Abstract
Cyclin-dependent kinase 5 (cdk5) is a serine/threonine kinase activated by associating with its neuron-specific activators p35 and p39. Analysis of cdk5–/– and p35–/– mice has demonstrated that both cdk5 and p35 are essential for neuronal migration, axon pathfinding and the laminar configuration of the cerebral cortex, suggesting that the cdk5–p35 complex may play a role in neuron survival. However, the targets of cdk5 that regulate neuron survival are unknown. Here, we show that cdk5 directly phosphorylates c-Jun N-terminal kinase 3 (JNK3) on Thr131 and inhibits its kinase activity, leading to reduced c-Jun phosphorylation. Expression of cdk5 and p35 in HEK293T cells inhibits c-Jun phosphorylation induced by UV irradiation. These effects can be restored by expression of a catalytically inactive mutant form of cdk5. Moreover, cdk5-deficient cultured cortical neurons exhibit increased sensitivity to apoptotic stimuli, as well as elevated JNK3 activity and c-Jun phosphorylation. Taken together, these findings show that cdk5 may exert its role as a key element by negatively regulating the c-Jun N-terminal kinase/stress-activated protein kinase signaling pathway during neuronal apoptosis.
Keywords: apoptosis/cdk5/c-Jun/JNK/phosphorylation
Introduction
Cdk5 and p35, which are highly expressed in post-mitotic neurons, are essential for neuronal migration, neurite outgrowth and laminar configuration of the cerebral cortex (Lew et al., 1994; Tsai et al., 1994; Humbert et al., 2000). Cdk5 and p35 have recently been shown to phosphorylate Munc-18 and amphiphysin, which in turn affect neuronal exocytosis and neurite outgrowth (Shuang et al., 1998; Fletcher et al., 1999; Rosales et al., 2000; Floyd et al., 2001), and DARPP-32, which regulates dopamine signaling (Bibb et al., 1999). Elevated cdk5 expression levels and kinase activity during apoptotic cell death have been reported (Kwon et al., 1999; Ahlijanian et al., 2000). Recently, cdk5 in association with p25, a truncated form of p35, is thought to disrupt the neuronal cytoskeleton and may be involved in neurodegenerative diseases such Alzheimer’s disease (AD) (Patrick et al., 1999; Lee et al., 2000; Kusakawa et al., 2000). In vivo data from cdk5 knockout mice exhibit a unique phenotype with perinatal mortality associated with extensive disrupted cerebral cortical layering due to abnormal neuronal migration, the lack of cerebellar foliation and degeneration of neurons in the brain stem and spinal cord (Ohshima et al., 1996, 1999). The p35–/– mice are viable and fertile (Chae et al., 1997; Kwon et al., 1999), with abnormalities of laminar structures in cerebral cortex, but less severe than those in cdk5 null mice (Ohshima et al., 2001). In addition, our recent studies on transgenic mice (TgKO) expressing cdk5 only in the p35-expressing brain regions in endogenous cdk5 null mice reverted the phenotypes observed in cdk5 null mice. These cdk5 transgenic animals were viable and fertile. These studies indicate that neuronal cdk5 activity is critical for embryonic development and neuron survival (Tanaka et al., 2001).
The c-Jun NH2-terminal kinase (JNK) family of protein kinases, also known as stress-activated protein kinases (SAPK), phosphorylate serine residues 63 and 73 of the transcription factor c-Jun in the activation domain, leading to increased AP-1 transcription activity and apoptosis (Derijard et al., 1994; Kyriakis et al., 1994). In addition to c-Jun, JNK also phosphorylates ATF2 and other Jun family proteins that function as components of the AP-1 transcription factor complex (Gupta et al., 1995, 1996). Although JNK1 and JNK2 are widely expressed in murine tissues, including the nervous system, JNK3 is selectively expressed in the brain (Martin et al., 1996). JNK3 has also been shown to phosphorylate neurofilament heavy chain side-arms (NF-H) (Brownlees et al., 2000). Such phosphorylation on the NF-H tail domain affects neurofilament assembly, dynamics and axonal transport rate (Heins et al., 1994; Sahlgren et al., 2001; Yabe et al., 2001). Mice lacking JNK3 exhibit increased resistance to kainic acid-induced apoptosis in the hippocampus (Yang et al., 1997), indicating a preferential role of JNK3 in stress-induced neuronal apoptosis. Analysis of JNK1 and JNK2 knockout mice has shown that JNK1 and JNK2 regulate region-specific apoptosis during early brain development (Kuan et al., 1999). Transfection studies using constitutively active and dominant-negative components of the JNK signaling pathway established that JNK activity and c-Jun phosphorylation were involved in apoptosis of nerve growth factor (NGF)-deprived sympathetic neurons (Eilers et al., 1998). These observations, together with the data from cdk5–/– mice, suggest that there may be a link between JNK3 and cdk5 in regulating neuronal apoptosis. This allowed us to assess the role played by cdk5 in neuronal apoptosis.
Here, we show that apoptotic cell death is prevalent in brains of cdk5-deficient mice and that cdk5–/– neurons display higher sensitivity to UV irradiation. We found that cdk5 can phosphorylate JNK3 on Thr131 and inhibit its activity. Furthermore, active cdk5 inhibits the JNK3-mediated c-Jun phosphorylation and c-Jun-mediated transactivation of a reporter construct. These effects can be inhibited by expression of a catalytically inactive mutant form of cdk5. Finally, we show an increase in c-Jun phosphorylation and JNK3 kinase activity in cdk5–/– neurons. Based on these observations, we propose that cdk5 plays a role in neuron survival by negatively regulating JNK3, thus furthering the current understanding of JNK-dependent apoptotic signaling pathways and the interplay between kinase cascades implicated in development and degeneration of the nervous system.
Results
JNK3 kinase is phosphorylated on Thr131 by cdk5
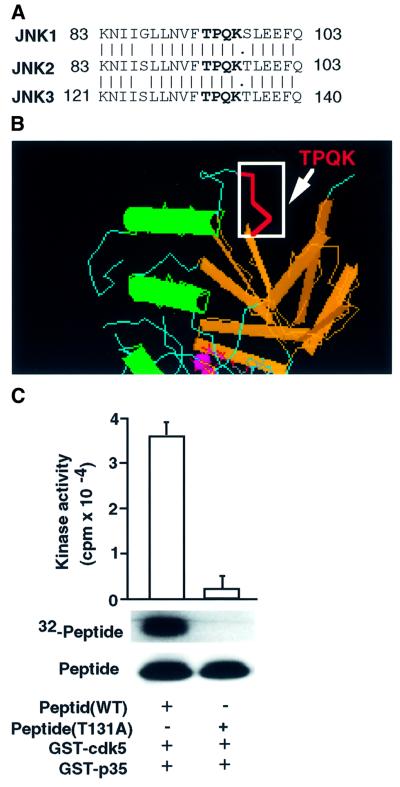
One putative cdk5 phosphorylation motif (T/SPXK) is present in JNK1, JNK2 and JNK3 (Thr93 in rat JNK1 and JNK2, Thr131 in rat JNK3; Figure 1A; Kyriakis et al., 1994). Analysis of the phosphorylated residues using various peptides and proteins revealed that XS/TPXK/R motifs (X = neutral/basic residues) are the most suitable substrates for cdk5 (Shetty et al., 1993). In our previous studies, we have analyzed the XS/TPXK motif and its variant peptides, indicating that cdk5 specifically phosphorylates peptides with XS/TPXK motifs (Veeranna et al., 1998). These studies suggest that FTPQK, the conserved motif in JNK1, JNK2 and JNK3 (Figure 1A), is an ideal substrate for cdk5–p35. The crystal structure of JNK3 revealed that the TPQK motif is in open configuration (Figure 1B; Xie et al., 1998). To test whether the Thr131 residue in the peptide derived from JNK3 is the site phosphorylated by cdk5, we incubated the wild-type peptide (LLNVFTPQK) or mutant peptide (LLNVFAPQK) with glutathione S-transferase (GST) fusion cdk5–p35 proteins and determined its phosphorylation by in vitro kinase assays (Figure 1C). These data provide direct evidence that the Thr131 residue containing the peptide derived from JNK3 is a target of cdk5.
