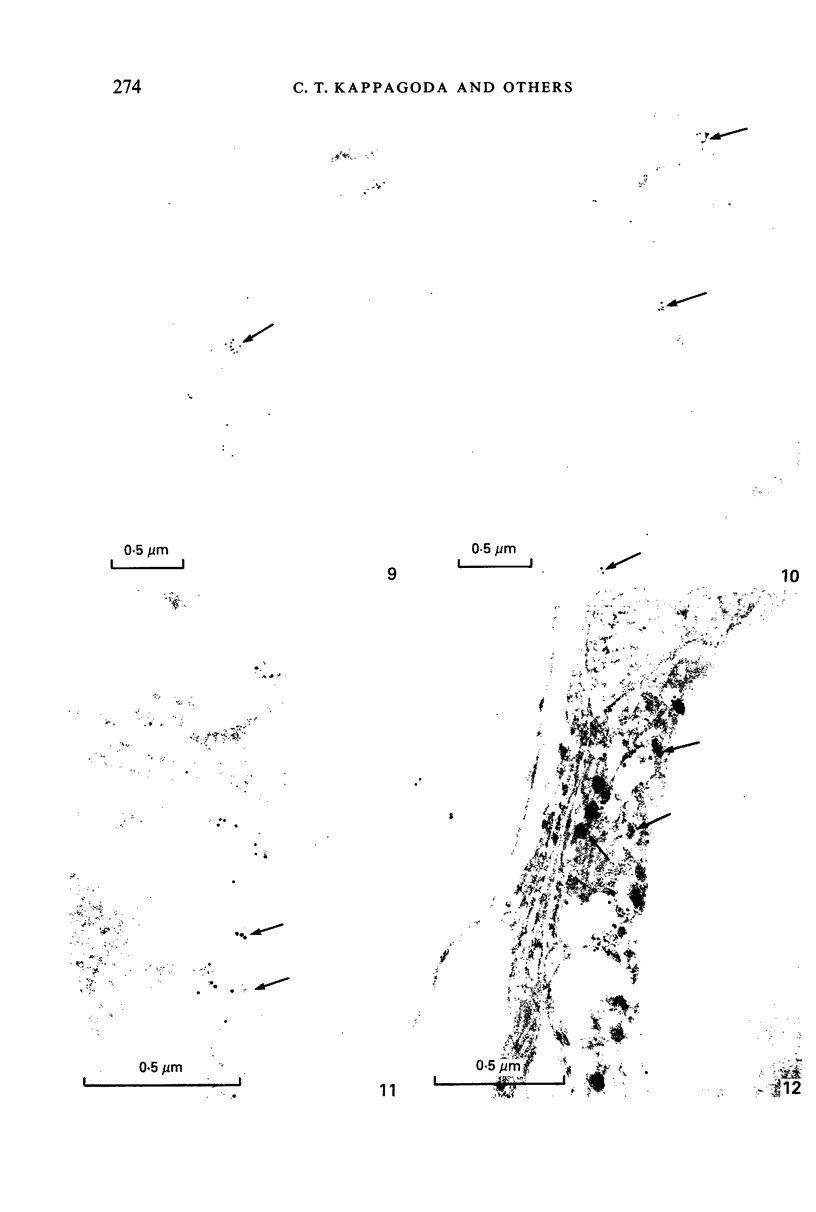
Abstract
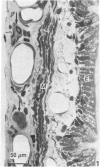
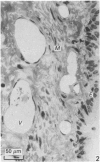
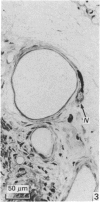
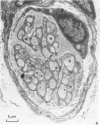
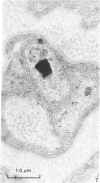
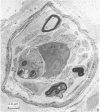
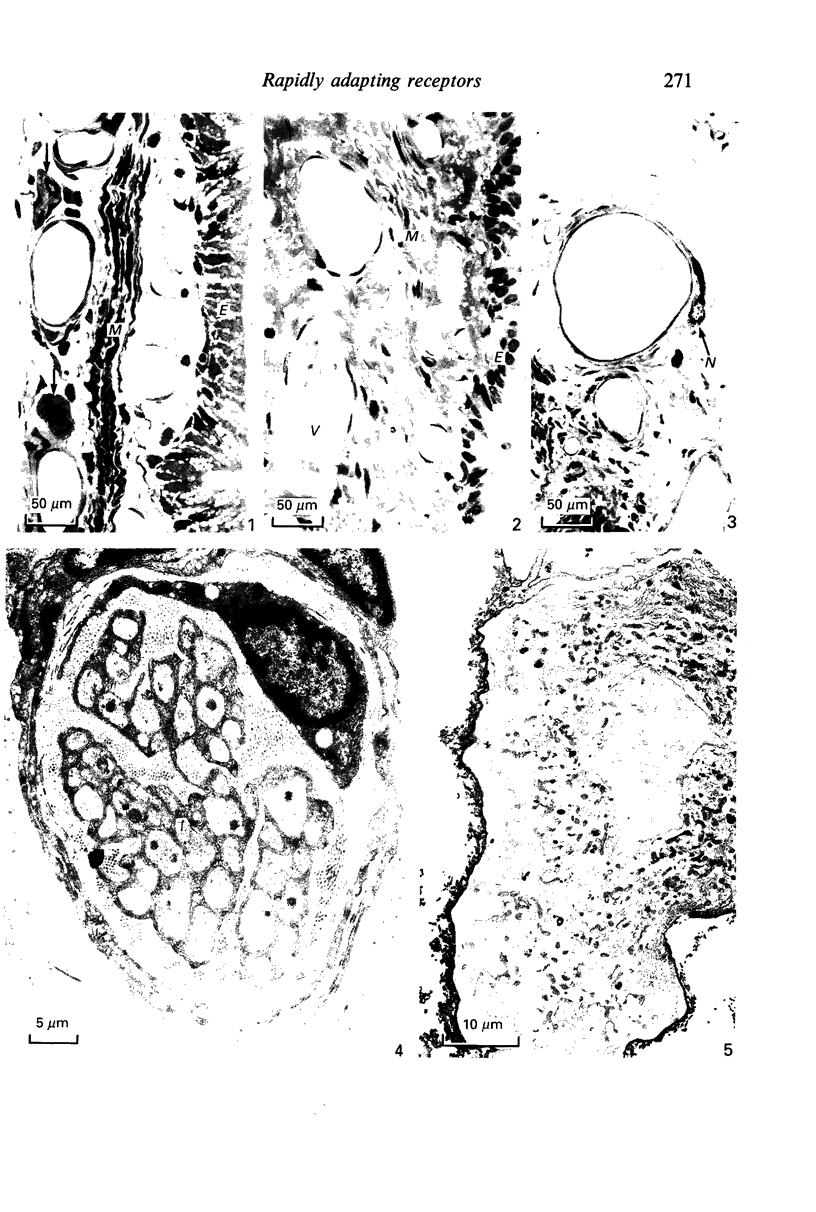
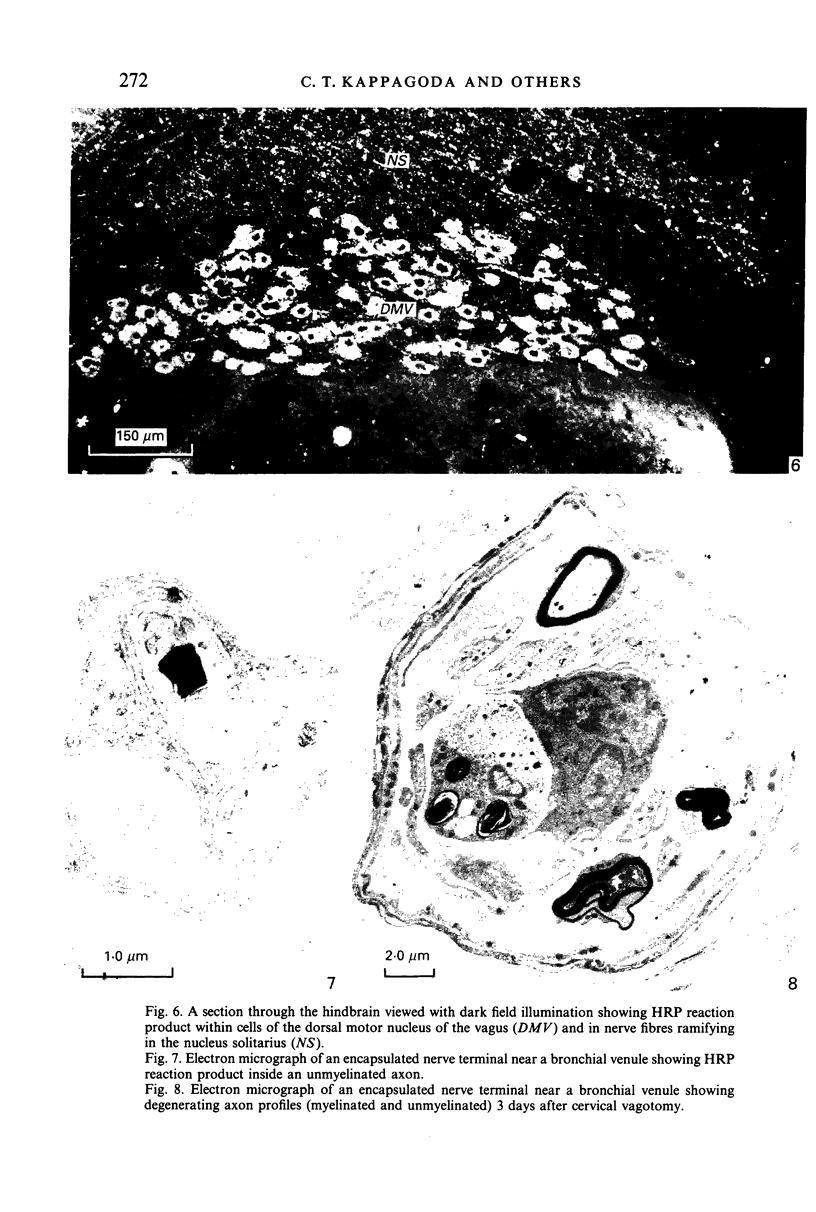
The present investigation was undertaken in rats to determine whether sensory nerves exist in apposition to the bronchial microvessels which may function as rapidly adapting receptors (RAR). The primary and secondary bronchi on both sides were removed and processed for light and electron microscopy. Nerves were frequently found in relation to venules external to the muscle coat of bronchi. They comprised myelinated axons which ended individually as non-myelinated convoluted terminals enclosed within a loose capsule of attenuated cells. Serial sections showed that these terminals were not related to ganglion cells. Cervical vagal section and injection of HRP-WGA into the nodose ganglion provided corroborative evidence of the sensory nature of these terminals. Vagal section caused degenerative changes in the encapsulated nerve terminals in the bronchial walls and horseradish peroxidase labelling was demonstrable in such terminals. Moreover, immunocytochemical