Abstract
The immunoglobulin heavy chain (IgH) gene locus spans several megabases. We show that IgH activation during B-cell differentiation, as measured by histone acetylation, occurs in discrete, independently regulated domains. Initially, a 120 kb domain of germline DNA is hyperacetylated, that extends from DFL16.1, the 5′-most DH gene segment, to the intergenic region between Cµ and Cδ. Germline VH genes were not hyperacetylated at this stage, which accounts for DH to JH recombination occurring first during B-cell development. Subsequent activation of the VH locus happens in at least three differentially regulated domains: an interleukin-7-regulated domain consisting of the 5′ J558 family, an intermediate domain and the 3′ VH genes, which are hyperacetylated in response to DJH recombination. These observations lead to mechanisms for two well-documented phenomena in B-cell ontogeny: the sequential rearrangement of DH followed by VH gene segments, and the preferential recombination of DH-proximal VH genes in pro-B cells. We suggest that stepwise activation may be a general mechanism by which large segments of the genome are prepared for expression.
Keywords: B cells/gene expression/hyperacetylation/immunoglobulin heavy chain
Introduction
The genome of higher eukaryotes is packaged into chromatin (Kornberg and Lorch, 1999; Wolffe and Guschin, 2000). The resulting compaction of DNA is not only essential to fit it into the cell nucleus, but also serves to suppress gene expression. Against this backdrop of primarily inactive genes, a restricted number of cell- and tissue-specific genes are activated in appropriate cells during differentiation. Regions of chromatin that contain active genes differ in several ways from those that contain inactive genes. For example, active genes are readily cleaved by DNase or micrococcal nuclease (MNase) (Gross and Garrard, 1988), and are usually hypomethylated at CpG dinucleotides (Siegfried and Cedar, 1997; Ng and Bird, 1999). Covalent modification of core histones recently has gained prominence as an important marker of chromatin domains. The N-termini of histones H3 and H4 associated with active genes are acetylated at several lysine residues (Workman and Kingston, 1998; Strahl and Allis, 2000). Conversely, inactive genes are associated with hypoacetylated histones. A mechanistic link between methylated DNA and hypoacetylated histones, both of which correlate with gene inactivity, was provided recently by the observation that methylcytosine-binding proteins interact with histone deacetylases (Jones et al., 1998; Nan et al., 1998). These parameters have been analyzed comprehensively in the β-globin locus with regard to differential gene expression (Hebbes et al., 1988, 1994; Litt et al., 2001).
Genes encoding B- and T-lymphocyte antigen receptors are assembled by DNA recombination events. The accessibility model of Alt and colleagues was proposed to explain the observation that the same machinery recombines all six antigen receptor loci, but that it does so in different cell types, and at different stages of differentiation (Sleckman et al., 1996, 1998). The crux of the accessibility model is that the recombinase machinery gains access to individual loci in a regulated manner. For example, when the immunoglobulin heavy chain (IgH) locus is rearranged in pro-B cells, the recombinase can only access IgH sequences, but not recombination signal sequences (RSSs) associated with the other five antigen receptor loci. Regulated changes in chromatin structure are believed to be the basis of gene-specific recombinase accessibility (Stanhope-Baker et al., 1996).
Locus-specific accessibility of the recombinase to antigen receptor genes is governed by cis-acting regulatory elements, including transcriptional enhancers. At the IgH locus, the µ heavy chain gene enhancer (µE) is one such element which activates recombination in stably transfected, or transgenic, substrates (Ferrier et al., 1990; Oltz et al., 1993; Fernex et al., 1994). Conversely, deletion of this enhancer from the endogenous locus abolishes VH to DJH recombination without significantly affecting DH to JH recombination (Chen et al., 1993; Serwe and Sablitzky, 1993; Sakai et al., 1999). Similarly, enhancers and sterile promoters associated with the T-cell receptor (TCR) loci also serve as recombination activators (Capone et al., 1993; Lauzurica and Krangel, 1994; McMurry et al., 1997; Sleckman et al., 1997; Whitehurst et al., 1999; Tripathi et al., 2000). Recent studies show a striking correlation between histone acetylation and V(D)J recombination at TCR loci (Mathieu et al., 2000; McBlane and Boyes, 2000; McMurry and Krangel, 2000). Recombinationally competent TCR loci were associated with hyperacetylated histones; deletion of TCRα or TCRβ enhancers that abolished recombination also diminished histone acetylation. In addition, deletion of the Dβ1 sterile promoter resulted in reduced recombination and enhanced methylation at proximal sites (Whitehurst et al., 2000).
The murine IgH locus comprises several hundred VH gene segments, 16 DH gene segments and four JH gene segments spread over 3 Mb (Figure 1A). During B-cell differentiation, two ordered DNA recombination events juxtapose a VH, DH and JH gene segment to generate functional IgH genes. Hardy et al. (1991) have classified B-cell precursors into six subsets, A2–F, using cell surface marker expression. In this nomenclature, A2 cells are the earliest B lineage-committed cells which have no gene rearrangements at either IgH or Ig light chain loci. IgH rearrangements are first evident in the B fraction which contains cells that have undergone DH to JH recombination to create DJH joins. VH gene recombination follows in fraction C to generate fully rearranged VDJ alleles. Because recombination is error prone, only a small proportion of cells that contain VDJ alleles can make IgH protein. These are selected to differentiate further to fraction D, which are the cells in which the bulk of light chain gene recombination occurs. Successful light chain recombination results in immature B cells that express immunoglobulin on the surface (fraction E).
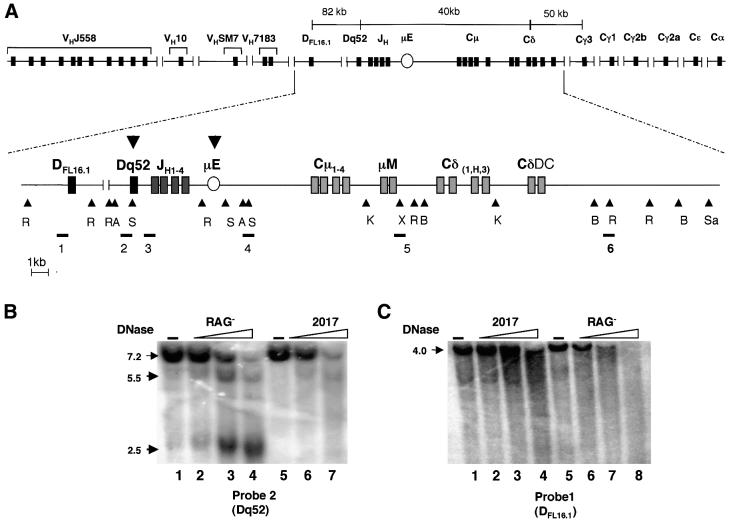
Fig. 1. DNase I-hypersensitive site analysis of the murine IgH gene locus. (A) Schematic representation of the IgH locus with DNase I-hypersensitive sites. The complete locus including VH gene segments and all heavy chain isotypes is shown on the top line. The second line is a detailed schematic of part of the locus starting from the 5′ DFL16.1 gene segment to 7 kb downstream of the Cδ membrane exon. The restriction endonuclease sites EcoRI (R), AseI (A), SacI (S), KpnI (K), SalI (Sa), XhoI (X) and BglII (B) were used for Southern blot analysis of DNase I-treated genomic DNA. Probes used in the study are labeled 1–6, and are shown below their corresponding hybridizing fragments. The position of the intronic µ enhancer is marked with an oval µE. The nuclease-hypersenstive sites identified are indicated by the bold arrowheads. (B and C) DNase I sensitivity assays. Nuclei from RAG– (pro-B) cells or 2017 (pro-T) cells were treated with increasing concentrations of DNase I as indicated, and purified genomic DNA analyzed by Southern blotting. DNA from untreated nuclei is shown in lanes 1 and 5. Restriction enzymes and probes used were as follows: (B) AseI, probe 2; (C) EcoRI, probe 1. DNase-hypersensitive sites are indicated by the bold arrows. The bands at 2.5 and 5.5 kb correspond to hypersensitive sites at the Dq52 region and the µE, respectively. Data shown are from one out of four independent experiments.
