubMed] [Google Scholar]
- White W.H. and Johnson,D.I. (1997) Characterization of synthetic-lethal mutants reveals a role for the Saccharomyces cerevisiae guanine-nucleotide exchange factor Cdc24p in vacuole function and Na+ tolerance. Genetics, 147, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. and Haas,A. (2000) Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem., 69, 247–275. [DOI] [PubMed] [Google Scholar]
- Wu W.J., Erickson,J.W., Lin,R. and Cerione,R.A. (2000) The γ-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature, 405, 800–804. [DOI] [PubMed] [Google Scholar]
- Wurmser A.E., Sato,T.K. and Emr,S.D. (2000) New component of the vacuolar class C–Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J. Cell Biol., 151, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.Y., Sato,K. and Wickner,W. (1998) LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell, 93, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Yang W. and Cerione,R.A. (1997) Cloning and characterization of a novel Cdc42-associated tyrosine kinase, ACK-2, from bovine brain. J. Biol. Chem., 272, 24819–24824. [DOI] [PubMed] [Google Scholar]
- Yang W., Lin,Q., Guan,J. and Cerione,R.A. (1999) Activation of the Cdc42-associated tyrosine kinase-2 (ACK-2) by cell adhesion via integrin β1. J. Biol. Chem., 274, 8524–8530. [DOI] [PubMed] [Google Scholar]
- Zerial M. and McBride,H. (2001) Rab proteins as membrane organizers. Nature Rev. Mol. Cell Biol., 2, 107–117. [DOI] [PubMed] [Google Scholar]
- Ziman M., O’Brien,J.M., Ouellette,L.A., Church,W.R. and Johnson,D.I. (1991) Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol., 11, 3537–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M., Preuss,D., Mulholland,J., O’Brien,J.M., Botstein,D. and Johnson,D.I. (1993) Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell, 4, 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]