Abstract
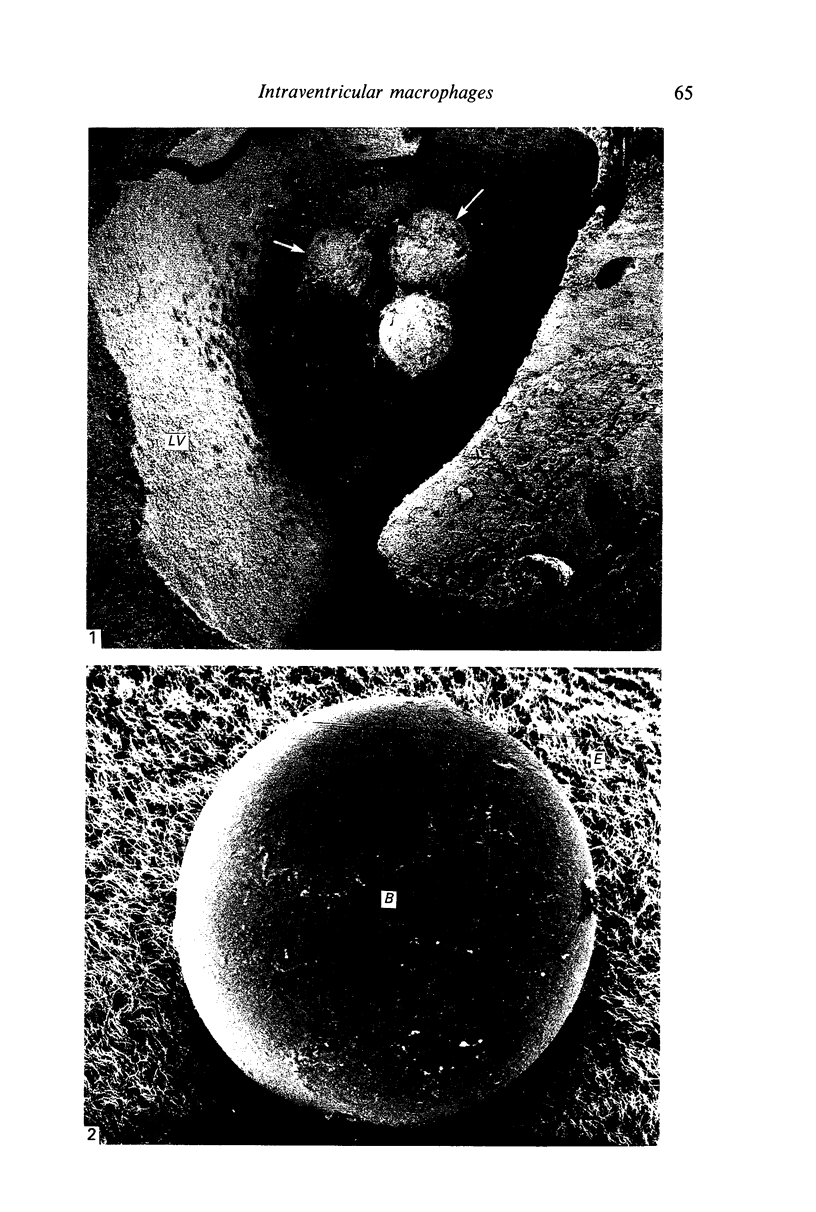
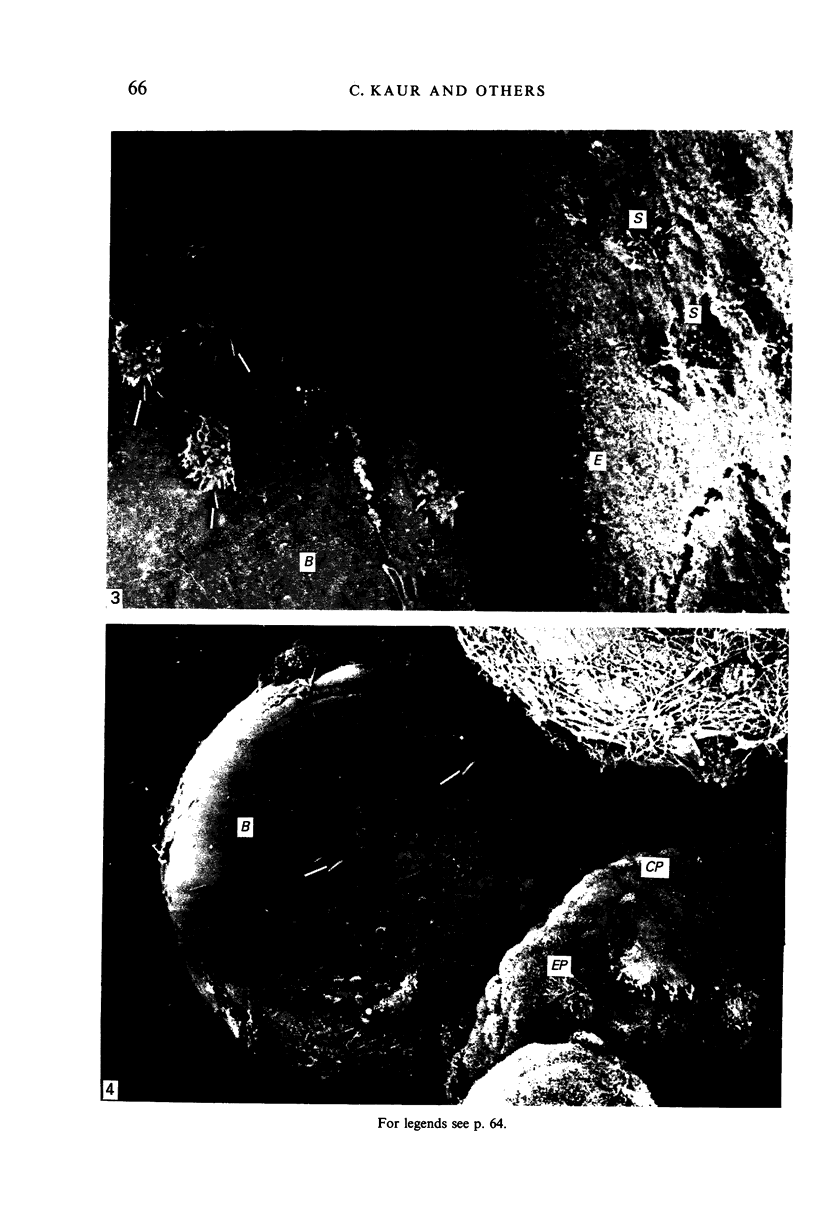
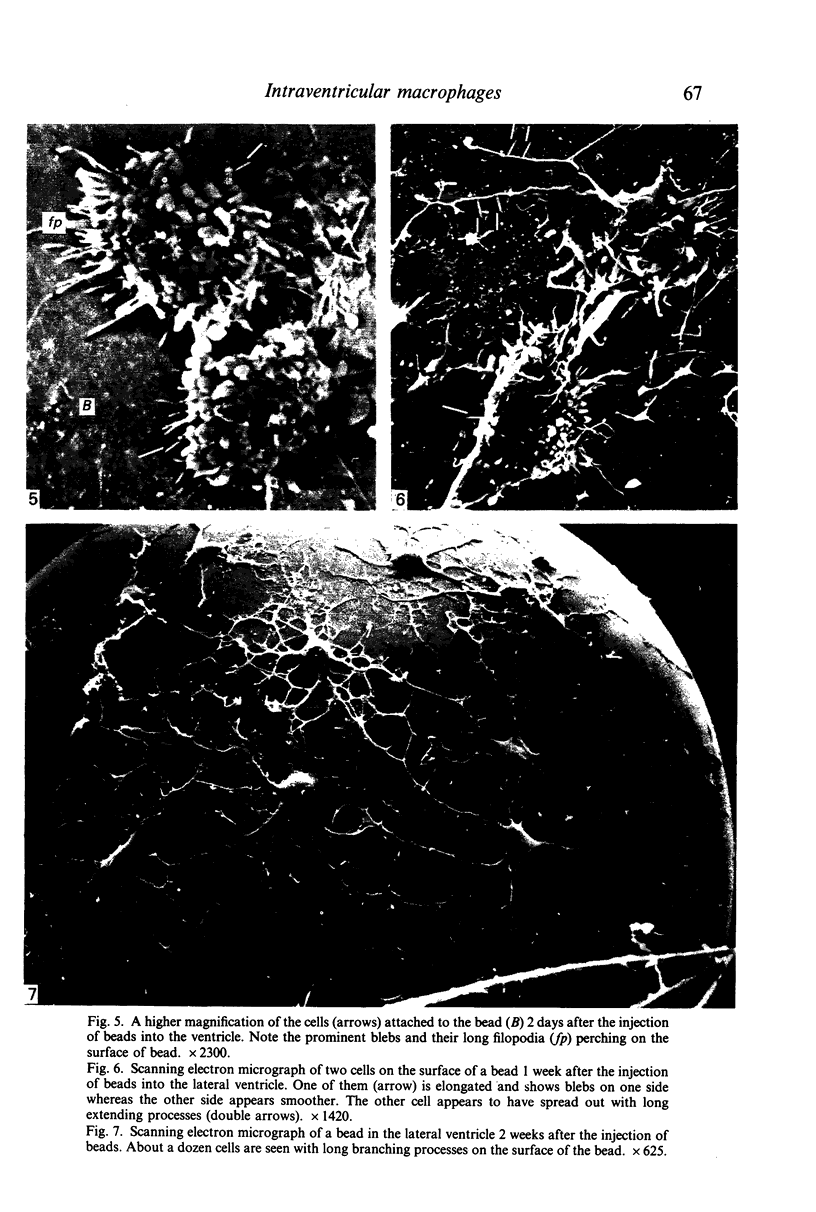
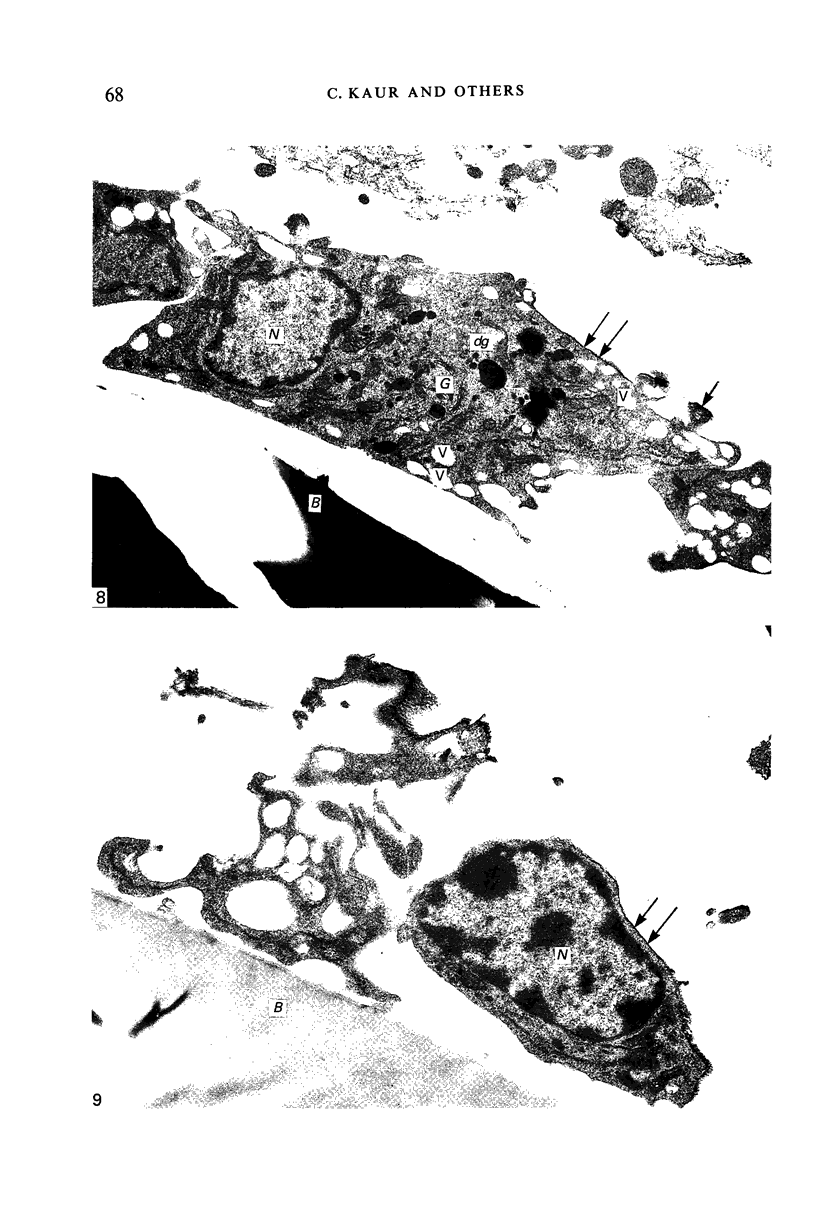
Crotoxin-coated microcarrier beads were injected into the lateral ventricles of 5 days old postnatal rats. The morphology of the cells attached to the beads at various time intervals was studied by scanning and transmission electron microscopy. Scanning electron microscopy showed that very few cells were associated with the surface of the beads 18 hours after the injection. After 2 days a large number of spherical cells showing blebs and filopodia were attached to the surface of the beads. One week after the injection, these cells became oval and, in longer survival periods between 2 weeks and 30 days after the injection, the cells developed a flattened or angular cell body bearing a number of radiating slender processes. Transmission electron microscopy of the re-embedded materials from animals killed 2 days after the injection showed many cells with an eccentric nucleus containing dense chromatin masses. Their abundant cytoplasm was endowed with a variable number of lysosome-like dense granules and vacuoles. In longer surviving animals, the cells became elongated with scanty cytoplasm showing relatively fewer dense granules and cytoplasmic vacuoles. It is postulated from this study that the cells attached to the crotoxin-coated beads are derived from the intraventricular macrophages. These are functionally active initially in response to the beads injected. With time, however, they undergo morphological alteration and regress into quiescent cells which are microglia-like.