Several aspects of the regulation of IgH gene recombination remain poorly understood. For example, despite appropriate RSSs flanking VH and DH gene segments, VH to DH recombination occurs only after DH to JH rearrangements. Secondly, VH genes that lie close to the DH cluster, such as the VH7183 family, have been shown to rearrange preferentially during B-cell ontogeny (Yancopoulos et al., 1984; Jeong and Teale, 1989; Malynn et al., 1990; ten Boekel et al., 1997). Therefore, these genes are over-represented at early stages of differentiation compared with the more distal (and more numerous) VHJ558 family. Moreover, in interleukin-7 receptor (IL-7R)-deficient mice, rearrangement of the J558 family is impaired relative to that of the DH-proximal V gene families (Corcoran et al., 1998). Finally, it is unclear why deletion of the µE located in the JH-Cµ intron affects VH to DJH recombination, rather than the more proximal DH to JH recombination.
Here we used histone hyperacetylation to assay activation of the IgH locus during B-cell differentiation. We show that the several megabase IgH locus is activated in discrete steps. An ∼120 kb domain, that includes the DH gene sements and extends till the Cµ exons, is hyperacetylated first prior to initiation of recombination. DH to JH recombination presumably occurs within this domain. The VH locus was inactive at this stage of differentiation, providing a simple explanation for the observation that DH to JH rearrangements precede VH to DH rearrangements. The VH locus itself contained at least three independently regulated domains; genes closest to DH/Cµ were associated with hyperacetylated histones only in cells that contained DJH recombined alleles, suggesting that they may be activated as a consequence of the first recombination event. The distal VH genes were IL-7 responsive, and genes within an intervening domain were activated by the v-abl tyrosine kinase. Independent regulation of segments of the IgH locus suggests a mechanism for preferential rearrangement of proximal VH genes, and provides a model for activation of large segments of the genome.
Results
Nuclease mapping
The observation that genetic deletion of the IgH µE (Figure 1A) does not affect DH to JH recombination hints at other recombination-activating regulatory sequences at this end of the locus. To search for additional regulatory sequences, we examined the chromatin structure of the locus prior to rearrangement using DNase I and MNase (see Figure 1A lower panel for probes and restriction sites). In these studies, we compared two cell lines whose characteristics resembled pre-rearrangement cells obtained from primary lymphoid tissue. RAG– is an Abelson virus-transformed cell line derived from the fetal liver of RAG2– mice and represents early B-lymphoid cells. 2017 cells were derived by intra-thymic injection of Moloney virus and represent early T-lymphoid cells (Spolski et al., 1988). DNase I digestion revealed a strong hypersensitive site in the vicinity of the µE in RAG– (pro-B) cells, but not in 2017 cells (pro-T) (Figure 1B, and summarized in Figure 1A, lower level, bold arrow). This site was evident using either AseI- or SacI-digested DNA and probes 2 or 3, respectively. In addition, pro-B-specific hypersensitivity was detected near Dq52, which probably corresponds to the Dq52 promoter where µo transcripts are initiated (Alessandrini and Desiderio, 1991; Kottmann et al., 1992, 1994). No other DNase I- or MNase-hypersensitive sites were detected in the region extending to ∼5 kb 3′ of the Cδ transmembrane exons (data not shown ).
Furthemore, no additional hypersensitive sites were detected in ∼10 kb surrounding DFL16.1, the most 5′ DH gene segment (Figure 1C), and the adjacent DSP2.2. That Dq52 contained a hypersensitive site but DFL16.1 and DSP2.2 did not indicated that the chromatin structure surrounding DH gene segments was not identical. We conclude that in a 40 kb region that encompasses Dq52, Cµ and Cδ, or in a 10 kb region encompassing DFL16.1 and DSP2.2, there are only two nuclease-hypersensitive sites which are likely to determine the structure of this part of the IgH locus.
Histone acetylation at the IgH locus
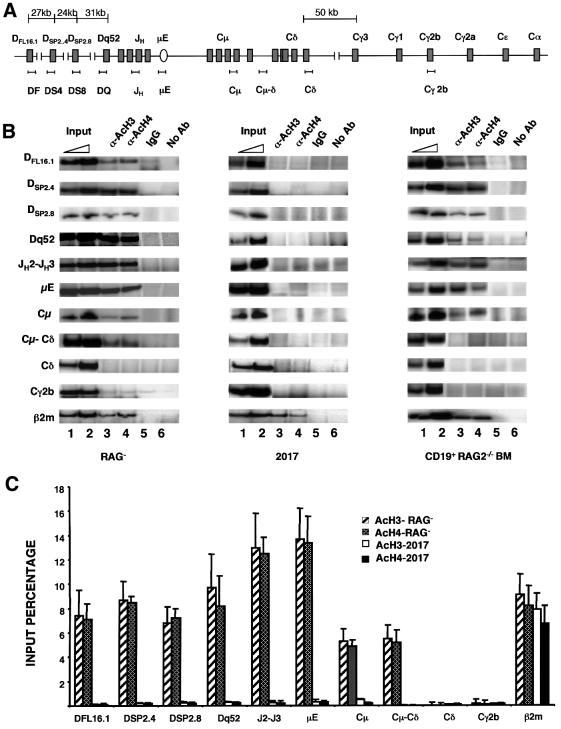
Association of genes with acetylated histones has been implicated in the regulation of gene expression and V(D)J recombination. To determine the extent of the IgH locus that was associated with acetylated histones prior to rearrangements, chromatin immunoprecipitations were carried out using anti-acetylated H3 or H4 antibodies followed by analysis of the co-precipitated DNA by PCR. As with the studies described above, we compared the acetylation status of the IgH locus in RAG– (pro-B) and 2017 (pro-T) cells. PCR primers that span the DH–Cδ regions are indicated in Figure 2A. The region close to the two identified nuclease-hypersensitive sites (Dq52 and µE), as well as the intervening region that contains the four JH gene segments, co-precipitated efficiently with anti-acetylated histone antibodies from RAG– cells where the IgH locus is accessible to recombinase (Figure 2B and C). Histone acetylation of the IgH locus was markedly diminished in 2017 (pro-T) cells. Comparable immunoprecipitation of β2-microglobulin (β2m) sequences from both cell lines served as a positive control.
