Abstract
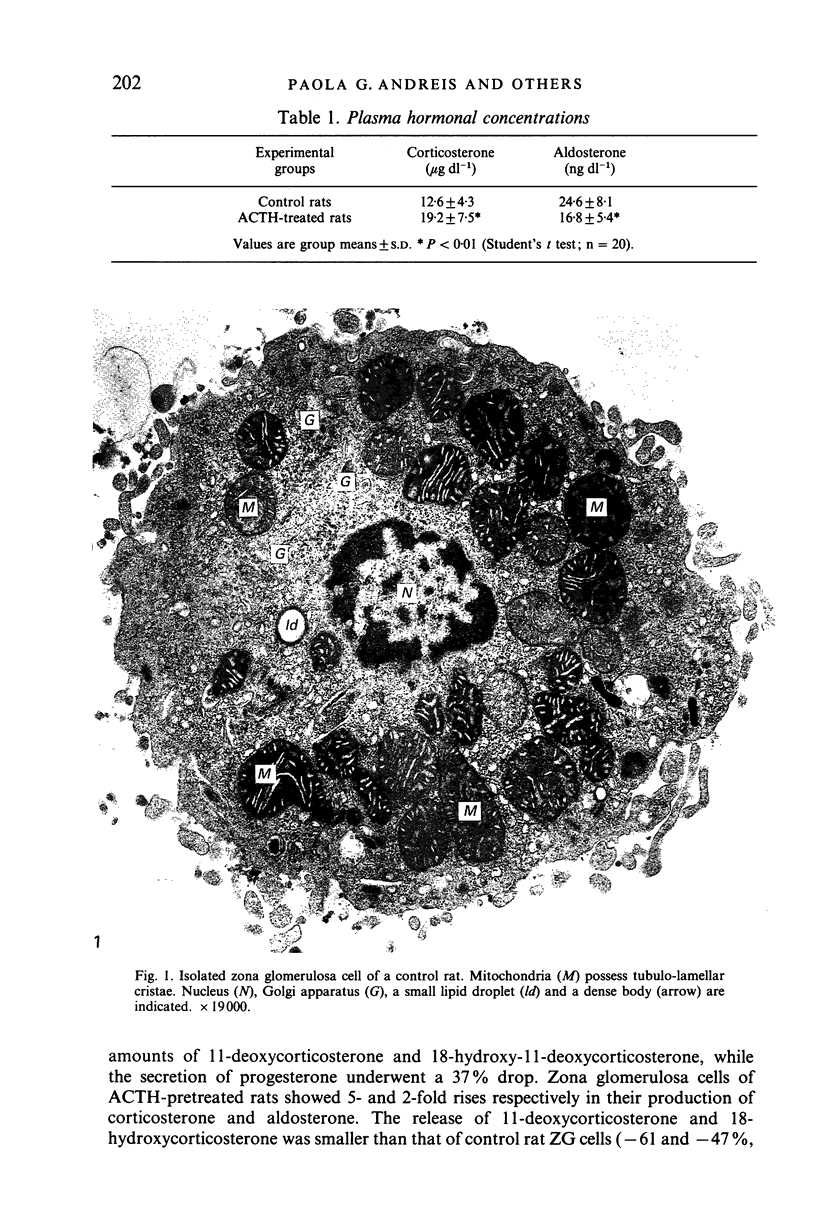
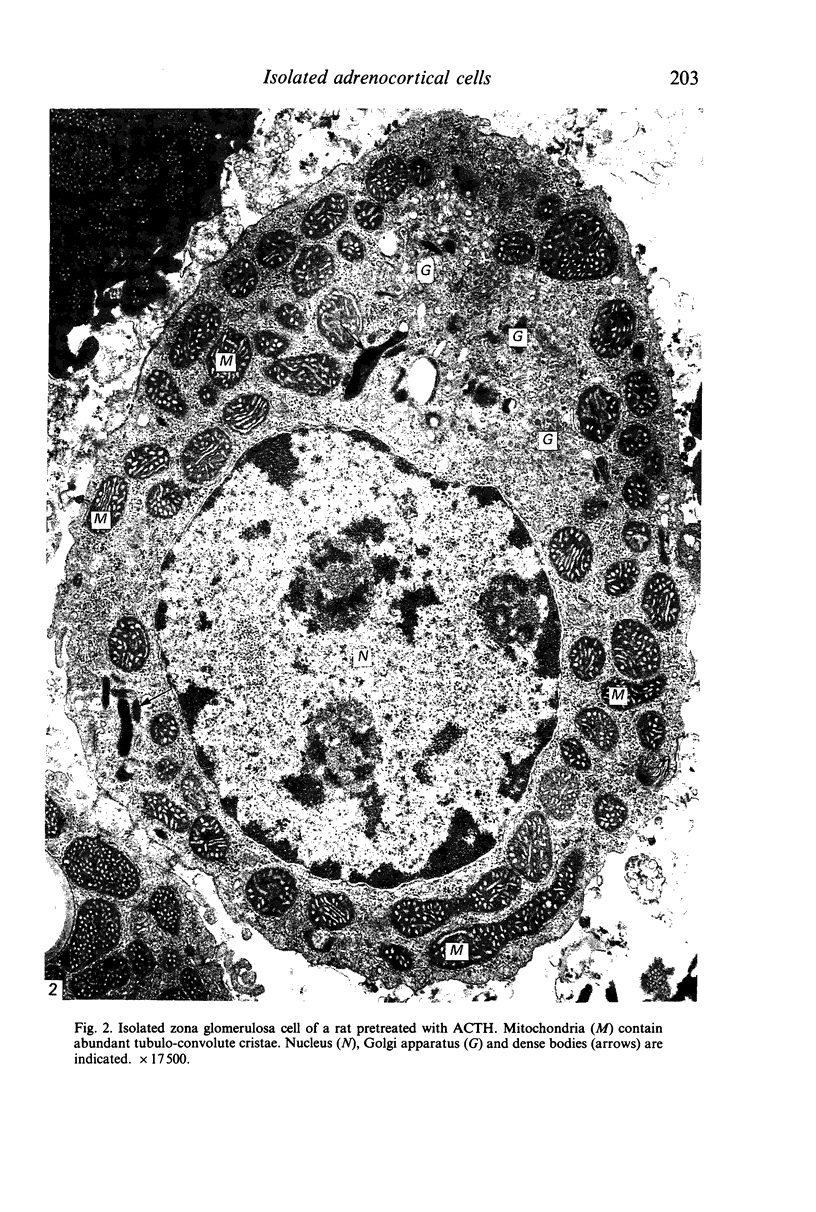
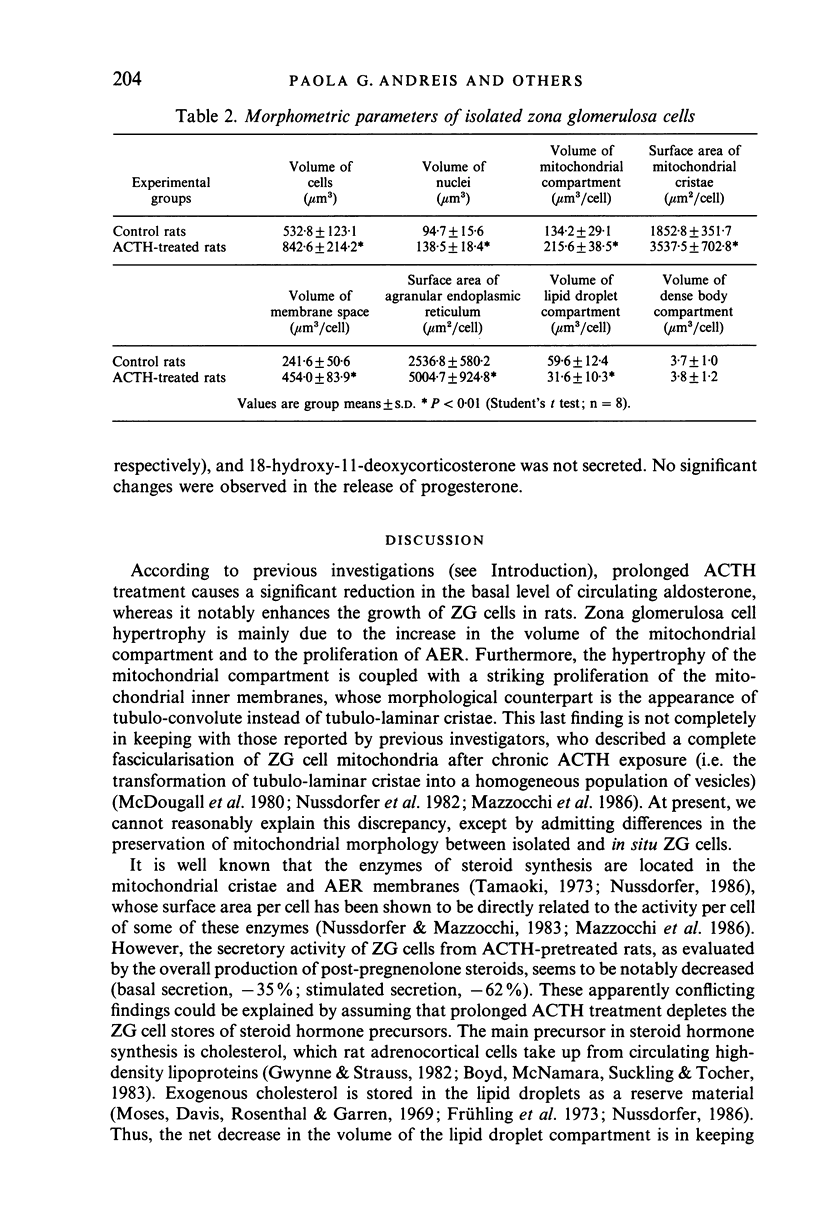
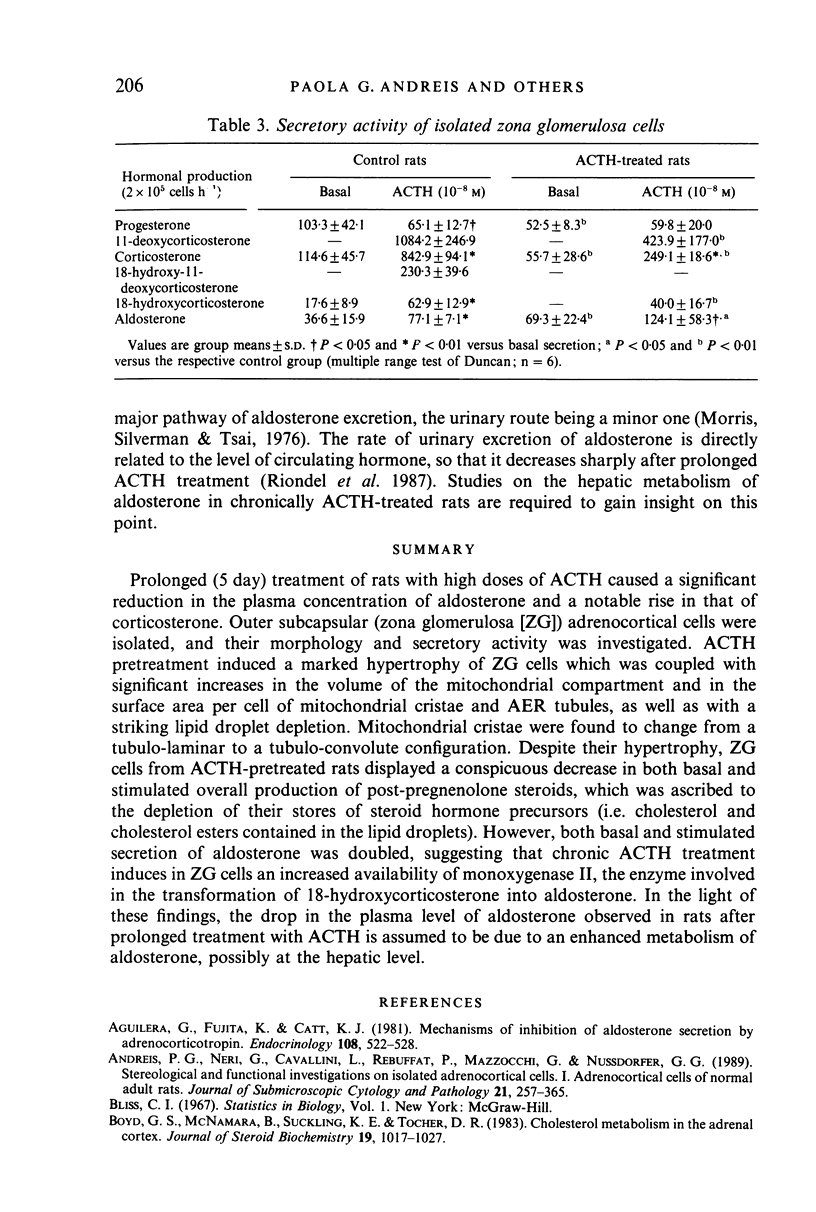
Prolonged (5 day) treatment of rats with high doses of ACTH caused a significant reduction in the plasma concentration of aldosterone and a notable rise in that of corticosterone. Outer subcapsular (zona glomerulosa [ZG]) adrenocortical cells were isolated, and their morphology and secretory activity was investigated. ACTH pretreatment induced a marked hypertrophy of ZG cells which was coupled with significant increases in the volume of the mitochondrial compartment and in the surface area per cell of mitochondrial cristae and AER tubules, as well as with a striking lipid droplet depletion. Mitochondrial cristae were found to change from a tubulo-laminar to a tubulo-convolute configuration. Despite their hypertrophy, ZG cells from ACTH-pretreated rats displayed a conspicuous decrease in both basal and stimulated overall production of post-pregnenolone steroids, which was ascribed to the depletion of their stores of steroid hormone precursors (i.e. cholesterol and cholesterol esters contained in the lipid droplets). However, both basal and stimulated secretion of aldosterone was doubled, suggesting that chronic ACTH treatment induces in ZG cells an increased availability of monoxygenase II, the enzyme involved in the transformation of 18-hydroxycorticosterone into aldosterone. In the light of these findings, the drop in the plasma level of aldosterone observed in rats after prolonged treatment with ACTH is assumed to be due to an enhanced metabolism of aldosterone, possibly at the hepatic level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Fujita K., Catt K. J. Mechanisms of inhibition of aldosterone secretion by adrenocorticotropin. Endocrinology. 