studies demonstrated the presence of calcitonin gene regulated peptide and substance P in these structures. It is suggested that they comprise the RAR. Encapsulated nerve terminals were not found in the epithelial layer, in the submucous coat or in the muscularis of bronchi.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Luck J. C. A comparative study of irritant and type J receptors in the cat. Respir Physiol. 1974 Jul;21(1):47–60. doi: 10.1016/0034-5687(74)90006-1. [DOI] [PubMed] [Google Scholar]
- Cadieux A., Springall D. R., Mulderry P. K., Rodrigo J., Ghatei M. A., Terenghi G., Bloom S. R., Polak J. M. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: effect of capsaicin treatment and surgical denervations. Neuroscience. 1986 Oct;19(2):605–627. doi: 10.1016/0306-4522(86)90285-x. [DOI] [PubMed] [Google Scholar]
- Das R. M., Jeffrey P. K., Widdicombe J. G. The epithelial innervation of the lower respiratory tract of the cat. J Anat. 1978 May;126(Pt 1):123–131. [PMC free article] [PubMed] [Google Scholar]
- El-Bermani A. W., Chang T. L. Cobalt iontophoresis of sensory nerves in the rat lung. Am J Anat. 1979 Feb;154(2):277–281. doi: 10.1002/aja.1001540211. [DOI] [PubMed] [Google Scholar]
- Fahim M., Jain S. K. The effect of bupivacaine aerosol on the activity of pulmonary stretch and 'irritant' receptors. J Physiol. 1979 Mar;288:367–378. [PMC free article] [PubMed] [Google Scholar]
