Abstract
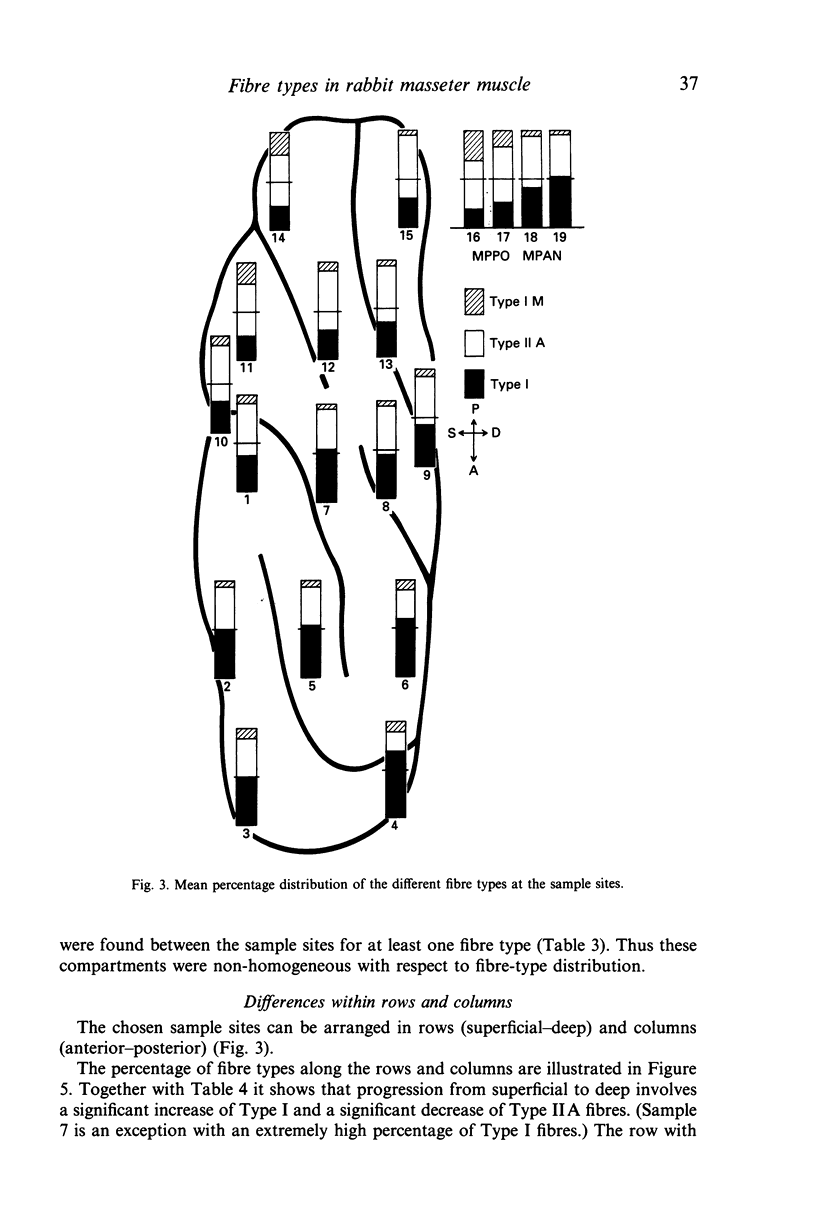
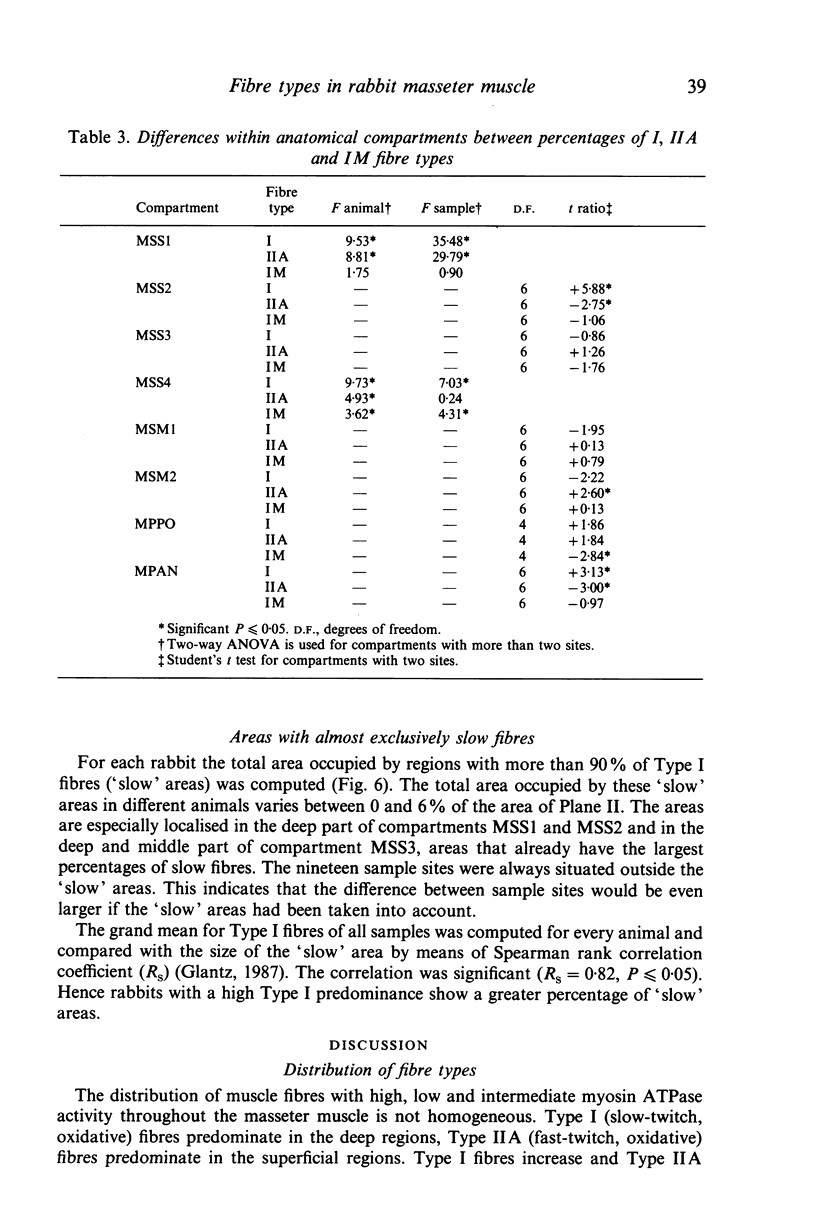
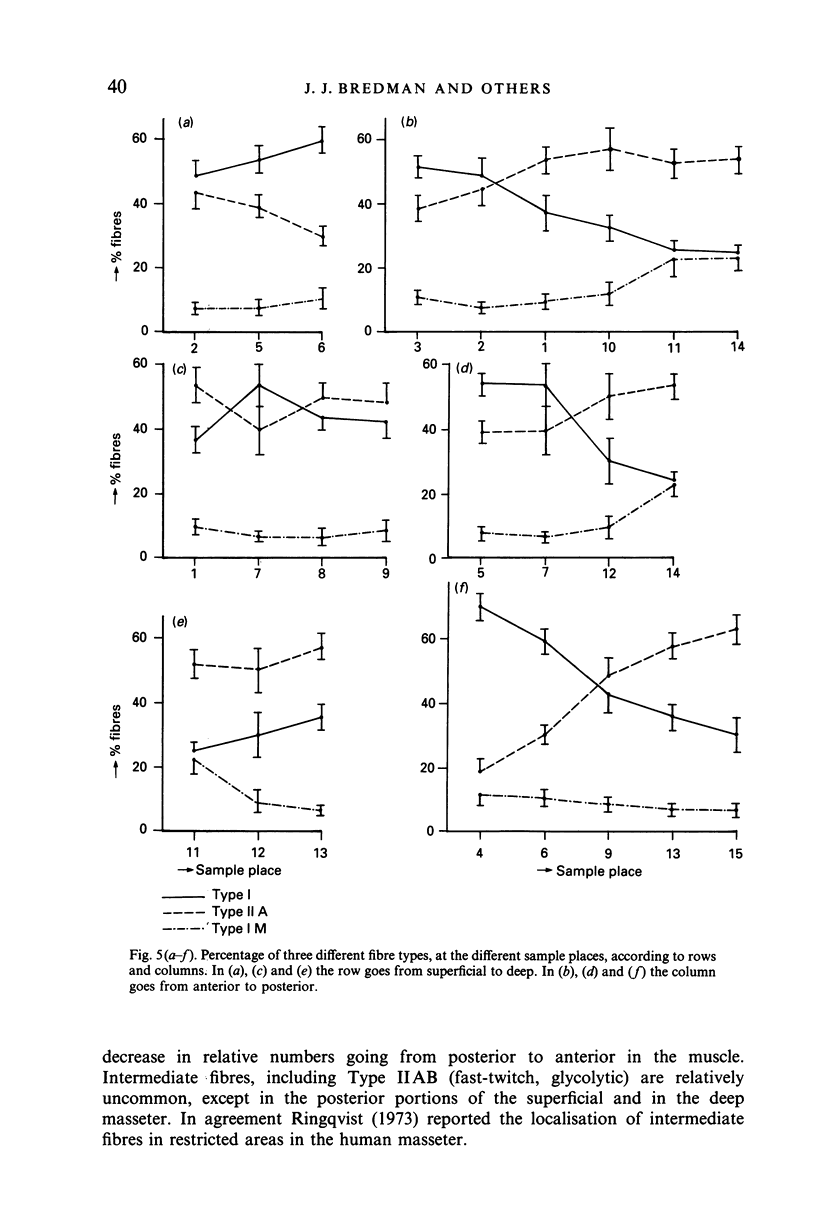
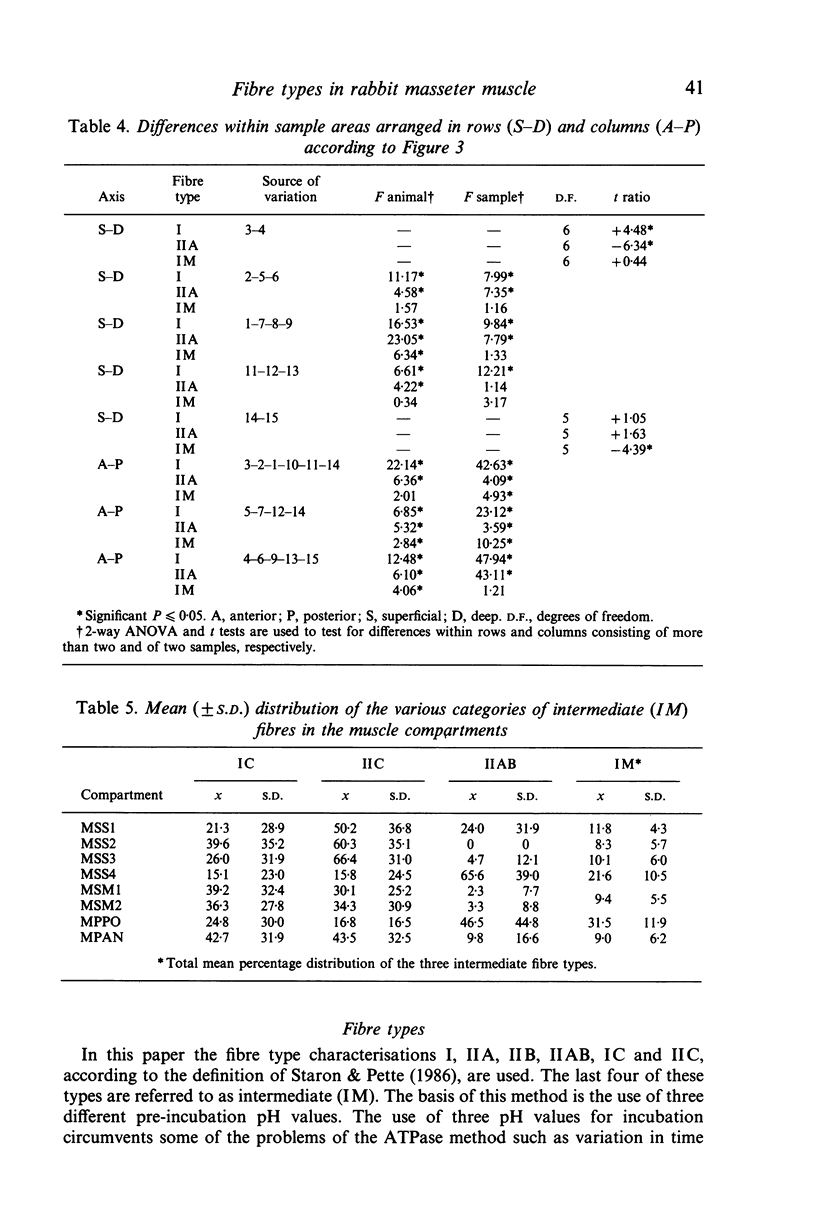
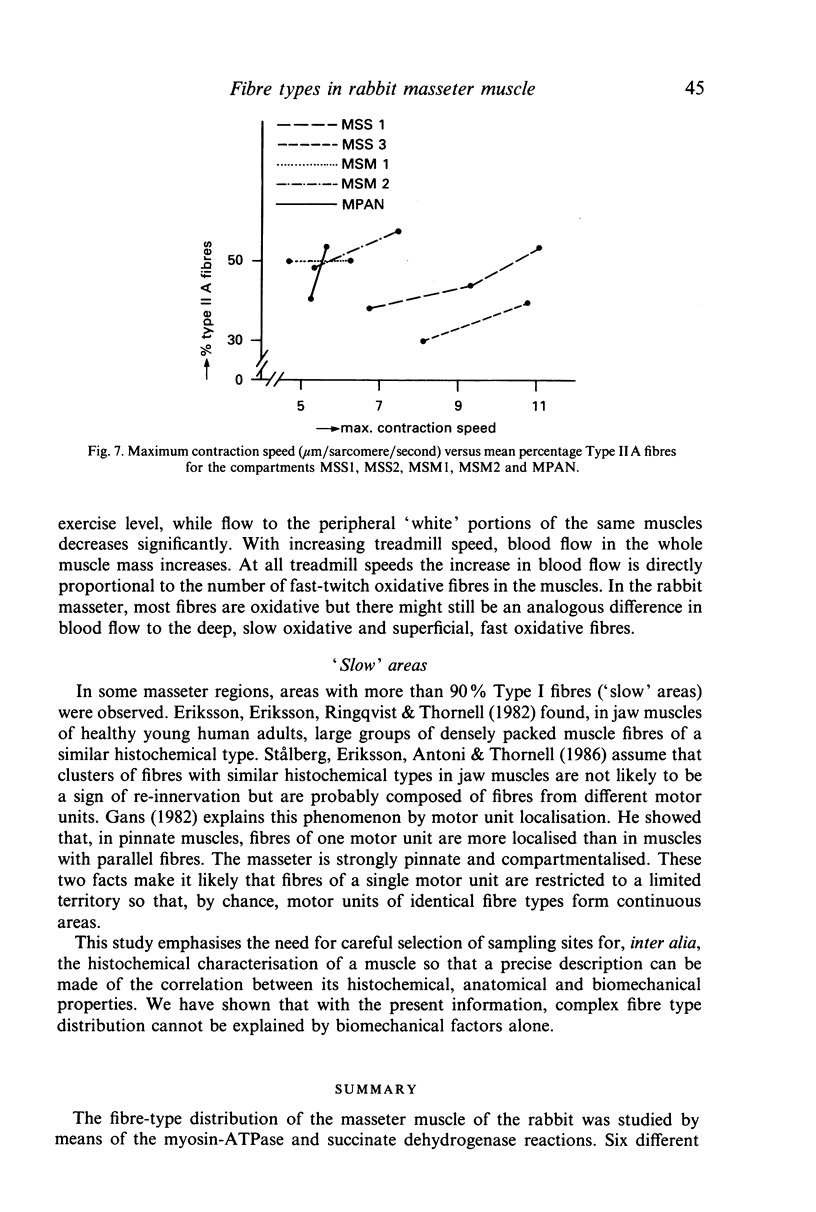
The fibre-type distribution of the masseter muscle of the rabbit was studied by means of the myosin-ATPase and succinate dehydrogenase reactions. Six different fibre types were found and these were unequally distributed between and within the anatomical compartments of the muscle. Most of the masseter consists of slow- and fast-twitch oxidative fibres. The slow fibres increase in numbers in the deeper and more anterior regions of the muscle. Fast-twitch glycolytic fibres were almost exclusively found in the most posterior portions of the superficial and deep masseter. The fibre composition within the sagittally orientated anatomical compartments was found to be correlated with maximal contraction speeds during natural mastication as estimated from a mechanical model. However, the differences in fibre composition between the anatomical compartments (and hence between superficial and deep layers) appeared not to be correlated with contraction speed. The regional and compartmental specialisation within the masseter permits the muscle to perform many different functional roles in the generation and control of the jaw movements, jaw position and bite forces.
