Abstract
We recently proposed that extracellular Ca2+ ions participate in a novel form of intercellular communication involving the extracellular Ca2+-sensing receptor (CaR). Here, using Ca2+-selective microelectrodes, we directly measured the profile of agonist-induced [Ca2+]ext changes in restricted domains near the basolateral or luminal membranes of polarized gastric acid-secreting cells. The Ca2+-mobilizing agonist carbachol elicited a transient, La3+-sensitive decrease in basolateral [Ca2+] (average ≈250 µM, but as large as 530 µM). Conversely, carbachol evoked an HgCl2-sensitive increase in [Ca2+] (average ≈400 µM, but as large as 520 µM) in the lumen of single gastric glands. Both responses were significantly reduced by pre-treatment with sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) pump inhibitors or with the intracellular Ca2+ chelator BAPTA-AM. Immunofluores cence experiments demonstrated an asymmetric localization of plasma membrane Ca2+ ATPase (PMCA), which appeared to be partially co-localized with CaR and the gastric H+/K+-ATPase in the apical membrane of the acid-secreting cells. Our data indicate that agonist stimulation results in local fluctuations in [Ca2+]ext that would be sufficient to modulate the activity of the CaR on neighboring cells.
Keywords: extracellular calcium microdomains/extracellular calcium-sensing receptor/intercellular signaling/PMCA/store-operated calcium channels
Introduction
It is indisputable that Ca2+ serves a fundamental role in signal transduction inside living cells (Bootman et al., 2001). Less importance has been given to the potential role of extracellular Ca2+ as a first messenger. In intact tissues, where extracellular fluid volumes are extremely limited compared with the cell volume, changes in intracellular [Ca2+] may also be predicted to have effects on extracellular [Ca2+], by virtue of pathways for cellular Ca2+ influx and efflux that become activated during intracellular signaling events. Specifically, it is well known that Ca2+ released from intracellular stores in response to Ca2+-mobilizing agonists is largely extruded from cells through the actions of the plasma membrane Ca2+ ATPase (PMCA; Muallem et al., 1988; Carafoli et al., 1989), and in some cell types, the Na+/Ca2+ exchanger (Sedova and Blatter, 1999). In addition, store emptying triggers the opening of store-operated Ca2+ entry pathways (SOCs) in the plasma membrane and the ensuing influx of Ca2+ may potentially result in localized depletion of extracellular [Ca2+]. Using a co-culture model system, we recently provided evidence that such extracellular [Ca2+] changes may be of sufficient magnitude to be detected by an extracellular Ca2+-sensing receptor (CaR; Hofer et al., 2000). This receptor, first described in the parathyroid gland (Brown et al., 1993), has since been identified in a variety of tissues and cell types, including neurons (Ruat et al., 1996), glia (Chattopadhyay et al., 1998b) and epithelia [e.g. lung (Ruat et al., 1996), pancreas (Bruce et al., 1999), ovary (McNeil et al., 1998), kidney (Riccardi et al., 1995), intestine (Chattopadhyay et al., 1998a) and stomach (Cheng et al., 1999)]; for review see Brown and MacLeod (2001).
Changes in extracellular [Ca2+] as a function of cellular activity have been recorded in a number of different tissue preparations using a variety of techniques. For example, Ca2+-sensitive metallochromic absorbance indicators have been used to measure external [Ca2+] in cardiac muscle during the contractile cycle (Hilgemann and Langer, 1984; Hilgemann, 1986). Microelectrode-based electrophysiological approaches have been applied to diverse cell and tissue types such as hair cells, cells of the zona pellucida, and the central nervous system (Knox et al., 1996; Yamoah et al., 1998; Pepperell et al., 1999; Smith et al., 1999). Mupanomunda et al. used microdialysis techniques in vivo to monitor extracellular [Ca2+] in the sub-epithelial spaces of the intestine (Mupanomunda et al., 1999) and kidney (Mupanomunda et al., 2000). An increased load of [Ca2+] in the gut lumen or perturbations in systemic Ca2+ homeostasis were shown to cause significant changes in the external [Ca2+] underlying the epithelium of these tissues.
Much less is known about the magnitudes and dynamic characteristics of changes in extracellular [Ca2+] occurring in response to agonists that mobilize intracellular Ca2+, particularly in epithelial cells, which may have a polarized distribution of influx and efflux pathways. Petersen, Tepikin and colleagues (Tepikin et al., 1994; Belan et al., 1996) introduced two clever fluorescence approaches to examine this question in pancreatic acinar cells, using either cells suspended in a microdroplet of solution containing a fluorescent Ca2+ indicator or heavy dextran-conjugated Ca2+ probes, to measure changes in extracellular [Ca2+]. Despite the elegant techniques employed, these investigators were obliged to conduct their experiments in nominally calcium-free conditions, due to the lack of sensitive, low-affinity Ca2+ indicators capable of reporting external [Ca2+] changes within the physiological range.
In the present study we applied Ca2+-selective microelectrodes in order to quantitatively assess dynamic changes in extracellular [Ca2+] in the extracellular fluids of the gastric gland lumen and the basolateral interstitial spaces of the intact amphibian gastric mucosa, taking advantage of an electrophysiological technique originally developed in our laboratory to measure pH in restricted extracellular compartments (Debellis et al., 1998). Sub stantial decreases and increases in extracellular [Ca2+] were recorded at the basolateral and apical microdomains, respectively, of the epithelium in response to a Ca2+-mobilizing agonist (carbachol). These localized extracellular [Ca2+] changes may be sufficient to regulate resident CaRs, as well as to modulate a host of other key biological functions.
Results
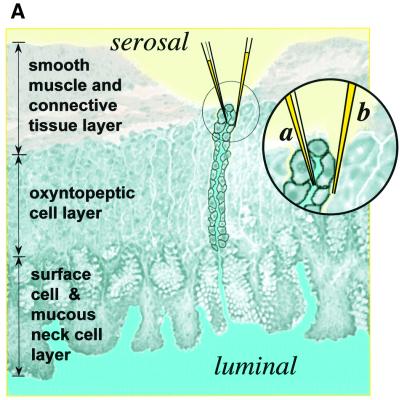
In amphibians, the gastric glands that make up the mucosa are comprised principally of oxyntopeptic cells, which secrete both pepsin and HCl. A few mucous neck cells are found within the gland near its opening at the surface. In addition to having been well characterized in terms of its ion transport properties (Kasbekar et al., 1965; Forte, 1967; Shoemaker and Sachs, 1972; Silen et al., 1975; Machen et al., 1977; Carlisle et al., 1978; Machen and McLennan, 1980; Schettino and Trischitta, 1989; Debellis et al., 1990, 1992, 1998; Schwartz et al., 1991; Supplisson et al., 1991; Ruiz et al., 1993; Seidler et al., 1995), this preparation also offers the advantage that it maintains its anatomical and functional properties intact for several hours in vitro, thus permitting long experiments like those described here. Moreover, using this same model, we recently developed a technique that, following microdissection of the muscular and connective layers, allows direct access of double-barreled, ion-sensitive microelectrodes to the lumen of single gastric glands.
Intracellular [Ca2+] increases in response to cholinergic stimulation as measured by fura-2
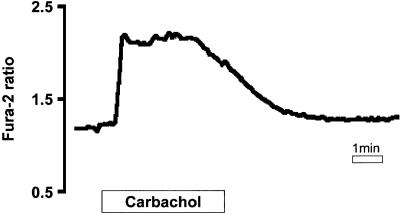
In many different species, cholinergic stimulation of acid- and pepsin-secreting cells results in an elevation in intracellular [Ca2+] (Muallem and Sachs, 1985; Chew, 1986; Negulescu and Machen, 1988; Maruyama et al., 1993). Microspectrofluorimetry of fura-2 signals from frog oxyntopeptic cells in hand-microdissected gastric glands showed that this was also the case in the amphibian stomach. Figure 1 shows that carbachol (100 µM) stimulation produced the typical ‘peak and plateau’ response in intracellular [Ca2+], which presumably reflects, by analogy with many other cell types, an initial phase of release of Ca2+ from intracellular stores, followed by Ca2+ influx through SOC entry pathways.