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. J., Low F. N. Scanning electron microscopy of the subarachnoid space in the dog. III. Cranial levels. J Comp Neurol. 1975 Jun 15;161(4):515–539. doi: 10.1002/cne.901610404. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Artault J. C., Rebel G., Dreyfus H., Massarelli R. Phagocytosis of polystyrene latex beads by rat brain microglia cell cultures is increased by treatment with gangliosides. Dev Neurosci. 1988;10(4):270–276. doi: 10.1159/000111977. [DOI] [PubMed] [Google Scholar]
- Carpenter S. J., McCarthy L. E., Borison H. L. Electron microscopic study of the epiplexus (Kolmer) cells of the cat choroid plexus. Z Zellforsch Mikrosk Anat. 1970;110(4):471–486. doi: 10.1007/BF00330099. [DOI] [PubMed] [Google Scholar]
- Coates P. W. Supraependymal cells: light and transmission electron microscopy extends scanning electron microscopic demonstration. Brain Res. 1973 Jul 27;57(2):502–507. doi: 10.1016/0006-8993(73)90157-1. [DOI] [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. The appearance of transition forms between monocytes and Kupffer cells in the liver of rats treated with glucan. A cytochemical and ultrastructural study. J Exp Med. 1979 Apr 1;149(4):883–897. doi: 10.1084/jem.149.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya Y., Fujita T. Scanning electron microscope observation of intraventricular macrophages (Kolmer cells) in the rat brain. Arch Histol Jpn. 1973 Jan;35(2):133–140. doi: 10.1679/aohc1950.35.133. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Fujiwara R., Nagai T., Maeda T. Distribution and fate of macrophagic ameboid cells in the rat brain. Arch Histol Jpn. 1982 Dec;45(5):505–518. doi: 10.1679/aohc.45.505. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in rats of various ages following an intravenous injection of horseradish peroxidase. Acta Anat (Basel) 1986;125(2):132–137. doi: 10.1159/000146150. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Origin and fate of neural macrophages in a stab wound of the brain of the young rat. J Anat. 1987 Oct;154:215–227. [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Transformation of amoeboid microglial cells into microglia in the corpus callosum of the postnatal rat brain. An electron microscopical study. Arch Histol Jpn. 1985 Feb;48(1):17–25. doi: 10.1679/aohc.48.17. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Gopalakrishnakone P., Tan C. K. Electron-microscopical study of the choroid plexus and epiplexus cells in cats following a cisternal injection of crotoxin complex. Acta Anat (Basel) 1988;131(3):241–248. doi: 10.1159/000146523. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Scanning electron microscopic study of epiplexus cells in the lateral ventricles of the monkey (Macaca fascicularis). J Anat. 1983 Dec;137(Pt 4):645–652. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Some aspects of amoeboid microglia in the corpus callosum and neighbouring regions of neonatal rats. J Anat. 1976 Feb;121(Pt 1):29–45. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Tseng C. Y., Voon F. C., Wong W. C. Isolation and culture of amoeboid microglial cells from the corpus callosum and cavum septum pellucidum in postnatal rats. J Anat. 1983 Sep;137(Pt 2):223–233. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Tseng C. Y., Wong W. C. An electron microscopical study of epiplexus and supraependymal cells in the prenatal rat brain following a maternal injection of 6-aminonicotinamide. J Anat. 1985 Jan;140(Pt 1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Ultrastruct and origin of epiplexus cells in the telencephalic choroid plexus of postnatal rats studied by intravenous injection of carbon particles. J Anat. 1979 Oct;129(Pt 3):479–492. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Ultrastructure and mode of formation of epiplexus cells in the choroid plexus in the lateral ventricles of the monkey (Macaca fascicularis). J Anat. 1981 Dec;133(Pt 4):555–569. [PMC free article] [PubMed] [Google Scholar]
- Malloy J. J., Low F. N. Scanning electron microscopy of the subarachnoid space in the dog. IV. Subarachnoid macrophages. J Comp Neurol. 1976 Jun 1;167(3):257–283. doi: 10.1002/cne.901670302. [DOI] [PubMed] [Google Scholar]
- Maxwell W. L., McGadey J. Response of intraventricular macrophages after a penetrant cerebral lesion. J Anat. 1988 Oct;160:145–155. [PMC free article] [PubMed] [Google Scholar]
- Merchant R. E. Scanning electron microscopy of epiplexus macrophages responding to challenge by bacillus Calmette-Guerin. Acta Neuropathol. 1979 Aug;47(3):183–188. doi: 10.1007/BF00690545. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969 Jan;135(1):57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L., Gauger G. E. Experimental hydrocephalus: surface alterations of the lateral ventricle. Scanning electron microscopic studies. Lab Invest. 1974 May;30(5):618–625. [PubMed] [Google Scholar]
- Parakkal P., Pinto J., Hanifin J. M. Surface morphology of human mononuclear phagocytes during maturation and phagocytosis. J Ultrastruct Res. 1974 Aug;48(2):216–226. doi: 10.1016/s0022-5320(74)80078-x. [DOI] [PubMed] [Google Scholar]