Full text
PDF

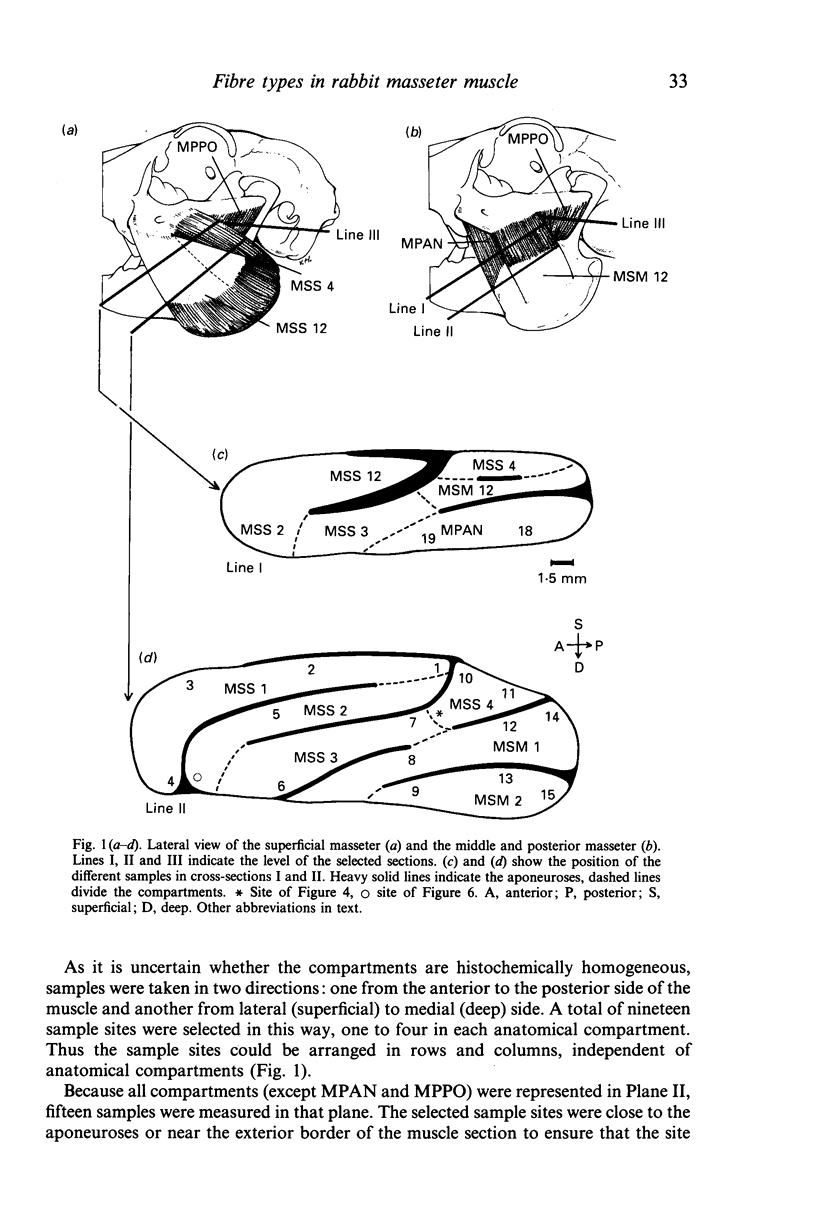

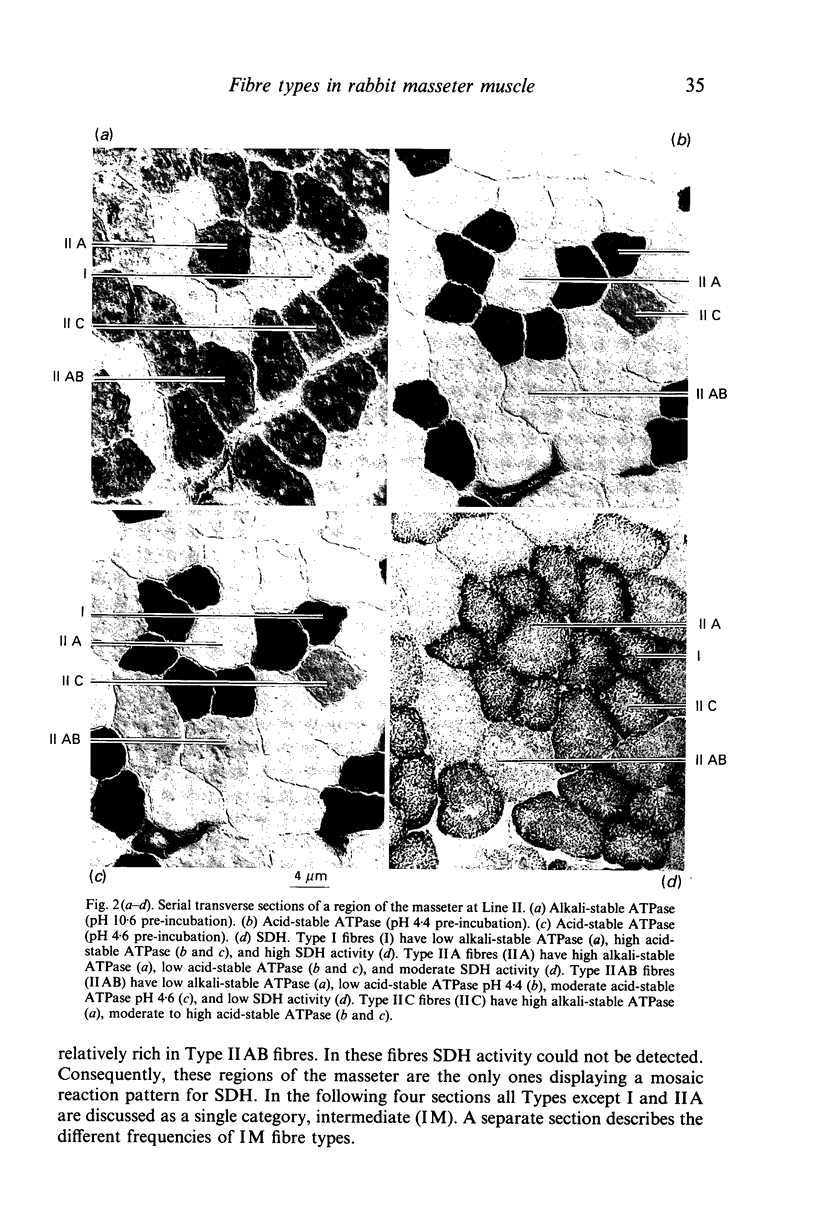






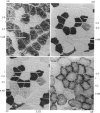
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B., Laughlin M. H. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983 Nov;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Bubb W. J., Sims M. H. Fiber type composition of rostral and caudal portions of the digastric muscle in the dog. Am J Vet Res. 1986 Aug;47(8):1834–1842. [PubMed] [Google Scholar]
- Clark R. W., Luschei E. S. Histochemical characteristics of mandibular muscles of monkeys. Exp Neurol. 1981 Dec;74(3):654–672. doi: 10.1016/0014-4886(81)90242-9. [DOI] [PubMed] [Google Scholar]
- English A. W., Letbetter W. D. A histochemical analysis of identified compartments of cat lateral gastrocnemius muscle. Anat Rec. 1982 Oct;204(2):123–130. doi: 10.1002/ar.1092040205. [DOI] [PubMed] [Google Scholar]
- English A. W., Wolf S. L. The motor unit. Anatomy and physiology. Phys Ther. 1982 Dec;62(12):1763–1772. doi: 10.1093/ptj/62.12.1763. [DOI] [PubMed] [Google Scholar]
- Eriksson P. O., Eriksson A., Ringqvist M., Thornell L. E. Histochemical fibre composition of the human digastric muscle. Arch Oral Biol. 1982;27(3):207–215. doi: 10.1016/0003-9969(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Eriksson P. O., Thornell L. E. Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol. 1983;28(9):781–795. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- Gans C. Fiber architecture and muscle function. Exerc Sport Sci Rev. 1982;10:160–207. [PubMed] [Google Scholar]
- Gorniak G. C. Correlation between histochemistry and muscle activity of jaw muscles in cats. J Appl Physiol (1985) 1986 Apr;60(4):1393–1400. doi: 10.1152/jappl.1986.60.4.1393. [DOI] [PubMed] [Google Scholar]
- Guth L., Yellin H. The dynamic nature of the so-called "fiber types" of nammalian skeletal muscle. Exp Neurol. 1971 May;31(2):227–300. doi: 10.1016/0014-4886(71)90196-8. [DOI] [PubMed] [Google Scholar]
