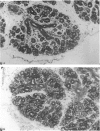
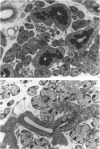
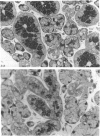
Abstract
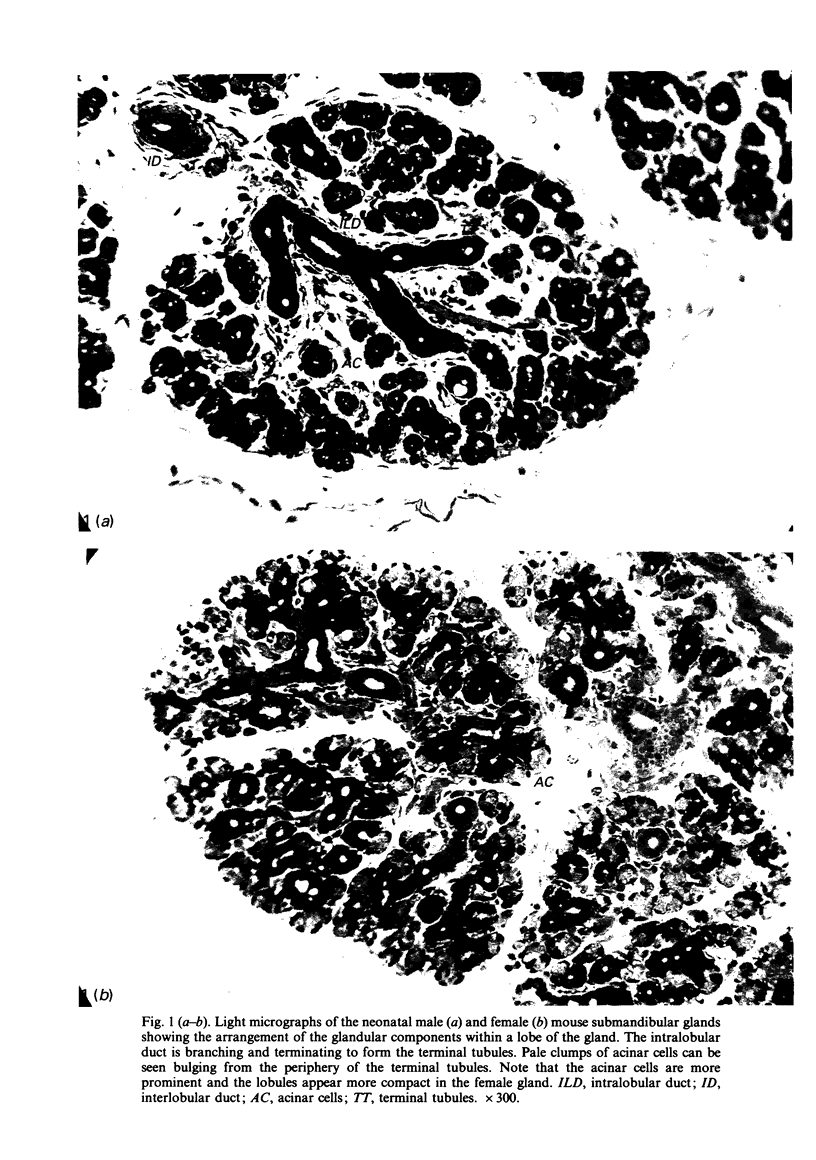
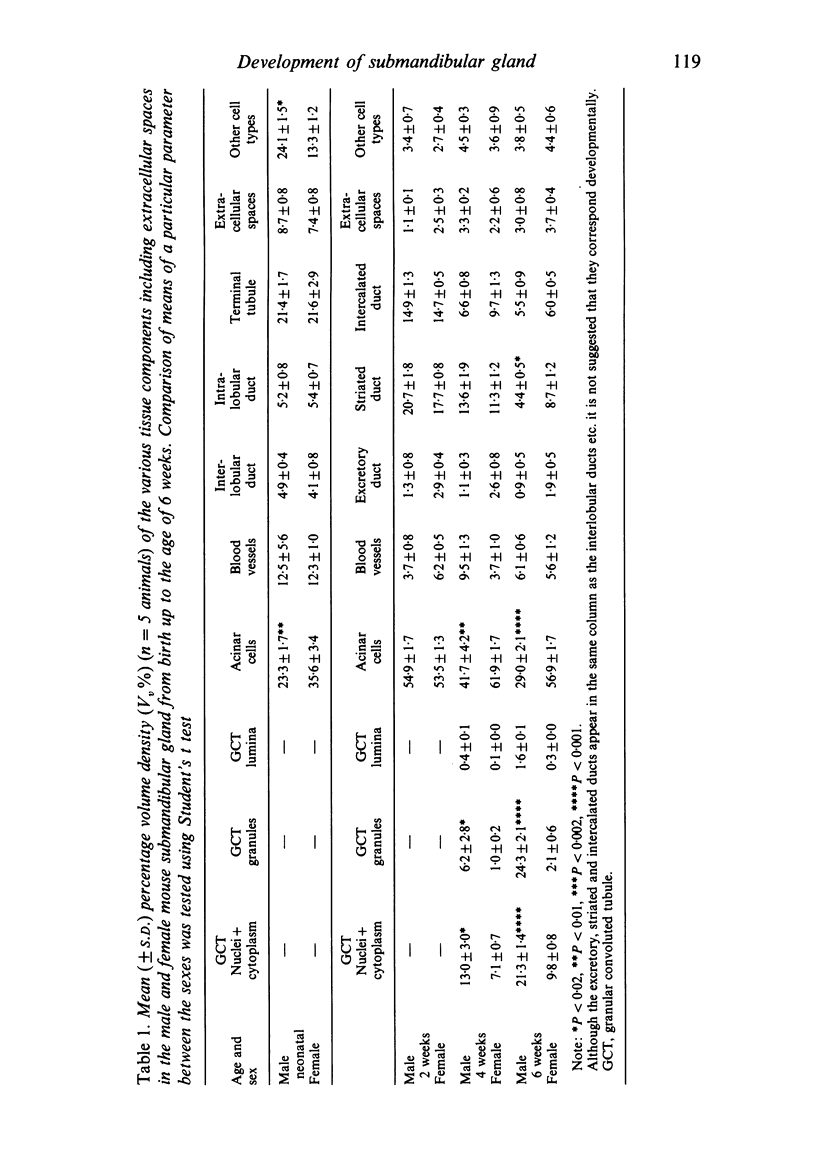
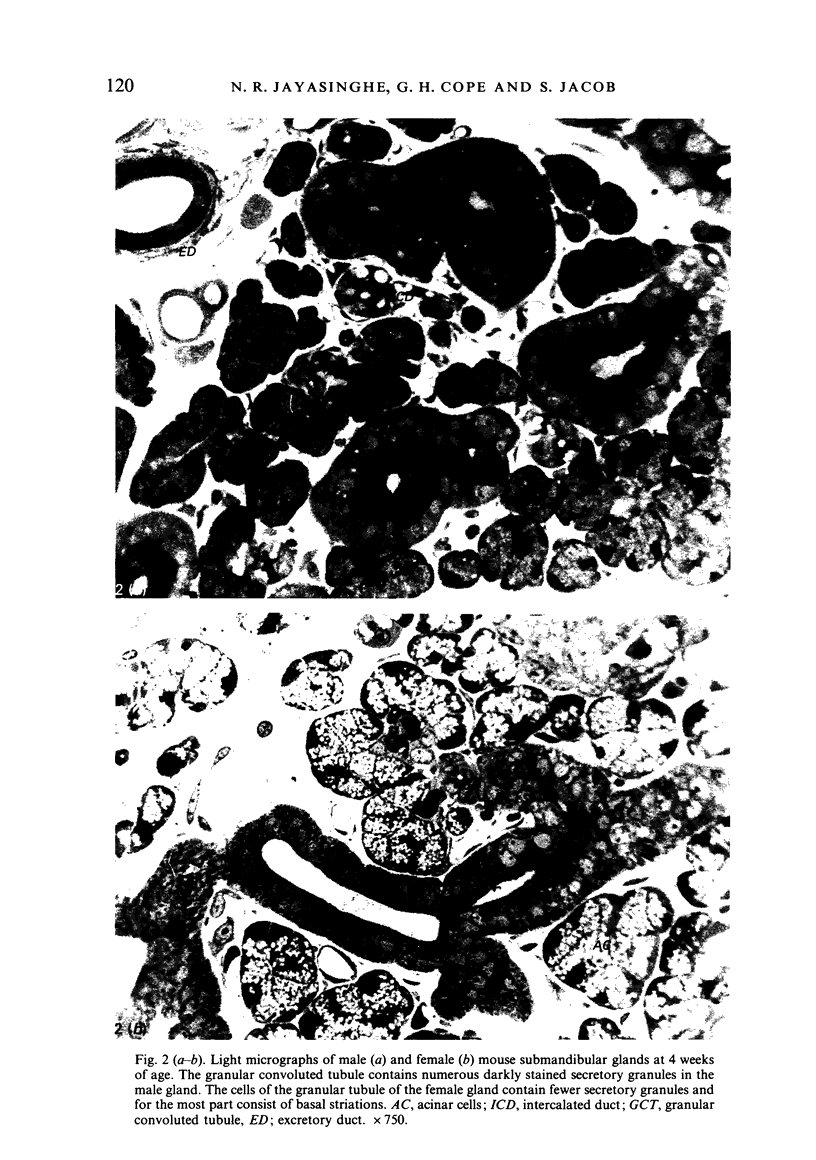
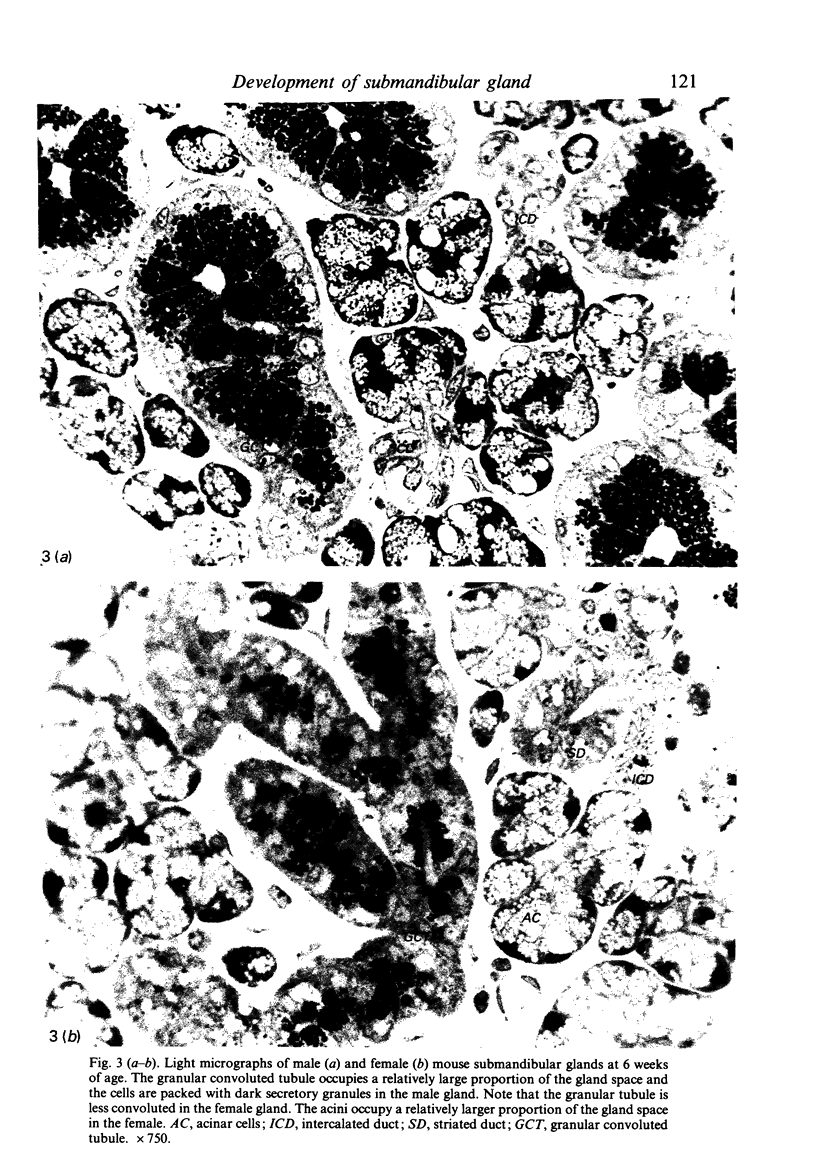
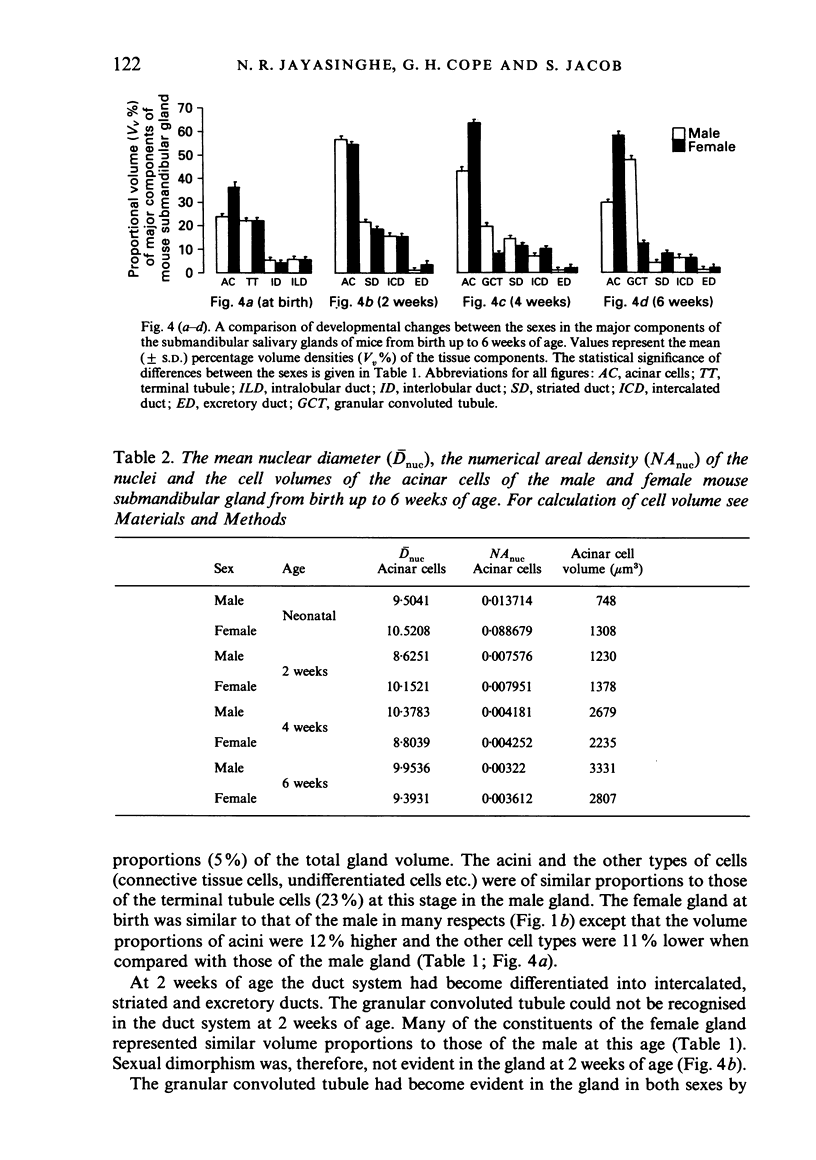
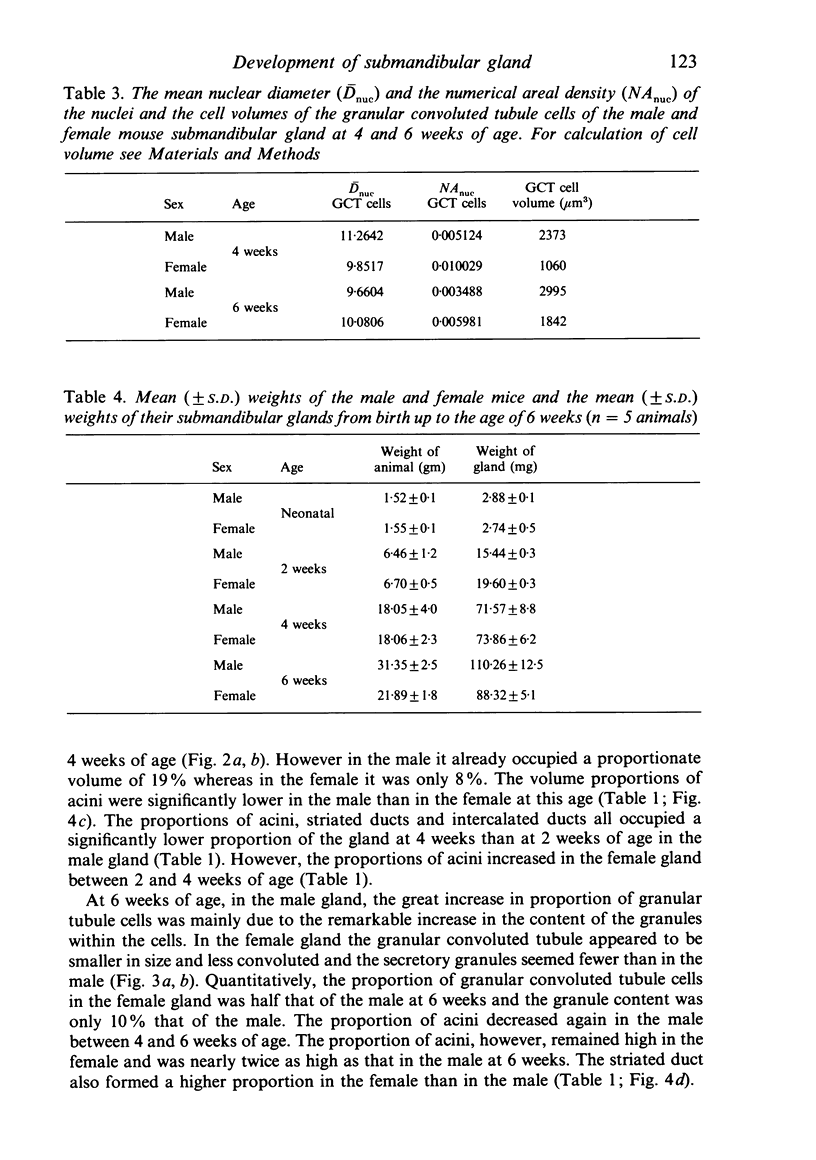
A light microscopic morphometric analysis of the development of the mouse submandibular gland has been carried out from birth up to the age of 6 weeks. At birth the bulk of the gland consists of approximately equal volume proportions of acinar, terminal tubule and non-secretory cells. The granular convoluted tubule is absent at birth. The neonatal female gland resembles that of the male in many respects. With the regression of the terminal tubule at 2 weeks of age the duct system of the gland is seen to differentiate into excretory, striated and intercalated ducts. The volume proportions of the gland constituents of the female are similar to those of the male at 2 weeks. At this age, the acini occupy 55%, the striated duct 20% and the intercalated duct 15% of the total gland volume. Sexual dimorphism is clearly evident in the gland at 4 weeks of age when the duct system