Fig. 2. Histone acetylation in the IgH locus. (A) Schematic representation of the DH–Cµ locus with approximate locations of the primer sets used in chromatin immunoprecipitation assays. Sequences of the primers are provided in Table I. (B) Chromatin immunoprecipitation assays using pro-B (RAG–) and pro-T (2017) cell lines, and primary pro-B cells from RAG2-deficient bone marrow. Formaldehyde-cross-linked chromatin prepared from cells as described in Materials and methods was incubated with anti-acetylated histone H3 (α-AcH3) or anti-acetylated histone H4 (α-AcH4) antibodies. Control immunoprecipitations were carried out using a 2-fold excess of non-specific rabbit IgG (lane 5) or no antibody (lane 6). Antibody-bound DNA was collected by adsorption to protein A–agarose, uncross-linked and amplified by PCR with the primer sets indicated in (A). The 3′ primer was radiolabeled for quantitation, and the products were visualized after fractionation through 6% polyacrylamide gels. Phosphoimager analysis was used to detect and quantitate reaction products. Lanes marked Input (lanes 1 and 2) correspond to DNA purified from chromatin before immunoprecipitation. The amount of input DNA used as the template in the PCR was one-tenth (lane 1) or one-fifth (lane 2) that used for immunoprecipitations (lanes 3–6). Primers hybridizing to the β2-microglobulin gene were used as a positive control. Results shown are representative of one out of three independent experiments. (C) Data in (B) of the RAG– and 2017 cell lines were quantitated by phosphoimager analysis and are represented graphically. Results shown are an average of three independent experiments, with the error bars representing the standard deviation. The input percentage was calculated taking the subsaturating PCR product of the input DNA (lane1) and also taking into account that the input had 10-fold less DNA template per PCR.
We examined the state of DFL16.1 and found that it was also associated with acetylated histones in RAG– (pro-B) cells, but not in 2017 (pro-T) cells (Figure 2B). Thus, gene segments at either extremity of the DH gene cluster were in an activated state as assessed by histone acetylation. To probe the middle of the DH gene cluster, we used primers specific for DSP2.4 and DSP2.8 which lie at similar distances from the 5′ and 3′ ends of the DH cluster, respectively (Figure 2A). Both regions co-precipitated with acetylated histones from RAG– cells, but not from 2017 cells (Figure 2B). These observations suggest that the entire DH cluster is activated simultaneously at the earliest stages of B-cell differentiation. It is interesting to note that DFL16.1 is associated with acetylated histones, though it is not marked by a nuclease-hypersensitive site like Dq52. The 5′ end of the acetylated domain has not been defined in these studies; however, the lower levels of DFL16.1 DNA detected in the immunoprecipitates suggest that the domain does not extend much further upstream.
To identify the 3′ end of the domain of hyperacetylation, we used primer sets that hybridized within and beyond the Cµ exons (Figure 2A). Histone acetylation was evident at Cµ, as well as in the intergenic region between Cµ and Cδ (Cµ–Cδ) exons (Figure 2B). Quantitation of the proportion of input DNA that immunoprecipitated with acetylated histone antibodies indicated that the level of acetylation between Cµ and Cδ was significantly reduced compared with the JH region (Figure 2C). Cδ sequences located only 3 kb 3′ of the intergenic Cµ–Cδ region did not co-precipitate with acetylated histones, and neither did downstream Cγ2b sequences. As expected, none of these regions were associated with acetylated histones in 2017 cells.
To confirm these observations, CD19+ cells were purified from the bone marrow of RAG2–/– mice (Figure 2B, third column) and used for chromatin immunoprecipitation assays. Most of the results paralleled those in cell lines. DFL16.1, DSP2.4, DSP2.8 and Dq52 regions were associated with acetylated histones, as were the JH cluster, the µE and the Cµ exons (Figure 2B). The main difference between the two populations of pro-B cells was that the Cµ–Cδ intergenic region did not precipitate from primary cells as had been seen in the RAG– cell line. As before, the Cδ and Cγ2b regions were not associated with acetylated histones. We conclude that the 3′ end of the activated IgH locus in pro-B cells terminates abruptly in the 5 kb region between Cµ and Cδ. This domain of hyperacetylation of the germline IgH locus spans ∼120 kb and includes all DH and JH gene segments, and the Cµ exons. Our observations suggest that V(D)J recombination at the IgH locus is initiated within this domain.
Distinction between the DH-Cµ and VH locus activation
The most µ-proximal VH gene family, VH7183, lies ∼40 kb upstream from DFL16.1 (Figure 1A). The remaining several hundred VH gene segments are spread over >2 Mb, with the largest VHJ558 family occupying almost half of this region (Haines and Brodeur, 1998). Two facets of the regulation of VH gene recombination are well known. First, VH gene recombination in Hardy fraction C follows DH to JH recombination (in fraction B) during B-cell development. Secondly, µ-proximal VH genes, particularly those of the VH7183 family, are over-represented in fraction C compared with the larger numbers of VHJ558 genes. These observations have led to the idea that VH7183 genes rearrange preferentially during B-cell ontogeny. The molecular mechanisms of neither phenomenon are clear. Wu and colleagues have proposed that part of the selectivity of VH gene rearrangements resides in the sequences of the genes and their flanks (Yu et al., 1998). Furthermore, a comprehensive analysis of the chromatin structure of the VH locus by DNase I sensitivity also did not reveal significant differences amongst family members, or stages of differentiation (Haines and Brodeur, 1998) that could explain VH gene regulation.
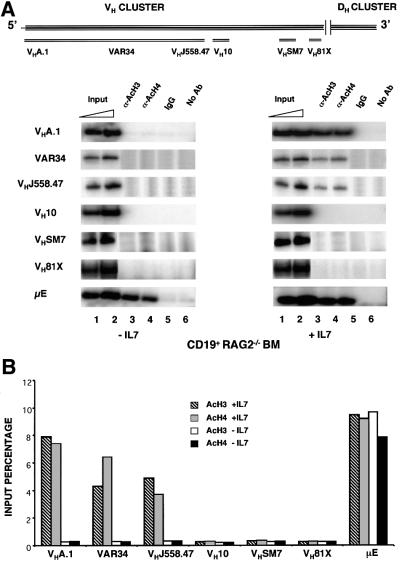
We assayed VH gene acetylation in CD19+ bone marrow cells from RAG2-deficient mice using representative examples of the VH genes from the 5′ end, the 3′ end and the middle of the VH cluster. The 5′ VH J558 family was represented by three genes: VHA.1, which is considered to be one of the 5′-most VH genes (Haines and Brodeur, 1998); VHJ558.47 which is one of the most 3′ J558 genes; and VAR34 which maps between A.1 and J558.47. DH/Cµ-proximal (3′) VH genes were represented by VH81X and VHSM7, and the middle of the VH cluster was represented by VH10.1A. An approximate map of these genes is shown in Figure 3A (upper panel). None of the VH genes that we examined co-precipitated with acetylated histones from CD19+ RAG2– bone marrow cells (Figure 3A, left panel) though the DH–Cµ locus was activated in these cells (Figure 2). The efficient precipitation of µE DNA in these assays served as a positive control. We conclude that the domain of hyperacetylation that reflects the earliest opening of the IgH locus includes the DH cluster, the JH gene segments and the Cµ exons, but excludes all VH gene segments. These observations suggest that the order of rearrangements at the IgH locus may be determined by the ordered activation of rearrangeable gene segments, i.e. DH to JH rearangements occur first because these segments are available first to the recombinase machinery.