Fig. 1. Carbachol (100 µM) increases cytoplasmic [Ca2+] in frog oxyntopeptic cells as measured with fura-2 fluorescence (n = 5).
Extracellular [Ca2+] changes in response to cholinergic stimulation
Basolateral measurements of extracellular [Ca2+] changes in response to carbachol. Single- or double-barreled, Ca2+-selective microelectrodes were positioned at the base of a group of glands that were exposed by microdissection of a limited area of the connective tissue layer (Figure 2A). Provided that the microelectrode tip was in a shielded location between a group of glands, it was possible to monitor extracellular [Ca2+] changes without stopping the flow of the perfusion fluid. Before starting the experiment, a test to document the appropriate positioning of the microelectrode was performed by perfusing the serosal compartment with calibration solutions containing varying levels of [Ca2+] (see Materials and methods). If the electrode was properly positioned, the change in the recorded [Ca2+]ext in the perfusion fluid equilibrated with a very slow time course compared with when the microelectrode tip was in the bath (194.4 ± 16.1 s versus 56.6 ± 5.4 s; n = 10).
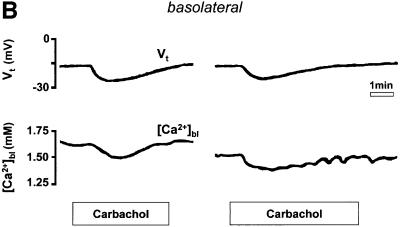
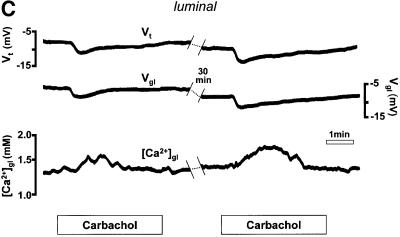
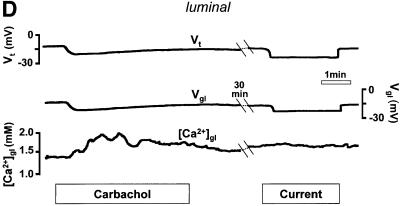
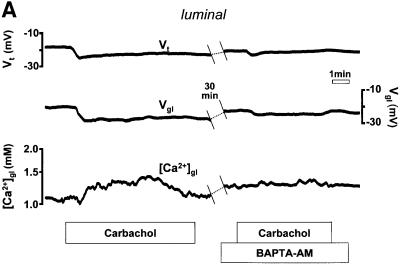
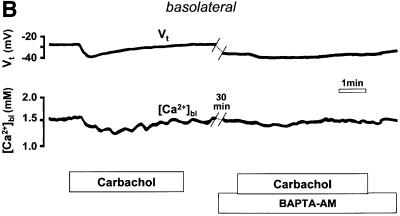
Fig. 2. (A) Schematic drawing of the structure of the amphibian gastric mucosa and of the technique employed to record [Ca2+] changes in the gastric gland lumen (a) or in the interstitial space at the basolateral side of the epithelium (b). (B) [Ca2+] changes recorded in the interstitial space underlying the basolateral membrane of oxyntopeptic cells in response to two sequential stimulations with carbachol (100 µM). Upper traces: transepithelial potential (Vt) in mV, mucosal surface negative; lower traces: local [Ca2+] measured in the basolateral space of the gland cells ([Ca2+]bl). (C) Recordings with double-barreled microelectrode in the gland lumen. Upper trace: transepithelial potential (Vt) in mV. Middle trace: gland lumen potential (Vgl) in mV. Bottom trace: [Ca2+] changes in the gland lumen ([Ca2+]gl) in response to carbachol. (D) Effect of hyperpolarization of the epithelium, induced by a current pulse to mimic the transepithelial response to carbachol, on gland lumen [Ca2+]; control response to carbachol is shown for comparison.
After stimulation with carbachol (Figure 2B), the [Ca2+] in the extracellular space underlying the basolateral membrane of the gastric cells ([Ca2+]bl) decreased transiently over several minutes. As shown in Figure 2B, it was possible to record two sequential responses to carbachol with similar appearance in the same epithelium, provided an interval of at least 30 min had passed between the two stimulations. The decrease in [Ca2+]bl (248 ± 21 µmol/l, n = 18, in 15 tissues, p <0.0001) was accompanied by an increase in the transepithelial potential (Vt, by 6.6 ± 0.7 mV, n = 18, p <0.0001). The change in transepithelial potential, already observed in previous studies (Debellis et al., 1994, 1998), has been shown to be due to an increase in the conductance of basolateral Ca2+-activated K+ channels (Ueda and Okada, 1989; Kotera et al., 1991; Supplisson et al., 1991).
Measurements of extracellular [Ca2+] in the lumen of the gastric gland. Double-barreled, Ca2+-sensitive microelectrodes were applied from the serosal side of a microdissected area of the epithelium. The microelectrode tip was advanced through a cell first and then positioned in the restricted space of the gland lumen, as shown in Figure 2A. The criteria adopted to ascertain the proper positioning of the microelectrodes are described in detail in Materials and methods.
In our previous study using pH-sensitive microelectrodes, it was shown that activation of the tissue with histamine, a potent stimulator of acid secretion, resulted in a profound, reversible acidification of the gland lumen, as expected. These studies further showed that carbachol actually caused a very slight alkalinization of the gastric gland lumen (from pH 7.36 to 7.46), thus it is unlikely that pH changes interfered with our Ca2+ measurements. As shown in Figure 2C, a substantial increase in the luminal extracellular Ca2+ concentration ([Ca2+]gl) was recorded in response to stimulation with carbachol (by 395 ± 27 µmol/l, n = 12, p <0.0001). As with the changes in basolateral [Ca2+], the luminal changes were transient and were paralleled by an increase in Vt (by 7.0 ± 0.8 mV, n = 12, p <0.0001). Similar responses were also recorded in these experiments after two sequential stimulations in the same gland lumen, as shown in Figure 2C.
Thus far, our data demonstrate that the epithelium responds to cholinergic stimulation with a considerable decrease in basolateral [Ca2+]ext and an even larger increase in the luminal [Ca2+]. Since both responses were accompanied by an increase in Vt, it is possible that Ca2+ moved passively from serosa to lumen, driven by the lumen-negative hyperpolarization. We therefore performed experiments in which the epithelium was hyperpolarized by a transepithelial current pulse to mimic the Vt response to carbachol. As shown in Figure 2D, [Ca2+] within the gland lumen did not change during the application of current, while in the same gland [Ca2+]gl had increased in response to an earlier application of carbachol (n = 3). Similar results were obtained when measurements of basolateral [Ca2+]ext were performed (n = 3, not shown).
Effects of SERCA inhibitors
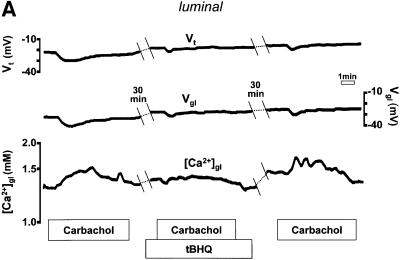
Another possible explanation for our data is that Ca2+ influx and efflux pathways in the plasma membrane were distributed in a polarized manner, and that these became activated following intracellular Ca2+ mobilization by carbachol. If the above-described increase in [Ca2+]gl was a direct consequence of Ca2+ extrusion following agonist-evoked release of the cation from internal stores, we would predict that prior emptying of the stores would abolish the [Ca2+]gl response. We therefore measured luminal [Ca2+] before and after pre-incubation with the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor, 2,5-di-(tert-butyl)-hydroquinone (15 µM tBHQ; applied for ∼20 min on the basolateral side). As shown in Figure 3A, tBHQ pre-treatment eliminated the rise in [Ca2+]gl evoked by carbachol (n = 4, p <0.0001) and reduced the transepithelial hyperpolarization (by 58 ± 18%, n = 4). Thus, preventing the agonist-induced increase in intracellular [Ca2+] inhibited the luminal response to cholinergic stimulation.