is seen to differentiate to form its granular convoluted tubule component. The granular tubule occupied 19% of the gland volume in the male but only 8% in the female at 4 weeks. The proportions of acini are only 41% in the total gland volume of the male mouse but 62% in the female at 4 weeks. In the male gland the proportions of granular convoluted tubule increase from 13% to 21% between 4 and 6 weeks and the secretory granule content of these cells from 6% to 24%. At 6 weeks of age the volume proportion of granular convoluted tubule in the male is 45% and that in the female is only 12%. At this age the acini occupy a proportion of 30% in the male gland as opposed to 57% in the female gland. At 6 weeks the volume of granular convoluted tubule cells is 40% lower in the female (1842 microns 3) than in the male gland (2995 microns 3).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980 Aug;28(8):836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Barthe P. L., Bullock L. P., Mowszowicz I., Bardin C. W., Orth D. N. Submaxillary gland epidermal growth factor: a sensitive index of biologic androgen activity. Endocrinology. 1974 Oct;95(4):1019–1025. doi: 10.1210/endo-95-4-1019. [DOI] [PubMed] [Google Scholar]
- Booth W. D. Metabolism of androgens in vitro by the submaxillary salivary gland of the mature domestic boar. J Endocrinol. 1977 Oct;75(1):145–154. doi: 10.1677/joe.0.0750145. [DOI] [PubMed] [Google Scholar]
- Bower A. J. A simple, inexpensive apparatus to allow sequential perfusion of an animal several different fluids without an intervening drop in perfusion pressure. J Microsc. 1981 Apr;122(Pt 1):103–104. doi: 10.1111/j.1365-2818.1981.tb01248.x. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Caramia F. Ultrastructure of mouse submaxillary gland. I. Sexual differences. J Ultrastruct Res. 1966 Dec;16(5):505–523. doi: 10.1016/s0022-5320(66)80003-5. [DOI] [PubMed] [Google Scholar]
- Chrétien M. Action of testosterone on the differentiation and secretory activity of a target organ: the submaxillary gland of the mouse. Int Rev Cytol. 1977;50:333–396. doi: 10.1016/s0074-7696(08)60101-1. [DOI] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disher L., Elias J. J. The effect of testosterone injections on submaxillary salivary gland structure of castrated mature and immature male mice. J Oral Ther Pharmacol. 1967 Sep;4(2):128–139. [PubMed] [Google Scholar]
