Abstract
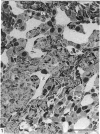
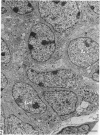
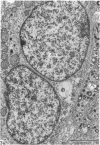
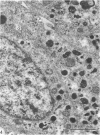
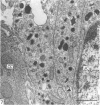
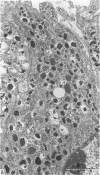
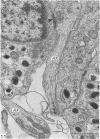
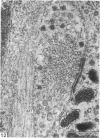
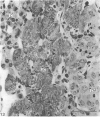
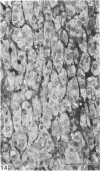
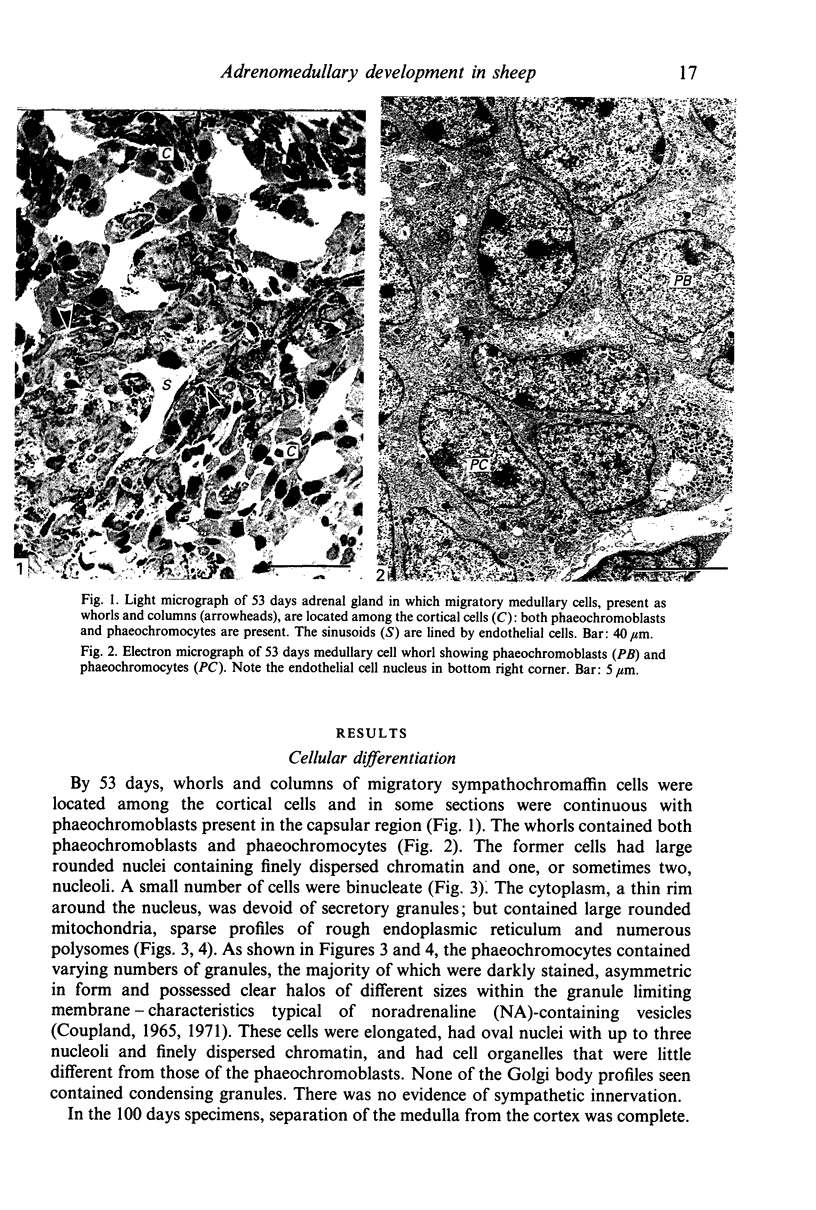
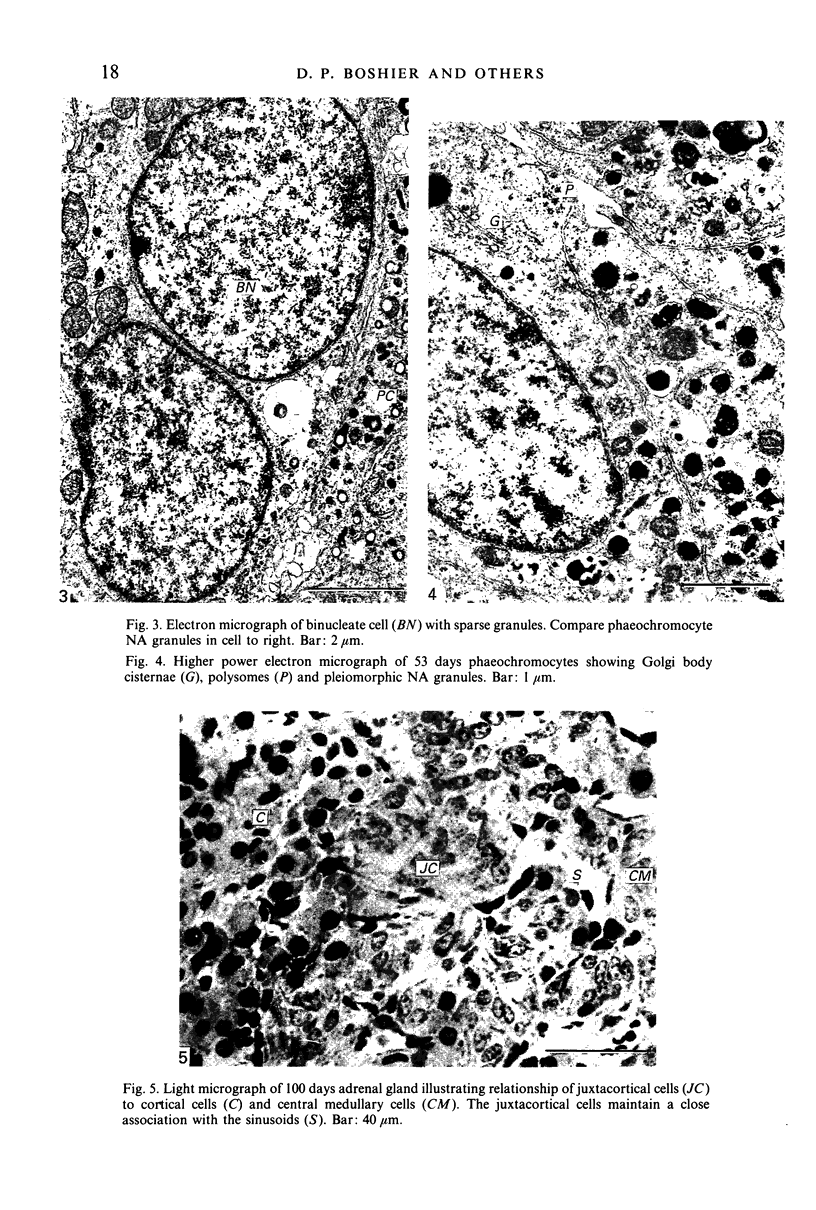
This account of fetal and neonatal sheep adrenomedullary development is the first such study in mammals using both morphometric and microscopic techniques. At 53 days gestation some cells in the migratory whorls and columns contained noradrenaline (NA) granules whereas by 100 days the medulla, now enclosed by the cortex, was composed of elongated juxtacortical cells and rounded central medullary cells, both populations of cells containing NA granules. In the 130 days glands, many of the juxtacortical cells contained adrenaline granules and had synaptic connection with axons of the preganglionic sympathetic nerve fibres. Later development was essentially growth-related. While the juxtacortical cells decreased from 33% of the medulla at 100 days to 22% at 144 days, the central medullary cells increased from 19% to 30% over the same period. Both cell populations exhibited hypertrophic growth over the study period; but the central cells multiplied at a faster rate. We conclude that the development of the cortical and medullary compartments of the adrenal gland are closely linked, for both showed rapid mid-gestational growth which slowed with the attainment of definitive tissue organisation. Then a second phase of growth, associated with increased and controlled catecholamine secretion in the medulla and cortisol secretion in the cortex, occurred in late gestation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdellatif M. M., Hollingsworth M. Effect of oxotremorine and epinephrine on lung surfactant secretion in neonatal rabbits. Pediatr Res. 1980 Aug;14(8):916–920. doi: 10.1203/00006450-198008000-00004. [DOI] [PubMed] [Google Scholar]