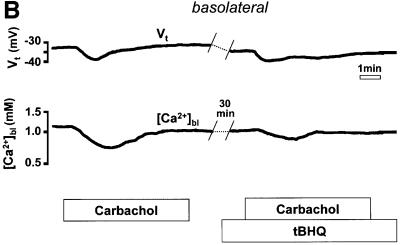
Fig. 3. Effect of pre-treatment with tBHQ (15 µM for 20 min) on the response of (A) luminal or (B) basolateral extracellular [Ca2+] to carbachol.
Likewise, if the agonist-induced decrease in [Ca2+]bl resulted from the entry of Ca2+ into the oxyntopeptic cells as a consequence of store emptying and the activation of SOC channels, we would expect that this response should also be affected by pre-treatment with tBHQ. In fact, as seen in Figure 3B, the transient decrease in [Ca2+]bl following carbachol stimulation was significantly attenuated in the presence of tBHQ (by 53 ± 9%, n = 4, p <0.01). A significant reduction of the Vt response to carbachol was again observed (by 36 ± 9%, n = 4, p = 0.01).
Interestingly, in three of the four experiments described above, a reduction in [Ca2+]bl (ranging between 80 and 240 µmol/l) was observed shortly after exposure to tBHQ (not shown). Therefore, a decrease in extracellular [Ca2+] in the space underlying the basolateral membrane is recorded every time that we induce store depletion (elicited either by agonists or by blockade of the SERCA pump).
Blockade of Ca2+ entry by La3+
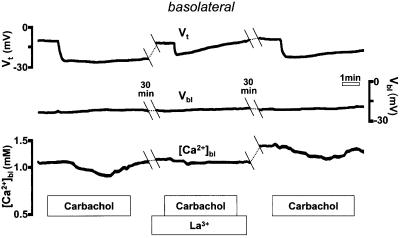
La3+ is a rather non-specific inhibitor of several cellular Ca2+ transport pathways, but it effectively blocks SOC channels in a variety of cell types, including gastric parietal cells (Muallem et al., 1986; Negulescu and Machen, 1988). As seen in Figure 4, serosal application of a low concentration of La3+ (10 µM) resulted in significant inhibition of both [Ca2+]bl and Vt in the response to cholinergic stimulation (by 78 ± 7%, n = 4, in three tissues p <0.01, and 45 ± 9%, n = 4, p <0.01, respectively). The effect of La3+ was at least partially reversible, and another response to carbachol could be recorded after La3+ wash-out. Thus, the decrease in basolateral Ca2+ observed during cholinergic stimulation appears to occur via an La3+-sensitive pathway that may represent the store-operated pathway.
Fig. 4. La3+ (10 µM) blocks the decrease in basolateral extracellular [Ca2+] following carbachol.
Buffering of cytoplasmic [Ca2+] with BAPTA-AM
In these experiments we used the Ca2+ chelator 1,2- bis-(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) to buffer increases in intracellular Ca2+ during cholinergic stimulation (Brown and Chew, 1989; Negulescu et al., 1989; Hofer et al., 1998). After recording the control response to carbachol, the mucosae were exposed to 35 µM BAPTA-AM for ∼30 min, washed, and then again stimulated with the agonist. As seen in Figure 5, both the basolateral and the luminal responses were reduced after BAPTA-AM treatment, although to differing extents. While the carbachol-induced [Ca2+]bl decrease was substantially attenuated (by 73 ± 7%, n = 5, p <0.001), the increase in [Ca2+]gl evoked by carbachol was effectively eliminated (by 98 ± 2%, n = 4, p <0.01). In both cases, the Vt responses to carbachol were also markedly reduced (by 62 ± 5%, n = 5, p <0.001, and 35 ± 12%, n = 4). The effect of BAPTA-AM on the increase in luminal [Ca2+]gl can be explained by the fact that trapping Ca2+ released from the internal stores by the chelator prevents extrusion of the cation into the extracellular space (Zhang et al., 1992; Hofer et al., 1998).
Fig. 5. Effect of pre-treatment with BAPTA-AM (35 µM) on the response of (A) luminal or (B) basolateral extracellular [Ca2+] to carbachol.
On the other hand, the effect of BAPTA-AM on the agonist-induced decrease in [Ca2+]bl is less easily explained by our model. Our expectation was that increasing the buffering of intracellular Ca2+ should facilitate the entry of Ca2+, and cause the extracellular [Ca2+] to be depleted to a greater extent. However, it is possible that BAPTA-AM, by preventing the hyperpolarization of the basolateral plasma membrane via Ca2+-activated K+ channels, has a more significant effect on Ca2+ entry at the basolateral membrane by reducing the driving force for Ca2+ entry. We do not have direct evidence from our preparation for Ca2+-activated K+ conductances. Such conductances have, however, been previously demonstrated in the basolateral membranes of the acid-secreting cells in both the amphibian (Supplisson et al., 1991) and in the mammalian stomach (Ueda and Okada, 1989; Kotera et al., 1991).
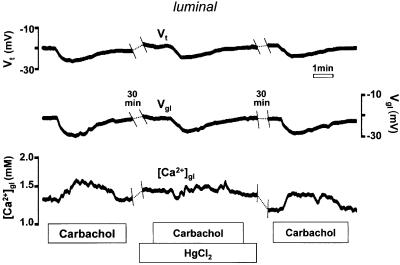
Effects of apical HgCl2
Apical application of HgCl2 (0.5 µM), a non-specific but potent blocker of the PMCA, strongly reduced (by 56 ± 10%, n = 4, p <0.05) the carbachol-induced increase in [Ca2+]gl, providing additional evidence for a role of the PMCA in this response (Figure 6). Interestingly, HgCl2 did not affect Vt, which suggests that other components of the Ca2+ signaling machinery responsible for activating Ca2+-dependent K+ channels are intact. Preliminary experiments conducted with other non- specific inhibitors of the PMCA vanadate and La3+ (applied luminally) gave similar results. The magnitude of the [Ca2+] increase in response to carbachol was reduced from 480 to 100 µM in the presence of vanadate (250 µM). Following wash-out of vanadate in the same tissue, the carbachol response was partially recovered (180 µM). La3+ (10 µM) also decreased the carbachol response (from 297 to 182 µM), and, like vanadate, was also partially reversible (magnitude of third response = 237 µM).
Fig. 6. Effect of HgCl2 (0.5 µM) on the response of luminal [Ca2+] to carbachol.
