Abstract
Opa adhesins of pathogenic Neisseria species target four members of the human carcinoembryonic antigen-related cellular adhesion molecule (CEACAM) family. CEACAM receptors mediate opsonization-independent phagocytosis of Neisseria gonorrhoeae by human granulocytes and each receptor individually can mediate gonococcal invasion of epithelial cells. We show here that gonococcal internalization occurs by distinct mechanisms depending on the CEACAM receptor expressed. For the invasion of epithelial cell lines via CEACAM1 and CEACAM6, a pathogen-directed reorganization of the actin cytoskeleton is not required. In marked contrast, ligation of CEACAM3 triggers a dramatic but localized reorganization of the host cell surface leading to highly efficient engulfment of bacteria in a process regulated by the small GTPases Rac1 and Cdc42, but not Rho. Two tyrosine residues of a cytoplasmic immune receptor tyrosine-based activating motif of CEACAM3 are essential for the induction of phagocytic actin structures and subsequent gonococcal internalization. The granulocyte-specific CEACAM3 receptor has properties of a single chain phagocytic receptor and may thus contribute to innate immunity by the elimination of Neisseria and other CEACAM-binding pathogens that colonize human mucosal surfaces.
Keywords: carcinoembryonic antigen-related cellular adhesion molecules/Cdc42/invasion/phagocytosis/Rac1
Introduction
Neisseria gonorrhoeae is a human-specific, Gram-negative pathogen that colonizes mucosal surfaces of the urogenital tract, but also infects the rectum, nasopharynx and the conjunctiva of the eye. For the colonization of such diverse human mucosal epithelia, gonococci rely on a combinatorial strategy that involves the phase-variable expresssion of a large panel of adhesive functions, including type IV pili with the PilC adhesin, colony opacity-associated (Opa) proteins, PorB and specific lipooligosaccharides (Dehio et al., 2000; Merz and So, 2000). Molecular mechanisms of invasion are only partly understood but appear to vary with the complement of adhesins expressed and with the host cell receptors involved in the interaction. The Opa proteins comprise a family of antigenically diverse outer membrane proteins of Neisseria that function as adhesins and invasins (Dehio et al., 1998). Up to 11 unlinked chromosomal alleles encoding distinct Opa variants (Kupsch et al., 1993) are regulated independently by phase variation, resulting in a heterogeneous population of bacteria expressing none, one or several Opa variants (Stern et al., 1986). An important role for the Opa adhesins during infection is suggested by the observation that mostly Opa+ bacteria are recovered during natural infection and following inoculation of human volunteers with Opa– bacteria (Swanson et al., 1988; Jerse et al., 1994). Some Opa proteins (e.g. Opa30 of strain MS11) bind cell surface-associated heparan sulfate proteoglycans (HSPGs), but most Opa variants characterized to date interact with the family of human carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs; for a review of Opa receptors see Dehio et al., 1998).
The CEACAM family belongs to the immunoglobulin (Ig) superfamily of adhesion molecules (Öbrink, 1997). It comprises seven members, four of which are receptors for Opa proteins: CEA (carcinoembryonic antigen; CD66e), CEACAM1 (biliary glycoprotein; BGP; CD66a), CEACAM3 (CEA gene family member 1; CGM1; CD66d) and CEACAM6 (non-specific cross-reacting antigen; NCA; CD66c). All CEACAM molecules share a conserved N-terminal Ig variable (Igv)-like domain that is followed by 0–6 Ig constant (Igc)-like domains. The Opa-binding CEACAM receptors are characterized by a particularly conserved Igv-like domain that contains the CD66 epitopes and is engaged in a protein–protein interaction by the Opa proteins (Bos et al., 1999; Popp et al., 1999; Virji et al., 1999). CEACAM1 is the Opa receptor with the widest tissue distribution. It is expressed by endothelial and epithelial cells of a wide range of human tissues and also on leukocytes, including granulocytes, activated T cells, B cells and CD16–/CD56+ natural killer cells (Grunert et al., 1998). Differential splicing of CEACAM1 generates at least eight transmembrane isoforms with different numbers of extracellular domains and either a long or a truncated cytoplasmic domain. The long cytoplasmic domain associates with Src family tyrosine kinases (Brummer et al., 1995; Skubitz et al., 1995) and with the tyrosine phosphatases SHP-1 and SHP-2 (Huber et al., 1999), and contains a functional immune receptor tyrosine-based inhibitory motif (ITIM; Chen et al., 2001b). This domain is also responsible for the growth-inhibitory effects of CEACAM1 in epithelial carcinoma cells (Fournes et al., 2001) and localizes the adhesion molecule to cell–cell contacts between epithelial cells in a way that depends on association with the actin cytoskeleton and that is subject to regulation by Rho-family GTPases (Sadekova et al., 2000). CEA, a widely used tumour marker, and CEACAM6 can be co-expressed with CEACAM1 on epithelial cells, where they engage in homophilic and heterophilic interactions with CEACAM molecules of neighbouring cells (Öbrink, 1997). CEA and CEACAM6 are inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. Granulocytes also express CEACAM1 and CEACAM6, together with CEACAM3 and CEACAM8, which are limited to this cell type. CEACAM3 has a cytoplasmic domain distinct from CEACAM1. It contains a functional immune receptor tyrosine-based activating motif (ITAM) that was shown recently to participate in gonococcal internalization when the recombinant receptor is expressed in a chicken B-cell line (Chen et al., 2001a). The specific cellular function of CEACAM3 is unknown, but all CEACAM family members of granulocytes have been implicated in cellular activation, resulting in degranulation, priming of oxidant production and increased β2 integrin-mediated adhesion (Skubitz et al., 1996; Stocks et al., 1996).
Neisseria expressing CEACAM-binding Opa variants adhere to and invade human epithelial cell lines expressing recombinant or endogenous CEACAM molecules and primary endothelial cells expressing CEACAM1 (Virji et al., 1996; Chen et al., 1997; Gray-Owen et al., 1997b; Muenzner et al., 2000). In polarized T84 epithelial monolayers, CEA, CEACAM1 and CEACAM6 are transported apically, where they mediate invasion and subsequent transcytosis of Opa+ gonococci by an intracellular route (Wang et al., 1998). CEACAM-binding Opa variants are also responsible for efficient, opsonization-independent phagocytosis of Neisseria by human granulocytes (Chen and Gotschlich, 1996; Virji et al., 1996; Gray-Owen et al., 1997b). Studying an in vitro differentiated myelomonocytic cell line expressing CEACAM1 and CEACAM6, we found that phagocytosis of gonococci expressing the CEACAM-binding Opa52 requires activation of Src-family tyrosine kinases and the small GTPase Rac (Hauck et al., 1998). Whether similar mechanisms are involved in epithelial cell invasion is not known.
In the present study, we use transfected epithelial cells for a comparative analysis of signalling through individual CEACAM receptors during neisserial invasion. This model circumvents complications arising from the ex pression of multiple, functionally diverse endogenous CEACAM family members and natural splice variants in many human tissues and cell lines. We provide evidence that ligation of CEACAM3 by gonococcal Opa proteins mediates bacterial internalization by a unique and highly efficient mechanism that is not induced by either CEACAM1 or CEACAM6. We show that upon receptor aggregation, tyrosine residues of the cytoplasmic ITAM motif of CEACAM3 are involved in triggering a localized reorganization of the host cell surface, leading to engulfment of adhering bacteria. We further identify key functions for the small GTP-binding proteins Rac1 and Cdc42 in regulating gonococcal internalization by this phagocytic mechanism and the underlying rearrangements of the cellular actin cytoskeleton. Our results suggest that the granulocyte-specific CEACAM3 receptor may have a phagocytic function in vivo. In contrast, epithelial cell invasion through CEACAM1 or CEACAM6 occurs by a less efficient mechanism that is insensitive to disruption of the F-actin cytoskeleton and does not require Rho-family GTPases.