Fig. 1. Analysis of peptides derived from the JNK3 corresponding to the potential phosphorylation site by cdk5 by mass spectrometry. (A) The cdk5 phosphorylation motif TPQK is indicated in JNKs. (B) TPQK motif in JNK3 crystal structure. (C) Peptide (LLNVFTPQK) derived from JNK3 was added to an in vitro kinase reaction with GST–cdk5 and GST–p35 (see Materials and methods). Top, incorporation of 32P into peptide; bottom, amount of peptide substrate by Coomassie Blue staining of the gel. The histogram (n = 3) reflects the relative amount of labeled peptide to the amount of peptide (Coomassie Blue) in the in vitro kinase reaction.
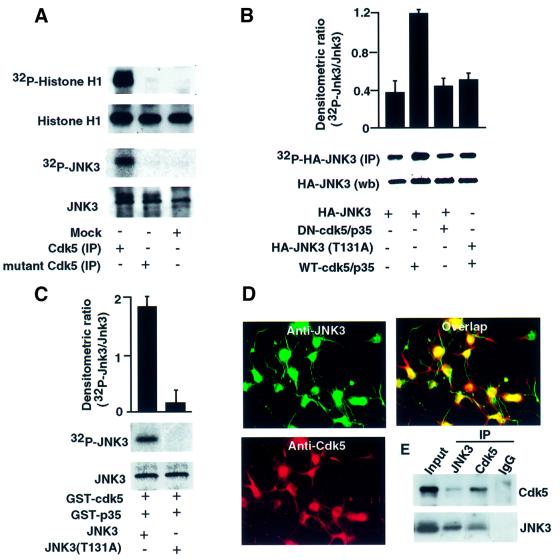
To establish whether JNK3 protein is a potential substrate for cdk5 phosphorylation in vitro, we co-transfected HEK293T cells with wild-type or the kinase-inactive mutant cdk5 (K33T) and assessed the status of phosphorylation of the recombinant JNK3. Cdk5 immunoprecipitate (IP) from cells transfected with active cdk5–p35 phosphorylated histone H1 and recombinant JNK3, whereas the IP from cells transfected with vector or mutant cdk5 did not (Figure 2A).
Fig. 2. Phosphorylation of JNK3 by cdk5 in vitro and in vivo. (A) HEK293T cells were transfected with cdk–p35 mutant cdk5 (K33T) or p35, and lysates were immunoprecipitated using anti-cdk5 antibody (C-8) and placed in an in vitro kinase reaction with histone H1 or recombinant JNK3 as substrates. Top, incorporation of 32P into the substrates; bottom, amount of substrate by Coomassie Blue staining of the gel. (C) Recombinant wild-type JNK3 or mutant JNK3 (T131A) was purified and equal amounts placed in an in vitro kinase reaction with GST–cdk5 and GST–p35 (see Materials and methods). Top, incorporation of 32P into JNK3; bottom, amount of substrate by Coomassie Blue staining of the gel. The histogram (n = 3) reflects the relative amount of labeled JNK3 to the amount of JNK3 (Coomassie Blue) in the in vitro kinase reaction. (B) 32P-labeled wild-type or threonine mutant (T131A) JNK3 was affinity purified from transfected HEK293T cells and subjected to autoradiography (top) or western blotting (bottom). The histogram shows the relative amount of labeled protein to the amount of immunoreactive JNK3 in the gel. (D) Co-localization of cdk5 with JNK3 in cortical neurons. Rat cortical neurons were stained with monoclonal cdk5 and polyclonal JNK3 antibodies; cdk5 and JNK3 staining was visualized with a rhodamine-coupled and fluorescein isothiocyanate-coupled secondary antibody, respectively. (E) Rat cortical neuronal extracts were prepared and immunoprecipitated with cdk5, JNK3 antibodies or control IgG (pre-immune). The immunoprecipitates were then immunoblotted for cdk5 and JNK3 antibodies.
To investigate the putative cdk5 phosphorylation site in JNK3, Thr131 in JNK3 was mutated to Ala (T131A). The ability of cdk5 to phosphorylate wild-type and mutant HA-JNK3 (T131A) was examined by an in vitro kinase assay with GST–cdk5–p35 (Figure 2B). Wild-type JNK3 was phosphorylated but not the mutant. These data were confirmed in intact cells. HEK293T cells were co-transfected with HA-JNK3 and wild-type cdk5–p35 or dominant-negative cdk5–p35 and then metabolically labeled with [32P]ortho-phosphate. The levels of phosphorylated HA-JNK3 were measured by immunoprecipitation using anti-HA-tagged antibody and its phosphorylation state and protein levels quantified. Co-expression of wild-type cdk5–p35 and HA-JNK3 resulted in a 3-fold enhancement of HA-JNK3 phosphorylation compared with co-expression of mutant cdk5–p35 and HA-JNK3 (Figure 2B). In addition, mutation of Thr131 to Ala in JNK3 significantly reduced cdk5-dependent phosphorylation of JNK3 compared with the wild-type protein (Figure 2B). IP using pre-immune antibody showed no detectable activity compared with HA antibody (data not shown).
To examine whether cdk5 was co-localized with JNK3, we performed double-labeled immunofluorescence staining of cultured rat cortical neurons and found that cdk5 specifically co-localizes with JNK3 in cortical neurons (Figure 2D). We also investigated whether cdk5 is associated with JNK3 by co-immunoprecipitation using JNK3 or cdk5 antibodies. Cell extracts from the cultured cortical neurons were precipitated with cdk5, JNK3 or control IgG antibodies. We found that cdk5 was present in JNK3 immunoprecipitate and vice versa. Control IgG antibody showed no detectable cdk5 or JNK3 (Figure 2E). These data suggest that cdk5 and JNK3 are co-localized and form a complex.
Cdk5 phosphorylation of JNK3 inhibits c-Jun phosphorylation
To examine the functional significance of the cdk5 phosphorylation site in JNK3, HEK293T cells were co-transfected with wild-type cdk5–p35 or inactive mutant cdk5–p35 and HA-JNK3 and exposed to UV irradiation (780 J/m2). Twenty-four hours post-UV irradiation produced no significant cell loss. Other investigators (Stambolic et al., 1998) have used a similar dose of UV irradiation. HA-JNK3 kinase activity was assessed using IPs with HA antibody from the cell lysates and GST–c-Jun as a substrate. The phosphorylated c-Jun was detected by autoradiography and western blot analysis (Figure 3A and B). Phosphorylation of GST–c–Jun was significantly inhibited by co-expression of wild-type cdk5–p35, but not by inactive mutant cdk5–p35 (Figure 3A). Co-transfection of wild-type cdk5–p35 and JNK3 significantly inhibited UV-induced c-Jun phosphorylation at Ser63 (Figure 3B) and Ser73 (data not shown). Co-transfection of inactive mutant cdk5–p35 also reduced the inhibition of c-Jun phosphorylation at Ser63 (Figure 3B). These results indicate that JNK3 is functionally important in down-regulating c-Jun phosphorylation by cdk5. The expression of JNK3 was unaffected by UV irradiation (data not shown). The increase in c-Jun phosphorylation by western blot analysis (shown in lane 3 of Figure 3B) is due to the activation of endogenous JNK1 and JNK2 upon UV irradiation. Transfection of cdk5–p35 reduced this stimulation by >50% (data not shown), whereas the expression of c-Jun was unaffected under these conditions. To exclude the possibility that cdk5 inhibition of UV-induced JNK3 kinase activity and c-Jun phosphorylation is due to UV-induced changes in cdk5 activity, we determined the kinase activity of cdk5–p35 upon UV irradiation in transfected HEK293T cells, using a cdk5 kinase assay with histone H1 as an in vitro substrate. Cdk5 kinase activity was unaffected by UV irradiation and kinase activity was similar to basal levels (Figure 3C).