- Alexander D. P., Britton H. G., James V. H., Nixon D. A., Parker R. A., Wintour E. M., Wright R. D. Steroid secretion by the adrenal gland of foetal and neonatal sheep. J Endocrinol. 1968 Jan;40(1):1–13. doi: 10.1677/joe.0.0400001. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986 Dec 26;47(6):1079–1090. doi: 10.1016/0092-8674(86)90823-8. [DOI] [PubMed] [Google Scholar]
- Artal R. Fetal adrenal medulla. Clin Obstet Gynecol. 1980 Sep;23(3):825–836. [PubMed] [Google Scholar]
- Axelrod J. Catecholamines: effects of ACTH and adrenal corticoids. Ann N Y Acad Sci. 1977 Oct 28;297:275–283. doi: 10.1111/j.1749-6632.1977.tb41860.x. [DOI] [PubMed] [Google Scholar]
- Boshier D. P., Holloway H. Morphometric analyses of adrenal gland growth in fetal and neonatal sheep. I. The adrenal cortex. J Anat. 1989 Dec;167:1–14. [PMC free article] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUPLAND R. E. On the morphology and adrenaline-nor-adrenaline content of chromaffin tissue. J Endocrinol. 1953 Apr;9(2):194–203. doi: 10.1677/joe.0.0090194. [DOI] [PubMed] [Google Scholar]
- Carballeira A., Fishman L. M. The adrenal functional unit: a hypothesis. Perspect Biol Med. 1980 Summer;23(4):573–597. doi: 10.1353/pbm.1980.0064. [DOI] [PubMed] [Google Scholar]
- Carmichael S. W., Spagnoli D. B., Frederickson R. G., Krause W. J., Culberson J. L. Opossum adrenal medulla: I. Postnatal development and normal anatomy. Am J Anat. 1987 Jul;179(3):211–219. doi: 10.1002/aja.1001790303. [DOI] [PubMed] [Google Scholar]
- Cheng J. B., Goldfien A., Ballard P. L., Roberts J. M. Glucocorticoids increase pulmonary beta-adrenergic receptors in fetal rabbit. Endocrinology. 1980 Nov;107(5):1646–1648. doi: 10.1210/endo-107-5-1646. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Silver M. Development of activity in the adrenal medulla of the foetus and new-born animal. Br Med Bull. 1966 Jan;22(1):16–20. doi: 10.1093/oxfordjournals.bmb.a070430. [DOI] [PubMed] [Google Scholar]
- Dvorák M. Adrenocortical function in foetal, neonatal and young pigs. J Endocrinol. 1972 Sep;54(3):473–481. doi: 10.1677/joe.0.0540473. [DOI] [PubMed] [Google Scholar]
- Elfvin L. G. The development of the secretory granules in the rat adrenal medulla. J Ultrastruct Res. 1967 Jan;17(1):45–62. doi: 10.1016/s0022-5320(67)80019-4. [DOI] [PubMed] [Google Scholar]
