Abstract
Here we analyze patterns of human infection with Onchocerca volvulus (the cause of river blindness) in different continents and ecologies. In contrast with some geohelminths and schistosome parasites whose worm burdens typically exhibit a humped pattern with host age, patterns of O. volvulus infection vary markedly with locality. To test the hypothesis that such differences are partly due to heterogeneity in exposure to vector bites, we develop an age- and sex-structured model for intensity of infection, with parasite regulation within humans and vectors. The model is fitted to microfilarial data from savannah villages of northern Cameroon, coffee fincas of central Guatemala, and forest-dwelling communities of southern Venezuela that were recorded before introducing ivermectin treatment. Estimates of transmission and infection loads are compared with entomological and epidemiological field data. Host age- and sex-heterogeneous exposure largely explains locale-specific infection patterns in onchocerciasis (whereas acquired protective immunity has been invoked for other helminth infections). The basic reproductive number,R0, ranges from 5 to 8, which is slightly above estimates for other helminth parasites but well below previously presented values.
Keywords: age, sex, helminth, mathematical model, onchocerciasis
Human helminth infections are a major cause of morbidity in low-income countries worldwide, with over one-third of the world's population infected and some 300 million people suffering consequences of severe schistosomiasis (bilharzia), intestinal helminthiases, lymphatic filariases (causing elephantiasis), and onchocerciasis (river blindness) (1–3). Large-scale control initiatives have been put in place to address these public health problems (3–5). However, the population and within-host processes underlying epidemiological patterns are complex and poorly understood. Among the remaining enigmas are the precise mechanisms regulating parasite abundance and determining host age–infection patterns. Understanding these mechanisms is important for explaining parasite resilience and, most crucially, for predicting postcontrol dynamics.
Analyses of age–infection patterns have been used to suggest processes underlying the population dynamics of helminth infections. The patterns may or may not be consistent with the (null) hypothesis of a simple immigration death process for the development of infection in hosts (6). Under this model, which assumes constant rates of parasite acquisition and mortality, infection intensity increases monotonically with age and saturates at a value determined by the ratio of these two rates. Departures from this pattern indicate that rates of parasite acquisition and/or mortality may depend on host age (through age-specific exposure or susceptibility), established parasite density (due to acquired immunity), or both (7, 8). A prediction of models incorporating long-lasting protective immunity is that age-intensity profiles would peak and subsequently decline with age (7). Also, peak infection intensities would be higher and shifted toward earlier ages as transmission increases (9). The detection of significant “peak shifts” in schistosomiasis is consistent with the acquisition of protective immunity elicited by repeated reinfection (9).
Depending on location, profiles of Onchocerca volvulus infection, measured by density of skin microfilariae (mf), are reported to plateau (10), peak (11), or increase (12) with host age. Although some age-dependency in exposure is assumed in epidemiological models (13–16), infection-facilitated parasite establishment has been proposed as another explanation for increasing age-intensity profiles (17). However, differences in infection between males and females can be as significant as those among age groups (18–20). Recent analyses suggest that excess mortality and blindness rates, for given mf loads, are higher in men than in women (21). However, most existing models consider the human population without regard for sex-specific characteristics.
In this paper, we present an age- and sex-structured model for human onchocerciasis. As in previous work (22), parasite establishment in humans is determined by exposure to infective stages. The model is fitted to cross-sectional survey data from northern Cameroon, central Guatemala, and southern Venezuela. We estimate entomological parameters (annual vector biting rate) and infection levels in humans and vectors and compare these outputs with observations. We focus on possible heterogeneity in exposure to explain age–infection patterns specific to each sex and geographic region and present estimates of the basic reproductive ratio, R0, of O. volvulus in each environment.
This full-lifecycle, host-structured, transmission model is fitted to O. volvulus infection data across continents in an attempt to capture major sex and geographical variations in intensity. We start by describing the study areas and data, then formulate the model and its assumptions, stating methods for parameter estimation and statistical analyses, and discuss the epidemiological implications of our results.
Methods
Study Areas and Parasitological Data. Cross-sectional surveys of skin mf load (mf per mg of skin) were conducted before introduction of mass ivermectin treatment in 37 Sudan savannah (Mboum and Dourou) villages of the Vina du Nord valley in northern Cameroon (23), Mayan populations living in nine coffee fincas of central Guatemala (24), and 21 Yanomami communities of the Venezuelan Upper Orinoco basin (12, 25, 26). The number of people (and percentage of the total population) examined was, respectively, 5,040 (50%), 872 (76–99%), and 995 (70%). The parasitological procedures applied have already been described (24, 25, 27). The average mf prevalence in each study area was ≈70%. Only villages mesoendemic and hyperendemic (mf prevalence, >20%) and individuals born or resident in each village (and aged 5–80 years) with mf loads ≤550 mf per mg of skin (in line with ref. 28) are included in the analyses.
Entomological Data. Annual vector biting rates (ABR) and number of third-stage infective larvae (L3) per fly for savannah forms of Simulium damnosum s.l. had been recorded before introduction of ivermectin in the Vina valley, an area without vector control (27). Preintervention entomological indices (biting and infection rates) have been collated for Simulium ochraceum s.l. in the Guatemalan fincas (29) and reported for Simulium guianense s.l. and Simulium incrustatum in mesoendemic and hyperendemic Amazonian areas (30–32).