Results
Internalization of Neisseria gonorrhoeae N313 (Opa57) by CEACAM3-expressing HeLa transfectants is a rapid process
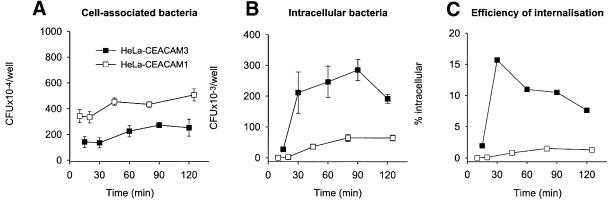
Previous studies consistently observed that Opa-mediated invasion of a HeLa cell line expressing CEACAM3 was more efficient than with other CEACAM receptors (Bos et al., 1997; Chen et al., 1997; Gray-Owen et al., 1997b), suggesting that different mechanisms of internalization may be involved. We first compared the time courses of neisserial invasion via CEACAM3 and CEACAM1. The gonococcal strain N313 expressing the recombinant CEACAM-binding Opa57 was used to infect HeLa cell lines stably transfected to express different CEACAM receptors. Bacterial adherence was determined in a plating assay and was found to be about twice as high with HeLa-CEACAM1 as compared with HeLa-CEACAM3 (Figure 1A). This difference reflects the different levels of CEACAM expression by the two cell lines, as shown by fluorescence-activated cell sorting (FACS) analysis using the cross-reactive monoclonal antibody D14HD11 (data not shown). An invasion assay, in which extacellular bacteria are killed selectively by treatment with gentamycin, showed that, in contrast to adherence, host cell invasion was increased in HeLa-CEACAM3 and occurred with markedly different kinetics as compared with HeLa-CEACAM1 (Figure 1B). In Figure 1C, we express numbers of viable intracellular bacteria as a percentage of total cell-associated bacteria to illustrate that the mechanism that mediates particularly efficient bacterial internalization via CEACAM3 may be largely limited to the first half hour after cell contact. Background adherence of N.gonorrhoeae N313 to a vector-transfected HeLa cell line was negligible and no invasion was observed (Gray-Owen et al., 1997a; data not shown).
Fig. 1. Different kinetics of internalization of N.gonorrhoeae N313 (Opa57) by HeLa cell lines expressing either CEACAM3 or CEACAM1. Cells were infected with a multiplicity of infection (m.o.i.) of 30 bacteria per cell. (A) Total cell-associated bacteria. (B) Gentamycin-resistant (i.e. intracellular, viable) bacteria. (C) Rate of internalization shown as the percentage of gentamycin-resistant bacteria calculated from data shown in (A) and (B). Data shown in (A) and (B) are mean results ± SD of triplicate samples for each time point. Data are representative of three independently performed experiments using either N313 (Opa57) or N309 (Opa52).
N313 (Opa57) triggers marked cell surface rearrangements in CEACAM3-expressing HeLa cells that engulf adherent bacteria
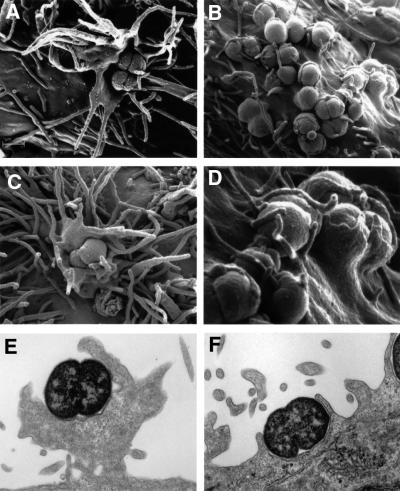
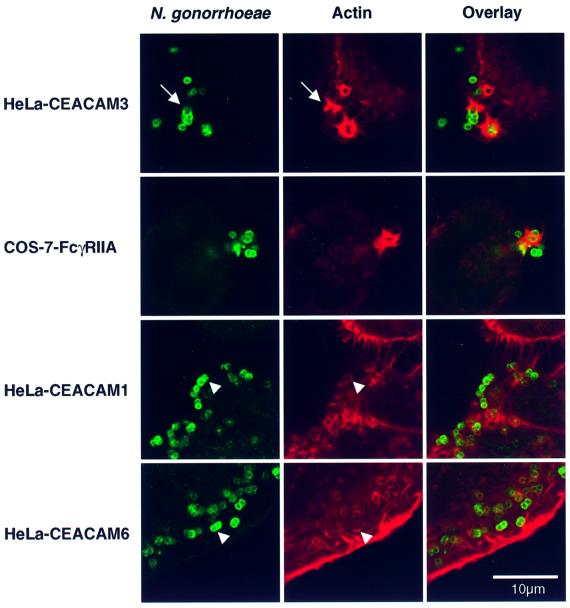
Having identified the first 30 min after infection as the critical time window for efficient uptake via CEACAM3, we were interested to study internalization at the ultrastructural level. Scanning electron microscopy (SEM) of HeLa-CEACAM3 cells infected for 30 min showed adhering diplococci in the centre of large, crown-like lamellipodial protrusions with long filopodial extensions emanating from the cell surface (Figure 2A and C). This marked reorganization of the cell surface was seen exclusively in association with individual gonococci or clusters of bacteria, suggesting that it was triggered by bacterial adherence. Transmission electron microscopy (TEM) of parallel cultures revealed corresponding structures extending up to 6 µm from the surface of the cells (Figure 2E). No such protrusions were triggered in HeLa lines expressing other CEACAM receptors. Instead, many bacteria adhering to HeLa-CEACAM1 or CEACAM6 were partially enclosed within modest pseudopods that enveloped bacteria without extending further from the cell (Figure 2B, D and F, and data not shown). TEM also confirmed that gonococci were capable of invading all HeLa cell lines in an Opa/CEACAM-dependent manner (data not shown) within 30 min after infection, albeit with different efficiencies depending on the CEACAM receptor expressed. Confocal laser scanning microscopy of phalloidin-stained infected cultures detected marked accumulations of F-actin around bacteria adhering to CEACAM3 (Figure 3) that corresponded well in shape and size to the lamellipodia-like cellular extensions seen by SEM. In many confocal sections, these F-actin-rich structures were highly reminiscent of ‘phagocytic cups’ engulfing adherent gonococci (arrow in Figure 3). These or similar structures were absent from vector-transfected control cells and from uninfected HeLa cells expressing CEACAM3 (data not shown). In contrast, infected HeLa cells expressing CEACAM1 or CEACAM6 showed only fine rings of F-actin that marked the circumference of cell-associated gonococci either partially or completely (Figure 3) and that were similar in frequency and size to the pseudopods seen by SEM.
Fig. 2. Neisseria gonorrhoeae N313 (Opa57) triggers different ultrastructural modifications of the cell surface in HeLa lines, depending on the expression of either CEACAM3 or CEACAM1. Subconfluent monolayers were infected with an m.o.i. of 100 for 30 min at 37°C. (A–D) SEMs of infected cells showing large cellular protrusions extending from the cell surface of HeLa-CEACAM3 (A and C) and tightly fitting pseudopods enclosing bacteria that adhere to HeLa-CEACAM1 (B and D). (D) shows a detail of (B). Interactions between N313 and a CEACAM6-expressing HeLa cell line were indistinguishable at the ultrastructural level from those with HeLa-CEACAM1 (not shown). (E and F) TEMs of infected HeLa-CEACAM3 (E) and HeLa-CEACAM1 (F). Images are representative of three independent experiments.
Fig. 3. Confocal sections showing adherent gonococci and the distinct F-actin structures they trigger in different epithelial cell lines. HeLa lines expressing recombinant CEACAM receptors were infected with N.gonorrhoeae N313 (Opa57), whereas transfected COS-7 cells expressing human FcγRIIa were incubated with immunoglobulin-opsonized N302 (Opa–). After 20 min, cells were fixed and processed for indirect immunofluorescence staining of gonococci (green). F-actin was visualized by staining with TRITC–phalloidin (red). F-actin structures induced by the interaction of Opa-expressing bacteria with HeLa-CEACAM3 were highly reminiscent of the phagocytic actin structures triggered by the FcγRIIa receptor. This similarity extended to the formation of distinct phagocytic cups (arrow). In contrast, most Opa-expressing gonococci adhering to CEACAM1- and CEACAM6-expressing cells were only associated with fine rings of F-actin (arrowheads). Images are representative of three or more independent experiments.