Immunostaining of PMCA, CaR and the gastric H+/K+-ATPase
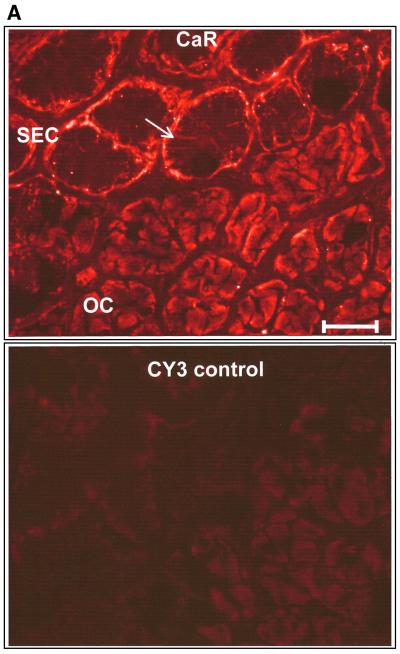
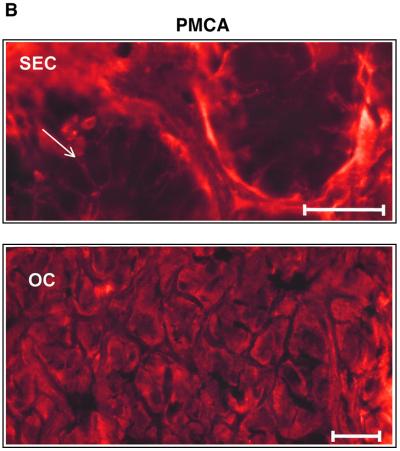
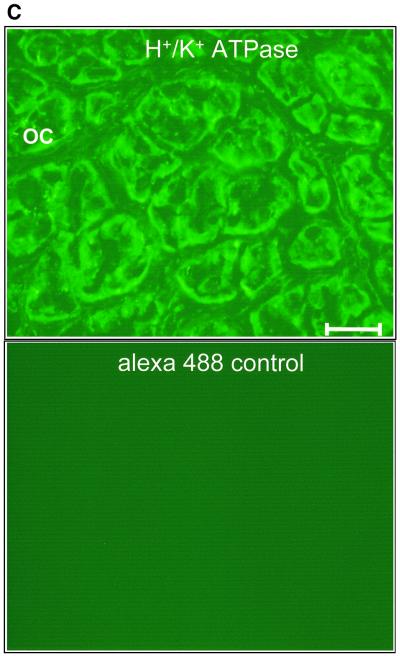
Our functional results strongly imply that oxyntopeptic cells possess a Ca2+ extrusion mechanism located predominantly at the apical face of the cell and entry pathways located basolaterally. While Ca2+ entry pathways in epithelia have only just begun to be identified at the molecular level (Hoenderop et al., 1999; Peng et al., 1999; Petersen and Fedirko, 2001; Yue et al., 2001), Ca2+ extrusion mechanisms, such as the PMCA, are better characterized. We used a PMCA antibody known to recognize all the major isoforms of the Ca2+ pump. Our immunostaining data showed that the PMCA was clearly localized to the basal and lateral membranes of the surface epithelial cells. Other cell types within the mucosa, such as smooth muscle cells, showed bright peripheral labeling of the plasma membrane, as expected (not shown). In contrast, the acid- and pepsinogen-secreting oxyntopeptic cells exhibited a peculiar localization of the PMCA, with a diffuse intracellular-like staining (Figure 7B). As seen in Figure 7B, the subcellular distribution of the PMCA exhibited a striking resemblance to that of the gastric H+/K+-ATPase (Figure 7C), the enzyme responsible for the elaboration of gastric acid from the oxyntopeptic cell. The proton pump is known to reside in an elaborate membrane network (the tubulovesicular system) that becomes fused to the apical membrane during acid secretion. Interest ingly, a very similar pattern of immunoreactivity was observed when antibodies directed against the extracellular CaR were used. As with the PMCA, distinct labeling of the basal and lateral aspects of the surface cells was noted but oxyntopeptic cells again displayed a diffuse ‘intracellular’ staining (Figure 7A). We performed this staining with two different antibodies directed against CaR using both fluorescent and HRP-conjugated secondary antibodies, with similar results (not shown).
Fig. 7. Immunofluorescence of CaR, PMCA (red) and H+/K+-ATPase (green) in transverse sections of amphibian gastric mucosa. (A) Positive immunofluorescence for the CaR in the basolateral membrane of surface epithelial cells (SEC, top portion of upper panel, arrow) and in an intracellular location of the oxyntopeptic cells (OC, bottom portion of upper panel). Lower panel: control staining with secondary antibody alone. Bar, 50 µm. (B) Similar localization was also found for PMCA immunofluorescence; SEC, upper panel and OC, lower panel. Bar, 50 µM. Note that for both CaR and PMCA the localization in the OC appears similar to that of the H+/K+-ATPase, as shown in the top panel of (C). Lower panel of (C) shows the control staining with secondary antibody alone.
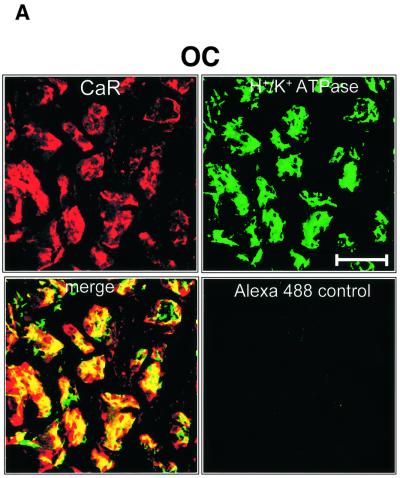
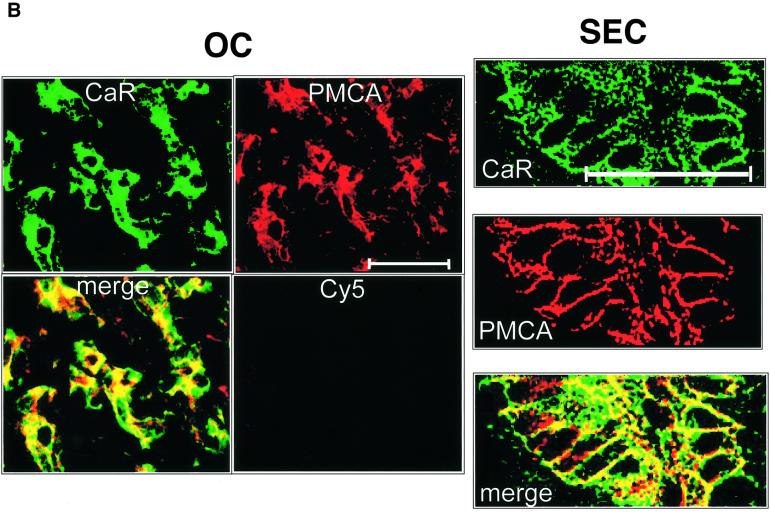
In order to assess whether these membrane proteins were co-localized, confocal imaging of double-labeled sections was performed. As shown in Figure 8A, there was substantial, although not exclusive, overlap in the staining for the H+/K+-ATPase and CaR in oxyntic cells. The PMCA and CaR also exhibited significant overlap (Figure 8B, left), although there were again many regions where the CaR and PMCA were found in isolation. Clear co-localization of PMCA and CaR was observed in the lateral membranes of the surface epithelial cells (Figure 8B, right).
Fig. 8. Confocal co-immunolocalization of CaR, H+/K+-ATPase and PMCA. (A) Oxyntic cells (OC) co-stained with CaR (CY5 labeling, red) and gastric H+/K+-ATPase (Alexa Fluor 488 label, green). Merged images depict areas of overlap in yellow. Control shows staining of Alexa Fluor 488 alone without primary antibody. (B) Left panels: co-staining of CaR (CY5) and PMCA (Alexa Fluor 488) in OC. Control: CY5 secondary antibody + peptide block (see Materials and methods for details). Right panels: co-immunostaining of SEC for CaR and PMCA. Bar, 50 µm.
Our data suggest the possibility that in oxyntic cells the PMCA, the H+/K+-ATPase and the CaR may all reside in the same membrane compartment(s), and that the Ca2+ extruded from stimulated oxyntopeptic cells may potentially provide signals that activate the apical CaR.
Discussion
Following agonist-induced Ca2+ mobilization, gastric acid-secreting cells appear to extrude Ca2+ through their apical membranes into the lumen and take up the cation from the basolateral or serosal compartment. The corresponding fluctuations in [Ca2+]ext were surprisingly large. Although our experiments were conducted with rapid, continuous superfusion, transient decreases and increases as great as 0.5 mM were recorded on the basal and apical aspects of the ex vivo tissue, respectively. How active blood supply to the tissue in the intact animal would influence this vectorial transfer of Ca2+ remains to be determined.
Our data suggest that the changes in extracellular [Ca2+] recorded in response to stimulation with carbachol were the result of the activation of plasma membrane pathways directly connected to intracellular Ca2+ signaling events. The basolateral [Ca2+] decrease was La3+ sensitive and was triggered in response to store emptying elicited by agonist or tBHQ. These data are consistent with the idea that Ca2+ uptake occurred through SOC channels located mainly at the basolateral membrane of the glandular cells.
The luminal [Ca2+] increase was prevented by prior store emptying with tBHQ, by chelation of intracellular Ca2+ with BAPTA-AM and by HgCl2. Our preliminary data also show that this response was sensitive to vanadate and luminal La3+. Considering that the gastric acid-secreting cells do not exhibit Na+/Ca2+ exchange activity (Negulescu and Machen, 1990), these findings support a model in which Ca2+ is expelled from the cell by an apical PMCA (Figure 9). Furthermore, our immunostaining studies of the PMCA suggest overlap with the gastric H+/K+-ATPase, which is known to be localized to the apical tubulovesicular acid secretory machinery in the oxyntopeptic cell. High resolution immunogold electron microscopy techniques will be required, however, to define the membrane localization of these two pumps precisely.