Fig. 3. Cdk5 inhibits c-Jun phosphorylation. (A) Cdk5 inhibits UV-irradiation-induced JNK3 activation. HEK293T cells were transfected with plasmids for HA-JNK3 and wild-type or mutant cdk5 (K33T)–p35 with or without UV irradiation, and JNK3 was immunoprecipitated using anti-HA antibody and subjected to an in vitro kinase reaction with GST–c-Jun as substrate. Top, incorporation of 32P into GST–c-Jun; bottom, amount of GST–c-Jun by Coomassie Blue staining of the gel. The histogram (n = 3) reflects the amount of labeled GST–c-Jun relative to the mass of GST–c-Jun (Coomassie Blue) in the in vitro kinase reaction. (B) Cdk5 inhibits c-Jun phosphorylation. Lysates from HEK293T cells as described above were subjected to western blotting analysis using anti-phospho-dependent c-Jun at Ser63 antibody. Top, phospho-c-Jun at Ser63; bottom, amount of c-Jun detected by western blotting using total c-Jun antibody. The histogram (n = 3) reflects the relative mean density of phospho-c-Jun relative to the amount of total c-Jun in the western blot analysis. (C) Cdk5 kinase activity is unaltered by UV irradiation in cdk–p35 transfected cells. Lysates from HEK293T cells as described above were immunoprecipitated and subjected to histone H1 substrate in vitro kinase assays.
Cdk5 inhibits JNK3-mediated c-Jun transcriptional activity
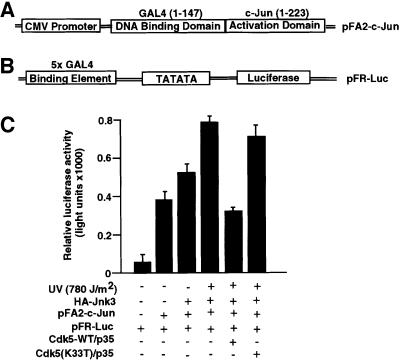
Since expression of cdk5 and p35 in HEK293T cells resulted in inhibition of JNK3 kinase activity and c-Jun phosphorylation at Ser63 and Ser73, we further investigated whether cdk5–p35 influences the transcriptional activity of c-Jun, a downstream effector of JNK family, using the PathDetect in vivo signal transduction pathway trans-reporting system. The c-Jun PathDetect trans-reporting system includes a unique fusion trans-activator plasmid that expresses a c-Jun fusion protein of the activation domain. The c-Jun fusion trans-activator protein fused with the DNA-binding domain of the yeast GAL4 (residues 1–147; Figure 4A and B). The transcription activator c-Jun is phosphorylated and activated by the JNK family. Such activity reflects the in vivo activation of JNK kinase and the corresponding signal transduction pathway (Hibi et al., 1993; Hill et al., 1993; Karin and Hunter, 1995). The HEK293T cells, which do not exhibit cdk5 activity and lack JNK3 but express JNK1 and JNK2, were co-transfected with JNK3, cdk5–p35 or the kinase-inactive mutant cdk5–p35 and GAL4–c-Jun fusion protein together with a reporter construct containing five tandem repeats of the yeast GAL4 binding sites and a TATA box that controls expression of the luciferase gene. Expression levels of luciferase reflect the activation status of JNK activity affected by cdk5. In addition to JNK3, c-Jun is also phosphorylated by JNK1 and JNK2. In the absence of JNK3 and UV, we observed activation of c-Jun (Figure 4C, lane 2), and transfection of JNK3 appeared to elevate this activation (Figure 4C, lane 3). However, co-transfection of cdk5–p35 produced a 50% reduction of these responses (data not shown). The increase in c-Jun transcription activity treated with UV irradiation was significantly (50%) inhibited by co-transfection of wild-type cdk5–p35 (Figure 4C, compare lanes 4 and 5). On the other hand, co-transfection of dominant-negative cdk5–p35 (inactive kinase) produced no reduction (Figure 4C, lane 6). The level of c-Jun expression was unaffected under these conditions (data not shown).
Fig. 4. Down-regulation of c-Jun transcriptional activity by cdk5. (A) Fusion protein construct consisting of the c-Jun activation domain (1–223) and GAL4 DNA-binding domain (1–147). (B) A reporter construct containing five tandem repeats of the yeast GAL4 binding sites and a TATA box that controls expression of the luciferase gene. (C) Luciferase activity was measured in HEK293T cells co-transfected with pFA2-c-Jun and pFR-luc, HA-JNK3 and with wild-type or mutant cdk–p35 expression constructs. Control experiments were performed with mock-transfected cells and the inactive cdk5 (K33T)–p35 mutant. Transfection efficiency was examined by co-transfection of a control plasmid that expressed β-galactosidase activity. The data are reported as the mean of three experiments (ratio of luciferase activity and β-galactosidase activity). The HEK293T cells were serum starved for 18 h and then exposed to UV irradiation (780 J/m2); 24 h post-UV irradiation, cells were assessed by expression of luciferase activity.
JNK3 activity and c-Jun phosphorylation are elevated in cdk5–/– mice
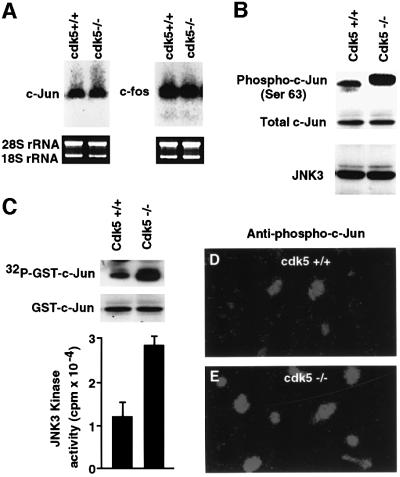
To further demonstrate the specificity of cdk5-inhibited JNK3 activity, we compared JNK3 kinase activity and c-Jun phosphorylation in wild-type and cdk5 knockout (cdk5–/–) mice. First, we examined the expression of c-Fos and c-Jun using total RNA from the cortex of wild-type and cdk5–/– mice. Northern blots were probed with murine c-Fos and c-Jun probes. Wild-type and cdk5–/– mice exhibited a similar level of mRNA expression (Figure 5A). Next, we compared JNK3 protein expression and c-Jun protein phosphorylation levels by western blot analysis with anti-JNK3 and anti-phospho-c-Jun (Ser63) antibody in the cortex of wild-type and cdk5–/– mice (Figure 5B). Phosphorylated c-Jun was confirmed by immunocytochemistry analysis with anti-phospho-c-Jun (Ser63; Figure 5D and E). Expression levels of JNK3 and c-Jun proteins were no different in either wild-type or cdk5 knockout mice (Figure 5B). However, there was a >3-fold increase in JNK3 activity (Figure 5C) and a robust c-Jun phosphorylation in cdk5–/– compared with wild-type mouse brain extracts (Figure 5B). The higher level of c-Jun phosphorylation in cdk5 null mice is evident by its shift in electrophoretic mobility (Figure 5B).