In time course experiments, the association of adherent gonococci with phagocytic actin structures in the HeLa-CEACAM3 cell line correlated well with gonococcal internalization as measured by the gentamycin protection assay. They were noticeable after 10 min of infection, became most prominent between 20 and 30 min, when internalization of bacteria was most efficient (Figure 1C), and had disappeared completely by 60 min of infection (data not shown). In marked contrast, in HeLa lines expressing CEACAM1 or CEACAM6, adherent bacteria remained associated with fine rings of F-actin throughout the 6 h study period and no other actin rearrangements were observed even after prolonged infection.
We next determined the role of the actin cytoskeleton in mediating internalization via the different CEACAM receptors. A specific inhibitor of F-actin polymerization, cytochalasin D, blocked internalization of N313 Opa57 by the HeLa-CEACAM3 cell line, with half-maximal inhibition by <0.1 µM (Figure 4A). In contrast, invasion of HeLa-CEACAM1 was not inhibited at high (5 µM) concentrations of cytochalasin D (Figure 4B). Comparable results were obtained with a different F-actin-disrupting agent, latrunculin A (data not shown).
Fig. 4. Cytochalasin D differentially inhibits CEACAM3-mediated internalization. Following a 30 min pre-incubation period with either cytochalasin D or carrier, HeLa cell lines were infected with N313 (Opa57). After 30 min, total cell-associated bacteria and gentamycin-protected bacteria were quantitated by dilution plating. Black bars show efficient inhibition of gonococcal internalization by HeLa-CEACAM3 (A; black bars) but not by HeLa-CEACAM1 (B), whereas gonococcal adherence (grey bars) to either cell line was not affected by the inhibitor.
Phagocytosis of Neisseria via CEACAM3 resembles Fc receptor-mediated phagocytosis and requires tyrosine residues of the ITAM motif in the cytoplasmic receptor domain
The ability of CEACAM3 to trigger phagocytic actin rearrangements prompted us to compare the mechanism by which it mediates neisserial internalization to the human single chain phagocytic Fc receptor, FcγRIIa. Recombinant expression of human FcγRIIa in COS epithelial cells was found to render these phagocytic, and the COS model has since been used to study different aspects of phagocytic receptor signalling (Indik et al., 1995; Downey et al., 1999). In preliminary experiments, phagocytosis of gonococci by transfected COS-7 cells expressing recombinant FcγRIIa was found to require opsonization of bacteria by specific IgG but no Opa expression. Significantly, phagocytic F-actin structures triggered by the interaction between IgG-opsonized N.gonorrhoeae N302 (Opa–) with COS-7 cells expressing human FcγRIIa were indistinguishable from those induced by the Opa–CEACAM3 interaction in the HeLa line or COS-7 cells expressing recombinant CEACAM3 (Figure 3 and data not shown). The phagocytic activity of FcγRIIa in the epithelial cell model and in phagocytes critically depends on cytoplasmic tyrosine residues that form part of an ITAM signalling motif (Daëron, 1997). The cytoplasmic receptor domain of CEACAM3 lacks significant amino acid sequence similarity to FcγRIIa. However, it does contain two tyrosine residues arranged in an YxxLx(7)YxxM motif that resembles an ITAM motif, except for a methionine residue instead of leucine or isoleucine in the last position. To examine the importance of this motif for the phagocytic activity of CEACAM3, we constructed receptor mutants in which either one or both tyrosine residues were replaced by phenylalanine (Figure 5A). We then produced polyclonal COS-7 cell lines expressing either CEACAM3, the tyrosine mutants of CEACAM3 or CEACAM3-1C1, a short natural splice variant of the receptor in which most of the cytoplasmic domain is replaced by an unrelated amino acid sequence and which therefore lacks the ITAM sequence entirely (Figure 5A). Similar levels of receptor expression by the different COS-7 populations were confirmed by FACS analysis (data not shown) and were reflected by similar levels of adherence of the Opa57-expressing gonococcal strain N313 (Figure 5B). Interactions between N313 and transfected COS-7 cells were Opa and CEACAM dependent since only low levels of background adherence and no internalization were observed with vector-transfected control cells and with CEACAM3 transfectants infected with the Opa-negative isogenic strain N302 (Figure 5B). In contrast, COS-7 cells expressing the different CEACAM3 constructs were all able to internalize adhering N313, but with marked differences in efficiency. The phagocytic activities of CEACAM3 receptor mutants and splice variants determined in three to five independent experiments are summarized in Table I. CEACAM3 had the highest phagocytic activity in all experiments. Single tyrosine replacements (Y230F or Y241F) each had a marked impact on phagocytic activity (Table I). The effect was even more pronounced in the Y230/241F double mutant. The most dramatic reduction of phagocytic activity was observed with the short natural splice variant CEACAM3-1C1, which failed almost completely to internalize adherent gonococci.
Fig. 5. Critical role of tyrosine residues in the cytoplasmic receptor domain of CEACAM3 for phagocytic uptake of Opa-expressing gonococci. (A) Cytoplasmic domain mutants and natural splice variants of CEACAM3. (B) Gentamycin protection assay to determine the phagocytic activity of CEACAM3 receptor mutants and splice variants. COS-7 cells expressing the receptor variants shown in (A) or vector transfected control cells were infected with N.gonorrhoeae N313 (Opa57) or N302 (Opa–) for 30 min before quantitation of cell-associated and gentamycin-protected bacteria.
Table I. Phagocytic activity of CEACAM3 splice variants and mutants expressed in COS-7 cells.
| Receptor | Phagocytic activitya | nb |
|---|---|---|
| CEACAM3 | 100.00 ± 0.00 | 5 |
| CEACAM3-Y230F | 55.90 ± 36.19 | 3 |
| CEACAM3-Y241F | 37.95 ± 15.28 | 3 |
| CEACAM3-Y230/241F | 25.41 ± 17.51 | 5 |
| CEACAM3-1C1 | 4.96 ± 2.69 | 5 |
| pCEP4 (vector) | 1.15 ± 2.09 | 5 |
aFollowing infection of cell lines with N.gonorrhoeae N313 (Opa57) for 30 min, the ratio between gentamycin-resistant (i.e. intracellular) and total cell-associated gonococci was determined and expressed as a percentage of the COS-7 cells expressing CEACAM3. Values are means ± SD.
bNumber of independent experiments.
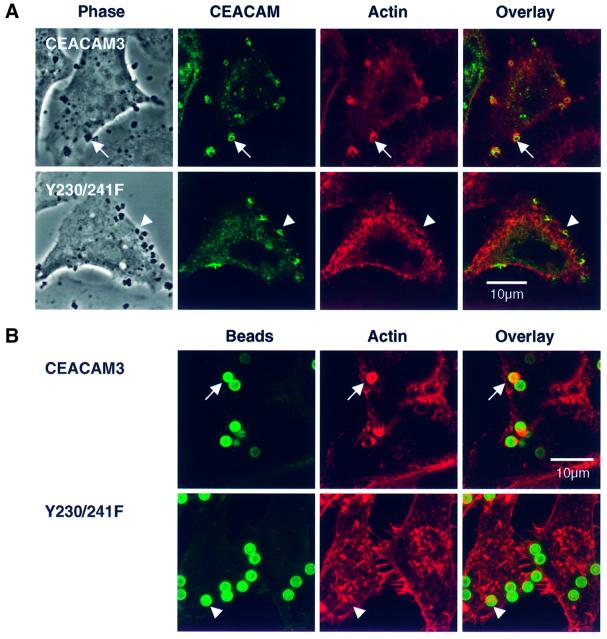
The strongly reduced ability of the Y230/241F double mutant to internalize adherent gonococci correlated with the inability to induce actin rearrangements, while receptor recruitment to the site of gonococcal adherence was preserved (Figure 6A). Receptor aggregation on its own was sufficient to trigger phagocytic actin rearrangements since magnetic beads coated with a monoclonal antibody against the N-terminal domain of CEACAM3 were capable of inducing F-actin accumulation in HeLa-CEACAM3 but not in HeLa cells expressing the Y230/241F double mutant (Figure 6B). These data show that aggregation of CEACAM3 by adherent gonococci triggers bacterial phagocytosis through actin rearrangements by a mechanism that critically depends on tyrosine residues in the cytoplasmic receptor domain of the long splice variant of CEACAM3 and that is independent of other neisserial factors.