Observations pertaining to each region (mf prevalence and intensity, ABR, and mean number of L3 larvae per fly) were averaged across study villages to obtain country- and vector-specific data with which to contrast predictions.
Ethical Clearance. Informed consent from participants (or their parents or guardians) was obtained before skin snip sampling. Communities in the study area are currently incorporated into national onchocerciasis control programs.
Model Assumptions and Formulation. We use an intensity model for mean parasite loads in an age- and sex-structured human population and in the vector population. The following assumptions and notation extend prior work (22).
The human population density, ρs(a) = ρsρ(a), is a truncated exponential function of age (a) (Table 1), with constant per capita mortality rate μH and sex-specific weights ρs(Σρs = 1), as supported by the data.
The mean number of adult worms, Ws(a), and density of mf per mg of skin, Ms(a), in a person depend on the person's age and sex. The mean number of L3 in a vector, Ls(a), depends on the age and sex of the person infecting the fly (assuming that each blood meal is taken from a single host and that larvae develop from surviving mf ingested during that blood meal).
- The contact rate per human with vectors is mβΩs(a), where m is the vector to human ratio, β is the biting rate per fly on humans, and
is an age- and sex-specific measure of exposure to vectors with parameters E0, Es, αs, and q, and with population average equal to 1 [ensured by ΣEsρs = 1 and factors γs]. Relative exposure (of males with respect to females) is defined by Q = EM/EF. After an increase in exposure approximated by a step function (E0 < 1) during childhood period q (13, 14), contacts can increase (αs < 0), decrease (αs > 0), or remain constant (αs = 0) with age.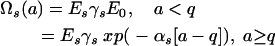
[1] There is a prepatent period, p, between acquisition of an infective larva from a vector and migration of offspring mf to human dermal tissues (after which transmission is possible). The value of p is fixed at 2 years (33, 34).
- The number of flies biting a host during p, mβΩs(a)p, is large; thus, these flies constitute a representative sample of the vector population. The number of parasites inoculated into a human at any time is therefore proportional to the mean number of L3 per fly in the vector population, given by
with probability ρ(a)Ωs(a) that a vector feeds on a host of given age and sex.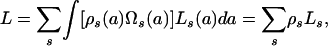
[2]
Table 1. Parameters of the model.
| Values and units
|
|||||
|---|---|---|---|---|---|
| Symbol | Description | Cameroon | Guatemala | Venezuela | Comments and sources |
| ks(a) | Overdispersion parameter of the negative binomial for mf load with host age a and sex s | Estimated (Fig. 1) | - | ||
| ρF/ρM | Fraction of human females/males | 0.45/0.55 | 0.42/0.58 | 0.40/0.60 | Sample |
| ρ(a) | Proportion of humans at age a (function of μH) | Estimated (Fig. 3) | |||
| μH | Per capita mortality rate of human host | 0.040 yr-1 | 0.040 yr-1 | 0.032 yr-1 | Fig. 3 |
| am | Maximum recorded human age | 80 yr | 80 yr | 70 yr | Sample |
| β | Biting rate per fly on humans = h/g | - | - | ||
| g | Time interval between consecutive blood meals | 0.0096 yr (3.5 days) | 22 | ||
| h | Fraction of blood meals taken on humans | 0.3 | 0.6* | 0.5† | 22, 29 |
| m | Average number of vectors per human | Estimated (Table 2) | - | ||
| Es | Sex-specific exposure | Estimated (Table 3) | - | ||
| E0 | Relative exposure at age 0 in relation to age q | 0.10 | 0.10 | 0.05 | ‡ |
| q | Period of initial increase in exposure to vector bites during childhood | Estimated (Table 3) | - | ||
| αs | Rate of change in contact rate with age in human of sex s | Estimated (Table 3) | - | ||
| Q | Relative male-to-female exposure = EM/EF. | Estimated (Table 3) | - | ||
| p | Prepatent period from infective bite to mf migration to human skin | 2 yr | 33, 34 | ||
| Δ | Per capita fecundity rate of female worms scaled per mg of skin assuming all females are mated and sex ratio is 1 | 0.337 yr-1 | 22 | ||
| σW/σM | Per capita mortality rates of adult worms/skin mf | 0.1 yr-1/0.8 yr-1 | 34 | ||
Assuming that parasite and host populations are at endemic equilibrium, as tested later, the dynamics of mean loads of adult worms and mf in humans are given by the following system of integro-differential equations:
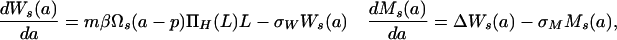 |
[3] |
with L given explicitly, in terms of human mf loads, by
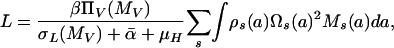 |
[4] |
where Ws(a) = Ms(a) = 0 for a ≤ p; ΠH(L) is the probability of establishment of acquired L3 larvae in humans; Δ is the per capita rate at which adult worms produce mf [assuming that half the worms are female and all females are mated (22)]; MV is the average mf density in the population of vectors feeding on human blood; ΠV(MV) is the probability of development of ingested mf into L3 larvae within the vector; σW and σM are, respectively, per capita mortality rates of adult worms and mf; σL(MV) is the rate of loss of L3 larvae per vector (including parasite-induced vector mortality); and 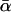 is the average of αs over the vector population. Regulatory constraints (nonlinearities) are present in both humans and vectors via ΠH, ΠV and σL (22, 29). At endemic equilibrium, an explicit solution is obtained (and used for efficient estimation) by ignoring density-dependent parasite uptake by vectors, i.e., with ΠV constant and parasite abundance within flies regulated solely through parasite-induced vector mortality. Model parameters are summarized in Table 1.