Fig. 5. Comparison of expression of c-Fos, c-Jun and JNK3 in wild-type and cdk5 knockout mice (cdk5–/–). (A) Total RNA was isolated from wild-type and cdk5–/– mouse embryo brains (E16) and probed with a murine c-Fos and c-Jun cDNA probe. Ethidium bromide staining of 18S and 28S ribosomal RNA monitors RNA loading and transfer efficiency. (B) Western blot analysis of total JNK3, c-Jun protein expression and phospho-c-Jun in cdk5–/– and wild-type mouse brain extracts. (C) Comparison of JNK3 kinase activity in wild-type and cdk5–/– mice. JNK3 was immunoprecipitated using anti-JNK3 antibody from wild-type and cdk5–/– mouse (E16) brain and subjected to an in vitro kinase assay with GST–c-Jun as a substrate. Top, incorporation of 32P into GST–c-Jun; bottom, amount of GST–c-Jun by Coomassie Blue staining of the gel. The histogram (n = 3) reflects the relative amount of labeled GST–c-Jun to the mass of GST–c-Jun (Coomassie Blue). (D and E) Immunocytochemical analysis of phospho-c-Jun in cdk5–/– compared with wild-type mice. Expression of phosphorylated c-Jun at Ser63 (E) was greatly increased in the cortex of cdk5–/– mice compared with the wild type (D).
Cdk5–/– mice show a higher incidence of apoptotic neuronal cell death
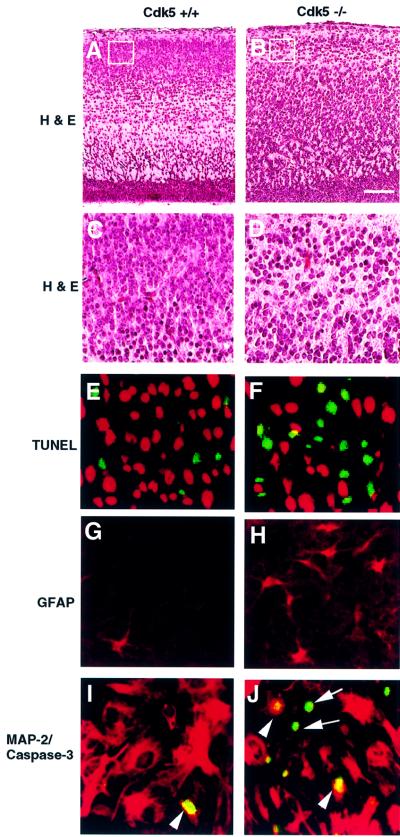
The above studies show that the phosphorylation of JNK3 by cdk5 resulted in inhibition of JNK3 activity and c-Jun phosphorylation. JNK3 and c-Jun are involved in neuronal apoptosis (Yang et al., 1997; Behrens et al., 1999; Oo et al., 1999; Chen et al., 2000), suggesting that cdk5 may play an important role in neuronal apoptosis through regulation of JNK3 activity. Therefore, we examined whether cdk5–/– mice would show a higher incidence of apoptotic neuronal cell death. TUNEL histochemistry staining, which detects DNA fragmentation in dying cells, was used to assess the apoptotic cells. Haematoxylin and eosin (HE) staining showed a decreased number of cells in cdk5–/– compared with the wild type in cortex plate (CP) subfields (Figure 6A–D), as well as migration defects as shown previously (Ohshima et al., 1996, 1999). Numerous TUNEL-positive cells were observed in cdk5–/– mice (Figure 6F) compared with the wild type (Figure 6E). Immunostaining of glial fibrillary acidic protein (GFAP) was used independently to assess the loss of or damaged neurons (Yang et al., 1997). Consistent with the patterns of HE and TUNEL histochemistry staining, increased numbers of strongly GFAP-positive astrocytes were detected in the cortex of the cdk5–/– mice (Figure 6H) compared with the wild type (Figure 6G).
Fig. 6. Comparison of cell death in the cortex of wild-type and E16-cdk5 knockout mice. The first (bar represents 100 µm) and the second rows (A–D) were stained by the HE method, which indicates that the cdk5 deficiency interferes with the laminar configuration; the third row (E and F) was stained by TUNEL histochemistry staining, which indicates increased apoptosis in the cdk5–/– mice (green). Sections were counterstained with propidium iodide (red) after TUNEL staining (green); the fourth row (G and H) was immunostained with GFAP antibody for neuron loss or damage-induced GFAP as an independent assessment of cell destruction; the fifth row (I and J) was double stained by anti-active caspase-3 (green) and anti-MAP-2 (red) antibodies and indicates that the loss of MAP-2 staining occurred in parallel with the activation of caspase-3. Double labeling of MAP-2 (red) and caspase-3 (green) showed that caspase-3-positive cells were almost exclusively found in the neurons displaying loss of MAP-2 (arrow) and some were also found in the damaged neurons (arrowhead).
Recent studies indicated that caspase-3 was the principal effector for developmental neuronal cell death (Ham et al., 1995; Eilers et al., 1998; Srinivasan et al., 1998; Xie et al., 1998). The active caspase-3 patterns were compared between cdk5–/– and wild-type mice by double staining with the recently characterized caspase-3 antibody, which recognizes the cleaved 17 kDa subunit (Eilers et al., 1998) but not the 32 kDa procaspase-3. Increased caspase-3 activity and more loss of MAP-2 immunostaining were simultaneously detected in the cortex of cdk5–/– (Figure 6J) compared with that of wild-type mice (Figure 6I).
Cdk5–/– neurons are more sensitive than their wild-type counterparts to UV-induced apoptosis
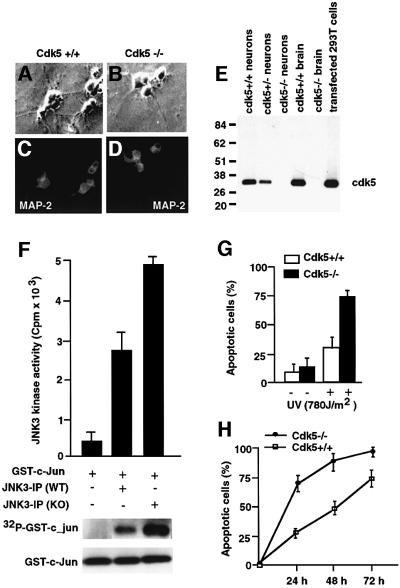
To investigate further the role of cdk5 in regulating apoptosis, we used cortical neurons derived from the embryonic brains (E16) of cdk5–/– and wild-type mice. Immunostaining showed MAP-2 expression, demonstrating that these cultured cells are neurons (Figure 7A–D). Western blot analysis of cell lysates from the established neuronal cultures confirmed the loss of cdk5 expression in cdk5–/– and its presence in wild-type cultured neurons (Figure 7E). The JNK3 kinase activity in these neurons was measured in the JNK3 IPs using GST–c-Jun as the substrate in the presence of [γ-32P]ATP (Figure 7F). Cortical neuronal cultures derived from cdk5–/– mice showed a 2-fold higher activity of JNK3 compared with the wild type (Figure 7F, lanes 2 and 3). To test whether cdk5-deficient cells exhibit increased sensitivity to apoptotic stimuli, we examined the rate of cell death upon UV irradiation in cdk5–/– and wild-type neurons. As measured by TUNEL staining, >70% of cdk5–/– cells were apoptotic following 24 h post-UV irradiation (780 J/m2). However, the same treatment resulted in 30% cell death in wild-type cells (Figure 7G). Prolonged monitoring of cell death indicated that the rate of apoptosis is faster following UV irradiation in cdk5–/– cells compared with the wild type (Figure 7H). The increased sensitivity of cdk5–/– cells to apoptotic stimuli suggests that cdk5 activity plays an important role in neuronal cell survival by regulating the JNK3 activity.