Fig. 6. The formation of phagocytic F-actin structures by CEACAM3 is triggered by receptor aggregation and critically depends on the tyrosine residues of the cytoplasmic receptor domain. (A) Confocal sections through COS-7 cells transiently transfected to express either CEACAM3 or the double mutant Y230/241F and infected with N313 (Opa57) for 20 min. Phase contrast shows cells and adhering bacteria. Immunofluorescence labelling of CEACAM receptors (green) reveals strong aggregation of both receptors at sites of bacterial adherence. Phagocytic F-actin structures as visualized by TRITC–phalloidin (red) are present in cells expressing wild-type CEACAM3 (arrows) but absent from cells expressing the Y230/241F double mutant (arrowheads). (B) Magnetic beads coated with the D14HD11 mouse monoclonal antibody against CEACAM receptors were incubated with HeLa cell lines for 20 min at 37°C at a ratio of five beads per cell. Receptor aggregation by anti-CEACAM beads is sufficient to induce phagocytic actin rearrangements in cells expressing CEACAM3 (arrows) but not in cells producing the Y230/241F double mutant. Beads were visualized in fixed and permeabilized samples by a dichlorotriazinylamino fluorescein (DTAF)-conjugated secondary antibody against the coating mouse monoclonal antibody.
Phagocytic activity of CEACAM3 is mediated by the GTPases Rac1 and Cdc42
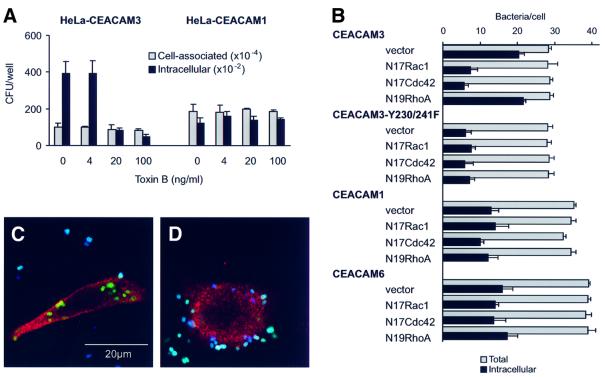
Members of the Rho family of small GTP-binding proteins are key regulators of cellular F-actin structures (Mackay and Hall, 1998). Receptor-mediated phagocytosis of opsonized particles invariably requires rearrangements of the actin cytoskeleton but, depending on the receptor involved, these may be regulated by different Rho-family GTPases. While phagocytosis of IgG-opsonized particles through FcγRIIa has been shown to require Rac and Cdc42, phagocytic uptake of complement-opsonized particles by the complement receptor 3 depends on Rho, both in mouse macrophages and in the COS epithelial cell model (Caron and Hall, 1998). We were interested to determine which Rho-family GTPases regulate CEACAM3-mediated internalization of Opa-expressing Neisseria. First we examined the sensitivity of neisserial internalization to toxin B from Clostridium difficile, which inhibits all members of the Rho subfamily of small GTPases by glucosylation (Lerm et al., 2000). Pre-incubation of HeLa-CEACAM3 with different concentrations of toxin B inhibited internalization of N.gonorrhoeae N313 (Opa57) in a dose-dependent manner (Figure 7A). Inhibition correlated with rounding of the cells that is indicative of effective inactivation of Rho-family GTPases. In contrast, invasion of the CEACAM1-expressing HeLa line was not affected by toxin B over the same range of concentrations. Next we examined the effect of the C3 exoenzyme of Clostridium botulinum, which specifically inactivates Rho by ADP-ribosylation (Lerm et al., 2000). Pre-incubation for 24 h with up to 40 µg/ml toxin did not inhibit bacterial entry into either cell line, suggesting that Rho activity was not required for internalization via CEACAM3 or CEACAM1 (data not shown).
Fig. 7. Different roles for Rho-family GTPases in neisserial internalization via different CEACAM receptors. (A) Gentamycin protection assay of HeLa cell lines infected with N.gonorrhoeae N313 (Opa57) for 30 min at 37°C showing differential inhibition of internalization via CEACAM3 by toxin B. (B) Effects of dominant-interfering GTPase constructs on neisserial internalization by COS-7 cells expressing different CEACAM receptors. Intracellular and extracellular bacteria were determined microscopically for 20 cells in each sample. The bars show the mean percentages of intracellular bacteria and standard deviations of three replicate samples from a single experiment. (C and D) Illustration of the microscopic readout of co-transfection experiments. Confocal sections of COS-7 cells are shown that were first co-transfected with constructs encoding CEACAM3 and either dominant-interfering N19RhoA (C) or N17Cdc42 (D) and then infected with FITC-labelled N313 (Opa57) before fixation and processing for immunofluorescence microscopy. Intracellular bacteria appear green because in non-permeabilized cells they are not accessible to a specific rabbit immune serum and Cy5-conjugated secondary antibody used to label extracellular bacteria, which appear white or blue. Expression of the dominant-interfering GTPase constructs is shown by immunofluorescence labelling against the myc epitope (red) after permeabilization of cells.
To determine the particular Rho-family GTPases involved, we co-expressed individual CEACAM receptors with dominant-interfering forms of Rac (N17Rac1), Cdc42 (N17Cdc42) and Rho (N19RhoA) in COS-7 cells. Cells were allowed to express for 48 h before infec tion with fluorescein isothiocyanate (FITC)-labelled N.gonorrhoeae N313 (Opa57). After a 30 min infec tion period, cells were washed, fixed and processed for immunostaining of extracellular gonococci and, after permeabilization of cells, epitope-tagged GTPases (Figure 7C and D). Quantitation of intracellular and extracellular bacteria by fluorescence microscopy confirmed the highly efficient internalization of N313 (Opa57) via CEACAM3 resulting in 70% of cell-associated bacteria in an intracellular location within 30 min of infection (Figure 7B). Internalization was strongly reduced in cells co-transfected with dominant-interfering constructs N17Rac1 and N17Cdc42, but not N19Rho. These results were consistent with the observed inhibition by toxin B but not C3 toxin and suggest that the phagocytic activity of CEACAM3 in COS-7 cells is mediated by Cdc42 and Rac1 but is independent of Rho activity. Invasion of CEACAM1- and CEACAM6-expressing cells was insensitive to dominant-interfering GTPase constructs, as was the residual phagocytic activity of the tyrosine double mutant Y230/241F of CEACAM3. This fits well with the inability of these receptors to trigger F-actin accumulation upon aggregation by adhering gonococci and suggests that tyrosine residues Y230 and Y241 within the putative ITAM motif of CEACAM3 are responsible for the efficient internalization of Opa-expressing Neisseria by regulating the induction of phagocytic actin rearrangements through Rac and Cdc42.
Discussion
In initial time course experiments with a CEACAM3-expressing HeLa cell line, we identified a narrow time window between 10 and 30 min after infection, during which most efficient gonococcal internalization correlated with the transient appearance of prominent, F-actin-rich cell surface protrusions that engulfed adherent gonococci. Cytochalasin D and C.difficile toxin B abrogated the formation of phagocytic actin structures on the cell surface and gonococcal internalization by CEACAM3-expressing cells, suggesting that the observed reorganization of the cellular actin cytoskeleton is required for uptake of adherent bacteria via CEACAM3 and that small GTPases of the Rho family have a critical regulatory function. Co-expression of CEACAM3 with dominant-interfering constructs known to inhibit individual Rho GTPases specifically identified essential roles for Rac1 and Cdc42 (but not Rho) in bacterial uptake via CEACAM3. Rho-family GTPases have various cellular functions, including the regulation of actin organization during cell motility, adherence and phagocytosis, but also gene expression and membrane trafficking (van Aelst and D’Souza-Schorey, 1997). They are also key regulators of epithelial cell invasion by Gram-negative pathogens such as Salmonella typhimurium and Shigella flexneri. In these species, invasion is accompanied by extensive membrane ruffling and requires different Rho GTPases including Rac and Cdc42, which are regulated directly through bacterial factors injected into the host cell by a type III secretion system (Galan and Zhou, 2000; Tran Van Nhieu et al., 2000). In contrast, we find that CEACAM3-mediated internalization of Neisseria, which lack a type III secretion system, is directed entirely by the cytoplasmic receptor domain. Ligation of CEACAM3 by antibody-coated beads was sufficient to trigger actin polymerization at the site of cell contact, suggesting that no bacterial factors other than the Opa adhesin are required. Our studies show that the two tyrosine residues of the cytoplasmic domain of CEACAM3, Y230 and Y241, are essential for induction of phagocytic actin structures and subsequent gonococcal internalization.