- Herring S. W., Grimm A. F., Grimm B. R. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979 Apr;154(4):563–576. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. The control and responses of mammalian muscle spindles during normally executed motor tasks. Exerc Sport Sci Rev. 1984;12:157–204. [PubMed] [Google Scholar]
- Mabuchi K., Pinter K., Mabuchi Y., Sreter F., Gergely J. Characterization of rabbit masseter muscle fibers. Muscle Nerve. 1984 Jul-Aug;7(6):431–438. doi: 10.1002/mus.880070603. [DOI] [PubMed] [Google Scholar]
- Maxwell L. C., Carlson D. S., McNamara J. A., Jr, Faulkner J. A. Histochemical characteristics of the masseter and temporalis muscles of the rhesus monkey (Macaca mulatta). Anat Rec. 1979 Mar;193(3):389–402. doi: 10.1002/ar.1091930306. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Ringqvist M. Histochemical enzyme profiles of fibres in human masseter muscles with special regard to fibres with intermediate myofibrillar ATPase reaction. J Neurol Sci. 1973 Feb;18(2):133–141. doi: 10.1016/0022-510x(73)90001-4. [DOI] [PubMed] [Google Scholar]
- Rokx J. T., van Willigen J. D., Jansen H. W. Muscle fibre types and muscle spindles in the jaw musculature of the rat. Arch Oral Biol. 1984;29(1):25–31. doi: 10.1016/0003-9969(84)90038-4. [DOI] [PubMed] [Google Scholar]
- Schiaffino S. Histochemical enzyme profile of the masseter muscle in different mammalian species. Anat Rec. 1974 Sep;180(1):53–61. doi: 10.1002/ar.1091800107. [DOI] [PubMed] [Google Scholar]
- Serratrice G., Pellissier J. F., Vignon C., Baret J. The histochemical profile of the human masseter. An autopsy and biopsy study. J Neurol Sci. 1976 Nov;30(1):189–200. doi: 10.1016/0022-510x(76)90266-5. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86(1):19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Stålberg E., Eriksson P. O., Antoni L., Thornell L. E. Electrophysiological study of size and fibre distribution of motor units in the human masseter and temporal muscles. Arch Oral Biol. 1986;31(8):521–527. doi: 10.1016/0003-9969(86)90145-7. [DOI] [PubMed] [Google Scholar]