- Peters A. The surface fine structure of the choroid plexus and ependymal lining of the rat lateral ventricle. J Neurocytol. 1974 Mar;3(1):99–108. doi: 10.1007/BF01111935. [DOI] [PubMed] [Google Scholar]
- Polliack A., Gordon S. Scanning electron microscopy of murine macrophages. Surface characteristics during maturation, activation, and phagocytosis. Lab Invest. 1975 Nov;33(5):469–477. [PubMed] [Google Scholar]
- Polliack A. The contribution of scanning electron microscopy in haematology: its role in defining leucocyte and erythrocyte disorders. J Microsc. 1981 Aug;123(Pt 2):177–187. doi: 10.1111/j.1365-2818.1981.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A light microscopic and scanning electron microscopic study of intraventricular macrophages in the brains of aged mice. J Anat. 1983 Jun;136(Pt 4):761–771. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A semithin light microscopic, transmission electron microscopic and scanning electron microscopic study of macrophages in the lateral ventricle of mice from embryonic to adult life. J Anat. 1979 Aug;129(Pt 1):31–44. [PMC free article] [PubMed] [Google Scholar]
- Tseng C. Y., Ling E. A., Wong W. C. Scanning electron microscopy of amoeboid microglial cells in the transient cavum septum pellucidum in pre- and postnatal rats. J Anat. 1983 Mar;136(Pt 2):251–263. [PMC free article] [PubMed] [Google Scholar]
- Walsh R. J., Brawer J. R., Lin P. S. Supraependymal cells in the third ventricle of the neonatal rat. Anat Rec. 1978 Feb;190(2):257–269. doi: 10.1002/ar.1091900209. [DOI] [PubMed] [Google Scholar]
- Warfel A. H., Elberg S. S. Macrophage membranes viewed through a scanning electron microscope. Science. 1970 Oct 23;170(3956):446–447. doi: 10.1126/science.170.3956.446. [DOI] [PubMed] [Google Scholar]