1981 Feb;108(2):522–528. doi: 10.1210/endo-108-2-522. [DOI] [PubMed] [Google Scholar]
- Andreis P. G., Neri G., Cavallini L., Rebuffat P., Mazzocchi G., Nussdorfer G. G. Stereological and functional investigations on isolated adrenocortical cells. I. Adrenocortical cells of normal adult rats. J Submicrosc Cytol Pathol. 1989 Apr;21(2):357–365. [PubMed] [Google Scholar]
- Boyd G. S., McNamara B., Suckling K. E., Tocher D. R. Cholesterol metabolism in the adrenal cortex. J Steroid Biochem. 1983 Jul;19(1C):1017–1027. doi: 10.1016/0022-4731(83)90048-1. [DOI] [PubMed] [Google Scholar]
- Frühling J., Sand G., Penasse W., Pecheux F., Claude A. Corrélation entre la morphologie et le contenu lipidique des corticosurrénales du cobaye, du rat et du boeuf. J Ultrastruct Res. 1973 Jul;44(1):113–133. doi: 10.1016/s0022-5320(73)90045-2. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Strauss J. F., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982 Summer;3(3):299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G., Malendowicz L. K., Rebuffat P., Robba C., Gottardo G., Nussdorfer G. G. Short- and long-term effects of ACTH on the adrenal zona glomerulosa of the rat. A coupled stereological and enzymological study. Cell Tissue Res. 1986;243(2):303–310. doi: 10.1007/BF00251044. [DOI] [PubMed] [Google Scholar]