Fig. 7. Characterization of cortical neuronal culture from cdk5–/– and wild-type mice. (A–D) Immunocytochemical analysis of wild-type and cdk5–/– cortical neurons, showing expression of neuron-specific protein MAP-2 in all cultured cells. (E) Western blot analysis of whole-cell lysates of wild-type, cdk5–/– and cdk5–/+ cultured cortical neurons. The positions of molecular weight markers (kDa) are shown on the left. The position of cdk5 is indicated. (F) JNK3 was immunoprecipitated from wild-type and cdk5–/– cortical neurons using anti-JNK3 antibody and subjected to an in vitro kinase reaction with GST–c-Jun as substrate in the presence of [γ-32P]ATP. (G) Cdk5-deficient cells are more sensitive to apoptotic stimuli. Apoptotic cells are normalized. Apoptosis was assessed by TUNEL staining. Open bars correspond to wild type; solid bars correspond to cdk5–/– cells. The data are presented from three independent experiments. Error bars represent the standard error of the mean expressed as a percentage. (H) Cdk5-deficient cells show increased sensitivity to apoptotic stimuli. The cells were treated as described in (G) and cell death was monitored for 3 days at different times after UV irradiation. Squares correspond to wild type and circles to cdk5–/–. The data are from three independent experiments. Error bars represent the standard error of the mean expressed as a percentage.
Discussion
The evidence presented here identifies a crucial regulator of cell survival, SAPK/JNK, as a putative target of cdk5. Cdk5 negatively regulates phosphorylation and JNK3 activity in cells, as indicated by the regulation of c-Jun phosphorylation. Activation of JNK3 induces apoptosis (Eilers et al., 1998). Higher activity of JNK3 in the cdk5–/– cells and the HEK293T cells transfected with inactive cdk5 mutant supports the role of active cdk5 in cell survival. The increased sensitivity of cdk5–/– neuronal cells to apoptotic stimuli was associated with increased phosphorylation of c-Jun. These results indicated that the observed neuronal protection in wild-type cells is, at least in part, due to the phosphorylation of JNK3 by cdk5, which, in turn, down-regulates JNK3 signaling cascades.
Programmed cell death is an active process occurring during both normal maturation of the nervous system and pathological situations, such as neurodegenerative diseases and stroke (Clarke, 1990; Oppenheim, 1991; Henderson, 1996; MacManus and Linnik, 1997; Pettmann and Henderson, 1998). The role of SAPK/JNK in the induction of and protection against apoptosis remains controversial (Kyriakis and Avruch, 1996; Nishina et al., 1997). However, activation of the SAPK/JNK pathway has been observed after induction of apoptosis by NGF withdrawal in rat PC12 cells (Xia et al., 1995), sympathetic neurons (Ham et al., 1995; Virdee et al., 1997), rat cerebellar granule neurons (Watson et al., 1998) and embryonic motoneurons (Maroney et al., 1998). Moreover, phosphorylation of c-Jun has been found after neuronal injury in the adult rat brain (Herdegen et al., 1998). Recently, Eilers et al. (1998) showed that expression of a constitutively active form of MEKK1, which strongly activates the JNK kinase pathway, increased c-Jun protein phosphorylation and induced apoptosis in the presence of NGF in sympathetic neurons. This induced apoptosis could be prevented by co-expression of the dominant-negative mutant SEK1, an activator of Jun kinase that is a target of MEKK1, suggesting that JNK/SAPK plays an important role in the regulation of c-Jun phosphorylation and apoptosis. Indeed, we have demonstrated that loss of cdk5 in cultured cortical neurons resulted in more sensitivity to UV-induced apoptosis together with increasing JNK3 activity and c-Jun phosphorylation. The evidence presented here identifies a crucial regulator of cell apoptosis, JNK3, as a target of cdk5 activity. Cdk5 with its activator p35 regulate phosphorylation and activity of JNK3 on Thr131 in neurons.
Cdk5-deficient mice (cdk5–/–) exhibited embryonic lethality associated with disruption of the cortical laminar structures in the cortex, olfactory bulb, hippocampus and cerebellar cortex (Ohshima et al., 1996). On the other hand, p35-deficient mice (p35–/–) were found to be viable and fertile with the abnormalities of laminar structure in cerebral cortex and subtle abnormalities in the laminar structures of olfactory bulb, hippocampus and cerebellum (Chae et al., 1997). These abnormalities are less severe compared with those in cdk5 null mice. To understand the biochemical basis of the phenotypic differences observed between p35–/– and cdk5–/– mice, we measured cdk5-specific kinase activity using cdk5 IPs of brain homogenates from cdk5–/– and p35–/– mice. No cdk5 activity was found in cdk5–/– brain homogenates, while a substantial amount of residual cdk5 activity was detected in cerebellum and cerebral cortex of p35–/– mice (Ohshima et al., 2001). The residual cdk5 activity and milder phenotypes in p35–/– were proposed to be due to overlapping expression of the cdk5 regulators p35 and p39. This hypothesis has been supported by recent studies (Ko et al., 2001). It was found that the double knockout of p35 and p39 genes in mice resulted in phenotypes notably identical to cdk5–/– mice. An identical phenotype is observed in cdk5–/– mice, and p35 and p39 double knockout mice indicated that p35 and p39 are necessary and sufficient for cdk5 activity during development. Our recent studies on transgenic mice using p35 promoter expressing cdk5 specifically in the regions where p35 was expressed in mice lacking endogenous cdk5 reverted almost all the cdk5–/– phenotypes. These transgenic mice were viable and fertile, and did not show defects in the neuronal migration as was observed in cdk5–/– mice (Tanaka et al., 2001). This study further supports the idea that cdk5 activity is important for survival and proper development of the nervous system. The present study provides the evidence that cdk5 may exert its protective role by negatively regulating the SAPK/JNK signal pathway during nervous system development.
In neurons, cdk5 activity requires the regulatory subunit p35 and p39 (Lew et al., 1994; Tsai et al., 1994). In a recent study, deregulation of cdk5 activity upon its binding with p25 has been implicated in neurodegenerative diseases. Patrick et al. (1999) have reported a 20- to 40-fold accumulation of p25 in brain lysates from patients with AD and thus concluded that p25 may contribute to the pathogenesis of neurodegeneration. In a similar study, Yoo and Lubec (2001) reported that the level of p25 in the frontal cortex of patients with AD or Down’s syndrome is actually lower than in controls. Patrick et al. (2001) have further reported higher levels of p25 in AD brains using higher numbers of AD brains. We have also conducted similar experiments but failed to detect the elevated levels of p25 in AD brains (P.Sharma, unpublished data). We do not understand the inconsistencies in these observations. However, in vitro as well as in vivo p25 transgenic mice studies have found that cdk5 in association with p25 has higher activity (Amin et al., 2001) and produces hyperphosphorylation of tau and neurofilament proteins (Ahlijanian et al., 2000). Interestingly, unlike p35, p25 is not readily degraded, and binding of p25 to cdk5 leads to constitutively active cdk5, resulting in hyperphosphorylation of neuronal cytoskeletal proteins such as tau, and may cause apoptosis. The cdk–p25 complex, like cdk–p35, could phosphorylate JNK3 in vitro or in vivo; however, the kinetics and extent of phosphorylation may be different. The hyperphosphorylation of cdk5 (deregulation) by p25 resulting in hyperphosphorylation of neuronal cytoskeletal proteins including tau may cause neurodegeneration. We propose that higher activity of cdk5 by its deregulation due to p25 will be destructive, as proposed by Patrick et al. (1999), while the basal levels are required for neuronal survival and development. The other possibility is that the cdk–p25 complex may not be able to phosphorylate JNK3, since the binding of p25 to cdk5 has been shown to alter its cellular localization and substrate specificity (Mandelkow, 1999). Thus, association of cdk5 with p35 or p25 may mediate different responses to environmental stimulation through different signaling pathways in cell survival and cell death in the nervous system. We propose that negative regulation of the JNK3 signaling pathway is one of the mechanisms by which cdk–p35 exerts its cell survival function. Thus, this study provides a possible link between two signaling kinase cascades. It furnishes the first mechanistic evidence of how the cdk5 kinase may regulate apoptotic events and provides a novel regulatory mechanism for JNK3-dependent apoptotic signaling.