Fig. 3. IL-7-dependent histone acetylation of VHJ558 gene segments. (A) Schematic representation of the murine VH gene locus showing the approximate location of gene segments analyzed in this study (adapted from Haines and Brodeur, 1998). CD19+ pro-B bone marrow cells from RAG2–/– mice were cultured for 4 days with or without IL-7 (20 ng/ml). Chromatin immunoprecipitation assays were done as described using primers from the VH region as shown in the top panel. The µ enhancer region (µE) was used as a positive control. Representative data from one of two experiments is shown. (B) The results in (A) were quantified by phosphoimager analysis and are represented as a proportion of the input DNA that was immunoprecipitated.
Activation of distal VHs
IL-7 and IL-7R interaction affects B lymphopoiesis by providing proliferative, survival and differentiative signals to pro-B cells. The earliest developmental defect in IL-7Rα-deficient (IL-7R–/–) mice is a 5- to 10-fold reduction in the cell numbers of pro-B cells, in which IgH gene rearrangements take place (Maraskovsky et al., 1998). In addition, VH genes at the 5′ end of the locus are significantly under-represented in VDJ recombined alleles in IL-7R–/– mice (Corcoran et al., 1998). To determine whether the effects of IL-7 on recombination were due to a direct effect on VH gene chromatin structure, CD19+ bone marrow cells from RAG2-deficient mice were cultured in vitro in the presence or absence of IL-7. After 4 days in culture, viable cells were recovered and used for chromatin immunoprecipitation assays with anti-acetylated histone antibodies. In response to IL-7, all three VH genes of the J558 family co-precipitated efficiently with acetylated histones (Figure 3A, right panel). However, VH10 located in the middle of the VH cluster, as well as VHSM7 and VH81X representing the 3′ end of the VH cluster, remained hypoacetylated under these conditions. Thus IL-7 induces histone hyperacetylation of a domain that encompasses the J558 genes and thereby may activate these genes for recombination. These results provide a plausible explanation for reduced VHJ558 gene rearrangements in the bone marrow of the IL7Rα-deficient mice.
Activation of proximal VHs
We found that the DH-proximal VH genes were inactive in RAG2– bone marrow cells and could not be activated by IL-7. Analysis of VH gene acetylation in an Abelson virus-transformed RAG-deficient cell line showed that VH J558 genes were acetylated, but the 3′ VHSM7 and VH81X genes were not (Figure 4, left panel). We attributed constitutive acetylation of the J558 genes to an earlier observation that the v-abl oncogene mimics aspects of IL-7R signaling (Banerjee and Rothman, 1998). However, lack of acetylation of the DH-proximal VH genes was surprising because these genes rearrange preferentially in early B cells, a phenomenon that was first described in Abelson transformants, and more recently reproduced after reconstitution of recombinase in RAG-deficient Abelson cell lines (Angelin-Duclos and Calame, 1998). We tested the possibility that the rearrangement state of the IgH locus played a role in activation of the proximal genes by comparing VH gene acetylation in three Abelson transformants: RAG2–, 22D6 and 38B9. The latter two cell lines contain DJH rearrangements on both alleles and undergo low frequency VH to DJH recombination in culture (Yancopoulos et al., 1984). In contrast to the observation in RAG– cells, proximal VH81X and VHSM7 genes co-precipitated with acetylated histone H3 in the two new cell lines (Figure 4A, middle and right columns). Efficient co-precipitation of the µE sequences served as a positive control, and Cγ2b sequences were unacetylated in all three lines. We suggest that DJH recombination may activate the proximal VH genes.
Fig. 4. Acetylation of the DH-proximal VH genes in pro-B- and pre-B-cell lines. (A) Chromatin immunoprecipitation assays were done using Abelson murine leukemia virus-transformed pro-B (RAG–) and pre-B cell lines (22D6 and 38B9). The IgH locus is in germline configuration in RAG– cells, whereas 22D6 and 38B9 cells contain DJH recombination on both alleles. Representative data from one of two experiments are shown. (B) Phosphoimager analysis and graphical representation of the data in (A).
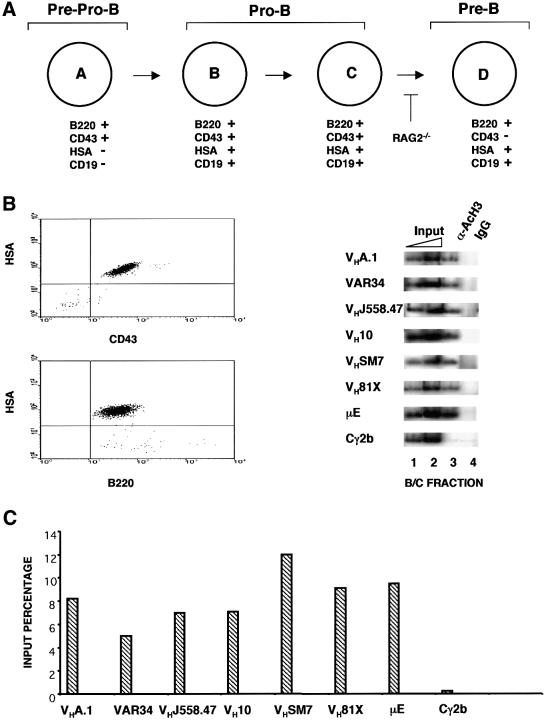
To confirm and extend these observations, we assayed the state of VH gene activation in Hardy fractions B/C (Figure 5A) purified from BALB/c bone marrow. These cells have been shown previously to contain DJH rearrangements on both alleles and are undergoing VH to DJH rearrangement (Ehlich et al., 1994). Total bone marrow depleted of myeloid, erythroid and T cells was sorted to obtain B220+HSA+CD43+ pro-B cells (Figure 5B). Proximal VH gene sequences were amplified easily in anti-acetylated histone immunoprecipitates from these cells (Figure 5B). Note that the 3′ primer used for amplification of the VH81X region is from germline 3′ non-coding sequences downstream of the RSS; therefore, the VH81X signal being detected arises only from the unrearranged gene. 5′ VH genes were also hyperacetylated in these cells, consistent with this cell population being the one in which the bulk of VH to DJH recombination takes place. The significant difference in the pattern of VH gene activation in B/C fraction cells from normal compared with RAG2– mice (Figures 3 and 5) strengthens the idea that DJH recombination is a prerequisite for activation of proximal VH gene segments.
Fig. 5. Histone acetylation of VH genes in primary bone marrow pro-B cells. (A) Hardy classification of early B-cell development listing some of the cell surface markers used to distinguish the different cell populations (Hardy et al., 1991). DJH recombination is seen in fraction B cells and VDJ recombination is seen in fraction C cells. (B and C) The B/C fraction pro-B cells were purified from bone marrow of wild-type BALB/c mice by magnetic depletion of erythroid, myeloid and T cells followed by sorting for the B220+ CD43+ HSA+ cells. Chromatin immunoprecipitation assays were carried out using the primers shown in Figure 3A. Phosphoimager quantitation was done as described in the legend to Figure 2. Representative data from one of two experiments using two preparation of bone marrow cells are shown.