- Elder J. B., Williams G., Lacey E., Gregory H. Cellular localisation of human urogastrone/epidermal growth factor. Nature. 1978 Feb 2;271(5644):466–467. doi: 10.1038/271466a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M. N., BURDMAN J. A. STUDIES OF THE NERVE GROWTH FACTOR IN SUBMANDIBULAR GLANDS OF FEMALE MICE TREATED WITH TESTOSTERONE. Anat Rec. 1965 Feb;151:199–207. doi: 10.1002/ar.1091510210. [DOI] [PubMed] [Google Scholar]
- Gresik E. W. Postnatal developmental changes in submandibular glands of rats and mice. J Histochem Cytochem. 1980 Aug;28(8):860–870. doi: 10.1177/28.8.6160181. [DOI] [PubMed] [Google Scholar]
- Gresik E. W. The postnatal development of the sexually dimorphic duct system and of amylase activity in the submandibular glands of mice. Cell Tissue Res. 1975;157(3):411–422. doi: 10.1007/BF00225529. [DOI] [PubMed] [Google Scholar]
- Jones R. O. The in vitro effect of epithelial growth factor on rat organ cultures. Exp Cell Res. 1966 Oct;43(3):645–656. doi: 10.1016/0014-4827(66)90036-x. [DOI] [PubMed] [Google Scholar]
- Kaiho M., Nakamura T., Kumegawa M. Morphological studies on the synthesis of secretory granules in convoluted tubules of mouse submandibular gland. Anat Rec. 1975 Nov;183(3):405–419. doi: 10.1002/ar.1091830305. [DOI] [PubMed] [Google Scholar]