Materials and methods
Constructs
JNK3 was generated by RT–PCR from rat cerebral cortex. Wild-type cdk5, inactive mutant cdk5 (K33T) and p35 in pcDNA3 were gifts from Dr Li-Huei Tsai. pFR-Luc plasmid, pFA-CMV plasmid, pFA2-c-Jun plasmid, GST–c-Jun and JNK were obtained from Stratagene. pcDNA3, HA-tagged wild-type, mutant (T131A) and recombinant JNK3 were generated by standard cloning methods. The putative phosphorylation site in JNK3 was mutated using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. The mutation was verified by DNA sequencing.
Cell culture
Cortical neurons from E16 cdk5 wild-type and knockout mice (cdk5–/–) were prepared as described by Ma et al. (2000). In brief, embryos were dissected and minced well with scissors. The dissociated cells were collected by centrifugation and resuspended in a serum-free neurobasal (NB) medium supplemented with B27 and 0.5 mM l-glutamine. Cells (25 × 103) were plated in 35 mm plastic dishes pre-coated with laminin (10 µg/ml; Gibco) for 5 days. HEK293T cells were cultured in Dulbecco’s modified Eagles’ medium (DMEM) with 5% fetal calf serum (FCS).
Transfection and luciferase assay
For measuring the transactivation activity by luciferase assay, HEK293T cells were grown in 6-well plates and transiently transfected with 5 µg of cdk5 or mutant cdk5 (K33T) and p35 construct, along with 2.5 µg of pFA-CMV, pFR-Lu, pFA2-c-Jun and HA-JNK3 or pcDNA3 as the control vector to equalize the amount of the transfected DNA using lipofectin. pSV40-β-gal (1 µg) was co-transfected for standardization of transfection efficiency by measurement of β-galactosidase activity (TROPLX Inc.). After 36 h transfection, cells were irradiated with UV (780 J/m2). Cells were assayed for luciferase (Stratagene) and β-galactosidase activity 24 h post-UV irradiation, in a Lumat LB 9510 luminometer according to the manufacturer’s instructions.
Expression and purification of GST–cdk5, GST–p35 and recombinant wild-type or mutant JNK3 from bacteria
GST fusion proteins were purified as described by Lee et al. (1997). In brief, cdk5 and p35 were subcloned into pGEX-4T-2 vector (Pharmacia) and transformed into Escherichia coli strain DH5α. The host cells were cultured in Luria–Bertani medium with 100 µg/ml ampicillin. The expression GST fusion proteins were induced with 1 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 3 h. The cells were harvested and lysed with lysis buffer [50 mM Tris–HCl pH 7.4, 2 mM EDTA, 1 mM dithiothreitol (DTT), 0.25 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 µg/ml antipain]. The lysates were centrifuged at 18 000 g for 30 min. The supernatant was incubated with glutathione–Sepharose 4B beads (Pharmacia). The column was washed with the washing buffer [1 × phosphate-buffered saline (PBS) supplemented with 0.25 M KCl, 0.1% Tween-20, 1 mM DTT, 0.25 mM PMSF, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 µg/ml antipain]. The expressed GST fusion proteins were eluted with 5 mM reduced glutathione in 50 mM Tris–HCl pH 8.0 and 1 mM DTT. GST–cdk5 and GST–p35 were confirmed by western blot analysis. Recombinant wild-type and mutant JNK3 were expressed and purified from E.coli as described by Ilardi et al. (1999).
Phosphorylation studies in vivo and in vitro
For in vivo phosphorylation studies, HEK293T cells were transfected with cDNA of wild-type HA-JNK3 or mutant JNK3 (T131A) and wild-type cdk–p35 or mutant cdk–p35 overnight. Thirty-six hours after transfection, phosphate-free Dulbecco’s minimum essential medium supplements with 80 µCi/ml [32P]ortho-phosphoric acid were added for 3 h. Lysates were collected, HA-JNK3 was immunoprecipitated using anti-HA antibody and 32P incorporation into JNK3 was visualized after SDS–PAGE (10–20%) by autoradiography. JNK3 levels were verified by western blotting. For in vitro phosphorylation studies, we incubated histone H1 or recombinant JNK3 purified from E.coli with wild-type or kinase-inactive cdk5 IP from transfected HEK293T cells as described by Li et al. (2001). Substrates were incubated with 0.1 mM [γ-32P]ATP in a buffer containing 50 mM Tris–HCl pH 7.4 with 1 mM EGTA, 1 mM DTT, 5 mM MgCl2, 0.5 mM microcystin L R and immunoprecipitated cdk5 for 30 min at room temperature. In experiments examining the in vitro phosphorylation of wild-type and mutant JNK3, GST–cdk5 and GST–p35 were incubated with wild-type or T131A JNK3 (2 µg) using essentially the same conditions as above. Proteins were resolved by SDS–PAGE and 32P incorporation, and the amount of protein was determined by Coomassie Blue staining as above. In order to evaluate the efficiency of cdk5 in phosphorylating the peptide motif corresponding to cdk5 consensus sequences (XS/TPXK) in JNK3, the conserved peptide sequences LLNVFTPQK and LLNVFAPQK were synthesized and their phosphorylation by cdk5 was analyzed. The standard assay mixture used is described by Li et al. (2000). In vitro kinase assays were carried out essentially as described above by incubating peptides (50 µg) with GST–cdk–p35. JNK3 kinase activity was measured in the same way as the cdk5 kinase assays, except that the substrate was GST–c-Jun (1–79; Stratagene).
Western blot analysis
HEK293T cells were transiently co-transfected with cdk5 (5 µg), p35 (5 µg) and HA-JNK3 (5 µg). Cells were stimulated with UV irradiation (780 J/m2) for the time indicated. Cells were lysed in a lysis buffer (5 mM HEPES pH 7.4, 150 mM NaCl, 1% Triton X-100, 10 mM glycerol, 1 mM EDTA, 2 mM Na3VO4, 5 mM PMSF, 5 µg/ml aprotinin, leupeptin and pepstatin). Proteins were resolved by 10–20% SDS–PAGE, blotted onto a PVDF membrane (Boehringer Mannheim), blocked in 5% skimmed milk, 1 × PBS, 0.05% Tween-20 and probed with primary antibodies. Anti-cdk5 polyclonal antibody (C-8; Santa Cruz), anti-phospho-Ser63 and Ser73 c-Jun and anti-total c-Jun were obtained from New England Biolabs. Following incubation with horseradish peroxidase-conjugated goat anti-rabbit antibody (Bio-Rad), bound immunoglobulins were detected using enhanced chemiluminescence (Amersham).