- Suzuki A. A comparative histochemical study of the masseter muscle of the cattle, sheep, swine, dog, guinea pig, and rat. Histochemistry. 1977 Mar 4;51(2-3):121–131. doi: 10.1007/BF00567218. [DOI] [PubMed] [Google Scholar]
- Tamari J. W., Tomey G. F., Ibrahim M. Z., Baraka A., Jabbur S. J., Bahuth N. Correlative study of the physiologic and morphologic characteristics of the temporal and masseter muscles of the cat. J Dent Res. 1973 May-Jun;52(3):538–543. doi: 10.1177/00220345730520032701. [DOI] [PubMed] [Google Scholar]
- Taylor A., Cody F. W., Bosley M. A. Histochemical and mechanical properties of the jaw muscles of the cat. Exp Neurol. 1973 Jan;38(1):99–109. doi: 10.1016/0014-4886(73)90011-3. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Hodgson J. A., Burke R. E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978 Sep;41(5):1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Weijs W. A., Brugman P., Klok E. M. The growth of the skull and jaw muscles and its functional consequences in the New Zealand rabbit (Oryctolagus cuniculus). J Morphol. 1987 Nov;194(2):143–161. doi: 10.1002/jmor.1051940204. [DOI] [PubMed] [Google Scholar]
- Weijs W. A., van der Wielen-Drent T. K. The relationship between sarcomere length and activation pattern in the rabbit masseter muscle. Arch Oral Biol. 1983;28(4):307–315. doi: 10.1016/0003-9969(83)90073-0. [DOI] [PubMed] [Google Scholar]
- van Noorden C. J., Bhattacharya R. D., Vogels I. M. Enzyme cytochemical staining of individual cells with the use of a polyacrylamide carrier. Studies on the synthetizing reaction technique, the indigogenic method, the metal salt method, the post-azo-coupling technique, and the tetrazolium salt technique. Acta Histochem. 1983;73(1):71–78. doi: 10.1016/S0065-1281(83)80077-4. [DOI] [PubMed] [Google Scholar]