In co-transfection experiments, dominant-interfering Rac1 and Cdc42 constructs strongly reduced CEACAM3-mediated internalization, but only to the level observed with the Y230/241F double mutant. Consistent with these results, the residual phagocytic activity of the double mutant was insensitive to further inhibition by dominant-interfering Rho-family GTPases. Our data support a model in which cross-linking of CEACAM3 initiates a sequence of events in which phosphorylation of cytoplasmic tyrosine residues Y230 and Y241 precedes either the local activation of Rac1 and Cdc42 or the recruitment of active GTPases, which then regulate the formation of transient cell surface protrusions that engulf adhering bacteria (Figure 8). This model is supported by recent findings of Chen et al. (2001a) who expressed CEACAM3 in DT-40 chicken B-cell lines and observed that cross-linking of the receptor resulted in an intracellular Ca2+ transient, followed by gonococcal internalization by a mechanism that required the cytoplasmic tyrosine residues of CEACAM3, Syk tyrosine kinase and phospholipase C. Syk kinase contains two SH2 domains that can interact specifically with ITAM motifs phosphorylated on both tyrosine residues (Futterer et al., 1998). Its involvement downstream of CEACAM3 activation is a strong indication that the cytoplasmic YxxLx(7)YxxM sequence of CEACAM3 indeed forms a functional ITAM, despite its deviation from the YxxI/ Lx(7/8)YxxI/L consensus (Reth, 1989; Cambier, 1995) in the last position.
Fig. 8. Model for CEACAM3 signalling leading to internalization of Neisseria. Following cell contact, CEACAM3-binding Opa variants recruit receptor molecules to the site of adherence. The four CEACAM3-binding Opa variants of the N.gonorrhoeae strain MS11 are shown as an example. Receptor ligation results in phosphorylation of tyrosine residues in the cytoplasmic ITAM motif of the receptor through kinases of the Src family. In granulocytes, Syk kinase may have a key function through its ability to interact specifically with phosphorylated ITAM motifs. However, in epithelial cells that lack Syk, this kinase is not essential. Downstream of receptor activation, the small GTPases Rac and Cdc42 regulate phagocytosis by initiating F-actin assembly, presumably through their downstream effectors such as WASP and the Arp2/3 complex.
ITAM motifs have been identified as key activating signalling motifs in a growing number of immunoreceptors, including signalling subunits of the T- and B-cell receptor complexes and members of the Fc receptor family (Cambier, 1995). COS epithelial cells have been used extensively as a model to study ITAM-containing receptors (Indik et al., 1995; Downey et al., 1999). Expression of the human single chain Fc receptor, FcγRIIa, renders these cells phagocytic. When we challenged COS-7 cells expressing FcγRIIa with IgG-opsonized Opa-negative gonococci, we observed local rearrangements of the F-actin cytoskeleton that were indistinguishable in kinetics and appearance from those triggered by ligation of CEACAM3 by Opa57-expressing gonococci. Consitent with our data on CEACAM3, phagocytosis through FcγRIIa requires the tyrosine residues of the cytoplasmic ITAM motif, Rac and Cdc42, but not Rho, both in macrophages and in the COS epithelial cell model (Indik et al., 1995; Cox et al., 1997; Daëron, 1997; Caron and Hall, 1998; Massol et al., 1998). In epithelial cells, ITAM signalling of CEACAM3 and FcγRIIa works despite the lack of Syk kinase expression in these cells although, at least in the case of FcγRIIa, phagocytosis is enhanced when Syk is co-expressed with the receptor (Indik et al., 1995). Tyrosine residues of the ITAM motif in FcγRIIa are substrates for tyrosine kinases of the Src family, which are believed to initiate ITAM signalling (May and Machesky, 2001), and similar mechanisms may mediate ITAM signalling of CEACAM3.
CEACAM3 thus exhibits key properties of a single chain phagocytic receptor: a functional ITAM motif that signals via Syk kinase and phospholipase C (Chen et al., 2001a) and that triggers typical phagocytic F-actin rearrangements via the same Rho-family GTPases activated by FcγRIIa (this study). Together with its expression exclusively in human granulocytes, these considerations suggest that CEACAM3 may also function as a phagocytic receptor in vivo. When considering possible natural ligands, it is interesting to note that adhesins targeting the CEACAM family have evolved independently in many bacterial species that colonize human mucosal epithelia, including pathogenic and commensal Neisseria species (Toleman et al., 2001), Haemophilus influenzae (Hill et al., 2001), uropathogenic Escherichia coli (Guignot et al., 2000) and Salmonella typhi (Leusch et al., 1991). Targeting of the CEACAM family by such diverse and sometimes highly human-specific pathogens may have contributed to the recent expansion and diversification of the CEACAM family during human evolution (Zhou et al., 2001). Whether CEACAM3 plays a role as an innate immune receptor controlling any of these human-specific pathogens remains to be determined. In addition to phagocytosis, ITAM signalling by CEACAM3 may affect phagosome maturation, the profile of cytokines produced or neutrophil apoptosis.
A strong inflammatory response and infiltration of neutrophils are hallmarks of gonorrhoea. In the purulent exudate produced during gonococcal infections, bacteria frequently are found intracellularly in neutrophils (Ward et al., 1972). However, it currently is not clear whether in vivo phagocytosis of gonococci by neutrophils contributes to pathogen control or whether intracellular survival of gonococci promotes dissemination and transmission. The roles of CEACAM receptors in gonococcal interactions with granulocytes are likely to be complex. Only a subset of Opa variants recognize CEACAM3 (Gray-Owen et al., 1997a) and those that do not are still subject to Opa-dependent, opsonin-independent phagocytosis (Kupsch et al., 1993), e.g. by targeting CEACAM1 and CEACAM6, which are also expressed on granulocytes. These receptors lack ITAM motifs and thus are likely to induce signalling events different from CEACAM3. Consistent with this notion, we have observed previously that ligation of CEACAM1 and CEACAM6 in the JOSK-M myelomonocytic cell line by Opa52-expressing gonococci activates the Src family kinases Hck and Fgr and the small GTPase Rac, but fails to activate Syk kinase (Hauck et al., 1998). These results highlight the ability of CEACAM1 and/or CEACAM6 to produce stimulatory signals that may share elements of CEACAM3 signalling. On the other hand, inhibitory functions of CEACAM1 are also likely to exist. A functional ITIM motif was demonstrated recently in the cytoplasmic domain of this receptor (Chen et al., 2001b). Inhibitory signalling through CEACAM1 may thus counteract ITAM signalling by CEACAM3 or other ITAM-containing receptors, thereby influencing the intracellular fate of the gonococci or modulating the immune response.
CEACAM1 and CEACAM6 are also naturally expressed on epithelial cells. Invasion of COS-7 and HeLa transfectants via these receptors was inhibited neither by F-actin-disrupting agents nor by co-expression of dominant-interfering Rho-family GTPases. Nevertheless, SEM showed bacteria adhering to CEACAM1 and CEACAM6 enclosed to varying degrees in tightly fitting sheet-like structures that emerged from the cell surface. Confocal sections showed adhering bacteria associated with fine rings of F-actin that correspond well to such pseudopods. Similar pseudopods were described previously in the COS-7 model as the end point of incomplete phagocytosis via FcγRIa, when ITAM signalling through the γ chain was lacking (Lowry et al., 1998). Interestingly, pseudopod extension around the target particles was not inhibited by cytochalasin D. Presumably, actin-independent movements of pseudopods around bacteria could be driven by a zipper-like sequential engagement of the abundantly expressed Opa proteins by their membrane-anchored cellular receptors. We currently do not know whether such a model is sufficient to explain gonococcal invasion of epithelial cell lines via CEACAM1 and CEACAM6, and possibly the residual ITAM-independent activity of the Y230/241F mutant of CEACAM3, or whether other biochemical mechanisms are involved.