is the average of αs over the vector population. Regulatory constraints (nonlinearities) are present in both humans and vectors via ΠH, ΠV and σL (22, 29). At endemic equilibrium, an explicit solution is obtained (and used for efficient estimation) by ignoring density-dependent parasite uptake by vectors, i.e., with ΠV constant and parasite abundance within flies regulated solely through parasite-induced vector mortality. Model parameters are summarized in Table 1.
Demography of the Human Population. Demographic parameters for each country (μH, ρs, s = F, M) are estimated directly from the population sample, which represents ≥50% of the total population. In the surveys, human age was ascertained by questioning participants during physical examination or estimated visually when exact age was not known. An exponential distribution of survival times adequately represents the proportion of individuals surviving at age a. Because, apart from scale factor ρs, there is similarity in age profiles between sexes, μH is estimated jointly (via least squares) for both sexes. Estimates of ρs and μH for each country are presented in Table 1.
Distribution of mf Counts. Although the dynamics of mean parasite loads are deterministic (i.e., not affected by the degree of variability about mean values), mf counts in individual humans are assumed to be independent random variables following a negative binomial (NB) distribution, with mean Ms(a) and aggregation index ks(a), which depend on an individual's country, age, and sex. The NB distribution is modified to allow for more flexible age- and sex-specific numbers of zero counts. The aggregation index, ks(a) = Ms(a)2/[σs(a)2 - Ms(a)], with σs(a)2 as the NB variance, is an inverse measure of overdispersion. Preliminary (moment) estimates of ks(a) [based on sample mean and variance within age groups (35)] suggest that aggregation does not depend strongly on host sex. Hence, we model aggregation with country-specific (logistic or lognormal) functions of age, k(a), with three free parameters (b0, b1, and b2).
Parameter Estimation and Predictions. We estimate exposure parameters m (vector-to-human ratio), q (human growth period when exposure to vector bites increases with age), Es, and αs [of the sex- and age-specific contact function Ωs(a)] and overdispersion parameters b0, b1, and b2 jointly by using maximum likelihood and individual mf-count data and by assuming a NB distribution of mf counts with age-, sex-, and country-specific mean and overdispersion, with demographic parameters as described and remaining parameters as in Table 1. Overall and sex-specific ABR per person are given by ABR = mβ and ABRs = mβEs, respectively. Predicted overall mean adult worm and mf loads in humans (W and M) and L3 larval load per fly in the vector population (L) are averages of model outputs over the human and vector populations. Mean number of palpable O. volvulus nodules per person is estimated assuming that the worm sex ratio is 1 and that each palpable nodule corresponds on average to 34 females anywhere in the host body (34). Predicted overall mf prevalence (proportion of mf carriers in the human population) combines the model for mean mf intensity, Ms(a), with the NB model for distribution of intensity (which determines the prevalence–mean relationship). Expressions for the basic reproductive ratio, R0 [average number of adult female worms in the human population produced by a mated female worm during reproductive lifetime in absence of regulation (6)], and effective reproductive ratio, Re (its equivalent in the presence of density dependence), are derived and evaluated. The latter is used to confirm endemic equilibrium status, i.e., when on average each female worm only replaces itself (Re ≅ 1) (6). Endemic equilibrium is not guaranteed, because it depends on the age structure and infection pattern of the communities not having been perturbed. The condition Re = 1 is only satisfied by some combinations of fixed and estimated parameter values, and estimated values are determined both by the model and the data. An estimate Re = 1 would show consistency of the data with the equilibrium assumption and give credit to R0 estimates.
Assessment. Confidence intervals (C.I.) for parameter estimates and predictions are evaluated using nonparametric bootstrap (36), i.e., by resampling the original number of individuals of each age and sex from the data set with replacement. Estimated C.I. are based on 1,000 simulations. Goodness of fit is assessed for each country by allowing a quadratic extension of the model for mean mf load: Ms(a) + Asa + Bsa2. Parameters As and Bs are estimated from the data, jointly with those in Ms(a), and improvements in fit are tested via likelihood ratio at 5% significance.
Supporting Information. For more information on methods and results, see Supporting Text, Table 4, and Fig. 3, which are published as supporting information on the PNAS web site.