Discussion
General features of the IgH locus
We used histone hyperacetylation as a measure of genome activation to examine the state of the IgH locus during B-cell differentiation. A domain of ∼120 kb, that included all DH gene segments, JH gene segments and Cµ exons, was activated first by this criterion. Given the reported correlation between acetylation and V(D)J recombination (Mathieu et al., 2000; McMurry and Krangel, 2000), it is likely that IgH rearrangements are initiated within this domain. The region containing the four JH gene segments precipitated more efficiently with anti-acetylated histone antibodies than other parts of the 120 kb domain. This microdomain may be the consequence of being flanked by two DNase I-hypersensitive sites corresponding to the Dq52 promoter and the µE. We speculate that the JH microdomain may help to initiate V(D)J recombination by targeting the recombinase to the JH gene segments. Microdomains of higher histone acetylation have also been detected in the murine β-globin locus (Litt et al., 2001) where they coincide with the expressed β-like gene.
Two other features of the DH–Cµ hyperacetylated domain are noteworthy. First, the domain terminates abruptly in the 5 kb region between the Cµ and Cδ exons. This intergenic region did not contain a nuclease-hypersensitive site that could indicate the presence of a classical insulator element. Presumably, the 3′ end of the domain is established by a different mechanism. Secondly, the chromatin structure surrounding DH gene segments was not identical. Whereas the level of histone acetylation of the five DH gene segments we examined was comparable, only the Dq52 region was marked by a closely associated DNase I-hypersensitive site. We conclude that a closely located hypersensitive site is not required for recombinase accessibility or histone acetylation.
Implications for VH gene rearrangements
DJH before VHDJH rearrangements. The 12/23 rule specifies that V(D)J recombination occurs only between gene segments whose RSSs contain 12 and 23 nucleotide spacers. In the IgH locus, the RSSs of murine JH and VH gene segments are associated with 22 bp spacers, while the DH gene segments are flanked by RSSs with 12 bp spacers. Therefore, VH can rearrange only to DH, but not JH gene segments. However, DH to JH rearrangements invariably occur before recombination of VH to DH gene segments. Our observations provide a plausible explanation for the order of rearrangements at the IgH locus. We found that VH genes were not associated with hyperacetylated histones in bone marrow pro-B cells, whereas DH and Cµ regions were. These observations suggest that the VH gene segments are not accessible to recombinase at the time that cells are undergoing DH to JH recombination. The later activation of VH ensures that VH rearrangement only occurs after DJH recombination.
VH subdomains. We find that the VH gene locus contains at least three independently regulated subdomains. The 5′-most genes were IL-7 responsive, genes lying in the middle of the cluster (represented by VH10) were activated by v-abl (unpublished data) and the 3′ most genes were only acetylated in cells that contained DJH recombinants. The close correlation between the DJH rearranged status of the IgH locus and proximal VH gene acetylation suggests that DJH recombination may be a prerequisite for activation of these genes. In this model, the state of DJH rearrangements must be sensed by proximal VH genes. An obvious possibility is that DH to JH rearrangement brings these genes closer to Cµ. By doing so, perhaps they come under the influence of Cµ-associated recombination enhancers, such as the µE. Obviously, how close they get depends on which DH segment rearranges on a particular allele. With the exception of Dq52, however, rearrangement of any other DH gene segment deletes, or inverts, at least 19 kb of genomic DNA. This is because the DH closest to Dq52, DSP2.10, is 19 kb away; every other DH gene segment is separated by a further 5–10 kb. Therefore, proximity changes as a result of DH to JH recombination can be significant, particularly in light of our observations that acetylation boundaries can be affected by even a few kilobases of DNA (e.g. between Cµ and Cδ).
Dysregulation of V gene recombination at the IgH and TCR γ chain loci has been noted in IL-7Rα-deficient mice (Corcoran et al., 1998; Schlissel et al., 2000). Lack of Vγ recombination in these animals has been attributed to reduced accessibility of the TCR-γ locus (Schlissel et al., 2000), but the mechanisms that operate at the IgH locus have not been clarified. IL-7-induced hyperacetylation of J558 genes indicates that impaired rearrangement of upstream VHs in IL-7R–/– pro-B cells (Corcoran et al., 1998) is likely to be a direct consequence of the control of chromatin structure of these VHs by IL-7, rather than the indirect result of the IL-7–IL-7R pathway in promoting proliferation, or survival, of early lymphoid precursors. We suggest that the genomic regions containing the upstream VHs never get hyperacetylated in IL-7R–/– mice and are therefore not accessible to the recombinase machinery. This direct effect on VDJ recombination in pro-B cells also explains why B lymphopoiesis cannot be rescued in IL-7R–/– mice by expression of a transgenic bcl-2 gene (Akashi et al., 1998; Maraskovsky et al., 1998).
Order of VH gene rearrangements. IL-7 responsiveness of the 5′ VH genes also suggests a model for the preferential rearrangement of DH-proximal VH genes in pro-B cells, a characteristic feature of IgH gene assembly. The model couples our results with the observation that developing pro-B cells become increasingly responsive to IL-7. This is manifest at the level of both receptor expression and receptor responsiveness. Though IL-7Rα is expressed at the earliest stages of B-cell development (Kim et al., 2000; Wei et al., 2000), highest levels are reached only in Hardy fractions C and D (Figure 6). However, high surface expression of the receptor is not sufficient for enhanced IL-7 responsiveness, as shown most clearly by the requirement for high concentrations of IL-7 to induce proliferation of RAG– pro-B cells (Marshall et al., 1998). It has been proposed that the threshold for IL-7 responsiveness may be regulated by the pre-B-cell receptor. We propose the following model for VH gene activation during B-cell ontogeny.
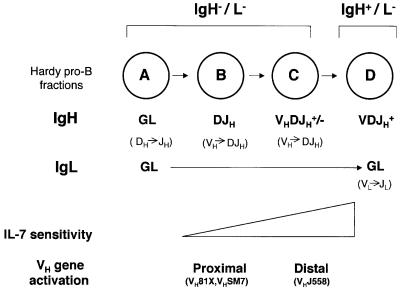
Fig. 6. Mechanism of ordered VH recombination. Pro-B cell subsets as defined by Hardy are shown in the circles. Genomic structures of the Ig loci are shown in bold below each circle (GL = germline). Presumed ongoing rearrangements in each subset are indicated in parentheses below the genomic structure. Recombination at the IgH locus is initiated in late A fraction cells; our observations suggest that only the DH–Cµ part of the locus is accessible to recombinase at this stage, resulting in DH to JH recombination. Proximal VH gene families are activated on DJH recombined alleles as outlined in the text. We propose that distal VH genes, that are activated by IL-7, lag behind the proximal VHs because IL-7 responsiveness is gained gradually during B-cell development based on Wei et al. (2000) (indicated by a triangle). Preferential rearrangement of proximal VH genes is therefore a consequence of the late activation of the 5′ VH genes via the IL-7 receptor.