The ability of GPI-anchored receptors, CEACAM6 and CEA, to mediate invasion of HeLa and COS-7 cell lines indicates that the cytoplasmic receptor domain of CEACAM1 may not be essential. This is confirmed by our unpublished data showing that natural splice variants and mutants of CEACAM1 lacking the long cytoplasmic domain are unaffected in their ability to mediate gonococcal invasion of epithelial cells. Some of the biological properties attributed to the cytoplasmic domain of CEACAM1, including its Rho-dependent interaction with the actin cytoskeleton (Sadekova et al., 2000), may thus not be related directly to epithelial cell invasion. On the other hand, our previous findings that myelomonocytic JOSK-M cells expressing CEACAM1 and CEACAM6 (but not CEACAM3) internalize Opa-expressing gonococci in an actin-dependent phagocytic process that requires Rac (Hauck et al., 1998) suggest that fundamental differences in CEACAM receptor functions may exist between cell types.
Gonococci can invade epithelial cells by various mechanisms that depend on the cellular receptors targeted by bacterial adhesins. In contrast to the CEACAM1- and CEACAM6-mediated process described here, other routes of invasion are sensitive to cytochalasin D (Grassmé et al., 1996; Song et al., 2000). Whether different epithelial invasion mechanisms entail different consequences for the intracellular bacteria is an interesting question, especially in view of the fact that CEACAM-binding Opa variants mediate particularly efficient bacterial transcytosis across monolayers of polarized epithelial cells (Wang et al., 1998), a process that is consistent with the ability of gonococci to penetrate deeply into target tissues as observed in more complex organ culture models (McGee et al., 1983).
Our comparative analysis of CEACAM receptor signalling shows that the cytoplasmic domain of CEACAM3 is sufficient to transform bacterial internalization from a cytochalasin-insensitive epithelial invasion process into an efficient phagocytic event, which, if present in granulocytes that normally express CEACAM3, may represent an innate immune mechanism contributing to pathogen control. The ongoing evolution of the highly dynamic repertoire of Opa proteins may thus be constrained by a selective advantage for variants that avoid binding to CEACAM3 while maintaining binding to the highly similar N-terminal domain of other CEACAM receptors. The reported failure of some CEACAM-binding Opa proteins of N.gonorrhoeae MS11 and all CEACAM-binding Opa variants of three strains of meningococci to recognize CEACAM3 (Gray-Owen et al., 1997b; Muenzner et al., 2000) is consistent with this notion.
Materials and methods
Cell culture and cell lines
HeLa cell lines expressing recombinant CEACAM receptors were obtained from Fritz Grunert (University of Freiburg, Germany) and were described previously (Nagel et al., 1993; Gray-Owen et al., 1997b). cDNAs used to create HeLa-CEACAM1 and HeLa-CEACAM3 encode the longest splice variants of the respective CEACAM family members (i.e. CEACAM1-4L and CEACAM3-1L). COS-7 green monkey kidney cells were obtained from the American Type Culture Collection (ATCC number CRL-1651). All cell lines were cultured in RPMI-1640 with l-glutamine (Life Technologies) and 10% fetal calf serum. Cell lines generated with the pRc/CMV or pCEP4 expression vectors (Invitrogen) were cultured in the presence of 500 µg/ml G418 (Calbiochem) or 200 µg/ml hygromycin B (Life Technologies), respectively. Polyclonal transfectants expressing recombinant CEACAM3 receptor constructs were generated by lipofection using LipofectAMINE 2000™ (Invitrogen) according to the manufacturer’s instructions. Transfected populations were selected using 200 µg/ml hygromycin B and receptor-expressing cells were enriched further using goat anti-mouse-conjugated Dynabeads® M-450 (Dynal) and the D14HD11 monoclonal antibody.
Bacterial strains
Strains N309, N313 and N302 carry conjugative pTH6a plasmids for the constitutive expression of Opa52, Opa57 or no Opa protein, respectively (Kupsch et al., 1993). Recombinant Opa52 and Opa57 as expressed by these strains bind to CEACAM1, CEACAM3, CEA and CEACAM6 but not to HSPGs (Gray-Owen et al., 1997b). All strains were derived from N297, a non-piliated, S-pilin-expressing variant of strain MS11-B1, in which the opaC locus is disrupted by a cat gene. These strains therefore cannot give rise to phase variants expressing the heparan sulfate-binding invasin Opa30. Strains were grown on GC agar (Life Technologies) supplemented with vitamins and appropriate antibiotics at 37°C and 5% CO2 and subcultured daily.
Antibodies and general immunocytochemistry procedures
The rabbit antiserum Ak213 was raised against gonococcal lysate. The D14HD11 mouse monoclonal antibody, which recognizes CEACAM1, CEACAM3, CEACAM6 and CEA, was obtained from Genovac (Freiburg, Germany). The chicken anti-c-myc antibody was purchased from Chemicon. Mouse IgG1 isotype control antibody MOPC-21 was purchased from Santa Cruz. All secondary antibodies were purchased from Jackson Immunoresearch (Baltimore, MD). Infected COS-7 or HeLa cells were fixed with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature. After permeabilization with 0.1% Triton X-100 in PBS, 1% bovine serum albumin (BSA), for 20 min at room temperature, coverslips were incubated with the first antibodies for 1 h before incubation with the second antibody. To label F-actin, tetramethylrhodamine isothiocyanate (TRITC)–phalloidin (Sigma) was included with the second antibody. After washing, specimens were mounted in Mowiol (Hoechst) and analysed by confocal laser scanning microscopy using a Leica TCS NT.
Plasmids and generation of CEACAM3 mutants
Epitope-tagged, dominant-interfering Rho-family GTPases were expressed from the pRK5 eukaryotic expression vector (Caron and Hall, 1998). A cDNA encoding the human IgG receptor FcγRIIa was PCR amplified from a pRK5-derived plasmid (Caron and Hall, 1998), recloned into the BamHI and HindIII restriction sites of the pCEP4 expression vector (Invitrogen) and confirmed by sequencing. The pRc/CMV-based constructs containing cDNAs for CEACAM6, CEACAM1, CEACAM3 and CEACAM3-1C1 were described previously (Nagel et al., 1993). Cytoplasmic domain mutants of CEACAM3 were generated by PCR-SOEing (Horton et al., 1990) using pRc/CMV-CEACAM3 as a tem plate for two PCRs. In reaction 1, the vector primer T7-5′ (AATACGACTCACTATAGGG) was combined with the mutagenesis primers Y230F-3′ (AGCAATTCCTCAAAGATGGAAGC) or Y241F-3′ (CCATCCGGCAGAAAATGTTTGTG). In reaction 2, the mutagenesis primers Y230F-5′ (GCTTCCATCTTTGAGGAATTGCT) or Y241F-5′ (CACAAACATTTTCTGCCGGATGG) were combined with the vector primer SP6-3′ (GAGCTCTAGCATTTAGGTG). PCR products were purified from agarose gels and the appropriate fragments from reactions 1 and 2 were combined in an overlap extension reaction to which T7-5′ to SP6-3′ primers were added after the fifth amplification cycle. The final PCR products were ligated into the NotI and XbaI sites of the pRc/CMV vector. The Y230/241F double mutant was then made by an analogous strategy using CEACAM3-Y230F as a template and Y241F-3′ and Y241F-5′ as mutagenesis primers. Finally, all CEACAM3 constructs were PCR amplified from start to stop and recloned into the XhoI and HindIII restriction sites of the pCEP4 expression vector, removing 3′-untranslated regions from the original cDNAs that appeared to reduce expression. Sequencing of the final constructs showed that the original pRc/CMV-CEACAM3 plasmid, and all mutants derived from it, differed from previously published CEACAM3 sequences (Kuroki et al., 1991; Nagel et al., 1993) in one amino acid, Gly48→Glu, which according to current models (Popp et al., 1999; Virji et al., 1999) is located in a loop of the extracellular receptor domain that does not participate in Opa binding.