Results
Parasitological and Entomological Estimates. Predicted ABR, overall mf prevalence, and overall parasite loads in humans and flies are presented in Table 2. Predicted confidence regions are narrowest for Cameroon (the largest data set) and contain the observed values, with two exceptions. First, C.I.s for mf prevalence contain the observed value of 70% in Venezuela but are 12–14% below observations for Cameroon and Guatemala. We attribute this small discrepancy to inaccuracy either in the model for mean intensity or, perhaps more likely, in the NB distribution assumption. As we fit intensity data, we expect more accurate predictions for intensity than for prevalence of infection, which is, nevertheless, in the range 60–70%, which is very close to observations. Second, mean mf load in the Amazonian focus is underestimated by ≈15% (counterbalanced by a better prediction of prevalence).
Table 2. Entomological and parasitological estimates.
| Country (vector) | Data type | ABR per person per yr | Mf prevalence, % | Mf load, mf per mg of skin | Infective larval load, L3 per fly |
|---|---|---|---|---|---|
| Cameroon (S. damnosum s.s./S. sirbanum) | Observed | 42,800 | 71.0 | 39.4 | 0.032 |
| Estimate | 42,500 | 61.0 | 38.1 | 0.031 | |
| 95% C.I. | 38,700, 46,400 | 60.0, 62.0 | 36.4, 39.8 | 0.029, 0.035 | |
| Guatemala (S. ochraceum s.l.) | Observed | 205,700 | 70.0 | 40.3 | 0.005 |
| Estimate | 202,800 | 63.0 | 40.2 | 0.006 | |
| 95% C.I. | 154,000, 245,600 | 61.0, 65.0 | 36.3, 44.5 | 0.005, 0.007 | |
| Venezuela (S. guianense s.l./S. incrustatum) | Observed | 63,970 | 70.0 | 38.7 | 0.012 |
| Estimate | 60,500 | 71.0 | 29.2 | 0.013 | |
| 95% C.I. | 47,500, 74,400 | 69.0, 73.0 | 26.1, 32.4 | 0.012, 0.014 |
Observations of ABR and L3 load are independent from the data to which the model has been fitted.
The average number of female adult worms per person is in the range 40–50, in line with nodulectomy data from Burkina Faso (savannah) and Liberia (forest) (17, 28). The mean number of palpable O. volvulus nodules per person is 1.42 (95% C.I. 1.36, 1.48) in Cameroon; 1.46 (95% C.I. 1.31, 1.60) in Guatemala; and 1.10 (95% C.I. 0.99, 1.22) in Venezuela. Because of overdispersion, individual hosts may carry considerably more or less nodules than indicated by these averages.
The predicted ABR is higher for S. ochraceum s.l. (Guatemala), than for Amazonian vectors S. guianense/S. incrustatum (Venezuela), or savannah vectors S. damnosum/Simulium sirbanum (Cameroon). L3 larval loads in the vector follow the opposite trend, whereas mf prevalence (≈60–70%) and mf intensity (≈40 mf per mg of skin) are very similar in all three areas. These results agree with the fact the meso-American vector is the least competent, requiring higher densities to attain similar endemicity levels (29).
Exposure. Model fits suggest that males are subject to a higher vector biting rate than females in the study areas of Cameroon and Guatemala. Predicted ABRM is 45,900 (95% C.I.: 40,900, 50,700) whereas ABRF is 38,300 (34,300, 42,700) in Cameroon. Corresponding predictions for Guatemala are ABRM = 241,900 (185,200, 292,300) and ABRF = 153,084 (108,100, 191,100). In contrast, males and females are more evenly bitten in Venezuela, with ABRM = 64,700 (50,700, 79,500) and ABRF = 54,300 (40,300, 69,200). The significantly high men-to-women relative exposure (Q) in Guatemala and different magnitude and sign of age-contact exponents (αM and αF) in Cameroon (Table 3) suggest that exposure differences in Guatemala are consistent across host ages, whereas relative exposure in Cameroon may change with age. Parameter αs is significantly negative for women in Cameroon and men and women in Venezuela, indicating that, after period q, exposure continues to increase with age. In contrast, αs ≈ 0 for men in Cameroon and men and women in Guatemala, suggesting that exposure reaches a plateau.
Table 3. Exposure and epidemiological estimates.