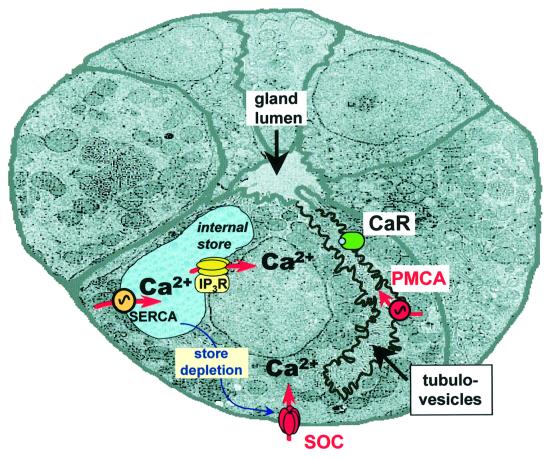
Fig. 9. Schematic illustration of a transverse section through a gastric gland and of the principal Ca2+ transporting pathways. The tubulovesicular compartment has been highlighted in order to better illustrate the putative localization of PMCA and CaR. Note that the tubulovesicles and the gland lumen are contiguous. PMCA, plasma membrane Ca2+-ATPase; SERCA, sarco-endoplasmic reticulum Ca2+ATPase; SOC, store-operated channels, CaR, extracellular Ca2+-sensing receptor; IP3R, inositol trisphosphate receptor.
Ample precedent exists for the polarization of Ca2+ signals and membrane transport pathways in several epithelial cell types (Belan et al., 1996; Lee et al., 1997; Kerstan et al., 1999; Petersen et al., 1999) including gastric cells (Matovcik et al., 1996). Ca2+ transients in pancreatic acinar cells display a characteristic spatial organization with an initial increase in [Ca2+] at the luminal pole, followed by a wave spreading toward the basolateral regions (Kasai and Augustine, 1990; Toescu et al., 1992). In the same cells, using confocal microscopy combined with a Ca2+-sensitive fluorescent dye coupled to heavy dextran, Belan et al. (1996) demonstrated that the apical membrane is the preferential pathway for agonist-induced Ca2+ extrusion. Immunolocalization studies showed high levels of expression of PMCA in the luminal plasma membrane of both pancreatic acinar and salivary gland cells (Lee et al., 1997). Furthermore, as demonstrated by Leipziger and collaborators, human renal, bronchial and colonic epithelial cells appear to have a discrete basolateral localization of capacitative calcium entry pathways (Gordjani et al., 1997; Kerstan et al., 1999).
Our data show that extracellular [Ca2+] changes in response to cholinergic stimulation are large enough to modulate a number of fundamental biological processes. For example, the localized depletion in [Ca2+]bl is sufficient to have an impact on capacitative Ca2+ entry, and may serve as a feedback regulatory mechanism to limit Ca2+ influx in the face of chronic agonist stimulation. Quist et al. (2000) recently demonstrated that gap-junctional hemichannels in epithelial cells can be modulated by a modest decrease in extracellular [Ca2+] (from 1.8 to 1.6 mM), well within the range of external [Ca2+] shifts found in our study. The opening of these hemichannels in response to decrements in extracellular [Ca2+] was coupled to cellular volume changes. In another example, increases in extracellular [Ca2+] have been shown to diminish the effectiveness of ATP (an important paracrine messenger) on purinergic receptors, likely through the formation of inactive ATP–Ca2+ complexes (Zanotti and Charles, 1997), although it remains to be determined whether this type of regulation would occur in vivo. Furthermore, it is plausible that extracellular [Ca2+] changes may affect, or regulate, other stomach-specific functions, such as pepsinogen or mucus secretion through effects on endocytosis and the recycling of secretory membranes (Belan et al., 1996).
Our microelectrode measurements of external [Ca2+] are particularly interesting in light of the accompanying immunolocalization data for the CaR. The CaR has been identified previously in the gastric mucosa by several laboratories (Cima et al., 1997; Ray et al., 1997; Cheng et al., 1999; Rutten et al., 1999). In particular, distinctive staining of the basolateral membranes of the surface cells has been documented, in agreement with our findings (Cima et al., 1997; Cheng et al., 1999; Rutten et al., 1999). Diffuse ‘cytoplasmic’ labeling of the acid-secreting cells has also been described (Cheng et al., 1999; Rutten et al., 1999), similar to that documented recently in several other cell types (Yamaguchi et al., 1998a,b, 2001; Sanders et al., 2000). On first inspection, our data in the amphibian would also suggest an ‘intracellular’ distribution for CaR in the oxyntopeptic cell. However, the conspicuous similarity in the staining pattern for CaR and the H+/K+-ATPase observed in our study is evocative of an apical or tubulovesicular localization for the CaR. Interestingly, in the inner medullary collecting duct of rat kidney, the CaR co-localizes with the aquaporin-2 water channel. Both are in the apical membrane and sub-apical endosomes, which are known to traffic into the apical membrane in response to vasopressin stimulation (Sands et al., 1997). Our data would imply that CaR, PMCA and the H+/K+-ATPase are co-localized. Therefore, we can speculate that Ca2+-sensing receptors on the oxyntopeptic cell are poised to respond to fluctuations in luminal [Ca2+] that are generated following stimulation with agonists that mobilize intracellular Ca2+. Our preliminary investigations have not shown any definitive action of mimicking the extracellular [Ca2+] changes elicited by carbachol (i.e. simultaneously decreasing basolateral [Ca2+] while elevating luminal [Ca2+]) on gastric acid secretion (R.Caroppo and S.Curci, unpublished observations). However, other preliminary experiments in our laboratory have shown that these maneuvers do enhance alkaline secretion (R.Caroppo and S.Curci, unpublished observations), although the role of the CaR in this essential protective response of the gastric epithelium remains to be determined.
The gastric epithelium forms a complex assemblage of CaR-expressing cells with many different functional roles. Intercellular communication is paramount if there is to be integration of function at the tissue level. Whether cells send or receive signals mediated by extracellular Ca2+ will depend on the specific localization of Ca2+ entry and exit pathways in the plasma membrane, as well as the location of the CaR and/or other extracellular Ca2+ sensors that may potentially detect the extracellular signal. However, additional studies will be necessary to ascertain whether Ca2+ can truly act in a ‘first messenger’ capacity in an intact system.
Materials and methods
Tissue and solutions
Experiments were performed on gastric fundus mucosa of Rana esculenta in accordance with the Italian guidelines for animal experiments. The frogs were kept in an aquarium at room temperature (RT) and fed with earthworms until 3–7 days prior to the experiment. After the animals had been killed by decapitation, followed by destruction of the spinal cord and brain, the stomach was isolated and the muscle layer and connective tissue removed by blunt dissection. The mucosa was then mounted horizontally as a flat sheet between two halves of a Lucite chamber (aperture 0.2 cm2) with the serosal side facing up. The connective tissue layer was further removed with sharpened watch-maker forceps under direct microscopic observation in order to expose the gastric glands in a limited area. Both the serosal and mucosal surfaces of the tissue were continuously superfused with oxygenated Ringer’s solution at RT. Fast fluid exchange in the chamber was achieved within seconds utilizing a shock-free, remote-control, eight-way manifold.
Control Ringer’s solution had the following composition: 102.4 mmol/l Na+, 4.0 mmol/l K+, 1.4 mmol/l Ca2+, 0.8 mmol/l Mg2+, 91.4 mmol/l Cl–, 17.8 mmol/l HCO3–, 0.8 mmol/l SO42–, 0.8 mmol/l H2PO4– and 11 mmol/l glucose. It was gassed with 5% CO2–95% O2 and had a pH of 7.36.
Tissues were maintained in the resting state by adding 100 µM cimetidine (SmithKline Beecham, Baranzate, Italy) to the serosal solution or stimulated with 100 µM carbachol. All chemicals were of reagent grade and purchased from Farmitalia Carlo Erba (Milan, Italy), Sigma Chemical Co. (St Louis, MO), Fluka Chemie AG (Buchs, Switzerland) or Molecular Probes (Eugene, OR).