Activation of the 120 kb DH–Cµ region takes place first; VH genes are inactive at this stage because low levels of IL-7R expression makes these cells non-responsive to IL-7 and DH to JH recombination has not yet occurred. This is presumably the state in late A fraction cells and allows initiation of DH to JH recombination (Figure 6). DJH recombination on both alleles (fraction B, Figure 6) primes the proximal VH genes to become hyperacetylated, and consequently recombinogenic, as described above. Though these cells steadily gain IL-7 responsiveness, the lag between DJH rearrangement and IL-7 signaling delays activation of the 5′ VH gene families. By default, the proximal VH gene families become accessible earlier, and therefore undergo preferential rearrangement in pro-B cells. Thus, preferential rearrangement of the VH 7183 family is the result of independent activation of different parts of the VH locus and the complex pattern of IL-7 responsiveness of developing pro-B cells. Our model also accounts for the observation that the frequency of VH81X utilization drops even in the absence of selection mediated by IgH (Marshall et al., 1996).
Materials and methods
DNaseI and MNase assays
The DNase hypersensitive assay was done as described (Landry et al., 1993), using 108 RAG– and 2017 cells, respectively. The MNase digestion was done similarly, with the addition of 2 mM CaCl2 in the RSB buffer (Landry et al., 1993). An 8–10 µg aliquot of DNase- or MNase-treated DNA was digested with appropriate restriction endonucleases, fractionated by agarose gel electrophoresis and analyzed by Southern blotting. Probes indicated in Figure1 were labeled by random priming in the presence of [α-32P]dCTP.
Chromatin immunoprecipitations
The method of Parekh and Maniatis (1999) was adapted as follows. RAG– (108), 2017 cells (108), 22D6 cells (5 × 106), 38B9 cells (5 × 106), B/C fraction pro-B bone marrow cells and CD19+ bone marrow cells (5 × 106) were cross-linked by addition of 1% formaldehyde to the medium for 5 min. Cells were lysed in hypotonic lysis buffer and nuclei resuspended in sonication buffer (Parekh and Maniatis, 1999). The nuclear suspension was sonicated to reduce DNA length to between 400 and 1000 bp, and debris removed by centrifugation. The chromatin solution was diluted 2-fold in immunoprecipitation buffer (Parekh and Maniatis, 1999) pre-cleared with non-specific IgG and protein A beads for 3 h at 4°C. The supernatant was incubated with 0.5 µg of α-acetylated H3 or H4 antibodies for 2 h at 4°C. The supernatant from B/C fraction cells was incubated with 5 µg of α-acetylated H3 antibody. Immune complexes were collected with protein A beads pre-absorbed with sonicated single-stranded DNA. Following washes and elution (Braunstein et al., 1993; Parekh and Maniatis, 1999), cross-links were reversed by heating at 65°C for 6 h; DNA was recovered after proteinase K treatment, two phenol extractions and ethanol precipitation. Specific sequences in the immunoprecipitates were detected by PCR under conditions in which product yield was dependent on input DNA dose. The sequences of the primers used are given in Table I. The reactions also contained 10 ng of 32P-radiolabeled 3′ primer as a tracer. The PCR products were fractionated through 6% polyacrylamide gels and quantified using a phosphoimager.
Table I. Primer sets used in chromatin immunoprecipitation assays.
| Primer | Sequence |
|---|---|
| VHA1-5′ | GGACCTGAGCATCCTGTTGC |
| VHA1-3′ | CGAGAGCACACTGATCATAGGTAGG |
| VAR34-5′ | CCTGGGATGTCACTGATATACACTCTG |
| VAR34-3′ | GTAGTAGCCAGTAAATGAGTAACCAGAAGC |
| VHJ558.47-5′ | GCAAGGCTTCTGGATACACATTCAC |
| VHJ558.47-3′ | CCTTGCCTTTGAACTTCTCATTGTACTTAG |
| VH10-5′ | CTCTCTGCCAATGTAGGACCAG |
| VH10-3′ | GCCTGAATTTCAGGGTCAGGG |
| VHSM7-5′ | CGTTATCCTCATTGCTACTACCACC |
| VHSM7-3′ | CCAAGTCCTAACTCTGTCTGAAGAAC |
| VH81X-5′ | GAGATGAGATTCTGTCTGTTGTATGCAC |
| VH81X-3′ | CTGCAAACAAAGAGTGCTGGTCAG |
| VH81X(G)-5′ | CTCCAGAGACAATACCAAGAAGACCC |
| VH81X(G)-3′ | CCCCCTGCTGGTCCTAGATG |
| DF-5′ | GGGCAGGCATGTCTCAAAGCACAATGC |
| DF-3′ | GGAATGGTCTGTTTCTGGGGGACTTCCTC |
| DS4-5′ | GGCAGGGATTTTTGTCAAGGGATC |
| DS4-3′ | GGGTTTTTGCTGGATATATCACTGTGG |
| DS8-5′ | GTTACCTTACTTGGCAGGGATTTTTGTC |
| DS8-3′ | GTCTGCTGGGCATAATGGGTTTTTG |
| DQ-5′ | CCCCACAGGCTCGAGAACTTTAGCGACTG |
| DQ-3′ | CAGTCAGAGACCACAGGGACTCTGAGGC |
| JH-5′ | GCTGATGCAGACAGACATCCTCAGCTCC |
| JH-3′ | GGGCTCCAGGATTATCTCAGATGGAGGCC |
| EN-5′ | GGAATGGGAGTGAGGCTCTCTC |
| EN-3′ | GCTGCAGGTGTTCCGGTTCTGATCGGCC |
| Cδ-5′ | GGATGGCCTCCTACCACCTC |
| Cδ-3′ | CGTGGAGCTACATAGGGCCC |
| Cµ-5′ | GGTAGGTATCCCCCCTTCCC |
| Cµ-3′ | GAAGACAGTAGTGAGGATAGGGTGG |
| Cµ-δ-5′ | CAGCCCACCATCTTGGGCTGGTG |
| Cµ-δ-3′ | CCCTAGGGCTTGTCATGTTGTGGGAGAG |
| Cγ-2b-5′ | GGGAGGAGGGAATCACCAGAGTTGTAGGC |
| Cγ-2b-3′ | CCCTGGTATGGGCTTAGTCCAGGATGATCC |
| β2-microglobulin-5′ | GCGGTCCCAGGCTGAACGACCAG |
| β2-microglobulin-3′ | GAGAGACCAGCTAGGGCGCGCG |
Purification of CD19+ pro-B cells
Bone marrow cells were recovered from 6-week-old RAG2-deficient (BALB/c) mice by flushing the femur and tibia with 10% calf serum in phosphate-buffered saline (PBS). The cell suspension was filtered through nylon mesh to remove debris, and the cells collected by centrifugation. From six mice, we obtained 2.5 × 108 cells. The cells were resuspended in 2.25 ml of magnetic cell sorting (MACS) buffer (PBS pH 7.2, 0.5% bovine serum albumin) and incubated with 250 µl of anti-mouse CD19 antibody-coated paramagnetic microbeads (Miltenyi Biotech.) at 4°C for 15 min. The cells were washed with 10–20 vols of MACS buffer. The resuspended cells were loaded onto positive selection (RS+) minimacs columns (Miltenyi Biotech.) and attached to a magnet at 4°C. Columns were washed twice with MACS buffer, and cells were collected from the flow through. These fractions typically contained <10% CD19+ cells. The column was removed from the magnet, and the cells were washed free with MACs buffer. A small aliquot of cells was removed and restained with phycoerythrin (PE)-conjugated anti-CD19 antibody (Pharmingen) to monitor the purity of the cells by flow cytometry. More than 90% of the cells were CD19+. Purity was also confirmed by simultaneous staining of independent aliquots of cells with anti-B220 antibodies (APC-conjugated anti-B220; Pharmingen). Recovery of CD19+ cells was ∼50%.