| Country | Data type | q, yr | αF | αM | Q = EM/EF | R0 | Re |
|---|---|---|---|---|---|---|---|
| Cameroon | Estimate | 0.0 | -0.023 | 0.007 | 1.20 | 7.7 | 0.99 |
| 95% C.I. | 0.0, 0.0 | -0.030, -0.017 | 0.002, 0.012 | 1.07, 1.33 | 7.0, 8.4 | 0.91, 1.04 | |
| Guatemala | Estimate | 2.6 | 0.004 | 0.007 | 1.61 | 7.3 | 1.02 |
| 95% C.I. | 2.0, 4.2 | -0.010, 0.020 | -0.002, 0.019 | 1.31, 2.04 | 5.6, 8.9 | 0.93, 1.06 | |
| Venezuela | Estimate | 2.3 | -0.023 | -0.039 | 1.19 | 5.3 | 0.97 |
| 95% C.I. | 1.6, 4.8 | -0.035, -0.011 | -0.048, -0.027 | 0.98, 1.47 | 4.1, 6.5 | 0.90, 1.03 |
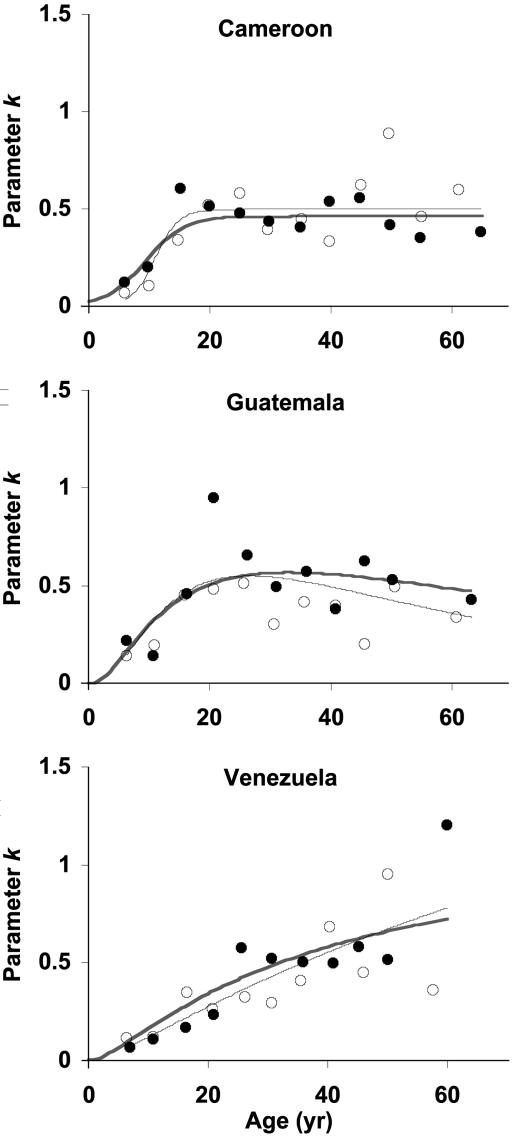
Age-Specific Overdispersion. In all studies, the endemic area dispersion index k(a) increases with host age until 25–30 years, when k(a) ≈ 0.5 (Fig. 1).
Fig. 1.
Observed and predicted age profiles of the overdispersion parameter, ka). Shown are moment estimates within 3-year age groups ○, females; •, males), a direct fit to moment estimates thick line), and the full model fitted to individual data jointly with the model for Msa) thin line). Direct and full-model fits use the same three-parameter function: logistic Cameroon) or lognormal Guatemala and Venezuela). The full model agrees with the moment estimates, supporting our model for overdispersion.
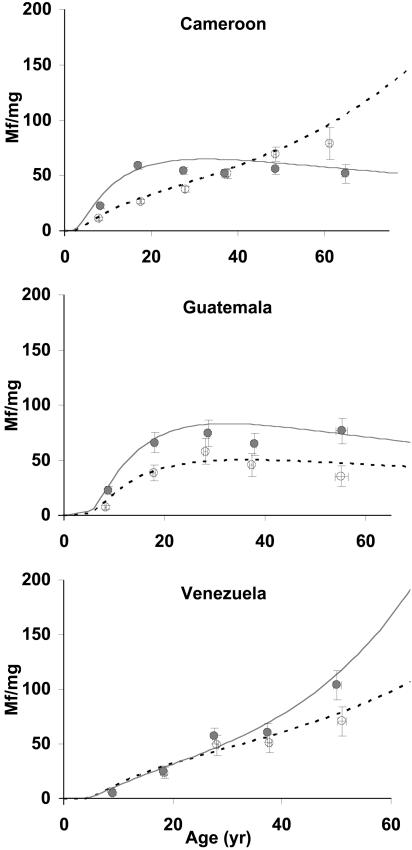
Age-Infection Profiles. Age-profiles of mf infection show marked differences among study areas and between sexes, consistent with the exposure results (Fig. 2). In northern Cameroon, mean mf intensity in men increases rapidly with age, leveling off at ≈60 mf per mg of skin by age 30 years. In contrast, women experience a much slower increase in infection intensity; for age <45, skin loads in females are consistently lower than those in males. Above 45 years, however, females surpass males as their mf loads rise at an increasing rate, reaching 140 mf per mg of skin at age 75. In central Guatemala and in agreement with other reports (11, 19), mf burden is higher in men than in women for all ages, reaching a plateau for both sexes at ≈30 years. In southern Venezuela, there is less difference between mf loads in men and women, with both sexes exhibiting an increase with age similar in shape and magnitude to that of women in the Vina valley. However, beyond age 30, skin infection in men tends to be higher than in women. Whereas mf levels can reach 150 mf per mg of skin for women living in endemic areas of northern Cameroon and among Yanomami males, they do not exceed 100 mf per mg of skin for either men or women in central Guatemala.
Fig. 2.