- McDougall J. G., Butkus A., Coghlan J. P., Denton D. A., Müller J., Oddie C. J., Robinson P. M., Scoggins B. A. Biosynthetic ad morphological evidence for inhibition of aldosterone production following administration of ACTH to sheep. Acta Endocrinol (Copenh) 1980 Aug;94(4):559–570. doi: 10.1530/acta.0.0940559. [DOI] [PubMed] [Google Scholar]
- Morris D. J., Silverman A., Tsai R. Fecal and urinary excretion of [3H]-aldosterone and its sex dependence in rats. J Steroid Biochem. 1976 Aug;7:561–564. doi: 10.1016/0022-4731(76)90077-7. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Davis W. W., Rosenthal A. S., Garren L. D. Adrenal cholesterol: localization by electron-microscope autoradiography. Science. 1969 Mar 14;163(3872):1203–1205. doi: 10.1126/science.163.3872.1203. [DOI] [PubMed] [Google Scholar]
- Müller J. Suppression of aldosterone biosynthesis by treatment of rats with adrenocorticotropin: comparison with glucocorticoid effects. Endocrinology. 1978 Dec;103(6):2061–2068. doi: 10.1210/endo-103-6-2061. [DOI] [PubMed] [Google Scholar]
- Nussdorfer G. G. Cytophysiology of the adrenal cortex. Int Rev Cytol. 1986;98:1–405. [PubMed] [Google Scholar]
- Nussdorfer G. G., Mazzocchi G. Long-term effects of ACTH on rat adrenocortical cells: a coupled stereological and enzymological study. J Steroid Biochem. 1983 Dec;19(6):1753–1756. doi: 10.1016/0022-4731(83)90354-0. [DOI] [PubMed] [Google Scholar]
- Nussdorfer G. G., Mazzocchi G., Rebuffat P. An ultrastructural stereologic study of the effects of ACTH and adenosine 3',5'-cyclic monophosphate on the zona glomerulosa of rat adrenal cortex. Endocrinology. 1973 Jan;92(1):141–151. doi: 10.1210/endo-92-1-141. [DOI] [PubMed] [Google Scholar]