- Hoyes A. D., Barber P. Morphology and response to vagus nerve section of the intra-epithelial axons of the rat trachea. A quantitative ultrastructural study. J Anat. 1981 May;132(Pt 3):331–339. [PMC free article] [PubMed] [Google Scholar]
- Jeffery P., Reid L. Intra-epithelial nerves in normal rat airways: a quantitative electron microscopic study. J Anat. 1973 Jan;114(Pt 1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Joosten E. A., Gribnau A. A., Dederen P. J. Ultrastructural visualization of anterogradely transported horseradish peroxidase in developing corticospinal tract of rat. J Histochem Cytochem. 1987 May;35(5):623–626. doi: 10.1177/35.5.2435786. [DOI] [PubMed] [Google Scholar]
- Kappagoda C. T., Man G. C., Teo K. K. Behaviour of canine pulmonary vagal afferent receptors during sustained acute pulmonary venous pressure elevation. J Physiol. 1987 Dec;394:249–265. doi: 10.1113/jphysiol.1987.sp016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs J. M. Morphology of presumptive slowly adapting receptors in dog trachea. Anat Rec. 1984 Sep;210(1):73–85. doi: 10.1002/ar.1092100111. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Van Lommel A. Ultrastructure of nerve endings and synaptic junctions in rabbit intrapulmonary neuroepithelial bodies: a single and serial section analysis. J Anat. 1987 Apr;151:65–83. [PMC free article] [PubMed] [Google Scholar]