Observed and predicted age profiles of mf load by sex and country. Shown are mean and standard error of observations within ≈10-year age groups ○, females; •, males) and the model fitted to individual data Cameroon, ≈5,000 individuals; Guatemala, ≈900 individuals; Venezuela, ≈1,000 individuals). Solid lines represent males, and dashed lines represent females.
Reproductive Ratios. Predicted Re values are very close to 1, and confidence regions include 1 (Table 3), which lends support to the assumption of endemic equilibrium. Predicted R0 values for O. volvulus range from 5 (Amazonian focus) to 8 (northern Cameroon), and confidence regions range from 4 to 9. R0 estimates for other helminth parasites are of the order of 1–4 for schistosomes and 1–6 for soil-transmitted nematodes (6). Previous village-specific R0 estimates for the same area of Cameroon ranged from 1 to ≥50 (13, 22).
Discussion
Endemic profiles of O. volvulus infection vary with host age and sex and geographic area quite unlike other directly and indirectly transmitted helminth parasites (possibly excepting lymphatic filarial nematodes). We have used a structured and full-lifecycle transmission model to explain such profiles across continents. Most models focus on onchocerciasis in West Africa (13–17), with some models focusing on Latin America (19), but few attempt to bring epidemiological commonalities and differences into a single and coherent mathematical framework (29). Age-specific exposure to vector bites (13–16), operation of parasite-related human mortality (13, 15), and parasite-induced immunosuppression (12, 17, 26) are among the mechanisms put forward to explain observed departures of O. volvulus patterns from predictions generated by simple immigration–death models (7). No model, however, has also attempted to capture sex-related heterogeneities, despite infection and morbidity in some endemic areas differing markedly between males and females (11, 18–22, 24). The relative contribution of ecological (behavior, environment, and body size) and physiological (hormonal and immunological) factors to sex-specific infection and disease has long been debated (35). Most studies, however, including ours, measure parasite load indirectly instead of directly through adult worms (but see ref. 17).
In this article, we have proposed a novel age- and sex-structured model for the population biology of human onchocerciasis, which largely explains differences in infection patterns among epidemiological settings and host ages and sexes by assuming heterogeneous exposure (rather than heterogeneous susceptibility/parasite establishment). Our formulation of exposure to vectors, in terms of host age and host sex yields infection patterns that may increase, saturate, or peak with host age [respectively, the so-called types I, II, and III age-intensity curves (35)]. The goodness of fit achieved for endemic equilibrium is very satisfactory, given that we (i) predict overall transmission and infection intensities compatible with averaged (precontrol) entomological and parasitological indices, (ii) reproduce age–infection profiles, and (iii) provide sensible composite measures of transmission success (R0 and Re).
Behavior, occupation, and clothing are factors that would determine exposure in different settings. Our results are compatible with knowledge of population activities in the study areas. Differences in exposure between boys (fishing and swimming) and girls (occupied in households) and between men (spending less time outside villages as they age) and women (working the fields and collecting water in rivers until old age) have been quantified in northern Cameroon and related to subsequent infection and ocular morbidity (20). Supporting the importance of behavioral and cultural determinants of exposure, a study conducted in the same villages and ethnic groups 10 years earlier (18) reported age and sex patterns of mf intensity very similar to those shown in Fig. 2A (although differences between sexes were ascribed to hormonal factors; see also ref. 37). In Guatemala, onchocerciasis is transmitted mainly in coffee growing, mountainous areas, with men generally more exposed to vectors while working in such areas (11, 19, 24). Among the Yanomami, use of clothing is minimal, traditional houses lack enclosing walls, and men and women engage in activities around and outside villages (26, 30). However, we cannot dismiss the possible influence of host genetics, because the study populations belong to distinct ethnic groups. In West Africa, larger excess mortality of males in relation to females because of onchocerciasis has been well documented (21) and could also help explain sex differences in age profiles of mf load in Cameroon. Possible causes for an increase or decrease in (effective) exposure to vector bites with age may include changes in host daily activity patterns or thickening of the skin with prolonged exposure to flies and/or mf infection (24).
We are not proposing that exposure is the only determinant of observed age-profiles of infection. O. volvulus has been shown to reduce host immunological responsiveness to parasite-specific and other antigens (reviewed in ref. 38), and it has been proposed that age- and/or parasite-specific immunosuppression may be responsible for increasing parasite loads with host age (12, 17). Models have been developed that make the rate of parasite establishment an increasing function of already established worms to fit adult worm nodulectomy data (8, 17, 28). However, these models exclude age-dependent exposure as a plausible hypothesis and do not deal with sex-related heterogeneities. The hypotheses of heterogeneity in host exposure or in parasite establishment rates are not mutually exclusive, and our model can easily be modified to include parasite-, age-, and sex-dependent susceptibility (35, 37, 39). In practice, given the lack of detailed exposure data, it may be difficult to disentangle exposure effects from those of within-host factors (e.g., hormonal changes and host genetics) (39). Model fitting to data categorized by endemicity level (a surrogate for transmission intensity) may help clarify the relative contribution of exposure and susceptibility.