- Nussdorfer G. G., Mazzocchi G., Robba C., Belloni A. S., Rebuffat P. Effects of ACTH and dexamethasone on the zona glomerulosa of the rat adrenal cortex: an ultrastructural stereologic study. Acta Endocrinol (Copenh) 1977 Jul;85(3):608–614. doi: 10.1530/acta.0.0850608. [DOI] [PubMed] [Google Scholar]
- Nussdorfer G. G., Neri G., Belloni A. S., Mazzocchi G., Rebuffat P., Robba C. Effects of ACTH on the zona glomerulosa of sodium-loaded timolol maleate-treated rats: stereology and plasma hormone concentrations. Acta Endocrinol (Copenh) 1982 Feb;99(2):256–262. doi: 10.1530/acta.0.0990256. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Nice E. C., Magee-Brown R., Bullman H. High-pressure liquid chromatography of steroids secreted by human adrenal and testis cells in monolayer culture. J Chromatogr. 1976 Sep 29;125(1):357–367. doi: 10.1016/s0021-9673(00)93831-7. [DOI] [PubMed] [Google Scholar]
- Riondel A. M., Rebuffat P., Mazzochi G., Nussdorfer G. G., Gaillard R. C., Bockhorn L., Nussberger J., Vallotton M. B., Muller A. F. Long-term effects of ACTH combined with angiotensin II on steroidogenesis and adrenal zona glomerulosa morphology in the rat. Acta Endocrinol (Copenh) 1987 Jan;114(1):47–54. doi: 10.1530/acta.0.1140047. [DOI] [PubMed] [Google Scholar]
- Sippell W. G., Bidlingmaier F., Becker H., Brünig T., Dörr H., Hahn H., Golder W., Hollmann G., Knorr D. Simultaneous radioimmunoassay of plasma aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, 11-deoxycortisol, cortisol and cortisone. J Steroid Biochem. 1978 Jan;9(1):63–74. doi: 10.1016/0022-4731(78)90104-8. [DOI] [PubMed] [Google Scholar]
- Szabó D., Szalay K. S., Tóth I. E. Correlation of lipid droplet content and steroidogenic capacity in zona glomerulosa and fasciculata cells from lipoprotein-deficient rats. Mol Cell Endocrinol. 1984 Jan;34(1):59–66. doi: 10.1016/0303-7207(84)90159-x. [DOI] [PubMed] [Google Scholar]