- Kasselberg A. G., Orth D. N., Gray M. E., Stahlman M. T. Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem. 1985 Apr;33(4):315–322. doi: 10.1177/33.4.3884705. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Hendry I., Short R. V. The submaxillary salivary glands as test organs for response to androgen in mice with testicular feminization. J Endocrinol. 1973 Sep;58(3):357–362. doi: 10.1677/joe.0.0580357. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Watson A. Y., McCarthy M., Papastavros M., Neutra M., Forssmann W. G. Submandibular glands in mice with muscular dystrophy: studies with nerve growth factor. Anat Rec. 1981 Jun;200(2):177–194. doi: 10.1002/ar.1092000207. [DOI] [PubMed] [Google Scholar]
- Poulsen S. S., Nexø E., Olsen P. S., Hess J., Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85(5):389–394. doi: 10.1007/BF00982668. [DOI] [PubMed] [Google Scholar]
- Reed H. C., Melrose D. R., Patterson R. L. Androgen steroids as an aid to the detection of oestrus in pig artificial insemination. Br Vet J. 1974 Jan-Feb;130(1):61–67. doi: 10.1016/s0007-1935(17)35991-2. [DOI] [PubMed] [Google Scholar]
- Rogers A. W., Brown-Grant K. The effects of castration on the ultrastructure and the iodide-concentrating ability of mouse submaxillary salivary glands. J Anat. 1971 May;109(Pt 1):51–62. [PMC free article] [PubMed] [Google Scholar]