Transepithelial and extracellular [Ca2+] measurements
The transepithelial potential difference (Vt) was measured with a model 610C high-impedance differential electrometer (Keithley, Cleveland, OH) using two flowing-boundary, calomel half-cells filled with 2.7 mol/l KCl solution, which were connected to each bath solution downstream of the tissue. The serosal bath was connected to ground.
Measurements of Ca2+ concentration in the basolateral space ([Ca2+]bl) were performed with single- or double-barreled Ca2+-selective microelectrodes (see below) positioned between gastric glands in the extracellular space close to the basolateral membrane of oxyntopeptic cells, as illustrated in Figure 2A.
Measurements of extracellular [Ca2+] in the lumen of single gastric glands were achieved by first impaling an oxyntopeptic cell and, after the basolateral cell membrane (Vm) had been recorded, gradually advancing the electrode until the tip entered the gland lumen as shown in Figure 2A. The correct positioning of the microelctrode tip in the gland lumen was established by the following criteria: (i) the near identity of the glandular luminal potential (Vgl) with the transepithelial potential (Vt); (ii) the near identity of the electrical resistance recorded between the microelectrode reference channel and serosal bath macroelectrode with the transepithelial resistance; and (iii) a delayed response to changes in [Ca2+] in the luminal bath.
All measurements were performed with a model FD 223 dual-channel electrometer (WPI, New Haven, CT) and recorded on a strip-chart recorder (Kipp & Zonen, Delft, The Netherlands).
Microelectrodes
Double-barreled, Ca2+-selective microelectrodes were constructed as described previously for pH-sensitive electrodes (Debellis et al., 1994, 1998). Briefly, two pieces of filament-containing aluminium silicate glass tubing (Hilgenberg, Malsfeld, Germany), which were of different diameters (1.5 mm outer/1.0 mm inner diameter and 1.1 mm outer/0.75 mm inner diameter) were twisted together during melting and then pulled (tip length ∼20 mm) in a PE2 vertical puller (Narishige, Tokyo, Japan). After pulling, the back of the smaller channel was closed, and the larger channel was exposed to dimethyldichlorosilane vapor (Fluka Chemie AG) for 1 min, then baked in an oven at 140°C for 4 h. After baking, the larger channel was back-filled with a small amount of the Ca2+ ligand (Calcium Ionophore I, Cocktail A; Fluka) and its shaft was later filled with a Ringer’s solution, containing 3.2 mmol/l KCl, 84.6 mmol/l NaCl, 3.2 mmol/l MgCl2, 25 mmol/l HEPES and 1.4 mmol/l CaCl2. The reference channel was filled with 2.7 mmol/l KCl, and an Ag–AgCl wire was inserted and sealed in place with wax to prevent leak currents.
All microelectrodes were calibrated in place prior to impalement by flushing the chamber with a HEPES-buffered Ringer’s solution containing different CaCl2 concentrations (0.5, 1.0, 2.0 or 4.0 mmol/l). After completing the experimental measurement on the tissue, the calibration was repeated in the chamber.
Average slopes of the electrodes were 28 ± 0.4 mV (mean ± SEM, n = 17) per decade change in [Ca2+]. The slope of the microelectrodes was not affected by pH (ranging between 4.0 and 8.4) or by La3+, Hg2+, BAPTA-AM or tBHQ at the concentrations employed in these experiments. The average resistances of the microelectrodes were 92.2 ± 23 GΩ (selective channel) and 125 ± 9 MΩ (reference channel), n = 6.
Intracellular Ca2+ measurements
Single gastric glands from frog mucosa isolated by free hand-dissection (Caroppo et al., 1994) were attached to a Cell-Tak®-coated coverslip and loaded with the Ca2+-sensitive fluorescent dye fura-2 AM (5 µM) for 30 min at RT. Glands were then transferred to a chamber on the stage of an inverted microscope (Zeiss IM 35, Oberkochen, Germany) for microspectrofluorimetric measurements of intracellular [Ca2+] in single oxyntopeptic cells. Fura-2-loaded cells were alternately excited at 340 and 380 nm, while monitoring the emission at 510 nm, and ratios were acquired every 6 s. The changes in fura-2 ratio (340:380) provide a measure of free cytoplasmic [Ca2+].
Immunofluorescence and immunohistochemistry
Thin frozen sections (5 µm thick) of frog stomach were fixed in acetone for 10 min at 20°C. After washing for ∼5 min in phosphate-buffered saline (PBS) at RT, the sections were incubated overnight at 4°C with a primary antibody against the PMCA (Affinity BioReagents, Golden, CO) or the gastric H+/K+-ATPase (kind gift of Dr Dennis Brown, Harvard Medical School, Boston, MA). Two different primary antibodies were used for immunolocalization of CaR: 4637, a rabbit polyclonal antibody directed against amino acids 344–358 of the human CaR; or ADD, a mouse monoclonal antibody directed against residues 214–235 of the human receptor. Afterwards, the sections were rinsed again for ∼5 min in PBS at RT and incubated for 2 h with the appropriate secondary antibody (1:500 dilution): anti-mouse or anti-rabbit Cy3-conjugated antibody (Sigma), anti-mouse Alexa Fluor 568 or Alexa Fluor 488 (Molecular Probes, Inc.). For double-labeling experiments, autofluorescence was quenched using 100 µM glycine in PBS for 10 min at RT, and sections were blocked using 1% normal goat serum for 30 min at RT. Following overnight incubation with primary antibodies at 4°C, sections were incubated with a mixture of secondary antibodies (1:500 dilution): goat anti-mouse Alexa Fluor 488-conjugated IgG and goat anti-rabbit affinity-purified CY5-conjugated IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). All sections were mounted with the Prolong Antifade Kit (Molecular Probes, Inc.). Controls for non-specific staining were performed by omission of the primary antibody, or in the case of the anti-CaR 4637 antibody by using a peptide block (co-incubation with the immunizing peptide). In some experiments, immunoperoxidase techniques were used as described previously (Cheng et al., 1999). Sections were viewed using a Zeiss Axioskop, and images such as those presented in Figure 7 were recorded with an attached 12-bit digital camera (Orca 100, Hamamatsu, Japan) controlled by OpenLab software (Improvision, Coventry, UK). Alternatively, confocal images (such as those presented in Figure 8) of sections co-stained with Alexa Fluor 488 (ex. 488 nm) and Cy5 (ex. 640 nm) were taken with a Bio-Rad MRC1024 multiphoton/confocal microscope using a 40× water immersion objective.
Data analysis and statistics
All measurements were quantified as mean values ± SEM of n individual recordings. The significance of the observations was evaluated by Student’s t-test for paired data with p <0.05 considered to be statistically different.
Acknowledgments
Acknowledgements
We thank Bonnie Lau for excellent assistance in immunofluorescence staining and Gregorio Fistetto for performing some of the micropuncture experiments. We are grateful to Dr Hemant Thatte of the West Roxbury VAMC Multiphoton Microscope Core Facility for help with confocal imaging. We also thank Drs Dennis Brown and Alfred Van Hoek for the kind gift of the H+/K+-ATPase antibody. Our thanks are also due to Dr E.Frömter for his critical reading of the manuscript. R.C. and S.C. were supported by Cofin, MURST1999–2000 (Rome, Italy) and by Finanziamenti di Ateneo (Bari, Italy). Partial support for this study was also provided by the Medical Research Service of the Veteran’s Administration and NIH Center Grant DK34854 (to A.M.H.), DK44571 (to D.I.S.) and DK 52005, 48330 and 4145 (to E.M.B. and O.K.), as well as the St Giles Foundation (to E.M.B.).