Apoptosis assays
DNA fragmentation associated with apoptosis was detected by TUNEL histochemistry staining. Tissue sections, cut frozen and mounted directly on salinated slides, were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100 [20 min at room temperature and then incubated for nick end-labeling for 2 h at 37°C with TdT according to the standard procedure (Boehringer Mannheim)]. In other experiments, wild-type and cdk5-deficient neurons were treated with UV irradiation (780 J/m2) for the times indicated. Apoptotic cells were detected by viability dye staining (Schmid et al., 1994) or TUNEL assays essentially as described above.
Northern blotting and immunocytochemistry
Northern blot analysis was performed on total RNA (10 µg) from brains of cdk5–/– and wild-type mice using the TRIzol Reagent (Gibco BRL). The blots were hybridized to a radiolabeled probe (199 bp) corresponding to nucleotides 891–1089 of the murine c-Jun cDNA. A 346 bp fragment corresponding to nucleotides 2173–2518 of the murine c-Fos cDNA was used to generate radiolabeled probes for northern hybridization analysis. Standard immunofluorescence procedures were used to stain frozen tissue sections. Antibodies used included monoclonal anti-MAP-2 antibody (MAB 378; Chemicon), JNK3 polyclonal antibody (Upstate Biotechnology), Ser63- and Ser73-phosphorylated c-Jun polyclonal antibodies (New England Biolabs), GFAP monoclonal antibody (Chemicon) and a polyclonal antibody against cleaved caspase-3 subunit (New England Biolabs).
Acknowledgments
Acknowledgements
We thank Dr Philip Grant and Mrs Devee Schoenberg for critically reading this manuscript. We also thank Dr Carolyn Smith in the NINDS Light Microscopy Facility for her assistance in confocal microscopy.
References
- Ahlijanian M.K. et al. (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl Acad. Sci. USA, 97, 2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin N.D., Albers,W. and Pant,H.C. (2001) Cyclin dependent kinase (cdk5) activation requires interaction with three domains of p35. J. Neurosci. Res., in press. [DOI] [PubMed] [Google Scholar]
- Behrens A., Sibilia,M. and Wagner,E.F. (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature Genet., 21, 326–329. [DOI] [PubMed] [Google Scholar]
- Bibb J.A. et al. (1999) Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature, 402, 669–671. [DOI] [PubMed] [Google Scholar]
- Brownlees J., Yates,A., Bajaj,N.P., Davis,D., Anderton,B.H., Leigh,P.N., Shaw,C.E. and Miller,C.C. (2000) Phosphorylation of neurofilament heavy chain side-arms by stress activated protein kinase-1b/Jun N-terminal kinase-3. J. Cell Sci., 113, 401–407. [DOI] [PubMed] [Google Scholar]
- Chae T., Kwon,Y.T., Bronson,R., Dikkes,P. and Tsai,L.H. (1997) Mice lacking p35, a neuronal specific activator of cdk5, display cortical lamination defects, seizures and adult lethality. Neuron, 18, 29–42. [DOI] [PubMed] [Google Scholar]
- Chen Y.R. and Tanm,T.H. (2000) The c-Jun N-terminal kinase pathway and apoptotic signaling. Int. J. Oncol., 16, 651–662. [DOI] [PubMed] [Google Scholar]
- Clarke P.G. (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol., 181, 195–213. [DOI] [PubMed] [Google Scholar]
- Derijard B., Hibi,M., Wu,I.H., Barrett,T., Su,B., Deng,T., Karin,M. and Davis,R.J. (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell, 76, 1025–1037. [DOI] [PubMed] [Google Scholar]
- Eilers A., Whitfield,J., Babij,C., Rubin,L.L. and Ham,J. (1998) Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J. Neurosci., 18, 1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A.I., Shuang,R., Giovannucci,D.R., Zhang,L., Bittner,M.A. and Stuenkel,E.L. (1999) Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J. Biol. Chem., 274, 4027–4035. [DOI] [PubMed] [Google Scholar]
- Floyd S.R., Porro,E.B., Slepnev,V.I., Ochoa,G.C., Tsai,L.H. and De Camilli,P. (2001) Amphiphysin binds the cdk5 regulatory subunit p35 and is phosphorylated by cdk5 and cdc2. J. Biol. Chem., 276, 8104–8110. [DOI] [PubMed] [Google Scholar]
- Gupta S., Campbell,D., Derijard,B. and Davis,R.J. (1995) Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science, 267, 389–393. [DOI] [PubMed] [Google Scholar]
- Gupta S., Barrett,T., Whitmarsh,A.J., Cavanagh,J., Sluss,H.K., Derijard,B., Martin,J.H., Mohit,A.A. and Miller,C.A. (1996) Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res. Mol. Brain Res., 35, 47–57. [DOI] [PubMed] [Google Scholar]
- Ham J., Babij,C., Whitfield,J., Pfarr,C.M., Lallemand,D., Yaniv,M. and Rubin,L.L. (1995) A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron, 14, 927–939. [DOI] [PubMed] [Google Scholar]
- Heins S. and Aebi,U. (1994) Making heads and tails of intermediate filament assembly, dynamics and networks. Curr. Opin. Cell Biol., 6, 25–33. [DOI] [PubMed] [Google Scholar]
- Henderson C.E. (1996) Programmed cell death in the developing nervous system. Neuron, 17, 579–585. [DOI] [PubMed] [Google Scholar]
- Herdegen T., Claret,F.X., Kallunki,T., Martin-Villalba,A., Winter,C., Hunter,T. and Karin,M. (1998) Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J. Neurosci., 18, 5124–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Lin,A., Smeal,T., Minden,A. and Karin,M. (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev., 7, 2135–2148. [DOI] [PubMed] [Google Scholar]
- Hill C.S., Marais,R., John,S., Wynne,J., Dalton,S. and Treisman,R. (1993) Functional analysis of a growth factor-responsive transcription factor complex. Cell, 73, 395–406. [DOI] [PubMed] [Google Scholar]
- Humbert S., Dhavan,R. and Tsai,L.-H. (2000) p39 activates cdk5 in neurons and is associated with the actin cytoskeleton. J. Cell Sci., 113, 975–983. [DOI] [PubMed] [Google Scholar]
- Ilardi J.M., Mochida,S. and Sheng,Z.H. (1999) Snapin: a SNARE-associated protein implicated in synaptic transmission. Nature Neurosci., 2, 119–124. [DOI] [PubMed] [Google Scholar]
- Karin M. and Hunter,T. (1995) Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr. Biol., 5, 747–757. [DOI] [PubMed] [Google Scholar]
- Ko J., Humbert,S., Bronson,R.T., Takahashi,S., Kulkarni,A.B., Li,E. and Tsai,L.H. (2001) p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J. Neurosci., 21, 6758–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C.Y., Yang,D.D., Samanta Roy,D.R., Davism,R.J., Rakic,P. and Flavell,R.A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron, 22, 667–676. [DOI] [PubMed] [Google Scholar]
- Kusakawa G., Saito,T., Onuki,R., Ishiguro,K., Kishimoto,T. and Hisanaga,S. (2000) Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J. Biol. Chem., 275, 17166–17172. [DOI] [PubMed] [Google Scholar]
- Kwon Y.T., Tsai,L.-H. and Crandall,J.E. (1999) Callosal axon guidance defects in p35–/– mice. J. Comp. Neurol., 415, 218–229. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch,J. (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem., 271, 24313–24316. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M., Banerjee,P., Nikolakaki,E., Dai,T., Rubie,E.A., Ahmad,M.F., Avruch,J. and Woodgett,J.R. (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature, 369, 156–160. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Helbing,C.C., Choi,K.S., Johnston,R.N. and Wang,J.H. (1997) Neuronal Cdc2-like kinase (Nclk) binds and phosphorylates the retinoblastoma protein. J. Biol. Chem., 272, 5622–5626. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Kwon,Y.T., Li,M., Peng,J., Friedlander,R.M. and Tsai,L.H. (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature, 405, 360–364. [DOI] [PubMed] [Google Scholar]