- Frydman R., Geffen L. B. Depletion and repletion of adrenal dopamine- -hydroxylase after reserpine. Immunohistochemical and fine structural correlates. J Histochem Cytochem. 1973 Feb;21(2):166–174. doi: 10.1177/21.2.166. [DOI] [PubMed] [Google Scholar]
- Galabov P., Schiebler T. H. The ultrastructure of the developing neural lobe. Cell Tissue Res. 1978 May 29;189(2):313–329. doi: 10.1007/BF00209280. [DOI] [PubMed] [Google Scholar]
- Graham A. D., Longo L. D., Cheung C. Y. Catecholamine secretion from the adrenal medulla of the fetus, regulation by hormones. J Dev Physiol. 1986 Aug;8(4):227–235. [PubMed] [Google Scholar]
- Grothe C., Hofmann H. D., Verhofstad A. A., Unsicker K. Nerve growth factor and dexamethasone specify the catecholaminergic phenotype of cultured rat chromaffin cells: dependence on developmental stage. Brain Res. 1985 Jul;353(1):125–132. doi: 10.1016/0165-3806(85)90030-6. [DOI] [PubMed] [Google Scholar]
- Hervonen A. Development of catecholamine--storing cells in human fetal paraganglia and adrenal medulla. A histochemical and electron microscopical study. Acta Physiol Scand Suppl. 1971;368:1–94. [PubMed] [Google Scholar]
- Ikeda Y., Lister J., Bouton J. M., Buyukpamukcu M. Congenital neuroblastoma, neuroblastoma in situ, and the normal fetal development of the adrenal. J Pediatr Surg. 1981 Aug;16(4 Suppl 1):636–644. doi: 10.1016/0022-3468(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Ritchie J. W. The metabolic and endocrine effects of circulating catecholamines in fetal sheep. J Physiol. 1978 Dec;285:395–408. doi: 10.1113/jphysiol.1978.sp012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. T., Robinson R. O. Plasma catecholamines in foetal and adult sheep. J Physiol. 1975 Jun;248(1):15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson E. E., Brown E. R., Torday J. S., Madansky D. L., Taeusch H. W., Jr The effect of epinephrine on tracheal fluid flow and surfactant efflux in fetal sheep. Am Rev Respir Dis. 1978 Dec;118(6):1023–1026. doi: 10.1164/arrd.1978.118.6.1023. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Day R., Elde R. P., Howe P. R. Co-storage of enkephalins and adrenaline in the bovine adrenal medulla. Neuroscience. 1982 May;7(5):1323–1332. doi: 10.1016/0306-4522(82)91138-1. [DOI] [PubMed] [Google Scholar]