- Schwab M. E., Stöckel K., Thoenen H. Immunocytochemical localization of nerve growth factor (NGF) in the submandibular gland of adult mice by light and electron microscopy. Cell Tissue Res. 1976 Jun 28;169(3):289–299. doi: 10.1007/BF00219602. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Frommer J. Quantitative morphology and carbohydrate histochemistry of the mouse submandibular gland following prepubertal castration. Am J Anat. 1975 Oct;144(2):137–147. doi: 10.1002/aja.1001440203. [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Chang W. W. The postnatal development of the submandibular gland of the mouse. Cell Tissue Res. 1979 May 18;198(2):363–371. doi: 10.1007/BF00232017. [DOI] [PubMed] [Google Scholar]
- Watson A. Y., Radie K., McCarthy M., Larsen P. R., Murphy R. A. Thyroxine reverses deficits of nerve growth factor and epidermal growth factor in submandibular glands of mice with muscular dystrophy. Endocrinology. 1982 Apr;110(4):1392–1401. doi: 10.1210/endo-110-4-1392. [DOI] [PubMed] [Google Scholar]
- Yohro T. Development of secretory units of mouse submandibular gland. Z Zellforsch Mikrosk Anat. 1970;110(2):173–184. doi: 10.1007/BF00335523. [DOI] [PubMed] [Google Scholar]