- Lew J., Huang,Q.Q., Qi,Z., Winkfein,R.J., Aebersold,R., Hunt,T. and Wang,J.H. (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature, 371, 423–425. [DOI] [PubMed] [Google Scholar]
- Li B.S., Zhang,L., Gu,J., Amin,N.D. and Pant,H.C. (2000) Integrin α1β1-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J. Neurosci., 20, 6055–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.S., Ma,W., Zhang,L., Barker,J.L., Stenger,D.A. and Pant,H.C. (2001) Activation of phosphatidylinositol-3 kinase (PI-3K) and extracellular regulated kinases (Erk1/2) is involved in muscarinic receptor-mediated DNA synthesis in neural progenitor cells. J. Neurosci., 21, 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W. et al. (2000) Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur. J. Neurosci., 12, 1227–1240. [DOI] [PubMed] [Google Scholar]
- MacManus J.P. and Linnik,M.D. (1997) Gene expression induced by cerebral ischemia: an apoptotic perspective. J. Cereb. Blood Flow Metab., 17, 815–832. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. (1999) The tangled tale of tau. Nature, 402, 588–589. [DOI] [PubMed] [Google Scholar]
- Maroney A.C. et al. (1998) Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J. Neurosci., 18, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.H., Mohit,A.A. and Miller,C.A. (1996) Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res. Mol. Brain Res., 35, 47–57. [DOI] [PubMed] [Google Scholar]
- Nishina H. et al. (1997) Stress-signaling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature, 385, 350–353. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward,M., Huh,C.G., Longenecker,G., Veeranna, Pant,H.C., Brady,R.O., Martin,L.J. and Kulkarni,A.B. (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and prenatal death. Proc. Natl Acad. Sci. USA, 93, 11173–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T., Gilmore,E.C., Longenecker,G., Jacobowitz,D.M., Brady,R.O., Herrup,K. and Kulkarni,A.B. (1999) Migration defects of cdk5–/– neurons in the developing cerebellum is cell autonomous. J. Neurosci., 19, 6017–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T. et al. (2001) Synergistic contributions of cyclin-dependent kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc. Natl Acad. Sci. USA, 98, 2764–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo T.F., Henchcliffe,C., James,D. and Burke,R.E. (1999) Expression of c-fos, c-Jun and c-Jun N-terminal kinase (JNK) in a developmental model of induced apoptotic death in neurons of the substantia nigra. J. Neurochem., 72, 557–564. [DOI] [PubMed] [Google Scholar]
- Oppenheim R.W. (1991) Cell death during development of the nervous system. Annu. Rev. Neurosci., 14, 453–501. [DOI] [PubMed] [Google Scholar]
- Patrick G.N., Zukerberg,L., Nikolic,M., de la Monte,S., Dikkes,P. and Tsai,L.H. (1999) Conversion of p35 to p25 deregulate Cdk5 activity and promote neurodegeneration. Nature, 402, 615–622. [DOI] [PubMed] [Google Scholar]
- Patrick G.N., Zukerberg,L., Nikolic,M., de la Monte,S., Dikkes,P. and Tsai,L.H. (2001) Reply: neurobiology p25 protein in neurodegeneration. Nature, 411, 764–765. [DOI] [PubMed] [Google Scholar]
- Pettmann B. and Henderson,C.E. (1998) Neuronal cell death. Neuron, 20, 633–647. [DOI] [PubMed] [Google Scholar]
- Rosales J.L., Nodwell,M.J., Johnston,R.N. and Lee,K.Y. (2000) Cdk–p25(nck5a) interaction with synaptic proteins in bovine brain. J. Cell. Biochem., 78, 151–159. [DOI] [PubMed] [Google Scholar]
- Sahlgren C.M., Mikhailov,A., Hellman,J., Chou,Y.H., Lendahl,U., Goldman,R.D. and Eriksson,J.E. (2001) Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J. Biol. Chem., 276, 16456–16463. [DOI] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart,C.H. and Giorgi,J.V. (1994) Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry, 15, 12–20. [DOI] [PubMed] [Google Scholar]
- Shetty K.T., Link,W.T. and Pant,H.C. (1993) Cdk2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc. Natl Acad. Sci. USA, 90, 6844–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuang R., Zhang,L., Fletcher,A., Groblewski,G.E., Pevsner,J. and Stuenkel,E.L. (1998) Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J. Biol. Chem., 273, 4957–4966 [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Roth,K.A., Sayers,R.O., Shindler,K.S., Wong,A.M., Fritz,L.C. and Tomaselli,K.J. (1998) In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ., 5, 1004–1016. [DOI] [PubMed] [Google Scholar]
- Stambolic V. et al. (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell, 95, 29–39. [DOI] [PubMed] [Google Scholar]
- Tanaka T. et al. (2001) Neuronal cyclin-dependent kinase 5 activity is critical for survival. J. Neurosci., 21, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.H., Delalle,I., Caviness,V.S.,Jr, Chae,T. and Harlow,E. (1994) p35 is a neural specific regulatory subunit of cycline-dependent kinase 5. Nature, 371, 419–423. [DOI] [PubMed] [Google Scholar]
- Veeranna Amin,N.D., Ahn,N.G., Jaffe,H., Winters,C.A., Grant,P. and Pant,H.C. (1998) Mitogen-activated protein kinases (Erk1,2) phosphorylated Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J. Neurosci., 18, 4008–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdee K., Bannister,A.J., Hunt,S.P. and Tolkovsky,A.M. (1997) Comparison between the timing of JNK activation, c-Jun phosphorylation and onset of death commitment in sympathetic neurons. J. Neurochem., 69, 550–561. [DOI] [PubMed] [Google Scholar]
- Watson A., Eilers,A., Lallemand,D., Kyriakis,J., Rubin,L.L. and Ham,J. (1998) Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons. J. Neurosci., 18, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Dickens,M., Raingeaud,J., Davis,R.J. and Greenberg,M.E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Xie X., Gu,Y., Fox,T., Coll,J.T., Fleming,M.A., Markland,W., Caron,P.R., Wilson,K.P. and Su,M.S. (1998) Crystal structure of JNK3: a kinase implicated in neuronal apoptosis. Structure, 6, 983–991. [DOI] [PubMed] [Google Scholar]
- Yabe J.T., Chylinski,T., Wang,F.S., Pimenta,A., Kattar,S.D., Linsley,M.D., Chan,W.K. and Shea,T.B. (2001) Neurofilaments consist of distinct populations that can be distinguished by C-terminal phosphorylation, bundling and axonal transport rate in growing axonal neurites. J. Neurosci., 21, 2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.D., Kuan,C.Y., Whitmarsh,A.J., Rincon,M., Zheng,T.S., Davis,R.J., Rakic,P. and Flavell,R.A. (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature, 389, 865–870. [DOI] [PubMed] [Google Scholar]
- Yoo B.C. and Lubec,G. (2001) p25 protein in neurodegeneration. Nature, 411, 763–765. [DOI] [PubMed] [Google Scholar]