- Malendowicz K. A correlated stereological and functional studies on the long-term effects of ACTH on rat adrenal cortex. Folia Histochem Cytobiol. 1986;24(3):203–211. [PubMed] [Google Scholar]
- McMillen I. C., Mulvogue H. M., Coulter C. L., Browne C. A., Howe P. R. Ontogeny of catecholamine-synthesizing enzymes and enkephalins in the sheep adrenal medulla: an immunocytochemical study. J Endocrinol. 1988 Aug;118(2):221–226. doi: 10.1677/joe.0.1180221. [DOI] [PubMed] [Google Scholar]
- Padbury J. F., Polk D. H., Newnham J. P., Lam R. W. Neonatal adaptation: greater sympathoadrenal response in preterm than full-term fetal sheep at birth. Am J Physiol. 1985 Apr;248(4 Pt 1):E443–E449. doi: 10.1152/ajpendo.1985.248.4.E443. [DOI] [PubMed] [Google Scholar]
- Phillippe M. Fetal catecholamines. Am J Obstet Gynecol. 1983 Aug 1;146(7):840–855. doi: 10.1016/0002-9378(83)91088-8. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Seidler F. J. Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J Dev Physiol. 1988 Feb;10(1):1–16. [PubMed] [Google Scholar]
- Stadnicka A., Van Wynsberghe D. Cytochemistry and ultrastructure of the prenatal porcine adrenal medulla. Z Mikrosk Anat Forsch. 1982;96(1):103–112. [PubMed] [Google Scholar]
- Tomlinson A., Durbin J., Coupland R. E. A quantitative analysis of rat adrenal chromaffin tissue: morphometric analysis at tissue and cellular level correlated with catecholamine content. Neuroscience. 1987 Mar;20(3):895–904. doi: 10.1016/0306-4522(87)90250-8. [DOI] [PubMed] [Google Scholar]
- Walters D. V., Olver R. E. The role of catecholamines in lung liquid absorption at birth. Pediatr Res. 1978 Mar;12(3):239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]
- Wilburn L. A., Goldsmith P. C., Chang K. J., Jaffe R. B. Ontogeny of enkephalin and catecholamine-synthesizing enzymes in the primate fetal adrenal medulla. J Clin Endocrinol Metab. 1986 Oct;63(4):974–980. doi: 10.1210/jcem-63-4-974. [DOI] [PubMed] [Google Scholar]
- Wintour E. M., Brown E. H., Denton D. A., Hardy K. J., McDougall J. G., Oddie C. J., Whipp G. T. The ontogeny and regulation of corticosteroid secretion by the ovine foetal adrenal. Acta Endocrinol (Copenh) 1975 Jun;79(2):301–316. doi: 10.1530/acta.0.0790301. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]