Purification of B/C fraction pro-B cells from wild-type mouse
Bone marrow cells were recovered from 6-week-old wild-type (BALB/c) mice as described above. From 10 mice we obtained 5 × 108 cells. The cells were incubated with biotinylated antibodies (Pharmingen, San Diego, CA) against Ter119, Mac1, Gr1, Thy1 and CD3, respectively. After repeated washing, the labeled cells were then incubated with streptavidin-coated paramagnetic microbeads (Miltenyi Biotech, Auburn, CA). Again after repeated washing, the cell suspension was loaded on to a depletion (CS+) column (Miltenyi Biotech). Approximately 10% of the cells were recovered and these were then sorted in a MoFlo cell sorter for CD43+B220+HSA+ cells. We obtained 8 × 106 cells with >95% purity.
Culturing CD19+ pro-B cells with IL-7
CD19+ pro-B cells were purified as described above with the following modification. The cell suspension recovered from the bone marrow was layered on lympholyte, and centrifuged at room temperature at 1800 r.p.m. for 20 min. Live cells were aspirated from the interphase, leaving the dead cells and red blood cells in the pellet. The live cells were then washed in several volumes of the MACS buffer and the CD19+ cells were purified. Cells were cultured in RPMI medium (Gibco) supplemented with 20% fetal calf serum, 5 × 10–5 M 2-mercaptoethanol, 100 U/ml penicillin and 100 U/ml streptomycin at a concentration of 106 cells/ml. Purified recombinant mouse IL-7 (Endogen, Woburn, MA) was added at a concentration of 20 ng/ml and replenished to the same level after 3 days. Cells were cultured for 4 days and then harvested for chromatin immunoprecipitation assays.
Acknowledgments
Acknowledgements
We thank Drs Bhavin Parekh for assistance with chromatin immunoprecipitation assays, Fay Young for a Cδ probe, Eugene Oltz for a Dq52 probe, Mark Schlissel for cell purification protocols, Peter Brodeur for the design of VH-specific primers, and Mr Phil Gnatowski for help in the preparation of the manuscript. This work was supported by NIH grant (GM38925) to R.S.
References
- Akashi K., Kondo,M. and Weissman,I.L. (1998) Role of interleukin-7 in T-cell development from hematopoietic stem cells. Immunol. Rev., 165, 13–28. [DOI] [PubMed] [Google Scholar]
- Alessandrini A. and Desiderio,S.V. (1991) Coordination of immunoglobulin DJH transcription and D-to-JH rearrangement by promoter–enhancer approximation. Mol. Cell. Biol., 11, 2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin-Duclos C. and Calame,K. (1998) Evidence that immunoglobulin VH–DJ recombination does not require germ line transcription of the recombining variable gene segment. Mol. Cell. Biol., 18, 6253–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. and Rothman,P. (1998) IL-7 reconstitutes multiple aspects of v-Abl-mediated signaling. J. Immunol., 161, 4611–4617. [PubMed] [Google Scholar]
- Braunstein M., Rose,A.B., Holmes,S.G., Allis,C.D. and Broach,J.R. (1993) Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev., 7, 592–604. [DOI] [PubMed] [Google Scholar]
- Capone M., Watrin,F., Fernex,C., Horvat,B., Krippl,B., Wu,L., Scollay,R. and Ferrier,P. (1993) TCRβ and TCRα gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO J., 12, 4335–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Young,F., Bottaro,A., Stewart,V., Smith,R.K. and Alt,F.W. (1993) Mutations of the intronic IgH enhancer and its flanking sequences differentially affect accessibility of the JH locus. EMBO J., 12, 4635–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran A.E., Riddell,A., Krooshoop,D. and Venkitaraman,A.R. (1998) Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature, 391, 904–907. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Martin,V., Muller,W. and Rajewsky,K. (1994) Analysis of the B-cell progenitor compartment at the level of single cells. Curr. Biol., 4, 573–583. [DOI] [PubMed] [Google Scholar]
- Fernex C., Caillol,D., Capone,M., Krippl,B. and Ferrier,P. (1994) Sequences affecting the V(D)J recombinational activity of the IgH intronic enhancer in a transgenic substrate. Nucleic Acids Res., 22, 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier P. et al. (1990) Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J., 9, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D.S. and Garrard,W.T. (1988) Nuclease hypersensitive sites in chromatin. Annu. Rev. Biochem., 57, 159–197. [DOI] [PubMed] [Google Scholar]
- Haines B.B. and Brodeur,P.H. (1998) Accessibility changes across the mouse Igh-V locus during B cell development. Eur. J. Immunol., 28, 4228–4235. [DOI] [PubMed] [Google Scholar]
- Hardy R., Carmack,C., Shinton,S., Kemp,J. and Hayakawa,K. (1991) Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med., 173, 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T.R., Clayton,A.L., Thorne,A.W. and Crane-Robinson,C. (1994) Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken β-globin chromosomal domain. EMBO J., 13, 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T.R., Thorne,A.W. and Crane-Robinson,C. (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J., 7, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H.D. and Teale,J.M. (1989) VH gene family repertoire of resting B cells. Preferential use of D-proximal families early in development may be due to distinct B cell subsets. J. Immunol., 143, 2752–2760. [PubMed] [Google Scholar]
- Jones P.L., Veenstra,G.J., Wade,P.A., Vermaak,D., Kass,S.U., Landsberger,N., Strouboulis,J. and Wolffe,A.P. (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet., 19, 187–191. [DOI] [PubMed] [Google Scholar]
- Kim D.W. et al. (2000) Activation of NF-κB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis, 21, 871–879. [DOI] [PubMed] [Google Scholar]
- Kornberg R.D. and Lorch,Y. (1999) Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell, 98, 285–294. [DOI] [PubMed] [Google Scholar]
- Kottmann A.H., Brack,C., Eibel,H. and Kohler,G. (1992) A survey of protein–DNA interaction sites within the murine immunoglobulin heavy chain locus reveals a particularly complex pattern around the DQ52 element. Eur. J. Immunol., 22, 2113–2120. [DOI] [PubMed] [Google Scholar]
- Kottmann A.H., Zevnik,B., Welte,M., Nielsen,P.J. and Kohler,G. (1994) A second promoter and enhancer element within the immunoglobulin heavy chain locus. Eur. J. Immunol., 24, 817–821. [DOI] [PubMed] [Google Scholar]