References
- Belan P., Gerasimenko,O., Tepikin,A. and Petersen,O. (1996) Localiza tion of Ca2+ extrusion sites in pancreatic acinar cells. J. Biol. Chem., 271, 7615–7619. [DOI] [PubMed] [Google Scholar]
- Bootman M.D. et al. (2001) Calcium signalling—an overview. Semin. Cell Dev. Biol., 12, 3–10. [DOI] [PubMed] [Google Scholar]
- Brown E.M. and MacLeod,R.J. (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev., 81, 239–297. [DOI] [PubMed] [Google Scholar]
- Brown E.M. et al. (1993) Cloning and characterization of an extra cellular Ca2+-sensing receptor from bovine parathyroid. Nature, 366, 575–580. [DOI] [PubMed] [Google Scholar]
- Brown M.R. and Chew,C.S. (1989) Carbachol-induced protein phosphoryl ation in parietal cells: regulation by [Ca2+]i. Am. J. Physiol., 257, G99–G110. [DOI] [PubMed] [Google Scholar]
- Bruce J.I., Yang,X., Ferguson,C.J., Elliott,A.C., Steward,M.C., Case,R.M. and Riccardi,D. (1999) Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J. Biol. Chem., 274, 20561–20568. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Verma,A.K., James,P., Strehler,E. and Penniston,J.T. (1989) The calcium pump of the plasma membrane: structure–function relationships. Adv. Exp. Med. Biol., 255, 61–70. [DOI] [PubMed] [Google Scholar]
- Carlisle K.S., Chew,C.S. and Hersey,S.J. (1978) Ultrastructural changes and cyclic AMP in frog oxyntic cells. J. Cell Biol., 76, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo R., Coppola,S. and Fromter,E. (1994) Electrophysiological investigation of microdissected gastric glands of bullfrog. I. Baso lateral membrane properties in the resting state. Pflugers Arch., 429, 193–202. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N., Cheng,I., Rogers,K., Riccardi,D., Hall,A., Diaz,R., Hebert,S.C., Soybel,D.I. and Brown,E.M. (1998a) Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am. J. Physiol., 274, G122–G130. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N., Ye,C.P., Yamaguchi,T., Kifor,O., Vassilev,P.M., Nishimura,R. and Brown,E.M. (1998b) Extracellular calcium-sensing receptor in rat oligodendrocytes: expression and potential role in regulation of cellular proliferation and an outward K+ channel. Glia, 24, 449–458. [PubMed] [Google Scholar]
- Cheng I. et al. (1999) Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology, 116, 118–126. [DOI] [PubMed] [Google Scholar]
- Chew C.S. (1986) Cholecystokinin, carbachol, gastrin, histamine and forskolin increase [Ca2+]i in gastric glands. Am. J. Physiol., 250, G814–G823. [DOI] [PubMed] [Google Scholar]
- Cima R.R., Cheng,I., Klingensmith,M.E., Chattopadhyay,N., Kifor,O., Hebert,S.C., Brown,E.M. and Soybel,D.I. (1997) Identification and functional assay of an extracellular calcium-sensing receptor in Necturus gastric mucosa. Am. J. Physiol., 273, G1051–G1060. [DOI] [PubMed] [Google Scholar]
- Debellis L., Curci,S. and Fromter,E. (1990) Effect of histamine on the basolateral K+ conductance of frog stomach oxyntic cells and surface epithelial cells. Am. J. Physiol., 258, G631–G636. [DOI] [PubMed] [Google Scholar]
- Debellis L., Curci,S. and Fromter,E. (1992) Microelectrode determin ation of oxyntic cell pH in intact frog gastric mucosa: effect of histamine. Pflugers Arch., 422, 253–259. [DOI] [PubMed] [Google Scholar]
- Debellis L., Iacovelli,C., Fromter,E. and Curci,S. (1994) Model of bicarbonate secretion by resting frog stomach fundus mucosa. II. Role of the oxyntopeptic cells. Pflugers Arch., 428, 655–663. [DOI] [PubMed] [Google Scholar]
- Debellis L., Caroppo,R., Fromter,E. and Curci,S. (1998) Alkaline secretion by frog gastric glands measured with pH microelectrodes in the gland lumen. J. Physiol., 513, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte J.G. (1967) The effect of inhibitors of HCl secretion on the unidirectional fluxes of chloride across bullfrog gastric mucosa. Biochim. Biophys. Acta, 150, 136–145. [DOI] [PubMed] [Google Scholar]
- Gordjani N., Nitschke,R., Greger,R. and Leipziger,J. (1997) Capacitative Ca2+ entry (CCE) induced by luminal and basolateral ATP in polarized MDCK-C7 cells is restricted to the basolateral membrane. Cell Calcium, 22, 121–128. [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W. (1986) Extracellular calcium transients at single excitations in rabbit atrium measured with tetramethylmurexide. J. Gen. Physiol., 87, 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W. and Langer,G.A. (1984) Trans-sarcolemmal calcium movements in arterially perfused rabbit right ventricle measured with extracellular calcium-sensitive dyes. Circ. Res., 54, 461–467. [DOI] [PubMed] [Google Scholar]
- Hoenderop J.G., van der Kemp,A.W., Hartog,A., van de Graaf,S.F., van Os,C.H., Willems,P.H. and Bindels,R.J. (1999) Molecular identific ation of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem., 274, 8375–8378. [DOI] [PubMed] [Google Scholar]
- Hofer A.M., Landolfi,B., Debellis,L., Pozzan,T. and Curci,S. (1998) Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO J., 17, 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A.M., Curci,S., Doble,M.A., Brown,E.M. and Soybel,D.I. (2000) Intercellular communication mediated by the extracellular calcium-sensing receptor. Nature Cell Biol., 2, 392–398. [DOI] [PubMed] [Google Scholar]
- Kasai H. and Augustine,G.J. (1990) Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature, 348, 735–738. [DOI] [PubMed] [Google Scholar]
- Kasbekar D.K., Durbin,R.P. and Lindley,D. (1965) An adenosine triphosphatase from frog gastric mucosa. Biochim. Biophys. Acta, 105, 472–482. [DOI] [PubMed] [Google Scholar]
- Kerstan D., Thomas,J., Nitschke,R. and Leipziger,J. (1999) Basolateral store-operated Ca2+-entry in polarized human bronchial and colonic epithelial cells. Cell Calcium, 26, 253–260. [DOI] [PubMed] [Google Scholar]
- Knox R.J., Jonas,E.A., Kao,L.S., Smith,P.J., Connor,J.A. and Kaczmarek,L.K. (1996) Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica. J. Physiol., 494, 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera T., Hashimoto,A., Ueda,S. and Okada,Y. (1991) Whole-cell K+ current activation in response to voltages and carbachol in gastric parietal cells isolated from guinea pig. J. Membr. Biol., 124, 43–52. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Xu,X., Zeng,W., Diaz,J., Kuo,T.H., Wuytack,F., Racymaekers,L. and Muallem,S. (1997) Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells: role in initiation and propagation of [Ca2+]i waves. J. Biol. Chem., 272, 15771–15776. [DOI] [PubMed] [Google Scholar]
- Machen T.E. and McLennan,W.L. (1980) Na+-dependent H+ and Cl– transport in in vitro frog gastric mucosa. Am. J. Physiol., 238, G403–G413. [DOI] [PubMed] [Google Scholar]
- Machen T.E., Clausen,C. and Diamond,J.M. (1977) Electrical events during stimulation of HCl secretion by frog gastric mucosa in vitro. Gastroenterology, 73, 970. [PubMed] [Google Scholar]
- Maruyama Y., Inooka,G., Li,Y.X., Miyashita,Y. and Kasai,H. (1993) Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO J., 12, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matovcik L.M., Maranto,A.R., Soroka,C.J., Gorelick,F.S., Smith,J. and Goldenring,J.R. (1996) Co-distribution of calmodulin-dependent protein kinase II and inositol trisphosphate receptors in an apical domain of gastrointestinal mucosal cells. J. Histochem. Cytochem., 44, 1243–1250. [DOI] [PubMed] [Google Scholar]
- McNeil L., Hobson,S., Nipper,V. and Rodland,K.D. (1998) Functional calcium-sensing receptor expression in ovarian surface epithelial cells. Am. J. Obstet. Gynecol., 178, 305–313. [DOI] [PubMed] [Google Scholar]