Changes in degree of parasite overdispersion with host age have been reported widely (35); in the case of bancroftian filariasis, overdispersion decreases with age for males and females (39). Operation of density-dependent constraints, clumped infection events, heterogeneities in parasite acquisition, negative correlation between overdispersion and intensity of infection, and sample-size biases have been proposed to explain observed patterns (35, 39).
Our overall estimates of R0 for O. volvulus (ranging from 5 to 8) are slightly above those for other helminths (between 1 and 6) but well below village-specific estimates previously derived without accounting for age or sex structure (13, 22). It would be important to contrast the overall estimates with those obtained by endemicity level.
Our model assumes that age profiles of mf infection reflect those of adult worms, because mf production is conjectured to be proportional to worm burden and mf mortality independent of density or host age (but see (28)). For simplicity and tractability, we included a reduction in parasite establishment in humans with exposure to L3 larvae (22, 29) and parasite-induced vector mortality but not density-dependent parasite uptake by vectors (22). The various density-dependent mechanisms, by acting on different processes in the parasite's life cycle, may differentially influence rates of reinfection after treatment (Churcher, T. S., J.A.N.F. & M.-G.B., unpublished data). The age- and sex-structure of the human population represented in the model is relatively simple and assumed to be stationary; other models have been developed, particularly in the context of HIV transmission, that allow more realistic population structure (40, 41).
Supplementary Material
Acknowledgments
We thank Tom Churcher and Paul Clarke for insightful discussions during model development and Sébastien Pion and John Williams for helpful comments on the manuscript. This work was supported in part by the Medical Research Council of the United Kingdom (J.A.N.F. and M.-G.B.).
Author contributions: M.-G.B. designed research; J.A.N.F. and M.-G.B. performed research; J.A.N.F. contributed new analytic tools; J.A.N.F. analyzed data; J.A.N.F. and M.-G.B. wrote the paper; M.B. collected data in Cameroon and gave general advice; A.R. collected data in Cameroon; R.C.C. collected data in Guatemala; S.V.-M., M.-E.G., and M.-G.B. collected data in Venezuela; and M.P.L. gave statistical and general advice.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: mf, microfilariae; L3, third-stage infective larvae; ABR, annual vector biting rate; C.I., confidence interval; NB, negative binomial.
References
- 1.Anonymous (2004) Lancet 364, 1993-1994.15582039 [Google Scholar]
- 2.Michael, E., Bundy, D. A. & Grenfell, B. T. (1996) Parasitology 112, 409-428. [DOI] [PubMed] [Google Scholar]
- 3.Richards, F. O., Jr., Boatin, B., Sauerbrey, M. & Sékétéli, A. (2001) Trends Parasitol. 17, 558-563. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux, D. H. (2004) Lancet 364, 380-383. [DOI] [PubMed] [Google Scholar]
- 5.Fenwick, A., Savioli, L., Engels, D., Bergquist, N. R. & Todd, M. H. (2003) Trends Parasitol. 19, 509-515. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, R. M. & May, R. M. (1991) Infectious Diseases of Humans: Dynamics and Control (Oxford Univ. Press, Oxford).
- 7.Anderson, R. M. & May, R. M. (1985) Nature 315, 493-496. [DOI] [PubMed] [Google Scholar]
- 8.Duerr, H. P., Dietz, K. & Eichner, M. (2003) Parasitology 126, 87-101. [DOI] [PubMed] [Google Scholar]
- 9.Woolhouse, M. E. J., Taylor, P., Matanhire, D. & Chandiwana, S. K. (1991) Nature 351, 757-758. [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood, B., Smith, P., Marshall, T. & Prost, A. (1983) Trans. R. Soc. Trop. Med. Hyg. 77, 857-861. [DOI] [PubMed] [Google Scholar]
- 11.Tada, I., Aoki, Y., Rimola, C. E., Ikeda, T., Matsuo, F., Ochoa, J. O., Recinos, M., Sato, S., Godoy, H. A., Orellana, J., et al. (1979) Am. J. Trop. Med. Hyg. 28, 67-71. [DOI] [PubMed] [Google Scholar]
- 12.Botto, C., Gillespie, A. J., Vivas-Martínez, S., Martínez, N., Planchart, S., Basáñez, M.-G. & Bradley, J. E. (1999) Trans. R. Soc. Trop. Med. Hyg. 93, 25-30. [DOI] [PubMed] [Google Scholar]
- 13.Dietz, K. (1982) in Population Dynamics of Infectious Diseases, ed. Anderson, R. M. (Chapman & Hall, London), pp. 209-241.