- Szalay K. S. Effect of pituitary intermediate lobe extract on steroid production by the isolated zona glomerulosa and fasciculata cells. Acta Physiol Acad Sci Hung. 1981;57(3):225–231. [PubMed] [Google Scholar]
- Tait J. F., Tait S. A., Gould R. P., Mee M. S. The properties of adrenal zona glomerulosa cells after purification by gravitational sedimentation. Proc R Soc Lond B Biol Sci. 1974 Feb 26;185(1081):375–407. doi: 10.1098/rspb.1974.0025. [DOI] [PubMed] [Google Scholar]
- Tamaoki B. Steroidogenesis and cell structure. Biochemical pursuit of sites of steroid biosynthesis. J Steroid Biochem. 1973 Jan;4(1):89–118. doi: 10.1016/0022-4731(73)90084-8. [DOI] [PubMed] [Google Scholar]
- Vazir H., Whitehouse B. J., Vinson G. P., McCredie E. Effects of prolonged ACTH treatment on adrenal steroidogenesis and blood pressure in rats. Acta Endocrinol (Copenh) 1981 Aug;97(4):533–542. doi: 10.1530/acta.0.0970533. [DOI] [PubMed] [Google Scholar]
- Vinson G. P., Hinson J. P., Raven P. W. The relationship between tissue preparation and function; methods for the study of control of aldosterone secretion: a review. Cell Biochem Funct. 1985 Oct;3(4):235–253. doi: 10.1002/cbf.290030402. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Paumgartner D. Integrated stereological and biochemical studies on hepatocytic membranes. II. Correction of section thickness effect on volume and surface density estimates. J Cell Biol. 1978 May;77(2):584–597. doi: 10.1083/jcb.77.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]