- Mortola J., Sant'Ambrogio G., Clement M. G. Localization of irritant receptors in the airways of the dog. Respir Physiol. 1975 Jun;24(1):107–114. doi: 10.1016/0034-5687(75)90125-5. [DOI] [PubMed] [Google Scholar]
- Ravi K., Teo K. K., Kappagoda C. T. Stimulation of rapidly adapting pulmonary stretch receptors by pulmonary lymphatic obstruction in dogs. Can J Physiol Pharmacol. 1988 May;66(5):630–636. doi: 10.1139/y88-098. [DOI] [PubMed] [Google Scholar]
- Sampson S. R., Vidruk E. H. Properties of 'irritant' receptors in canine lung. Respir Physiol. 1975 Oct;25(1):9–22. doi: 10.1016/0034-5687(75)90047-x. [DOI] [PubMed] [Google Scholar]
- Skepper J. N., Navaratnam V. Analysis of the apparent heterogeneity of specific heart granules in rat atrial myocytes; an ultrastructural study including immunocytochemistry. Histochem J. 1988 Jan;20(1):1–10. doi: 10.1007/BF01745963. [DOI] [PubMed] [Google Scholar]
- Terenghi G., McGregor G. P., Bhuttacharji S., Wharton J., Bloom S. R., Polak J. M. Vagal origin of substance P-containing nerves in the guinea pig lung. Neurosci Lett. 1983 Apr 29;36(3):229–235. doi: 10.1016/0304-3940(83)90005-8. [DOI] [PubMed] [Google Scholar]