Infection of transfected cell lines
Cells were grown in 24-well cell culture plates to 70–80% confluency on the day of infection. Gonococci were suspended in PBS containing 1 mM Ca2+, 1 mM Mg2+, 10 mM glucose, at an OD550 of 0.5. Monolayers were washed twice with serum-free growth medium, pre-incubated with inhibitors or not and then inoculated with 1 × 107 bacteria corresponding to a multiplicity of infection (m.o.i.) of 50 bacteria per cell. Infected monolayers were then centrifuged for 5 min at 120 g to synchronize infections, followed by incubation in a humidified atmosphere at 37°C, 5% CO2. After different infection periods, monolayers were washed three times with 1 ml of medium and either fixed for microscopic analysis or processed further to determine cell-associated and intracellular colony-forming units. To quantitate total cell-associated bacteria, washed monolayers were lysed with 1% saponin in RPMI-1640 for 10 min. Gonococci were suspended by vigorous pipetting and colony-forming units in the lysates were determined by plating of serial dilutions. To obtain a measure for bacterial internalization, extracellular bacteria were killed selectively by incubating infected monolayers with 50 µg/ml gentamycin in growth medium for 2 h at 37°C, 5% CO2, prior to saponin lysis and plating of viable, intracellular bacteria. In experiments with F-actin-disrupting agents, cytochalasin D (Sigma), latrunculin A (Biomol) or carrier (dimethylsulfoxide; DMSO) were added 30 min before addition of bacteria and were present throughout the assay until saponin lysis. Toxin B, which was generously provided by K.Aktories (University of Freiburg, Germany), was pre-incubated with cells for 1 h prior to infection, whereas pre-treatment of monolayers with C3 toxin (generously provided by M.Aepfelbacher, Max von Pettenkofer Institut, Munich, Germany) was for 24 h. In some experiments, Dynabeads® M-280 (diameter 2.8 µm), coated with sheep anti-mouse IgG, were incubated with D14HD11 or mouse IgG1 control antibodies, washed and added to transfected HeLa cell lines at a ratio of five beads per cell.
Electron microscopy
Subconfluent monolayers of HeLa cell lines were infected 24 h after seeding onto 12 mm coverslips (SEM) or 24-well cell culture plates (TEM) essentially as described above, except that the centrifugation step was omitted and the m.o.i. increased to 100 bacteria per cell. After 30 min, cells were washed twice with pre-warmed (37°C) medium and then fixed overnight in 2.5% glutaraldehyde diluted in pre-warmed medium. For TEM, cells were post-fixed in 1% OsO4, treated with 0.1% tannic acid and contrasted with 2% uranyl acetate. After dehydration in a graded series of ethanol, cells were infiltrated with PolyBed using styrol as intermedium. Cells were concentrated by centrifugation and embedded in PolyBed. After polymerization (36 h, 65°C), 60 nm sections were cut on a Leica Ultracut R (Bensheim, Germany) ultramicrotome, contrasted with lead citrate and analysed in a LEO 906E (Oberkochen, Germany) electron microscope equipped with a Megaview II (SIS, Muenster, Germany) slow scan digital camera. For SEM, cells were post-fixed in 0.5% OsO4 and treated with 1% tannic acid, followed by a second OsO4 fixation step and dehydration in a graded series of ethanol. Following critical-point drying, specimens were coated with a 10 nm layer of gold/palladium and analysed in a LEO 1550 field emission scanning electron microscope (LEO, Oberkochen, Germany).
Co-transfection of CEACAM receptors and GTPase constructs
A total of 4 × 104 COS-7 cells were seeded onto coverslips in 24-well cell culture plates and after 24 h were co-transfected using 0.3 µg of receptor DNA, 0.1 µg of dominant-interfering GTPase construct or empty pRK5-myc vector and 0.5 µl of LipofectAMINE 2000™ reagent (Invitrogen) per well, according to the manufacturer’s instructions. Neisseria gonorrhoeae N313 were pre-labelled by adding 2 µl of a solution of the succinimidyl esters of 5- (and-6)-carboxyfluorescein (10 mg/ml in DMSO; Molecular Probes) to 1 ml of a bacterial suspension (OD550 0.5 in PBS, 1 mM Ca2+, 1 mM Mg2+, 10 mM glucose), followed by incubation for 10 min at 37°C, and washed three times by centrifugation at 1000 g for 2 min. At 24 h after transfection, cells were infected with 4 × 106 FITC-labelled bacteria for 30 min at 37°C, washed three times with RPMI1640 to remove unbound bacteria, and then fixed and processed for immunofluorescence microscopy. First, extracellular bacteria were labelled with Ak213, followed by Cy5-conjugated goat anti-rabbit. After permeabilization of cells, epitope-tagged GTPases were stained using chicken anti-c-myc antibody followed by Cy3-conjugated donkey anti-chicken. To quantify internalization, extracellular and intracellular gonococci were counted by confocal fluorescence microscopy in 20 cells randomly selected among those that expressed both the receptor (as indicated by association with gonococci) and the myc epitope of the dominant-interfering GTPase. The pre-labelling procedure that allowed easy detection of intracellular bacteria did not interfere with the specificity of the assay since it neither inhibited the Opa–CEACAM interaction nor resulted in CEACAM-independent binding to cells.
Acknowledgments
Acknowledgements
We would like to thank Klaus Aktories and Martin Aepfelbacher for their generous gifts of bacterial toxins, and Fritz Grunert for generously providing cell lines. We are grateful to Thomas Rudel for helpful discussions, and to Christof Hauck for critical reading of the manuscript. O.B. was supported by an EMBO Long Term Fellowship.
References
- Bos M.P., Grunert,F. and Belland,R.J. (1997) Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect. Immun., 65, 2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos M.P., Hogan,D. and Belland,R.J. (1999) Homologue scanning mutagenesis reveals CD66 receptor residues required for neisserial Opa protein binding. J. Exp. Med., 190, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer J., Neumaier,M., Gopfert,C. and Wagener,C. (1995) Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene, 11, 1649–1655. [PubMed] [Google Scholar]
- Cambier J.C. (1995) Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J. Immunol., 155, 3281–3285. [PubMed] [Google Scholar]
- Caron E. and Hall,A. (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science, 282, 1717–1721. [DOI] [PubMed] [Google Scholar]
- Chen T. and Gotschlich,E.C. (1996) CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl Acad. Sci. USA, 93, 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Grunert,F., Medina-Marino,A. and Gotschlich,E.C. (1997) Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med., 185, 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Bolland,S., Chen,I., Parker,J., Pantelic,M., Grunert,F. and Zimmermann,W. (2001a) The CGM1a (CEACAM3/CD66d) mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity (Opa) proteins is also a pathway to cell death. J. Biol. Chem., 276, 17413–17419. [DOI] [PubMed] [Google Scholar]
- Chen T., Zimmermann,W., Parker,J., Chen,I., Maeda,A. and Bolland,S. (2001b) Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J. Leukoc. Biol., 70, 335–340. [PubMed] [Google Scholar]
- Cox D., Chang,P., Zhang,Q., Reddy,P.G., Bokoch,G.M. and Greenberg,S. (1997) Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med., 186, 1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daëron M. (1997) Fc receptor biology. Annu. Rev. Immunol., 15, 203–234. [DOI] [PubMed] [Google Scholar]
- Dehio C., Gray-Owen,S.D. and Meyer,T.F. (1998) The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol., 6, 489–495. [DOI] [PubMed] [Google Scholar]
- Dehio C., Gray-Owen,S.D. and Meyer,T.F. (2000). Host cell invasion by pathogenic Neisseriae. In Oelschlaeger,T.A. and Hacker,J. (eds), Subcellular Biochemistry, Vol. 33. Bacterial Invasion into Eukaryotic Cells. Kluwer Academic/Plenum Publishers, New York, NY, pp. 61–96. [DOI] [PubMed]
- Downey G.P., Botelho,R.J., Butler,J.R., Moltyaner,Y., Chien,P., Schreiber,A.D. and Grinstein,S. (1999) Phagosomal maturation, acidification and inhibition of bacterial growth in nonphagocytic cells transfected with FcγRIIA receptors. J. Biol. Chem., 274, 28436–28444. [DOI] [PubMed] [Google Scholar]
- Fournes B., Sadekova,S., Turbide,C., Letourneau,S. and Beauchemin,N. (2001) The CEACAM1-L Ser503 residue is crucial for inhibition of colon cancer cell tumorigenicity. Oncogene, 20, 219–230. [DOI] [PubMed] [Google Scholar]
- Futterer K., Wong,J., Grucza,R.A., Chan,A.C. and Waksman,G. (1998) Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J. Mol. Biol., 281, 523–537. [DOI] [PubMed] [Google Scholar]
- Galan J.E. and Zhou,D. (2000) Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl Acad. Sci. USA, 97, 8754–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmé H.U.C., Ireland,R.M. and Putten,J.P.M. (1996) Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect. Immun., 64, 1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen S.D., Dehio,C., Haude,A., Grunert,F. and Meyer,T.F. (1997a) CD66 carcinoembryonic antigens mediate interactions beween Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J., 16, 3435–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen S.D., Lorenzen,D.R., Haude,A., Meyer,T.F. and Dehio,C. (1997b) Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol., 26, 971–980. [DOI] [PubMed] [Google Scholar]
- Grunert F., Kuroki,M. and Stocks,S.C. (1998) CEA family members expressed on hematopoietic cells and their possible role in cell adhesion and signaling. In Stanners,C.P. (ed.), Cell Adhesion and Communication Mediated by the CEA Family—Basic and Clinical Perspectives. Harwood Academic Publishers, Amsterdam, The Netherlands, pp. 99–120.