- Landry D.B., Engel,J.D. and Sen,R. (1993) Functional GATA-3 binding sites within murine CD8α upstream regulatory sequences. J. Exp. Med., 178, 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzurica P. and Krangel,M.S. (1994) Enhancer-dependent and -independent steps in the rearrangement of a human T cell receptor δ transgene. J. Exp. Med., 179, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M.D., Simpson,M., Recillas-Targa,F., Prioleau,M. and Felsenfeld,G. (2001) Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J., 20, 2224–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn B.A., Yancopoulos,G.D., Barth,J.E., Bona,C.A. and Alt,F.W. (1990) Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J. Exp. Med., 171, 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E., Peschon,J.J., McKenna,H., Teepe,M. and Strasser,A. (1998) Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in IL-7 receptor-deficient mice but can enhance survival of mature B cells. Int. Immunol., 10, 1367–1375. [DOI] [PubMed] [Google Scholar]
- Marshall A.J., Wu,G.E. and Paige,G.J. (1996) Frequency of VH81x usage during B cell development: initial decline in usage is independent of Ig heavy chain cell surface expression. J. Immunol., 156, 2077–2084. [PubMed] [Google Scholar]
- Marshall A.J., Fleming,H.E., Wu,G.E. and Paige,C.J. (1998) Modulation of the IL-7 dose–response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J. Immunol., 161, 6038–6045. [PubMed] [Google Scholar]
- Mathieu N., Hempel,W.M., Spicuglia,S., Verthuy,C. and Ferrier,P. (2000) Chromatin remodeling by the T cell receptor (TCR)-β gene enhancer during early T cell development. Implications for the control of TCR-β locus recombination. J. Exp. Med., 192, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBlane F. and Boyes,J. (2000) Stimulation of V(D)J recombination by histone acetylation. Curr. Biol., 10, 483–486. [DOI] [PubMed] [Google Scholar]
- McMurry M.T. and Krangel,M.S. (2000) A role for histone acetylation in the developmental regulation of VDJ recombination. Science, 287, 495–498. [DOI] [PubMed] [Google Scholar]
- McMurry M.T., Hernandez-Munain,C., Lauzurica,P. and Krangel,M.S. (1997) Enhancer control of local accessibility to V(D)J recombinase. Mol. Cell. Biol., 17, 4553–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Ng,H.H., Johnson,C.A., Laherty,C.D., Turner,B.M., Eisenman,R.N. and Bird,A. (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature, 393, 386–389. [DOI] [PubMed] [Google Scholar]
- Ng H.H. and Bird,A. (1999) DNA methylation and chromatin modification. Curr. Opin. Genet. Dev., 9, 158–163. [DOI] [PubMed] [Google Scholar]
- Oltz E.M., Alt,F.W., Lin,W.C., Chen,J., Taccioli,G., Desiderio,S. and Rathbun,G. (1993) A V(D)J recombinase-inducible B-cell line: role of transcriptional enhancer elements in directing V(D)J recombination. Mol. Cell. Biol., 13, 6223–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh B.S. and Maniatis,T. (1999) Virus infection leads to localized hyperacetylation of histones H3 and H4 at the IFN-β promoter. Mol. Cell, 3, 125–129. [DOI] [PubMed] [Google Scholar]
- Sakai E., Bottaro,A., Davidson,L., Sleckman,B.P. and Alt,F.W. (1999) Recombination and transcription of the endogenous Ig heavy chain locus is effected by the Ig heavy chain intronic enhancer core region in the absence of the matrix attachment regions. Proc. Natl Acad. Sci. USA, 96, 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M.S., Durum,S.D. and Muegge,K. (2000) The interleukin 7 receptor is required for T cell receptor γ locus accessibility to the V(D)J recombinase. J. Exp. Med., 191, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwe M. and Sablitzky,F. (1993) V(D)J recombination in B cells is impaired but not blocked by targeted deletion of the immunoglobulin heavy chain intron enhancer. EMBO J., 12, 2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried Z. and Cedar,H. (1997) DNA methylation: a molecular lock. Curr. Biol., 7, R305–R307. [DOI] [PubMed] [Google Scholar]
- Sleckman B.P., Gorman,J.R. and Alt,F.W. (1996) Accessibility control of antigen-receptor variable-region gene assembly: role of cis-acting elements. Annu. Rev. Immunol., 14, 459–481. [DOI] [PubMed] [Google Scholar]
- Sleckman B.P., Bardon,C.G., Ferrini,R., Davidson,L. and Alt,F.W. (1997) Function of the TCRα enhancer in αβ and γδ T cells. Immunity, 7, 505–515. [DOI] [PubMed] [Google Scholar]
- Sleckman B.P., Bassing,C.H., Bardon,C.G., Okada,A., Khor,B., Bories,J.C., Monroe,R. and Alt,F.W. (1998) Accessibility control of variable region gene assembly during T-cell development. Immunol. Rev., 165, 121–130. [DOI] [PubMed] [Google Scholar]
- Spolski R., Miescher,G., Erard,F., Risser,R., MacDonald,H.R. and Mak,T.W. (1988) Regulation of expression of T cell γ chain, L3T4 and Ly-2 messages in Abelson/Moloney virus-transformed T cell lines. Eur. J. Immunol., 18, 295–300. [DOI] [PubMed] [Google Scholar]
- Stanhope-Baker P., Hudson,K.M., Shaffer,A.L., Constantinescu,A. and Schlissel,M.S. (1996) Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell, 85, 887–897. [DOI] [PubMed] [Google Scholar]
- Strahl B.D. and Allis,C.D. (2000) The language of covalent histone modifications. Nature, 403, 41–45. [DOI] [PubMed] [Google Scholar]
- ten Boekel E., Melchers,F. and Rolink,A.G. (1997) Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity, 7, 357–368. [DOI] [PubMed] [Google Scholar]
- Tripathi R.K. et al. (2000) Definition of a T-cell receptor β gene core enhancer of V(D)J recombination by transgenic mapping. Mol. Cell. Biol., 20, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Zeff,R. and Goldschneider,I. (2000) Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7Rα, terminal deoxynucleotidyltransferase and Cµ expression. J. Immunol., 164, 1961–1970. [DOI] [PubMed] [Google Scholar]
- Whitehurst C.E., Chattopadhyay,S. and Chen,J. (1999) Control of V(D)J recombinational accessibility of the Dβ1 gene segment at the TCRβ locus by a germline promoter. Immunity, 10, 313–322. [DOI] [PubMed] [Google Scholar]
- Whitehurst C.E., Schlissel,M.S. and Chen,J. (2000) Deletion of germline promoter PDβ1 from the TCRβ locus causes hypermethylation that impairs Dβ1 recombination by multiple mechanisms. Immunity, 13, 703–714. [DOI] [PubMed] [Google Scholar]
- Wolffe A.P. and Guschin,D. (2000) Review: chromatin structural features and targets that regulate transcription. J. Struct. Biol., 129, 102–122. [DOI] [PubMed] [Google Scholar]
- Workman J.L. and Kingston,R.E. (1998) Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem., 67, 545–579. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G.D., Desiderio,S.V., Paskind,M., Kearney,J.F., Baltimore,D. and Alt,F.W. (1984) Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature, 311, 727–733. [DOI] [PubMed] [Google Scholar]
- Yu C.C., Larijani,M., Miljanic,I.N. and Wu,G.E. (1998) Differential usage of VH gene segments is mediated by cis elements. J. Immunol., 161, 3444–3454. [PubMed] [Google Scholar]