- 14.Remme, J., Ba, O., Dadzie, K. Y. & Karam, M. (1986) Bull. W. H. O. 64, 667-681. [PMC free article] [PubMed] [Google Scholar]
- 15.Plaisier, A. P., Van Oortmarssen, G. J., Habbema, J. D. F., Remme, J. & Alley, E. S. (1990) Comput. Methods Programs Biomed. 31, 43-56. [DOI] [PubMed] [Google Scholar]
- 16.Davies, J. B. (1993) Ann. Trop. Med. Parasitol. 87, 41-63. [DOI] [PubMed] [Google Scholar]
- 17.Duerr, H. P., Dietz, K., Schulz-Key, H., Büttner, D. W. & Eichner, M. (2003) Trans. R. Soc. Trop. Med. Hyg. 97, 242-250. [DOI] [PubMed] [Google Scholar]
- 18.Anderson, J., Fuglsang, H., Hamilton, P. J. S. & Marshall, T. F. D. C. (1974) Trans. R. Soc. Trop. Med. Hyg. 68, 209-222. [DOI] [PubMed] [Google Scholar]
- 19.Wada, Y. (1982) Jpn. J. Med. Sci. Biol. 35, 183-196. [DOI] [PubMed] [Google Scholar]
- 20.Renz, A., Fuglsang, H. & Anderson, J. (1987) Ann. Trop. Med. Parasitol. 81, 253-262. [DOI] [PubMed] [Google Scholar]
- 21.Little, M. P., Breitling, L. P., Basáñez, M.-G., Alley, E. S. & Boatin, B. A. (2004) Lancet 363, 1514-1521. [DOI] [PubMed] [Google Scholar]
- 22.Basáñez, M.-G. & Boussinesq, M. (1999) Philos. Trans. R. Soc. London B 354, 809-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussinesq, M., Prod'hon, J. & Chippaux, J. P. (1997) Trans. R. Soc. Trop. Med. Hyg. 91, 82-86. [DOI] [PubMed] [Google Scholar]
- 24.Brandling-Bennett, A. D., Anderson, J., Fuglsang, H. & Collins, R. (1981) Am. J. Trop. Med. Hyg. 30, 970-981. [PubMed] [Google Scholar]
- 25.Basáñez, M.-G. & Yarzábal, L. (1989) in Parasitic Diseases: Treatment and Control, eds. Miller, M. J. & Love, E. J. (CRC Press, Boca Raton, FL), pp. 231-256.
- 26.Vivas-Martínez, S., Basáñez, M.-G, Botto, C., Rojas, S., García, M., Pacheco, M. & Curtis, C. F. (2000) Parasitology 121, 513-525. [DOI] [PubMed] [Google Scholar]
- 27.Renz, A. (1987) Ann. Trop. Med. Parasitol. 81, 239-252. [DOI] [PubMed] [Google Scholar]
- 28.Duerr, H. P., Dietz, K., Schulz-Key, H., Büttner, D. W. & Eichner, M. (2004) Int. J. Parasitol. 34, 463-473. [DOI] [PubMed] [Google Scholar]
- 29.Basáñez, M.-G., Collins, R. C., Porter, C. H., Little, M. P. & Brandling-Bennett, D. (2002) Am. J. Trop. Med. Hyg. 67, 669-679. [DOI] [PubMed] [Google Scholar]
- 30.Vivas-Martínez, S., Basáñez, M.-G., Grillet, M.-E., Weiss, H., Botto, C., García, M., Villamizar, N. J. & Chavasse, D. C. (1998) Trans. R. Soc. Trop. Med. Hyg. 92, 613-620. [DOI] [PubMed] [Google Scholar]
- 31.Grillet, M.-E., Basáñez, M.-G., Vivas-Martínez, S., Villamizar, N., Frontado, H., Cortez, J., Coronel, P. & Botto, C. (2001) J. Med. Entomol. 38, 520-530. [DOI] [PubMed] [Google Scholar]
- 32.Gowtage-Sequeira, S., Higazi, T., Unnasch, T. R. & Basáñez, M.-G. (2002) Br. Simuliid Group Bull. 18, 13-15. [Google Scholar]
- 33.Prost, A. (1980) Bull. W. H. O. 58, 923-925. [PMC free article] [PubMed] [Google Scholar]
- 34.Duke, B. O. L. (1993) Trop. Med. Parasitol. 44, 61-68. [PubMed] [Google Scholar]
- 35.Wilson, K., Bjørnstad, O. N., Dobson, A. P., Merler, S., Poglayen, G., Randolph, S. E., Read, A. F. & Skorping, A. (2003) in Ecology of Wildlife Diseases, eds. Hudson, P. J., Rizzoli, A., Grenfell, B. T., Heesterbeek, H. & Dobson, A. P. (Oxford Univ. Press, Oxford), pp. 6-44.
- 36.Efron, B. & Tibshirani, R. J. (1993) An Introduction to the Bootstrap (Chapman & Hall–CRC Press, London).
- 37.Brabin, L. (1990) Acta Leiden. 59, 413-426. [PubMed] [Google Scholar]
- 38.Bradley, J. E., Whitworth, J. & Basáñez, M.-G. (2005) in Topley and Wilson's Microbiology and Microbial Infections, eds. Wakelin, D., Cox, F. E. G., Despommier, D. & Gillespie, S. (Hodder Arnold, London) 10th Ed., in press.
- 39.Alexander, N. D. E & Grenfell, B. T. (1999) Parasitology 119, 151-156. [DOI] [PubMed] [Google Scholar]
- 40.Hadeler, K. P. & Castillo-Chavez, C. (1995) Math. Biosci. 128, 45-55. [DOI] [PubMed] [Google Scholar]
- 41.Martcheva, M. (1999) Math. Biosci. 157, 1-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.