- Guignot J., Peiffer,I., Bernet-Camard,M.F., Lublin,D.M., Carnoy,C., Moseley,S.L. and Servin,A.L. (2000) Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun., 68, 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck C.R., Meyer,T.F., Lang,F. and Gulbins,E. (1998) CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J., 17, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D.J., Toleman,M.A., Evans,D.J., Villullas,S., van Alphen,L. and Virji,M. (2001) The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol. Microbiol., 39, 850–862. [DOI] [PubMed] [Google Scholar]
- Horton R.M., Cai,Z.L., Ho,S.N. and Pease,L.R. (1990) Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques, 8, 528–535. [PubMed] [Google Scholar]
- Huber M., Izzi,L., Grondin,P., Houde,C., Kunath,T., Veillette,A. and Beauchemin,N. (1999) The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J. Biol. Chem., 274, 335–344. [DOI] [PubMed] [Google Scholar]
- Indik Z.K., Park,J.G., Hunter,S. and Schreiber,A.D. (1995) The molecular dissection of Fcγ receptor mediated phagocytosis. Blood, 86, 4389–4399. [PubMed] [Google Scholar]
- Jerse A.E., Cohen,M.S., Drown,P.M., Whicker,L.G., Isbey,S.F., Seifert,H.S. and Cannon,J.G. (1994) Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med., 179, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsch E.-M., Knepper,B., Kuroki,T., Heuer,I. and Meyer,T.F. (1993) Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J., 12, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M., Arakawa,F., Matsuo,Y., Oikawa,S., Misumi,Y., Nakazato,H. and Matsuoka,Y. (1991) Molecular cloning of nonspecific cross-reacting antigens in human granulocytes. J. Biol. Chem., 266, 11810–11817. [PubMed] [Google Scholar]
- Lerm M., Schmidt,G. and Aktories,K. (2000) Bacterial protein toxins targeting rho GTPases. FEMS Microbiol. Lett., 188, 1–6. [DOI] [PubMed] [Google Scholar]
- Leusch H.G., Drzeniek,Z., Markos-Pusztai,Z. and Wagener,C. (1991) Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic α-glycosides of mannose. Infect. Immun., 59, 2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry M.B., Duchemin,A.M., Robinson,J.M. and Anderson,C.L. (1998) Functional separation of pseudopod extension and particle internalization during Fcγ receptor-mediated phagocytosis. J. Exp. Med., 187, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D.J. and Hall,A. (1998) Rho GTPases. J. Biol. Chem., 273, 20685–20688. [DOI] [PubMed] [Google Scholar]
- Massol P., Montcourrier,P., Guillemot,J.C. and Chavrier,P. (1998) Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J., 17, 6219–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R.C. and Machesky,L.M. (2001) Phagocytosis and the actin cytoskeleton. J. Cell Sci., 114, 1061–1077. [DOI] [PubMed] [Google Scholar]
- McGee Z.A., Stephens,D.S., Hoffmann,L.H., Schlech,W.F.,III and Horn,R.G. (1983) Mechanisms of mucosal invasion by pathogenic Neisseria. Rev. Infect. Dis., 5, S708–S714. [DOI] [PubMed] [Google Scholar]
- Merz A.J. and So,M. (2000) Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell. Dev. Biol. 16, 423–457. [DOI] [PubMed] [Google Scholar]
- Muenzner P., Dehio,C., Fujiwara,T., Achtman,M., Meyer,T.F. and Gray-Owen,S.D. (2000) Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun., 68, 3601–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Grunert,F., Kuijpers,T.W., Watt,S.M., Thompson,J. and Zimmermann,W. (1993) Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Eur. J. Biochem., 214, 27–35. [DOI] [PubMed] [Google Scholar]
- Öbrink B. (1997) CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell Biol., 9, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp A., Dehio,C., Grunert,F., Meyer,T.F. and Gray-Owen,S.D. (1999) Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell. Microbiol., 1, 169–181. [DOI] [PubMed] [Google Scholar]
- Reth M. (1989) Antigen receptor tail clue. Nature, 338, 383–384. [PubMed] [Google Scholar]
- Sadekova S., Lamarche-Vane,N., Li,X. and Beauchemin,N. (2000) The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell–cell contact through activation of Rho-like GTPases. Mol. Biol. Cell, 11, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz K.M., Campbell,K.D., Ahmed,K. and Skubitz,A.P. (1995) CD66 family members are associated with tyrosine kinase activity in human neutrophils. J. Immunol., 155, 5382–5390. [PubMed] [Google Scholar]
- Skubitz K.M., Campbell,K.D. and Skubitz,A.P. (1996) CD66a, CD66b, CD66c and CD66d each independently stimulate neutrophils. J. Leukoc. Biol., 60, 106–117. [DOI] [PubMed] [Google Scholar]
- Song W., Ma,L., Chen,R. and Stein,D.C. (2000) Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J. Exp. Med., 191, 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown,M., Nickel,P. and Meyer,T.F. (1986) Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell, 47, 61–71. [DOI] [PubMed] [Google Scholar]
- Stocks S.C., Ruchaud-Sparagano,M.H., Kerr,M.A., Grunert,F., Haslett,C. and Dransfield,I. (1996) CD66: role in the regulation of neutrophil effector function. Eur. J. Immunol., 26, 2924–2932. [DOI] [PubMed] [Google Scholar]
- Swanson J., Barrera,O., Sola,J. and Boslego,J. (1988) Expression of outer membrane protein II by gonococci in experimental gonorrhea. J. Exp. Med., 168, 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleman M., Aho,E. and Virji,M. (2001) Expression of pathogen-like Opa adhesins in commensal Neisseria: genetic and functional analysis. Cell. Microbiol., 3, 33–44. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Bourdet-Sicard,R., Dumenil,G., Blocker,A. and Sansonetti,P.J. (2000) Bacterial signals and cell responses during Shigella entry into epithelial cells. Cell. Microbiol., 2, 187–193. [DOI] [PubMed] [Google Scholar]
- van Aelst L. and D’Souza-Schorey,C. (1997) Rho GTPases and signaling networks. Genes Dev., 11, 2295–2322. [DOI] [PubMed] [Google Scholar]
- Virji M., Makepeace,K., Ferguson,D.J.P. and Watt,S.M. (1996) Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol., 22, 941–950. [DOI] [PubMed] [Google Scholar]
- Virji M., Evans,D., Hadfield,A., Grunert,F., Teixeira,A.M. and Watt,S.M. (1999) Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol., 34, 538–551. [DOI] [PubMed] [Google Scholar]
- Wang J., Gray-Owen,S.D., Knorre,A., Meyer,T.F. and Dehio,C. (1998) Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol. Microbiol., 30, 657–671. [DOI] [PubMed] [Google Scholar]
- Ward M.E., Glynn,A.A. and Watt,P.J. (1972) The fate of gonococci in polymorphonuclear leucocytes: an electron microscopic study of the natural disease. Br. J. Exp. Pathol., 53, 289–294. [PMC free article] [PubMed] [Google Scholar]
- Zhou G.Q., Zhang,Y. and Hammarstrom,S. (2001) The carcinoembryonic antigen (CEA) gene family in non-human primates. Gene, 264, 105–112. [DOI] [PubMed] [Google Scholar]