- Muallem S. and Sachs,G. (1985) Ca2+ metabolism during cholinergic stimulation of acid secretion. Am. J. Physiol., 248, G216–G228. [DOI] [PubMed] [Google Scholar]
- Muallem S., Fimmel,C.J., Pandol,S.J. and Sachs,G. (1986) Regulation of free cytosolic Ca2+ in the peptic and parietal cells of the rabbit gastric gland. J. Biol. Chem., 261, 2660–2667. [PubMed] [Google Scholar]
- Muallem S., Pandol,S.J. and Beeker,T.G. (1988) Calcium mobilizing hormones activate the plasma membrane Ca2+ pump of pancreatic acinar cells. J. Membr. Biol., 106, 57–69. [DOI] [PubMed] [Google Scholar]
- Mupanomunda M.M., Ishioka,N. and Bukoski,R.D. (1999) Interstitial Ca2+ undergoes dynamic changes sufficient to stimulate nerve-dependent Ca2+-induced relaxation. Am. J. Physiol., 276, H1035–H1042. [DOI] [PubMed] [Google Scholar]
- Mupanomunda M.M., Tian,B., Ishioka,N. and Bukoski,R.D. (2000) Renal interstitial Ca2+. Am. J. Physiol., 278, F644–F649. [DOI] [PubMed] [Google Scholar]
- Negulescu P.A. and Machen,T.E. (1988) Release and reloading of intracellular Ca stores after cholinergic stimulation of the parietal cell. Am. J. Physiol., 254, C498–C504. [DOI] [PubMed] [Google Scholar]
- Negulescu P.A. and Machen,T.E. (1990) Lowering extracellular sodium or pH raises intracellular calcium in gastric cells. J. Membr. Biol., 116, 239–248. [DOI] [PubMed] [Google Scholar]
- Negulescu P.A., Reenstra,W.W. and Machen,T.E. (1989) Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am. J. Physiol., 256, C241–C251. [DOI] [PubMed] [Google Scholar]
- Peng J.B., Chen,X.Z., Berger,U.V., Vassilev,P.M., Tsukaguchi,H., Brown,E.M. and Hediger,M.A. (1999) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem., 274, 22739–22746. [DOI] [PubMed] [Google Scholar]
- Pepperell J.R., Kommineni,K., Buradagunta,S., Smith,P.J. and Keefe,D.L. (1999) Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biol. Reprod., 60, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Petersen O.H. and Fedirko,N.V. (2001) Calcium signalling: store-operated channel found at last. Curr. Biol., 11, R520–R523. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Burdakov,D. and Tepikin,A.V. (1999) Polarity in intracellular calcium signaling. BioEssays, 21, 851–860. [DOI] [PubMed] [Google Scholar]
- Quist A.P., Rhee,S.K., Lin,H. and Lal,R. (2000) Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol., 148, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J.M., Squires,P.E., Curtis,S.B., Meloche,M.R. and Buchan,A.M. (1997) Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J. Clin. Invest., 99, 2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi D., Park,J., Lee,W.S., Gamba,G., Brown,E.M. and Hebert,S.C. (1995) Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc. Natl Acad. Sci. USA, 92, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M., Snowman,A.M., Hester,L.D. and Snyder,S.H. (1996) Cloned and expressed rat Ca2+-sensing receptor: differential co-operative responses to calcium and magnesium. J. Biol. Chem., 271, 5972–5975. [DOI] [PubMed] [Google Scholar]
- Ruiz M.C., Acosta,A., Abad,M.J. and Michelangeli,F. (1993) Non-parallel secretion of pepsinogen and acid by gastric oxyntopeptic cells of the toad (Bufo marinus). Am. J. Physiol., 265, G934–G941. [DOI] [PubMed] [Google Scholar]
- Rutten M.J. et al. (1999) Identification of a functional Ca2+-sensing receptor in normal human gastric mucous epithelial cells. Am. J. Physiol., 277, G662–G670. [DOI] [PubMed] [Google Scholar]
- Sanders J.L., Chattopadhyay,N., Kifor,O., Yamaguchi,T., Butters,R.R. and Brown,E.M. (2000) Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology, 141, 4357–4364. [DOI] [PubMed] [Google Scholar]
- Sands J.M., Naruse,M., Baum,M., Jo,I., Hebert,S.C., Brown,E.M. and Harris,H.W. (1997) Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J. Clin. Invest., 99, 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettino T. and Trischitta,F. (1989) Transport properties of the basolateral membrane of the oxyntic cells in frog fundic gastric mucosa. Pflugers Arch., 414, 469–476. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Holloman,T.L., Zhang,J. and Carrasquer,G. (1991) Further evidence for a simple Cl– conductance pathway in nutrient membrane of frog stomach. Biochim. Biophys. Acta, 1062, 113–115. [DOI] [PubMed] [Google Scholar]
- Sedova M. and Blatter,L.A. (1999) Dynamic regulation of [Ca2+]i by plasma membrane Ca2+-ATPase and Na+/Ca2+ exchange during capacitative Ca2+ entry in bovine vascular endothelial cells. Cell Calcium, 25, 333–343. [DOI] [PubMed] [Google Scholar]
- Seidler U., Stumpf,P. and Classen M. (1995) Interstitial buffer capacity influences Na+/H+ exchange kinetics and oxyntic cell pHi in frog intact mucosa. Am. J. Physiol., 268, G496–G504. [DOI] [PubMed] [Google Scholar]
- Shoemaker R.L. and Sachs,G. (1972) Microelectrode studies of Necturus gastric mucosa. Gastric Secretion. Academic Press, New York, NY, pp. 147–163.
- Silen W., Machen,T.E. and Forte,J.G. (1975) Acid–base balance in amphibian gastric mucosa. Am. J. Physiol., 229, 721–730. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Hammar,K., Porterfield,D.M., Sanger,R.H. and Trimarchi,J.R. (1999) Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc. Res. Tech., 46, 398–417. [DOI] [PubMed] [Google Scholar]
- Supplisson S., Loo,D.D. and Sachs,G. (1991) Diversity of K+ channels in the basolateral membrane of resting Necturus oxyntic cells. J. Membr. Biol., 123, 209–221. [DOI] [PubMed] [Google Scholar]
- Tepikin A., Liopis,J., Snitsarev,V., Gallacher,D. and Petersen,O. (1994) The droplet technique: measurement of calcium extrusion from single isolated mammalian cells. Pflugers Arch., 428, 664–670. [DOI] [PubMed] [Google Scholar]
- Toescu E.C., Lawrie,A.M., Petersen,O.H. and Gallacher,D.V. (1992) Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J., 11, 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S. and Okada,Y. (1989) Acid secretagogues induce Ca2+ mobilization coupled to K+ conductance activation in rat parietal cells in tissue culture. Biochim. Biophys. Acta, 1012, 254–260. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Chattopadhyay,N., Kifor,O. and Brown,E.M. (1998a) Extracellular calcium (Ca2+o)-sensing receptor in a murine bone marrow-derived stromal cell line (ST2): potential mediator of the actions of Ca2+(o) on the function of ST2 cells. Endocrinology, 139, 3561–3568. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Kifor,O., Chattopadhyay,N. and Brown,E.M. (1998b) Expression of extracellular calcium (Ca2+o)-sensing receptor in the clonal osteoblast-like cell lines, UMR-106 and SAOS-2. Biochem. Biophys. Res. Commun., 243, 753–757. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Chattopadhyay,N., Kifor,O., Ye,C., Vassilev,P.M., Sanders,J.L. and Brown,E.M. (2001) Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am. J. Physiol., 280, C382–C393. [DOI] [PubMed] [Google Scholar]
- Yamoah E.N., Lumpkin,E.A., Dumont,R.A., Smith,P.J., Hudspeth,A.J. and Gillespie,P.G. (1998) Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J. Neurosci., 18, 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Peng,J.B., Hediger,M.A. and Clapham,D.E. (2001) CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature, 410, 705–709. [DOI] [PubMed] [Google Scholar]
- Zanotti S. and Charles,A. (1997) Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J. Neurochem., 69, 594–602. [DOI] [PubMed] [Google Scholar]
- Zhang B.X., Zhao,H., Loessberg,P. and Muallem,S. (1992) Activation of the plasma membrane Ca2+ pump during agonist stimulation of pancreatic acini. J. Biol. Chem., 267, 15419–15425. [PubMed] [Google Scholar]